Abstract
Background:
Atrial fibrillation (AF) is a common cardiac arrhythmia that increases the risk of stroke. Medical therapy for decreasing stroke risk involves anticoagulation, which may increase bleeding risk for certain patients. In determining optimal therapy for stroke prevention for patients with AF, clinicians use tools with various clinical, imaging, and patient characteristics to weigh stroke risk against therapy-associated bleeding risk.
Aim:
Review published literature and summarize available risk stratification tools for stroke and bleeding prediction in patients with AF.
Methods:
We searched for English-language studies in PubMed®, Embase®, and the Cochrane Database of Systematic Reviews published between January 1, 2000, and February 14, 2018. Two reviewers screened citations for studies that examined tools for predicting thromboembolic and bleeding risks in patients with AF. Data regarding study design, patient characteristics, interventions, outcomes, quality and applicability were extracted.
Results:
61 studies were relevant to predicting thromboembolic risk and 38 to predicting bleeding risk. Data suggest that CHADS2, CHA2DS2-VASc, and ABC risk scores have the best evidence predicting thromboembolic risk (moderate strength of evidence for limited prediction ability of each score) and that HAS-BLED has the best evidence for predicting bleeding risk (moderate strength of evidence).
Limitations:
Studies were heterogeneous in methodology and populations of interest, setting, interventions, and outcomes analyzed.
Conclusion:
CHADS2, CHA2DS2-VASc, and ABC stroke have the best prediction for stroke events, and HAS-BLED provides the best prediction for bleeding risk. Future studies should define the role of imaging tools and biomarkers in enhancing the accuracy of risk prediction tools.
Primary Funding Source:
Patient-Centered Outcomes Research Institute (PROSPERO #CRD42017069999)
Keywords: nonvalvular atrial fibrillation, stroke risk, bleeding risk
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia seen in clinical practice, occurring in up to 6.1 million people in the United States and accounting for approximately one-third of hospitalizations for cardiac rhythm disturbances.1-3 Further, AF is associated with significant morbidity and mortality, including increased risk of embolic stroke, heart failure, and cognitive impairment; reduced quality of life; and higher overall mortality.4-6
Optimal clinical management of AF is critical to reducing this associated morbidity and mortality, and includes prevention of AF-related thromboembolic events in at risk patients. Vitamin K antagonists or direct oral anticoagulants have been shown to reduce thromboembolic events, but long-term use of these medications puts certain patients at higher risk for serious bleeding events. As such, accurate risk stratification for both thromboembolic and bleeding risk is paramount in identifying patients for whom antithrombotic therapy would achieve maximum treatment benefit with the lowest risk of complications.
Unfortunately, it is challenging to estimate the tradeoff between stroke risk and risk of bleeding complications from long-term anticoagulation therapy because many risk factors for stroke are also associated with increased risk of bleeding. There are several available risk stratification tools used to determine thromboembolic and bleeding risk that incorporate diagnostic imaging as well as patient factors such as age, sex, and history of heart disease to aid in clinical decisionmaking around treatment strategies for AF. Of the many available risk stratification tools, the 2014 AHA/ACC/HRS guideline for patients with AF recommends the use of the CHADS2-VASc score to estimate the stroke risk and the HAS-BLED score for bleeding risk.7-9 However, these risk scores have been previously categorized as poor to moderate predictors of risk, and are just two of many different published and validated methods for assessing stroke and bleeding risk in patients with AF. Because patients, providers, and policymakers have numerous decision tools that could inform treatment decisions and policy recommendations, there is a need for a compilation and analysis of the currently available data. This systematic review was commissioned by the Patient-Centered Outcomes Research Institute (PCORI) to update a 2013 Agency for Healthcare Research and Quality (AHRQ) review,10 with a focus on evaluating the comparative diagnostic accuracy and impact on clinical decisionmaking of available clinical and imaging tools and associated risk factors for predicting thromboembolic and bleeding risk in U.S. patients with AF. Our findings related to stroke prevention treatments are discussed in a companion paper.
METHODS
Methods for this updated comparative effectiveness review (CER) follow the AHRQ’s Methods Guide for Effectiveness and Comparative Effectiveness Reviews (hereafter referred to as the Methods Guide)11 and Methods Guide for Medical Test Reviews (hereafter referred to as the Medical Test Guide).12 This paper is part of the larger updated review; complete details of our methods, including exact search strings, and full results and conclusions can be found in the full report, available at www.efectivehealthcare.ahrq.gov.
Defining the Key Questions
PCORI convened two multi-stakeholder virtual workshops in December 2016 and January 2017 to (1) gather input from end users and clinical, content, and methodological experts on scoping for the updated review; (2) prioritize the key questions; (3) discuss changes in the evidence base since the 2013 review; and (4) explore emerging issues in AF. The protocol for this systematic review was informed by discussion at the January 2017 workshop and builds upon the original report. The final protocol for this review is posted on the Effective Healthcare (EHC) website (www.effectivehealthcare.ahrq.gov) and registered at PROSPERO (CRD42017069999).
In this paper we summarize the evidence and findings related to two key questions (KQs): (1) In patients with nonvalvular AF, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of available clinical and imaging tools and associated risk factors for predicting thromboembolic risk? and (2) In patients with nonvalvular AF, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
Data Sources and Study Selection
In consultation with an expert medical librarian, we searched PubMed®, Embase®, and the Cochrane Database of Systematic Reviews for relevant literature published from August 1, 2011, to February 14, 2018 (exact search strings in Appendix Table 1). We supplemented electronic searches with a manual search of citations from a set of systematic review articles. Our findings were combined with those from the 2013 review, and so the literature summarized here reflects evidence back through January 1, 2000.10 Due to updates in inclusion criteria, any studies excluded from the original review were also re-reviewed for eligibility. We used search criteria to identify relevant ongoing clinical trials through ClinicalTrials.gov as well as citations to guide the conclusions (Appendix Table 1).
Our prespecified inclusion and exclusion criteria are in Appendix Table 2. We included English-language studies of adults with nonvalvular AF (including atrial flutter) that reported the efficacy of clinical or imaging tools, or patient risk factors, on predicting thromboembolic and/or bleeding outcomes. Clinical or imaging tools considered for predicting thromboembolic events were CHADS2 score, CHADS2-VASc score, Framingham risk score, ABC stroke score, transthoracic and transesophageal echocardiography, CT scans, and cardiac MRIs. Clinical or imaging tools considered for predicting bleeding events were HAS-BLED score, HEMORR2HAGES score, ATRIA score, Bleeding Risk Index (BRI), and ABC bleeding risk score. Thromboembolic outcomes included cerebrovascular infarction, transient ischemic attack (TIA), and systemic embolism (excluding pulmonary embolism and deep vein thrombosis). Bleeding outcