Abstract
Background
Major depressive disorder is a worldwide neuropsychiatric disorder associated with various symptoms, but current antidepressants used in clinical practice have various side effects and high failure rates. Andrographolide is the main bioactive ingredient of Andrographis paniculata and exhibits numerous pharmacological actions. This study aimed to evaluate the antidepressant-like effects of andrographolide in male C57BL/6J mice.
Methods
The antidepressant-like effects of andrographolide in mice were explored in a forced swim test, tail suspension test, and chronic unpredictable mild stress model of depression. Western blotting and immunofluorescence were further performed to assess the effects of chronic unpredictable mild stress and andrographolide on the brain-derived neurotrophic factor signalling cascade and hippocampal neurogenesis. Moreover, a pharmacological inhibitor (K252a) and a lentiviral-short hairpin RNA (LV-TrkB-shRNA) were used to clarify the antidepressant-like mechanism of andrographolide.
Results
Andrographolide exhibited antidepressant-like potential in the forced swim test and tail suspension test without influencing the locomotor activity of mice. Repeated andrographolide treatment not only produced significant antidepressant-like effects in the chronic unpredictable mild stress model but also prevented the decreasing effects of chronic unpredictable mild stress on hippocampal brain-derived neurotrophic factor signalling and neurogenesis in mice. Importantly, blockade of the hippocampal brain-derived neurotrophic factor system by K252a and TrkB-shRNA fully abolished the antidepressant-like effects of andrographolide in mice.
Conclusions
Andrographolide exerts antidepressant-like effects in mice via promoting the hippocampal brain-derived neurotrophic factor signalling cascade.
Keywords: andrographolide, brain-derived neurotrophic factor, depression, hippocampus
Significance Statement.
It is now popular and necessary to develop novel antidepressants beyond monoaminergic targets. As a natural diterpene lactone, Andro has numerous bioactivities, including hepatoprotective, anti-inflammatory, anti-tumour, antibacterial and anti-cardiovascular activities. This study is the first comprehensive study to our knowledge showing that Andro has antidepressant-like effects in mice that involve hippocampal BDNF signalling and neurogenesis, extending the knowledge of its pharmacological effects and supporting it as a novel potential antidepressant.
Introduction
Major depressive disorder is a neuropsychiatric disorder associated with loss of interest, helplessness, anhedonia, and even thoughts of suicide (Cui, 2015). According to published reports from the World Health Organization, major depressive disorder is projected to be a major reason for disability worldwide by 2030 (Yang et al., 2015). Selective serotonin reuptake inhibitors are the most commonly prescribed drugs for depression treatment but have various side effects and high failure rates (Gordon and Melvin, 2013). Therefore, more efforts should be made to explore antidepressants with increased effectiveness against depression and enhanced safety. In recent years, the development of new antidepressants from natural herbal medicine has become one of the important research hotspots (Liu et al., 2015).
Although monoamine deficiency was thought to be involved in the pathogenesis of depression, numerous recent studies have demonstrated that the brain-derived neurotrophic factor (BDNF) signalling pathway in various brain regions participates in the pathophysiology of depression (Neto et al., 2011; Yu and Chen, 2011; Adlam and Zaman, 2013; Jiang and Salton, 2013). BDNF induces the phosphorylation and activation of cyclic adenosine monophosphate response element-binding protein (CREB) by binding tyrosine receptor kinase B (TrkB) and subsequently promoting the activity of the downstream mitogen-activated protein kinase-extracellular regulated protein kinase (ERK) and phosphoinositide 3-kinase-protein kinase B (AKT) signalling pathways (Sasi et al., 2017). Clinical trials and animal studies have revealed that depression is accompanied by decreased expression of BDNF and pCREB in the hippocampus, whereas long-term treatments with antidepressant reverse these molecular changes (Blendy, 2006; Kozisek et al., 2008). Additionally, chronic stress reduces the levels of BDNF and pCREB in the medial prefrontal cortex (mPFC) while enhancing their expression in the nucleus accumbens (NAc) of rodents (Nestler and Carlezon, 2006; Shirayama and Chaki, 2006).
Andrographolide (Andro), a natural diterpene lactone, is the main active compound distributed in Andrographis paniculata. Andro has been explored for its numerous bioactivities including immunological, hepatoprotective, anti-inflammatory, anti-tumour, and anti-cardiovascular activities in humans and animals (Wen et al., 2014; Kishore et al., 2017; Li et al., 2017; Nie et al., 2017; Islam et al., 2018). Recent studies have demonstrated that Andro also has numerous pharmacological actions on the central nervous system, including anti-dementia, anti-neuroinflammation, anti-stroke, pro-neurogenic, and neuroprotective actions in rodents (Chan et al., 2010; Zhang et al., 2014; Varela-Nallar et al., 2015; Geng et al., 2018;). In 2016, Xu et al. reported that Andro treatment was able to promote the gene expression of several neurotrophic factors, including BDNF, in RSC96 Schwann cells (Xu et al., 2016). In 2015, Varela-Nallar et al., showed that Andro stimulated neurogenesis in the hippocampus of adult mice (Varela-Nallar et al., 2015). According to these reports, we speculated that Andro may have antidepressant-like actions. To test this assumption, various methods were used in this study, and the aim was to extend the knowledge of Andro’s pharmacological effects, supporting it as a novel potential antidepressant.
Methods
Animals
Adult male C57BL/6J mice (8 weeks old) were obtained from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Before use, all mice were acclimatized to their housing for 1 week under standard conditions (5 per cage, 12-hour-light/-dark cycle, lights on from 7:00 am to 7:00 pm; 23°C ± 1°C ambient temperature; 55% ± 10% relative humidity; noise <50 db; ammonia concentration <14 mg/m3; bedding replacement twice a week), with ad libitum access to food and water. The behavioral experiments were carried out during the light phase. For animal sacrifice, all mice were anaesthetized using carbon dioxide and then killed by cervical dislocation. The experimental procedures involving the care and use of animals were conducted in accordance with the ARRIVE guidelines (Kilkenny al., 2010; McGrath and Lilley, 2015) and approved by the Animal Welfare Committee of Nantong University (approval no. 20170420-001).
Materials
Andro, fluoxetine, K252a (a pharmacological inhibitor of TrkB), and 5-Bromo-2-deoxyUridine (BrdU) were purchased from Sigma (St. Louis, MO). Lentiviral (LV)-TrkB-short hairpin RNA (shRNA)-enhanced green fluorescent protein (EGFP) and LV-Control-shRNA-EGFP were provided by GeneChem Co., Ltd (Shanghai, China). The doses of Andro (10, 20, 50, and 100 mg/kg), fluoxetine (20 mg/kg), and K252a (25 μg/kg) were selected based on previous reports (Chen et al., 2014; Serrano et al., 2014; Jiang et al., 2017; Ren et al., 2017; Wang et al., 2017; Ni et al., 2018). Andro, fluoxetine, and K252a were dissolved in 0.9% sodium chloride (NaCl) containing 10% dimethyl sulfoxide and 20% Cremaphor EL (Vehicle). BrdU was dissolved in 0.9% NaCl. All compounds were i.p. injected at a concentration of 10 mL/kg. LV-TrkB-shRNA-EGFP and LV-Control-shRNA-EGFP were stereotaxically injected into the hippocampus.
Forced Swim Test (FST)
The FST is a widely used test for assessing potential antidepressant-like medications. This test was performed according to Porsolt et al. (1977) with slight modifications. Briefly, the test mice were placed individually in glass cylinders (45 cm height, 20 cm internal diameter) containing fresh water (15 cm height, 25 ± 1°C) and forced to swim for 6 minutes. The water was replaced after each trial. The immobility time was scored (for the last 4 minutes) when the mice were floating in the water without struggling with only slight movements to keep the nose above the water.
Tail Suspension Test (TST)
Similar to the FST, the TST is also widely used to screen potential antidepressants. This test was performed according to Steru et al., (1985) with slight modifications. Briefly, the test mice were individually suspended with adhesive tape 60 cm above the floor for 6 minutes (approximately 1 cm from the tip of tail). The duration of immobility was scored when the mice hung passively and were completely motionless.
Open Field Test (OFT)
This test was performed according to Covington et al. (2009) with slight modifications. Briefly, the test mice were individually placed in an open field apparatus (100 × 100 × 40 cm) containing 25 equal squares (5 × 5 cm) for 5 minutes under dim light conditions. The amounts of square crossing, grooming, and rearing performed by each mouse during the test period were scored. Before each trial, the apparatus was cleaned.
Sucrose Preference Test (SPT)
This test lasted for 4 days and was performed according to Pothion et al. (2004) with some modifications. On the first day, the mice were free to drink 2 bottles of 1% sucrose water. On the next day, 1 bottle of sucrose water was replaced by pure water. After 18 hours of fasting, the test mice were individually given 1 bottle of 1% sucrose water (pre-weighed) and 1 bottle of pure water (pre-weighed). After the mice were allowed to drink for 6 hours, the consumption by each mouse was calculated by weighing the 2 bottles. Sucrose preference = (sucrose consumption/water consumption + sucrose consumption) × 100%
Chronic Unpredictable Mild Stress (CUMS)
The CUMS model of depression was established as previously described (Ren et al., 2017; Ni et al., 2018). Briefly, mice were exposed to different stressors for 8 weeks. The stressors included light/dark cycle inversion, food/water deprivation for 24 hours, having the cage titled at 45° for 24 hours, behavior restrictions for 2 hours (using 50-mL conical plastic centrifuge tubes containing vent holes at both ends), damp bedding, rotation on a shaker for 30 minutes, and exposure to 4°C for 1 hour. Each stress stimulus was discontinuous and irregular. Administration of Andro/fluoxetine/K252a/vehicle was performed daily during the final 2 weeks (Zu et al., 2017; Sanna et al., 2018; Villas Boas et al., 2018; Wang et al., 2018). To assess the depressive-like behaviors of mice, the FST, TST, and SPT were performed.
Virus-Mediated Gene Transfer
Mice were anaesthetized with 0.5% sodium pentobarbital and fixed in stereotactic frames (Stoelting) (Ren et al., 2017; Wang et al., 2017; Ni et al., 2018). The scalp of each mouse was cut, and the skull was exposed using 75% ethanol and 1% H2O2. Five-mL microsyringes were used to deliver the LV. After a small drill hole was made on the skull of each mouse, the microsyringes were positioned at the following coordinates determined according to Paxinos and Franklin (2001): anteroposterior = −2.3 mm, mediolateral = ±1.5 mm, and dorsoventral = +1.4 mm for CA1 and 1.8 mm for the dentate gyrus (DG). The Control-shRNA/TrkB-shRNA lentiviral constructs were injected bilaterally into the hippocampus at a rate of 0.5 µL/min (2 µL/side: 1 µL for CA1, 1 µL for DG). The microsyringes were maintained in place for 4 minutes to limit reflux of the LV. The incision of each mouse was sutured, and the mice were allowed to recover for 3 days before further experiments. The production of LV-TrkB-shRNA-EGFP and LV-Control-shRNA-EGFP was described in our previous reports (Ren et al., 2017; Wang et al., 2017; Ni et al., 2018). The sequences of TrkB-shRNA and control-shRNA were 5’-GCAACCTGCGGCACATAAA-3’ and 5’-TTCTCCGAACG TGTCACGT-3’, respectively. They were diluted to 5 × 109 TU/mL before use.
Western Blotting
After mice were sacrificed, the tissues (hippocampus, mPFC, and NAc) were immediately dissected from each mouse and stored at −80°C as previously described (Jiang et al., 2017; Ren et al., 2017; Wang et al., 2017; Ni et al., 2018). Protein was extracted from the tissues using NP-40 lysis buffer. After quantification and denaturation, the protein samples were loaded and separated by 10/12% SDS/PAGE and were then transferred to nitrocellulose membranes. Membranes were incubated overnight at 4°C with primary antibodies against BDNF (1:500; Abcam), TrkB (1:1000; Abcam), p-TrkB (1:500; Abcam), ERK1 and 2 (1:500; Cell Signaling), p-ERK1 and 2 (1:500; Cell Signaling), AKT (1:500; Cell Signaling), p-AKT (1:500; Cell Signaling), CREB (1:500; Cell Signaling), p-CREB (1:500; Cell Signaling), and β-actin (1:5000; Santa Cruz, CA). After washing, membranes were incubated with IR-Dye 680-labelled secondary antibodies (1:10 000) for 2 hours at room temperature. An Odyssey CLx system (LI-COR) was used to detect the bands.
Immunofluorescence
Mice were anesthetized and perfused transcardially using 4% paraformaldehyde in 0.1 M phosphate buffer (Jiang et al., 2017; Wang et al., 2017). The brain of each mouse was dissected, postfixed, and dehydrated. Then, hippocampal slices of 25 µm were cut using a freezing microtome (Leica). For hippocampal doublecortin (DCX) staining, the sections were processed as follows: 1, incubated in 0.3% Triton X-100 for 30 minutes; 2, incubated in 3% bovine serum albumin for 30 minutes; 3, incubated in anti-DCX antibody (1:100; Cell Signaling) overnight at 4°C; 4, washed; 5, incubated in fluorescein isothiocyanate-labelled secondary antibody (1:50; Pierce) for 1 hour at room temperature; 6, washed; 7, incubated with 4’,6-diamidino-2-phenylindole (DAPI) for 10 minutes; 8, washed; and 9, cover slipped and observed.
For hippocampal neuronal nuclei (NeuN)+/BrdU+ co-labelling, mice were first injected with BrdU (4 × 75 mg/kg at 2-hour intervals per day) for 2 days, and hippocampal slices of 25 µm were cut after 28 days. The sections were processed as follows: 1, incubated in 50% formamide/2 × SSC at 65°C for 2 hours; 2, incubated in 2 N HCl at 37°C for 30 minutes; 3, incubated in 0.1 M boric acid buffer (pH 8.5) at room temperature for 10 minutes; 4, incubated in 0.3% Triton X-100 for 30 minutes; 5, incubated in 3% bovine serum albumin for 30 minutes; 6, incubated with mouse anti-BrdU (2 µg/mL, Roche) and rabbit anti-NeuN (1:500; Abcam) primary antibodies overnight at 4°C; 7, washed; 8, incubated with fluorescein isothiocyanate-conjugated anti-rabbit and rhodamine-conjugated anti-mouse secondary antibodies (1:50; Pierce) at room temperature for 1 hour; 9, washed; and 10, cover slipped and observed.
Experimental Protocols
Experiment 1
This experiment aimed to preliminarily assay the antidepressant-like potential of Andro in mice. Briefly, naive mice received a single injection of vehicle/fluoxetine (20 mg/kg)/Andro (10, 20, 50, or 100 mg/kg). After 30 minutes, the FST, TST, or OFT was performed (Farzin and Mansouri, 2006; Wang et al., 2008; Guan et al., 2014). Separate groups of mice were used for the 3 tests (n = 10).
Experiment 2
This experiment was performed to determine the antidepressant-like actions of Andro in mice using CUMS. Briefly, naive mice were subjected to 8 weeks of CUMS and received a daily injection of vehicle/fluoxetine (20 mg/kg)/Andro (20 or 50 mg/kg) during the final 2 weeks (n = 10). Then, the FST, TST, and SPT were performed successively. Furthermore, mice from each group were randomly selected for western blot (n= 5) and immunofluorescence (n = 5) assays.
Experiments 3 and 4
These 2 experiments were performed to explore the antidepressant-like mechanism of Andro in mice using K252a. In experiment 3, naive mice were first injected with K252a (25 μg/kg) and then with Andro (50 mg/kg, 30 minutes later) followed by the FST or TST (60 minutes later) (Jiang et al., 2017; Wang et al., 2017; Ni et al., 2018). Separate groups of mice were used for the 2 tests (n = 10).
In experiment 4, naive mice were subjected to 8 weeks of CUMS and received daily treatment with vehicle/K252a (25 μg/kg)/Andro (50 mg/kg)/Andro + K252a during the final 2 weeks (n = 10). Then, the FST, TST, and SPT were performed successively. Furthermore, mice from each group were randomly selected for western blot (n = 5) and immunofluorescence (n = 5) assays.
Experiments 5 and 6
These 2 experiments were performed to explore the antidepressant-like mechanism of Andro in mice using TrkB-shRNA. In experiment 5, naive mice received hippocampal infusion of LV-Control-shRNA/TrkB-shRNA and were housed for 14 days until the lentiviral expression was stable. On the 15th day, a single injection of vehicle/Andro (50 mg/kg) was administered, and after 30 minutes the FST or TST was performed. Separate groups of mice were used for the 2 tests (n = 10).
In experiment 6, naive mice infused with LV-Control-shRNA/TrkB-shRNA were housed for 14 days and were then subjected to 8 weeks of CUMS and 2 weeks of vehicle/Andro (50 mg/kg) administration. Then, the FST, TST, and SPT were performed successively. Furthermore, mice from each group were randomly selected for western blot (n = 5) and immunofluorescence (n = 5) assays.
Statistical Analysis
The results are presented as the means ± SEMs and were analyzed using SPSS 13.0 software. Multiple group comparisons were performed using 1-way ANOVA followed by the least significant difference (LSD) test. Pearson correlation coefficients (r) were determined to examine the correlation between the antidepressant-like effects of Andro and its effects on hippocampal BDNF using Graph Pad Prism version 5.0. A value of P < .05 was considered as significant.
Results
The Antidepressant-Like Effects of Andro Were Preliminarily Detected in Naive Mice via the FST, TST, and OFT
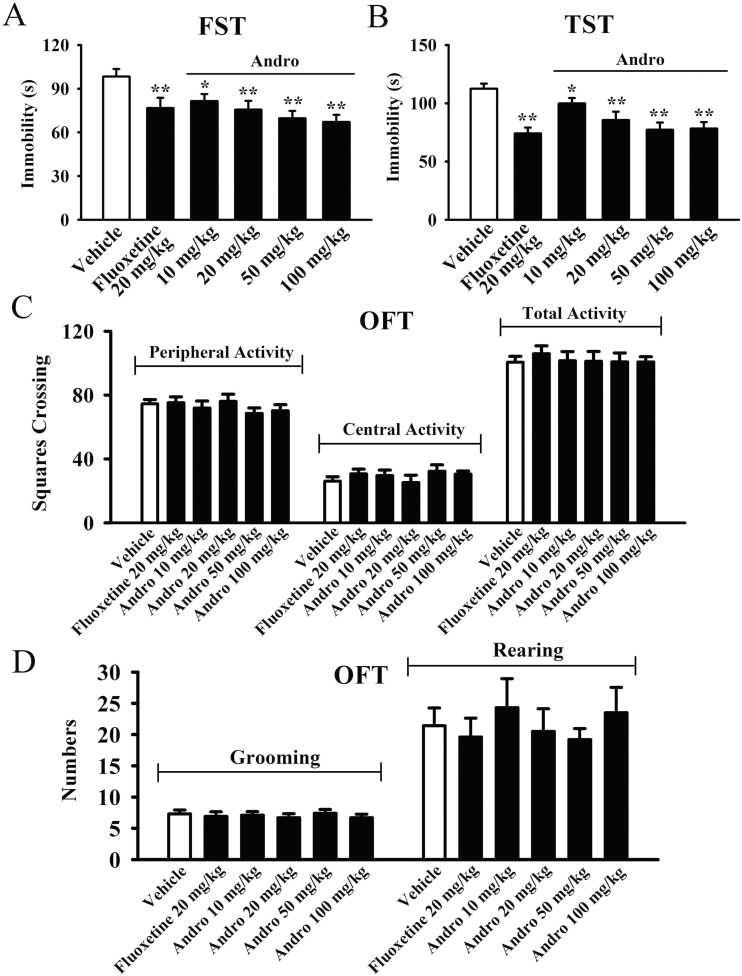
The antidepressant-like potential of Andro was first examined via the FST and TST. The FST results are shown in Figure 1A. Our data demonstrated that the immobility times of the vehicle-treated mice were significantly longer than those of the fluoxetine-treated and Andro-treated mice (n = 10, P < .01). Detailed analysis showed that compared with the vehicle, 10-, 20-, and 50-mg/kg Andro treatment induced a 17.2 ± 3.4%, 23.2 ± 2.8%, and 29.1 ± 3.5% decrease in immobility in the FST, respectively. The effects of 100 mg/kg Andro were similar to those of 50 mg/kg Andro, and 1-way ANOVA analysis revealed a significant effect of drug treatment [F(5, 54) = 23.827, P < .01]. Figure 1B also shows that administration of 10, 20, and 50 mg/kg Andro induced a 11.2 ± 2.6%, 23.9 ± 4.1%, and 31.3 ± 4.5% reduction in immobility in the TST, respectively (n = 10, P < .01). The effects of 100 mg/kg Andro were comparable with those of 50 mg/kg Andro, and 1-way ANOVA analysis also indicated a significant effect of drug treatment [F(5, 54) = 27.413, P < .01]. Therefore, 20 mg/kg and 50 mg/kg were selected as the doses of Andro for the following studies.
Figure 1.
A single andrographolide (Andro) injection produced antidepressant-like activity in naive mice, as seen in the forced swim test (FST) (A) and tail suspension test (TST) (B) without affecting their locomotor activity (C and D). All results are expressed as the means ± SEMs (n = 10); *P < .05, **P < .01 vs Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
In addition, no significant effects of Andro on the locomotor activity of mice were observed in the OFT. There was no difference among the groups in the number of squares that an animal crossed in the central area or peripheral area (n = 10; Figure 1C). One-way ANOVA analysis revealed no significant effects of drug treatments [F(5, 54) = 1.875, P = .213]. Additionally, all groups of mice displayed similar grooming [F(5, 54) = 1.834, P = .251] and rearing [F(5, 54) = 3.273, P = .106] behaviors (n = 10, Figure 1D). Collectively, these findings indicate that Andro may have antidepressant-like effects in mice.
Repeated Andro Administration Reversed the CUMS-Induced Depressive-Like Behaviors in Mice
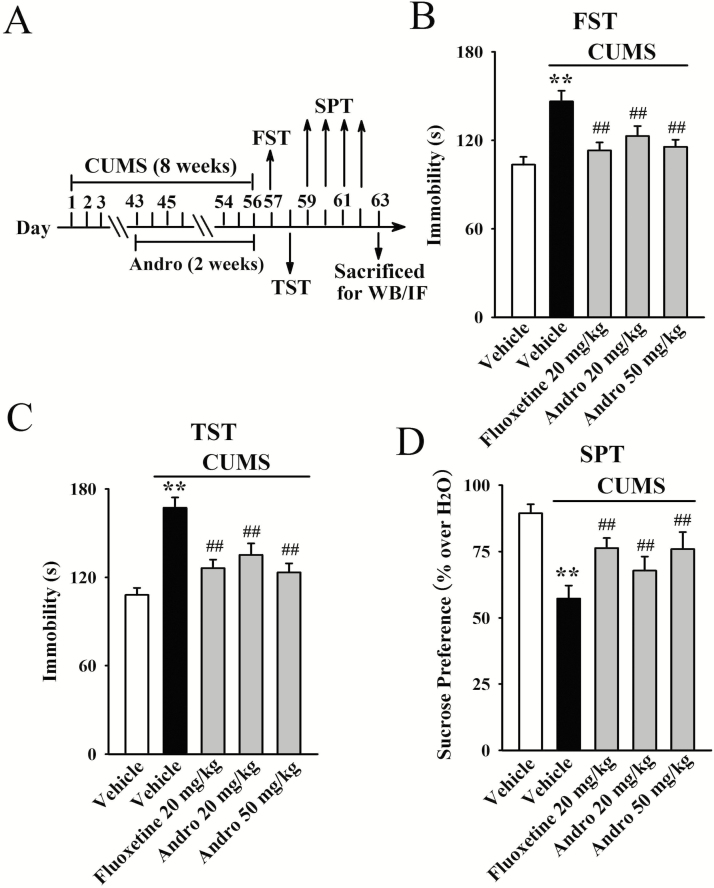
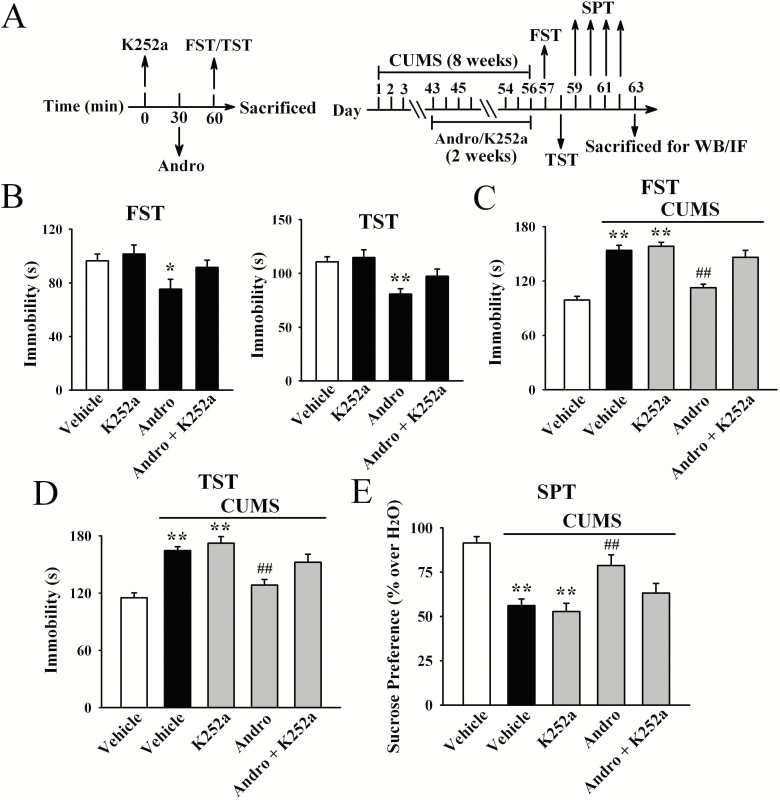
We further investigated the antidepressant-like effects of Andro using the CUMS model of depression. As shown in Figure 2B and C, the stressed mice had significantly greater immobility than the vehicle-treated control mice in the FST and TST (n = 10, P < .01), verifying the effectiveness of our model. Repeated administration of both fluoxetine and Andro fully reversed the effects of CUMS on the immobility of mice in the FST [ANOVA: F(4, 45) = 26.143, P < .01] and TST [ANOVA: F(4, 45) = 32.783, P < .01] (n = 10, P < .01). Repeated Andro treatment also decreased the immobility of naive control mice, as shown in supplementary Figure 1A (n = 10, P < .01).
Figure 2.
Repeated andrographolide (Andro) administration induced significant antidepressant-like effects in the chronic unpredictable mild stress (CUMS) model of depression, as revealed by the forced swim test (FST) (B), tail suspension test (TST) (C), and sucrose preference test (SPT) (D). A schematic of the procedural timeline is shown in A. All results are represented as the means ± SEMs (n = 10); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
Figure 2D illustrates the SPT results. CUMS induced a 35.9% ± 4.6% decrease in sucrose preference in the stressed mice compared with that in vehicle-treated mice (n = 10, P < .01), while this change was fully prevented by repeated Andro treatment (n = 10, P < .01). Specifically, the sucrose preference of the stressed mice was enhanced by 18.3% ± 2.9% and 32.5% ± 5.1% under administration of 20 and 50 mg/kg Andro, respectively. One-way ANOVA analysis revealed a significant overall effect [F(4, 45) = 22.086, P < .01]. In contrast, repeated Andro treatment barely affected the sucrose preference of naive control mice (n = 10; supplementary Figure 1A). Taken together, these findings indicate that Andro has antidepressant-like effects in mice.
Repeated Andro Treatment Reversed the CUMS-Induced Decrease in the Levels of Hippocampal BDNF Signalling Pathway Proteins
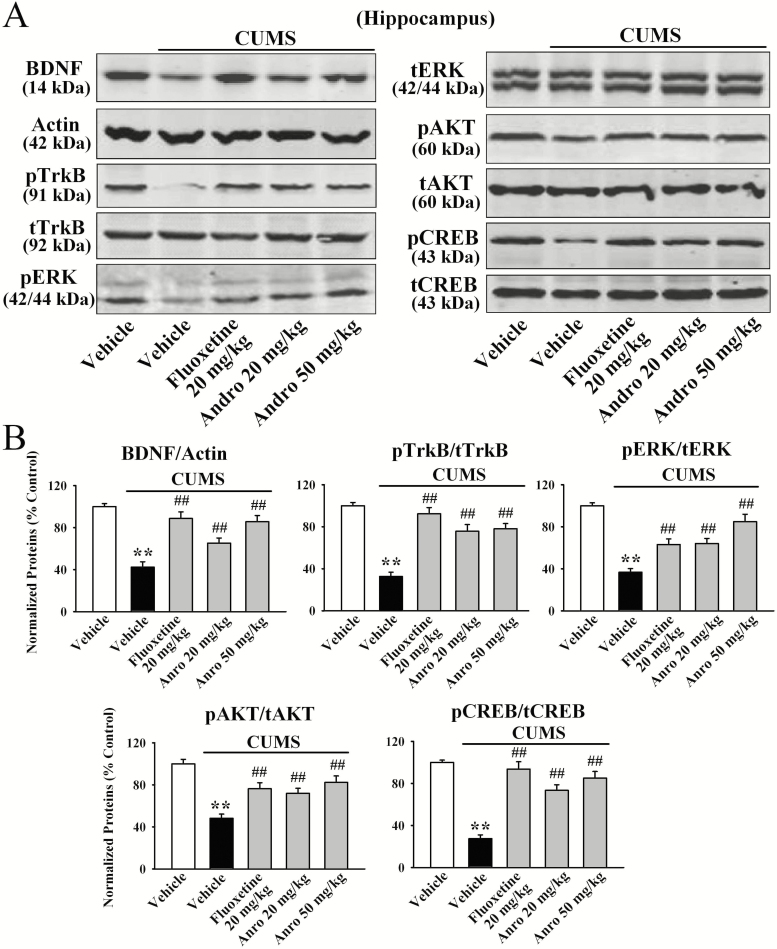
Western blotting was then performed to examine the protein level of BDNF in different brain regions following CUMS and drug treatments. Figure 3 shows that the protein level of hippocampal BDNF was significantly decreased in the mice exposed to CUMS compared with that in vehicle-treated mice (n = 5, P < .01), while treatment with 20 mg/kg and 50 mg/kg Andro enhanced it by 53.8 ± 6.8% and 101.9 ± 8.9%, respectively (n = 5, P < .01). One-way ANOVA analysis indicated a significant overall effect [F(4, 20) = 28.746, P < .01]. The data in supplementary Figure S3 further reveal that the variation in hippocampal BDNF levels was highly correlated with the variations in the FST (r = 0.7312, P = .0163; n = 10), TST (r = 0.8516, P = .0018; n = 10), and SPT (r = 0.8241, P = .0034; n = 10) scores shown in Figure 2.
Figure 3.
Repeated andrographolide (Andro) treatment fully reversed the effects of chronic unpredictable mild stress (CUMS) on the hippocampal brain-derived neurotrophic factor (BDNF) signalling cascade in mice. Representative western-blot images and the quantitative analyses are shown in A and B, respectively. All results are represented as the means ± SEMs (n = 5); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
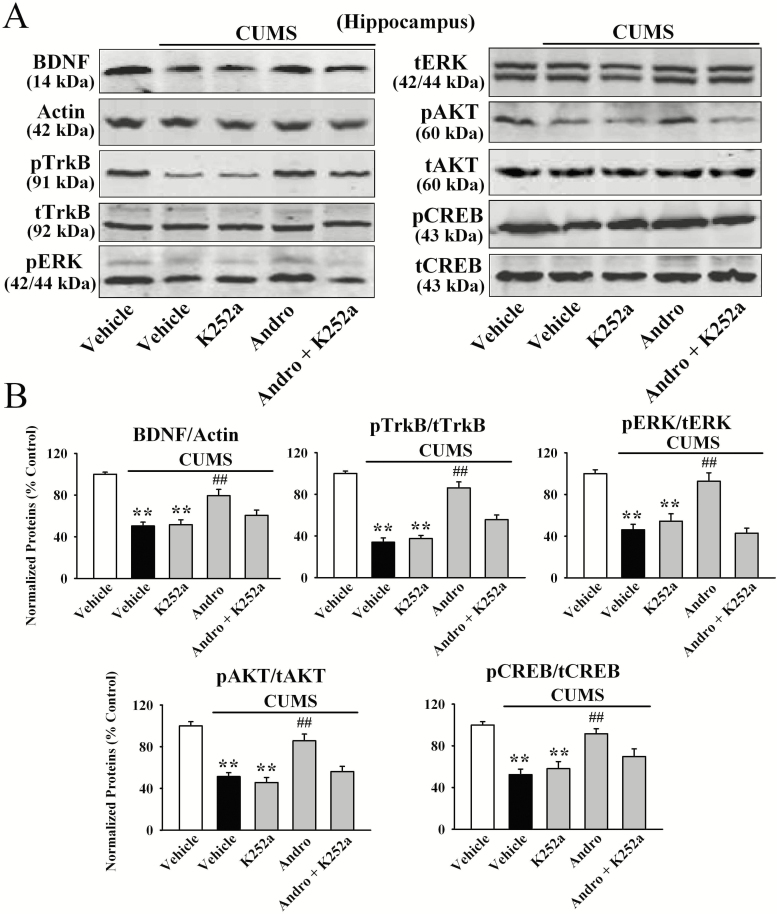
Next, we examined the protein phosphorylation of TrkB, ERK1 and 2, AKT, and CREB, the downstream signalling molecules of BDNF. Andro treatment fully reversed the CUMS-induced decrease in the protein levels of hippocampal pTrkB [ANOVA: F(4, 20) = 33.782, P < .01], pERK1 and 2 [ANOVA: F(4, 20) = 31.885, P < .01], pAKT [ANOVA: F(4, 20) = 24.112, P < .01], and pCREB [ANOVA: F(4, 20) = 35.862, P < .01] (n = 5, P < .01). Moreover, Andro administration enhanced the protein levels of hippocampal BDNF [ANOVA: F(2, 12) = 27.263, P < .01], pTrkB [ANOVA: F(2, 12) = 29.142, P < .01], pERK1 and 2 [ANOVA: F(2, 12) = 22.623, P < .01], pAKT [ANOVA: F(2, 12) = 14.622, P < .01], and pCREB [ANOVA: F(2, 12) = 20.633, P < .01] in naive control mice (n = 5, P < .01; supplementary Figure 1B–C). In contrast, the protein levels of total TrkB, ERK1 and 2, AKT, CREB, and β-actin were unchanged among the groups.
Supplementary Figure 2A and B show the mPFC data and NAc data, respectively. CUMS significantly reduced the protein levels of BDNF and pCREB in the mPFC (n = 5, P < .01 vs vehicle) and enhanced these levels in the NAc (n = 5, P < .01), consistent with previous reports (Nestler and Carlezon, 2006; Shirayama and Chaki, 2006). However, Andro treatment did not reverse these stress-induced changes (n = 5). Taken together, these results indicate that the antidepressant-like effects of Andro may involve the hippocampal BDNF system.
Repeated Andro Treatment Reversed the CUMS-Induced Decrease in Hippocampal Neurogenesis
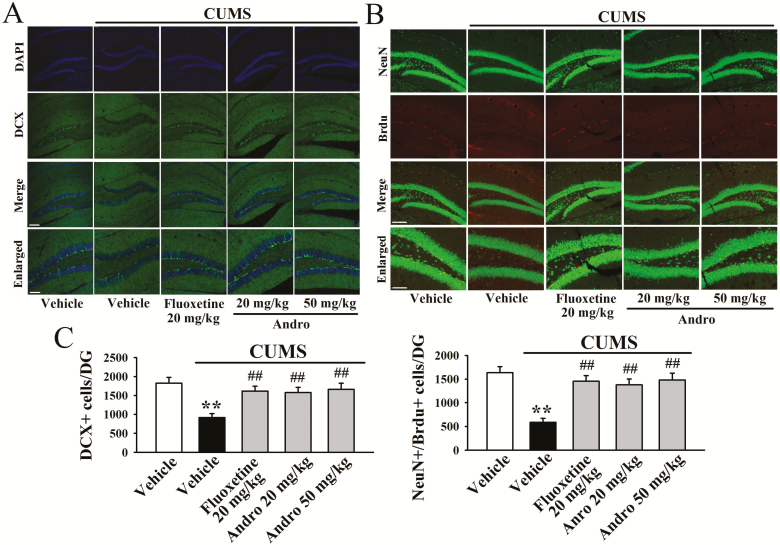
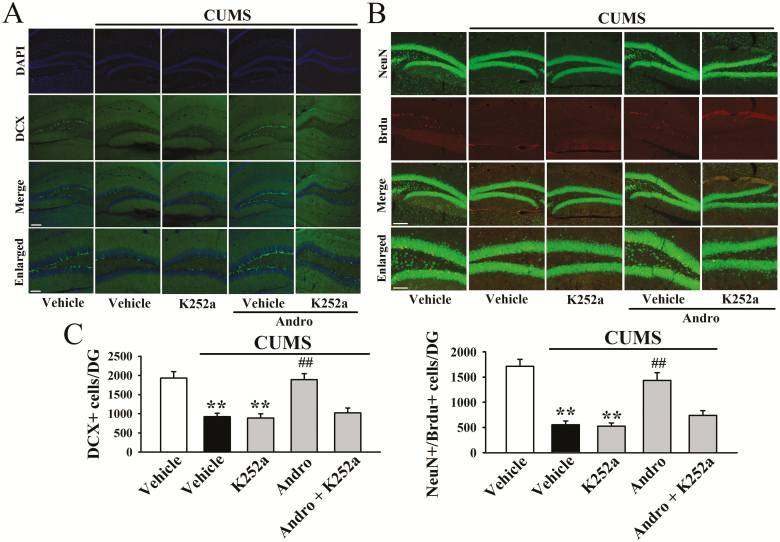
Chronic stress induces not only depressive-like behaviors and BDNF signalling dysfunction but also decreases neuronal proliferation and differentiation in the DG of mice (Lagace et al., 2010). In addition, hippocampal neurogenesis is required for the effects of many antidepressants including fluoxetine (Santarelli et al., 2003). We thus examined whether Andro treatment can prevent the effects of CUMS on hippocampal neurogenesis. In this study, neuronal proliferation was assessed by DCX immunofluorescence in the DG region. As shown in Figure 4A and C, CUMS induced a 49.9% ± 6.3% reduction in the number of DCX+ cells in the DG (n = 5, P < .01), while Andro treatment completely reversed this change (n = 5, P < .01), similar to fluoxetine. One-way ANOVA analysis indicated a significant overall effect [F(4, 20) = 36.735, P < .01].
Figure 4.
Repeated andrographolide (Andro) treatment fully reversed the effects of chronic unpredictable mild stress (CUMS) on hippocampal neurogenesis in mice. (A) Representative confocal microscopy images showing the localization of doublecortin (DCX; green) and 4’,6-diamidino-2-phenylindole (DAPI; blue) in the dentate gyrus (DG). The scale bar represents 150 µm for the representative images and 50 µm for the enlarged images. (B) Representative microscopic images showing the co-staining (yellow) of neuronal nuclei (NeuN; green) and 5-Bromo-2-deoxyUridine (BrdU; red) in the DG. The scale bar represents 150 µm for the representative images and 75 µm for the enlarged images. The quantitative analyses are shown in (C). All results are represented as the means ± SEMs (n = 5); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
Newly-generated cells in the DG differentiate into mature neurons within 28 days after their birth (Kempermann et al., 2003). To determine whether the Andro-induced newborn cells differentiated into mature neurons, BrdU was administered to label proliferating cells and NeuN was employed as a marker of mature neurons. As shown in Figure 4B and C, CUMS resulted in a 64.1 ± 9.6% reduction in the number of NeuN+/BrdU+ co-labelled cells in DG (n = 5, P < .01), while this change was fully reversed by Andro (n = 5, P < .01). One-way ANOVA analysis also revealed a significant overall effect [F(4, 20) = 32.659, P < .01]. These data indicate that Andro has promoting effects on hippocampal neurogenesis.
Blockade of Hippocampal BDNF Signalling Abolished the Antidepressant-Like Actions of Andro in Mice
To determine whether hippocampal BDNF was critical for the antidepressant-like actions of Andro in mice, we used K252a, as we have frequently done (Jiang et al., 2017; Ren et al., 2017; Wang et al., 2017; Ni et al., 2018). As shown in Figure 5B, although K252a alone did not influence the immobility of mice, pretreatment with K252a significantly attenuated the effects of Andro on naive mice in the FST [ANOVA: F(3, 36) = 18.648, P < .01] and TST [ANOVA: F(3, 36) = 19.331, P < .01] (n = 10). Moreover, co-treatment with K252a and Andro significantly prevented the reversal effects of Andro on mice exposed to CUMS in the FST [ANOVA: F(4, 45) = 27.407, P < .01; Figure 5C], TST [ANOVA: F(4, 45) = 31.225, P < .01; Figure 5D], and SPT [ANOVA: F(4, 45) = 23.087, P < .01; Figure 5E] (n = 10). In addition, K252a prevented the reversing effects of Andro on the levels of hippocampal BDNF protein [ANOVA: F(4, 20) = 25.774, P < .01; Figure 6], pTrkB protein [ANOVA: F(4, 20) = 34.699, P < .01; Figure 6], pERK1 and 2 protein [ANOVA: F(4, 20) = 23.524, P < .01; Figure 6], pAKT protein [ANOVA: F(4, 20) = 21.838, P < .01; Figure 6], pCREB protein [ANOVA: F(4, 20) = 28.112, P < .01; Figure 6], neuronal proliferation [ANOVA: F(4, 20) = 35.096, P < .01; Figure 7A,C] and differentiation [ANOVA: F(4, 20) = 38.429, P < .01; Figure 7B–C] in the stressed mice (n = 5).
Figure 5.
The usage of K252a attenuated the antidepressant-like effects of andrographolide (Andro) both in naive mice (B) and in the chronic unpredictable mild stress (CUMS) model of depression (C–E). Schematics showing the timelines of the procedures are shown in A. All results are represented as means ± SEM (n = 10); *P < .05, **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The comparisons were made by 1-way ANOVA followed by the least significant difference (LSD) test.
Figure 6.
K252a blocked the promoting effects of andrographolide (Andro) on hippocampal brain-derived neurotrophic factor (BDNF) signalling in chronic unpredictable mild stress (CUMS)-treated mice. Representative western-blot images and the quantitative analyses are shown in A and B, respectively. All results are represented as the means ± SEMs (n = 5); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
Figure 7.
K252a blocked the enhancing effects of andrographolide (Andro) on hippocampal neurogenesis in chronic unpredictable mild stress (CUMS)-treated mice. (A) Representative confocal microscopy images showing the localization of doublecortin (DCX; green) and 4’,6-diamidino-2-phenylindole (DAPI; blue) in the dentate gyrus (DG). The scale bar represents 150 µm for the representative images and 50 µm for the enlarged images. (B) Representative microscopic images showing the co-staining (yellow) of neuronal nuclei (NeuN; green) and 5-Bromo-2-deoxyUridine (BrdU; red) in the DG. The scale bar represents 150 µm for the representative images and 75 µm for the enlarged images. The quantitative analyses are shown in (C). All results are represented as the means ± SEMs (n = 5); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
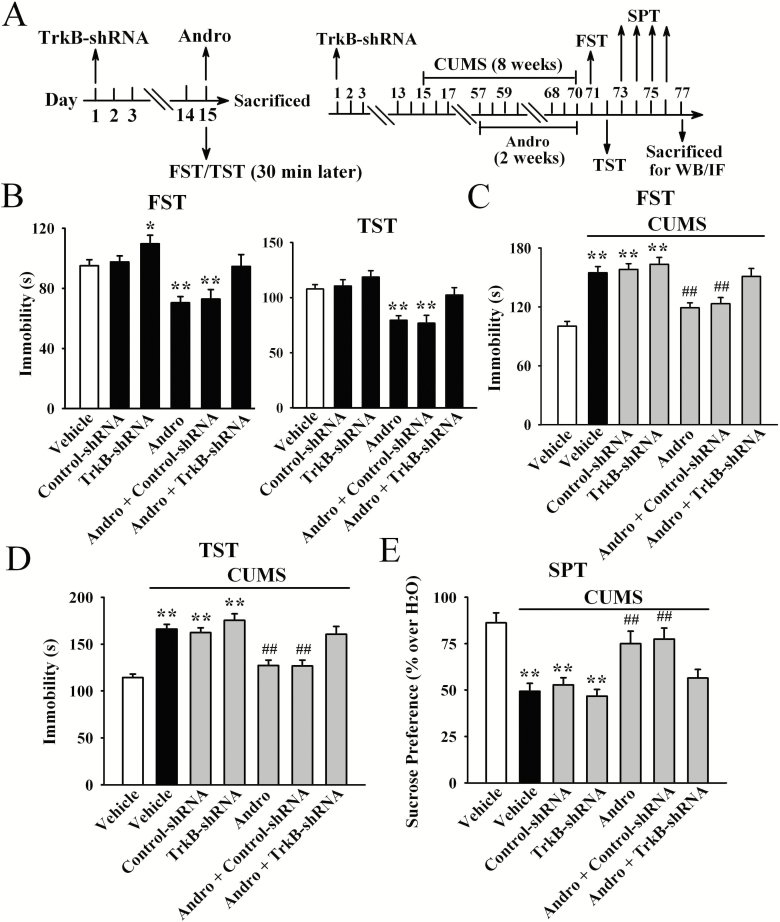
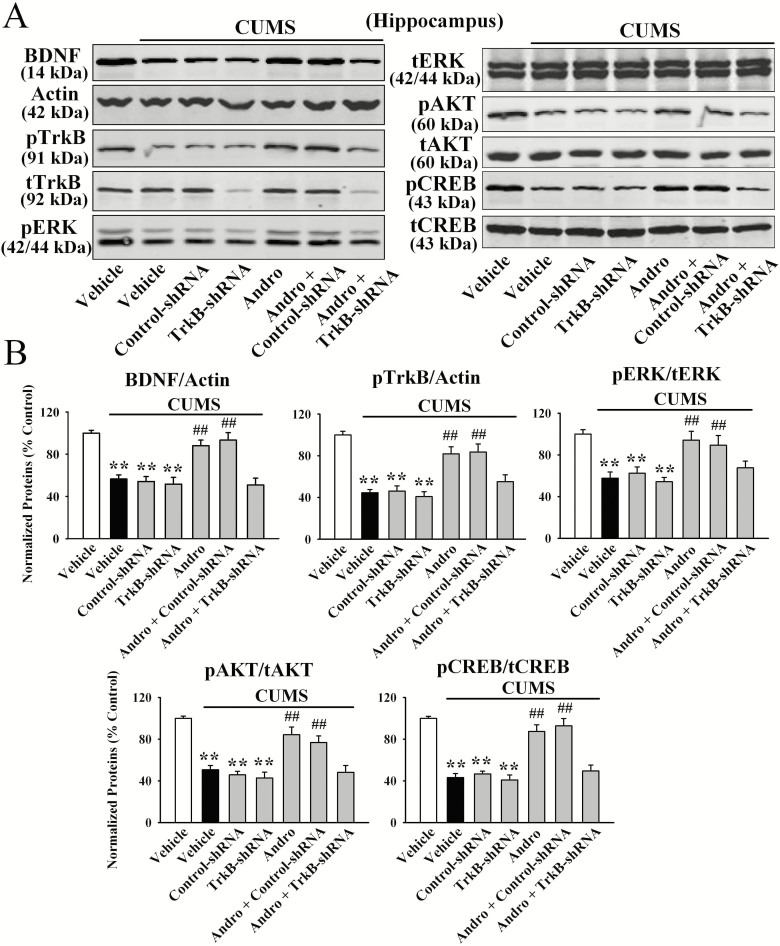
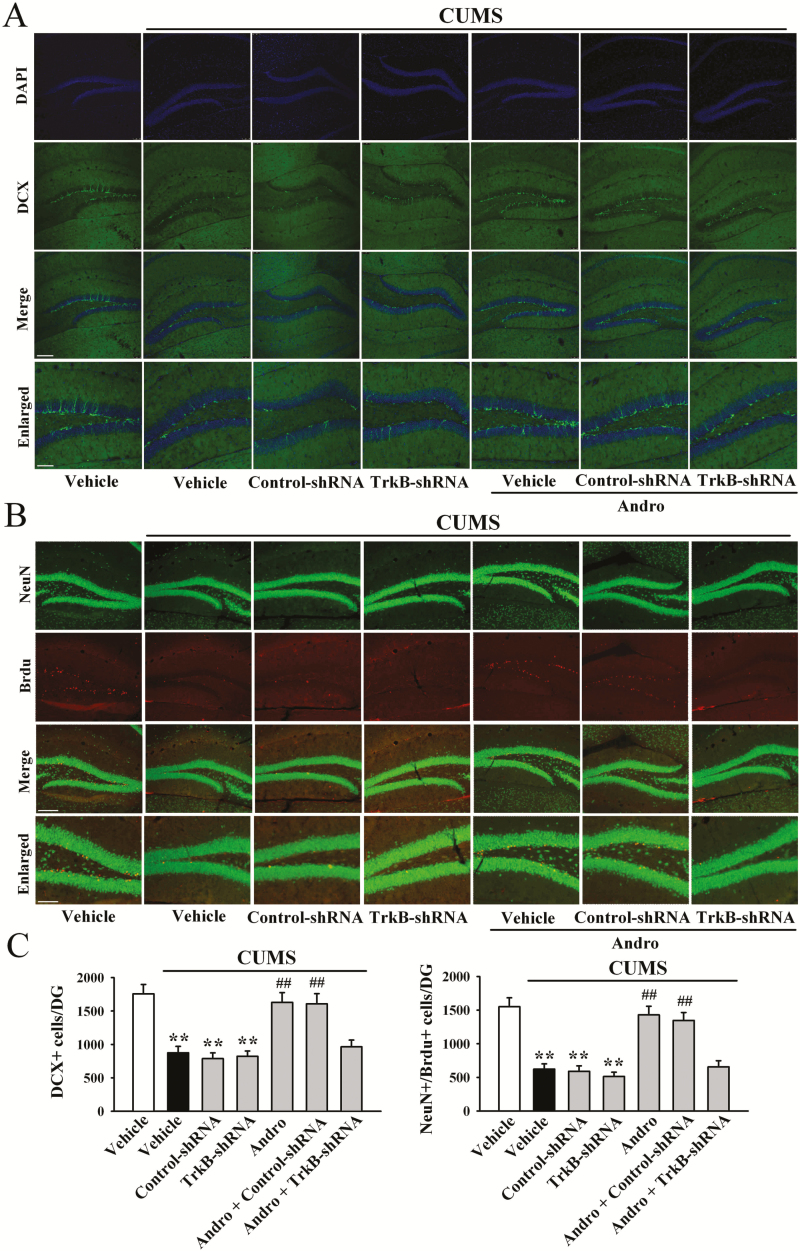
Furthermore, LV-TrkB-shRNA-EGFP was used to specifically knock down hippocampal TrkB expression. The efficacy of LV-TrkB-shRNA-EGFP was demonstrated in our previous reports (Ren et al., 2017; Wang et al., 2017; Ni et al., 2018). TrkB knockdown significantly abolished the antidepressant-like effects of Andro on naive mice in the FST [ANOVA: F(5, 54) = 14.542, P < .01] and TST [ANOVA: F(5, 54) = 16.708, P < .01] (n = 10; Figure 8B). Additionally, TrkB-shRNA fully blocked the reversal effects of Andro on mice exposed to CUMS in the FST [ANOVA: F(6, 63) = 22.015, P < .01; Figure 8C], TST [ANOVA: F(6, 63) = 27.503, P < .01; Figure 8D], and SPT [ANOVA: F(6, 63) = 19.887, P < .01; Figure 8E] (n = 10). Moreover, the reversing effects of Andro on the levels of hippocampal BDNF protein [ANOVA: F(6, 28) = 26.957, P < .01; Figure 9], pTrkB protein [ANOVA: F(6, 28) = 32.164, P < .01; Figure 9], pERK1 and 2 protein [ANOVA: F(6, 28) = 24.763, P < .01; Figure 9], pAKT protein [ANOVA: F(6, 28) = 23.227, P < .01; Figure 9], pCREB protein [ANOVA: F(6, 28) = 30.506, P < .01; Figure 9], neuronal proliferation [ANOVA: F(6, 28) = 33.663, P < .01; Figure 10A,C], and differentiation [ANOVA: F(6, 28) = 38.491, P < .01; Figure 10B,C] in the stressed mice were abolished by TrkB-shRNA (n = 5). Taken together, these results indicate that Andro requires the hippocampal BDNF system to exert its antidepressant-like effects in mice.
Figure 8.
The usage of TrkB-shRNA fully abolished the antidepressant-like effects of andrographolide (Andro) both in naive mice (B) and in the chronic unpredictable mild stress (CUMS) model of depression (C–E). Schematics showing the timelines of the procedures are shown in (A). All results are represented as the means ± SEMs (n = 10); *P < .05, **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
Figure 9.
TrkB-shRNA completely abolished the promoting effects of andrographolide (Andro) on hippocampal brain-derived neurotrophic factor (BDNF) signalling in chronic unpredictable mild stress (CUMS)-treated mice. Representative western-blot images and the quantitative analyses are shown in (A) and (B), respectively. All results are represented as the means ± SEM (n = 5); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the (LSD) test.
Figure 10.
Tyrosine receptor kinase B- short hairpin RNA (TrkB-shRNA) completely abolished the enhancing effects of andrographolide (Andro) on hippocampal neurogenesis in chronic unpredictable mild stress (CUMS)-treated mice. (A) Representative confocal microscopy images showing the localization of doublecortin (DCX; green) and 4’,6-diamidino-2-phenylindole (DAPI; blue) in the dentate gyrus (DG). The scale bar represents 150 µm for the representative images and 50 µm for the enlarged images. (B) Representative microscopic images showing the co-staining (yellow) of neuronal nuclei (NeuN; green) and 5-Bromo-2-deoxyUridine (BrdU; red) in the DG. The scale bar represents 150 µm for the representative images and 75 µm for the enlarged images. The quantitative analyses are shown in C. All results are represented as the means ± SEMs (n = 5); **P < .01 vs Vehicle; ##P < .01 vs CUMS + Vehicle. The data were compared by 1-way ANOVA followed by the least significant difference (LSD) test.
Discussion
From previous studies, we have learned that Andro exerts some interesting pharmacological actions such as enhancing the expression of BDNF in vitro and promoting hippocampal neurogenesis in mice (Varela-Nallar et al., 2015; Xu et al., 2016). Since depression is accompanied by decreased BDNF biosynthesis and downregulated hippocampal neurogenesis (Aldam et al., 2013; Lee et al., 2013), it is possible that Andro has antidepressant-like effects. In this study, the effects of Andro were first investigated in the FST and TST, 2 methods widely used to detect potential antidepressant activities. Our results in the FST and TST showed that Andro had properties similar to those of fluoxetine. It is possible that the effects of Andro observed in the FST and TST were due to enhanced spontaneous locomotor activity in mice (Bourin et al., 2001). To exclude this possibility, the OFT was performed, and no locomotion-stimulating effects were observed in mice treated with Andro. Furthermore, the effects of Andro were explored by exposing mice to CUMS, a very widely used and accepted model of depression in rodents. As expected, Andro significantly prevented CUMS-induced depressive-like behaviors in mice.
Many natural antidepressants are great alternatives/supplements to prescription antidepressant drugs (Liu et al., 2015). They work as well or even better and have fewer side effects than prescription antidepressants. Well-known natural antidepressants include St. John’s wort, omega-3 fatty acids, and saffron. As the primary active component of Andrographis paniculata, Andro has been widely used in China and other parts of Asia for treating upper respiratory tract infections due to its potent anti-inflammatory activity. This study provides the first experimental evidence to our knowledge that Andro has beneficial effects against depression, which is interesting and stimulating as it extends the knowledge of Andro’s pharmacological effects and supports Andro as a new potential antidepressant. Andro can easily cross the blood-brain-barrier in rats (Chan et al., 2010), and a pharmacokinetic study using rats revealed the highest tissue concentration of Andro in the kidney followed by the liver, spleen, and brain (Bera et al., 2014). Although the kidney is likely to be the organ most susceptible to Andro toxicity, some reports have demonstrated a good safety profile of Andro as a drug. For example, a daily oral dose of 1 g/kg A. paniculata extracts (with 10% Andro) for 86 days showed no adverse reproductive and fertility effects in rats (Allan et al., 2009). An oral dose of up to 5 g/kg Andro administered daily for up to 14 days in rats had no observable adverse effects in an acute toxicity study (Bothiraja et al., 2012). The LD50 of Andro administered via i.p. injection to mice is 11.46 g/kg (Handa and Sharma, 1990; Bothiraja et al., 2012). Additionally, in a clinical trial, oral tablets containing up to 170 mg of purified A. paniculata extracts (about 85 mg Andro) were administered every 12 hours for 12 months and found to be well tolerated (Bertoglio et al., 2016). Thus, in our study, 50 mg/kg Andro was a safe dose, producing minimal adverse effects in mice.
Our western blotting and immunofluorescence data showed that Andro also protected against the inhibitory effects of CUMS on the hippocampal BDNF system and neurogenesis in mice, consistent with the findings of Varela-Nallar et al. (2015) and Xu et al. (2016). Considering these results with the behavioral results, it can be observed that the greater Andro’s promoting effects on hippocampal BDNF, the greater were its antidepressant-like actions. Here, we studied not only the hippocampal region but also the mPFC and NAc regions. The results for the mPFC and NAc samples were very interesting, indicating that the effects of Andro on the central BDNF system are region selective, and in-depth studies are ongoing in our group to clarify this region selectivity. Although the usage of K252a and TrkB-shRNA collectively showed that the antidepressant-like actions of Andro required BDNF, it could not exclude other antidepressant targets for Andro. For example, Peng et al. (2016) reported that Andro ameliorated ovalbumin-induced lung injury in mice by suppressing reactive oxygen species-mediated nuclear factor-kappa B (NF-κB) signalling and NLRP3 inflammasome activation. Several in vitro and in vivo studies suggested that Andro was able to activate the Wnt/β-Catenin signalling pathway, inducing the transcription of Wnt target genes and inhibiting glycogen synthase kinase3β (GSK3β) by dephosphorylation (Jiang et al., 2015; Tapia-Rojas et al., 2015; Varela-Nallar et al., 2015). Many studies have implicated the role of β-Catenin, GSK3β, NF-κB, and the NLRP3 inflammasome in the pathogenesis of depression, as (1) chronic stress significantly reduced the total and nuclear levels of β-Catenin in the hippocampus of rats (Hui et al., 2018); (2) GSK3β was highly expressed and phosphorylated in the brain of chronically stressed mice, while inhibition of GSK3β led to antidepressant-like actions (Peng et al., 2018); and (3) several antidepressants exerted antidepressant-like effects in mice via the inhibition of NF-κB and the NLRP3 inflammasome (Jia et al., 2018; Song et al., 2018a, 2018b). Therefore, it is possible that these molecules also contribute to the antidepressant-like actions of Andro in mice, and more antagonists/shRNAs will be used in our further study.
How does Andro activate the hippocampal BDNF system? It has been demonstrated that Andro is a competitive inhibitor of GSK3β, and interestingly, a correlation between GSK3β inhibition and BDNF-mediated signal transduction has been found (Mai et al., 2002; Gupta et al., 2014). Therefore, GSK3β may underlie the effects of Andro on BDNF. Moreover, BDNF is initially synthesized as a precursor protein (proBDNF). ProBDNF is converted to BDNF either intracellularly by furin and/or prohormone convertases or extracellularly by matrix metalloproteinase enzymes and/or plasmin (Qiao et al., 2017). Both proBDNF and BDNF are biologically active but have opposite functions. For example, BDNF binds with TrkB and induces neuronal survival, while proBDNF binds with the apoptotic receptor p75 and induces neuronal death (Rahman et al., 2018). It is also possible that Andro has promoting effects on the conversion of proBDNF to BDNF, which needs further study.
In addition to dysfunction in hippocampal neurogenesis and the BDNF system, depression is accompanied by many other pathological symptoms, including monoaminergic deficiency, cognitive impairments, neuronal death, and neuroinflammation (Adlam and Zaman, 2013; Brites and Fernandes, 2015; Natarajan et al., 2017; Marathe et al., 2018). It is possible that Andro can also ameliorate these symptoms since it has anti-dementia, anti-neuroinflammatory, and neuroprotective effects in rodents (Chan et al., 2010; Serrano et al., 2014; Zhang et al., 2014). Moreover, there are some other acknowledged models of depression besides the CUMS model, such as the chronic social defeat stress and chronic restraint stress models. The antidepressant-like actions of Andro will be further assessed using chronic social defeat stress and chronic restraint stress in our next study.
In conclusion, our study provides new insight into the pharmacological effects of Andro and sheds light on the development of new antidepressants with improved efficacy and fewer side effects.
Supplementary Material
Acknowledgments
This work was supported by 2 grants from the National Natural Science Foundation of China to Dr Bo Jiang (no. 81401116) and Dr Jian-Feng Liu (no. 81601163), a grant from the Natural Science Foundation of Jiangsu Province to Dr Bo Jiang (no. BK20161284), a grant from the Science and Technology Projects of Nantong City to Dr Bo Jiang (no. MS12018076), and 2 grants from the Graduate Student Scientific Research Innovation Projects of Jiangsu Province to Ying-Jie Wang (KYCX17-1929) and Cheng-Niu Wang (KYCX18-2400).
Statement of Interest
None.
References
- Adlam J, Zaman R (2013) The role of BDNF and memory in major depressive disorder. Psychiatr Danub 25:S368–S369. [PubMed] [Google Scholar]
- Bera R, Ahmed SK, Sarkar L, Sen T, Karmakar S (2014) Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm Biol 52:321–329. [DOI] [PubMed] [Google Scholar]
- Bertoglio JC, Baumgartner M, Palma R, Ciampi E, Carcamo C, Cáceres DD, Acosta-Jamett G, Hancke JL, Burgos RA (2016) Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: a 12-month double-blind placebo-controlled pilot study. BMC Neurol 16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendy JA. (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59:1144–1150. [DOI] [PubMed] [Google Scholar]
- Bothiraja C, Pawar AP, Shende VS, Joshi PP (2012) Acute and subacute toxicity study of andrographolide bioactive in rodents: evidence for the medicinal use as an alternative medicine. Comp Clin Pathol 22:1123–1128. [Google Scholar]
- Bourin M, Fiocco AJ, Clenet F (2001) How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16:9–21. [DOI] [PubMed] [Google Scholar]
- Brites D, Fernandes A (2015) Neuroinflammation and depression: microglia activation, extracellular microvesicles and microrna dysregulation. Front Cell Neurosci 9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SJ, Wong WS, Wong PT, Bian JS (2010) Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol 161:668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HW, Huang CS, Li CC, Lin AH, Huang YJ, Wang TS, Yao HT, Lii CK (2014) Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol Appl Pharmacol 280:1–9. [DOI] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ (2009) Antidepressant actions of histone deacetylase inhibitors. J Neurosci 29:11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R. (2015) Editorial: a systematic review of depression. Curr Neuropharmacol 13:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin D, Mansouri N (2006) Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test. Eur Neuropsychopharmacol 16:324–328. [DOI] [PubMed] [Google Scholar]
- Geng J, Liu W, Xiong Y, Ding H, Jiang C, Yang X, Li X, Elgehama A, Sun Y, Xu Q, Guo W, Gao J (2018) Andrographolide sulfonate improves alzheimer-associated phenotypes and mitochondrial dysfunction in APP/PS1 transgenic mice. Biomed Pharmacother 97:1032–1039. [DOI] [PubMed] [Google Scholar]
- Gordon M, Melvin G (2013) Selective serotonin re-uptake inhibitors–a review of the side effects in adolescents. Aust Fam Physician 42:620–623. [PubMed] [Google Scholar]
- Guan LP, Tang LM, Pan CY, Zhao SL, Wang SH (2014) Evaluation of potential antidepressant-like activity of chalcone-1203 in various murine experimental depressant models. Neurochem Res 39:313–320. [DOI] [PubMed] [Google Scholar]
- Gupta V, Chitranshi N, You Y, Gupta V, Klistorner A, Graham S (2014) Brain derived neurotrophic factor is involved in the regulation of glycogen synthase kinase 3β (GSK3β) signalling. Biochem Biophys Res Commun 454:381–386. [DOI] [PubMed] [Google Scholar]
- Handa SS, Sharma A (1990) Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J Med Res 92:276–283. [PubMed] [Google Scholar]
- Hui J, Zhang J, Pu M, Zhou X, Dong L, Mao X, Shi G, Zou J, Wu J, Jiang D, Xi G (2018) Modulation of GSK-3β/β-catenin signaling contributes to learning and memory impairment in a rat model of depression. Int J Neuropsychopharmacol 21:858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MT, et al. (2018) Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett 420:129–145. [DOI] [PubMed] [Google Scholar]
- Jia KK, Ding H, Yu HW, Dong TJ, Pan Y, Kong LD (2018) Huanglian-wendan decoction inhibits NF-κb/NLRP3 inflammasome activation in liver and brain of rats exposed to chronic unpredictable mild stress. Mediators Inflamm 2018:3093516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Wang YJ, Wang H, Song L, Huang C, Zhu Q, Wu F, Zhang W (2017) Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br J Pharmacol 174:177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Salton SR (2013) The role of neurotrophins in major depressive disorder. Transl Neurosci 4:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Zhou B, Huang L, Wu H, Huang J, Liang T, Liu H, Zheng L, Zhao J (2015) Andrographolide exerts pro-osteogenic effect by activation of Wnt/β-catenin signaling pathway in vitro. Cell Physiol Biochem 36:2327–2339. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003) Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130:391–399. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore V, Yarla NS, Bishayee A, Putta S, Malla R, Neelapu NR, Challa S, Das S, Shiralgi Y, Hegde G, Dhananjaya BL (2017) Multi-targeting andrographolide and its natural analogs as potential therapeutic agents. Curr Top Med Chem 17:845–857. [DOI] [PubMed] [Google Scholar]
- Kozisek ME, Middlemas D, Bylund DB (2008) Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther 117:30–51. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ (2010) Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A 107:4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Reif A, Schmitt AG (2013) Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci 14:153–179. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu C, Wang Y, Wang P, Li Y, Li B (2015) Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol 13:481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He S, Tang J, Ding N, Chu X, Cheng L, Ding X, Liang T, Feng S, Rahman SU, Wang X, Wu J (2017) Andrographolide inhibits inflammatory cytokines secretion in LPS-stimulated RAW264.7 cells through suppression of NF-κB/MAPK signaling pathway. Evid Based Complement Alternat Med 2017:8248142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai L, Jope RS, Li X (2002) BDNF-mediated signal transduction is modulated by GSK3BETA and mood stabilizing agents. J Neurochem 82:75–83. [DOI] [PubMed] [Google Scholar]
- Marathe SV, D’almeida PL, Virmani G, Bathini P, Alberi L (2018) Effects of monoamines and antidepressants on astrocyte physiology: implications for monoamine hypothesis of depression. J Exp Neurosci 12:1179069518789149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015) Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172:3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Forrester L, Chiaia NL, Yamamoto BK (2017) Chronic-stress-induced behavioral changes associated with subregion-selective serotonin cell death in the dorsal raphe. J Neurosci 37:6214–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E (2011) Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol 9:530–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YF, Wang H, Gu QY, Wang FY, Wang YJ, Wang JL, Jiang B (2018) Gemfibrozil has antidepressant effects in mice: involvement of the hippocampal brain-derived neurotrophic factor system. J Psychopharmacol 32:469–481. [DOI] [PubMed] [Google Scholar]
- Nie X, Chen SR, Wang K, Peng Y, Wang YT, Wang D, Wang Y, Zhou GC (2017) Attenuation of innate immunity by andrographolide derivatives through NF-κB signaling pathway. Sci Rep 7:4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. 2nd ed. San Diego, CA: Academic Press. [Google Scholar]
- Peng H, Wang HB, Wang L, Zhou B, Li XY, Tan J (2018) Gsk3β aggravates the depression symptoms in chronic stress mouse model. J Integr Neurosci 17:169–175. [DOI] [PubMed] [Google Scholar]
- Peng S, Gao J, Liu W, Jiang C, Yang X, Sun Y, Guo W, Xu Q (2016) Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget 7:80262–80274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336. [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C (2004) Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155:135–146. [DOI] [PubMed] [Google Scholar]
- Qiao H, An SC, Xu C, Ma XM (2017) Role of probdnf and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 1663:29–37. [DOI] [PubMed] [Google Scholar]
- Rahman M, Luo H, Sims NR, Bobrovskaya L, Zhou XF (2018) Investigation of mature BDNF and proBDNF signaling in a rat photothrombotic ischemic model. Neurochem Res 43:637–649. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wang JL, Zhang X, Wang H, Ye Y, Song L, Wang YJ, Tu MJ, Wang WW, Yang L, Jiang B (2017) Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res Bull 134:211–219. [DOI] [PubMed] [Google Scholar]
- Sanna MD, Quattrone A, Galeotti N (2018) Antidepressant-like actions by silencing of neuronal ELAV-like RNA-binding proteins HuB and HuC in a model of depression in male mice. Neuropharmacology 135:444–454. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Sasi M, Vignoli B, Canossa M, Blum R (2017) Neurobiology of local and intercellular BDNF signaling. Pflugers Arch 469:593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano FG, Tapia-Rojas C, Carvajal FJ, Hancke J, Cerpa W, Inestrosa NC (2014) Andrographolide reduces cognitive impairment in young and mature aβppswe/PS-1 mice. Mol Neurodegener 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S (2006) Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MT, Ruan J, Zhang RY, Deng J, Ma ZQ, Ma SP (2018) Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol Sin 39:1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Sun R, Ji Z, Li X, Fu Q, Ma S (2018) Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci 206:117–124. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370. [DOI] [PubMed] [Google Scholar]
- Tapia-Rojas C, Schüller A, Lindsay CB, Ureta RC, Mejías-Reyes C, Hancke J, Melo F, Inestrosa NC (2015) Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3β: autoregulation of GSK-3β in vivo. Biochem J 466:415–430. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Arredondo SB, Tapia-Rojas C, Hancke J, Inestrosa NC (2015) Andrographolide stimulates neurogenesis in the adult hippocampus. Neural Plast 2015:935403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villas Boas GR, Stefanello da Silveira AP, Feitosa Farinelli BC, Lima Cardoso CA, Arce E, Oesterreich SA (2018) The ethanolic extract obtained from Campomanesia pubescens (D.C.) O.BERG fruits exerts anxiolytic and antidepressant effects on chronic mild stress model and on anxiety models in Wistar rats: behavioral evidences. Nutr Neurosci 1:1–11. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhao Y, Wang YJ, Song L, Wang JL, Huang C, Zhang W, Jiang B (2017) Antidepressant-like effects of tetrahydroxystilbene glucoside in mice: involvement of BDNF signaling cascade in the hippocampus. CNS Neurosci Ther 23:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li X, He S, Hu L, Guo J, Huang X, Hu J, Qi Y, Chen B, Shang D, Wen Y (2018) Regulation of the kynurenine metabolism pathway by Xiaoyao San and the underlying effect in the hippocampus of the depressed rat. J Ethnopharmacol 214:13–21. [DOI] [PubMed] [Google Scholar]
- Wang W, Hu X, Zhao Z, Liu P, Hu Y, Zhou J, Zhou D, Wang Z, Guo D, Guo H (2008) Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry 32:1179–1184. [DOI] [PubMed] [Google Scholar]
- Wen L, Xia N, Chen X, Li Y, Hong Y, Liu Y, Wang Z, Liu Y (2014) Activity of antibacterial, antiviral, anti-inflammatory in compounds andrographolide salt. Eur J Pharmacol 740:421–427. [DOI] [PubMed] [Google Scholar]
- Xu F, Wu H, Zhang K, Lv P, Zheng L, Zhao J (2016) Pro‑neurogenic effects of andrographolide on RSC96 Schwann cells in vitro. Mol Med Rep 14:3573–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, Cui R (2015) The effects of psychological stress on depression. Curr Neuropharmacol 13:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chen ZY (2011) The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin 32:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lai D, Wang L, Yu P, Zhu L, Guo B, Xu L, Zhou L, Sun Y, Lee SM, Wang Y (2014) Neuroprotective effects of the andrographolide analogue AL-1 in the MPP⁺/MPTP-induced Parkinson’s disease model in vitro and in mice. Pharmacol Biochem Behav 122:191–202. [DOI] [PubMed] [Google Scholar]
- Zu X, Zhang M, Li W, Xie H, Lin Z, Yang N, Liu X, Zhang W (2017) Antidepressant-like effect of bacopaside I in mice exposed to chronic unpredictable mild stress by modulating the hypothalamic-pituitary-adrenal axis function and activating BDNF signaling pathway. Neurochem Res 42:3233–3244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.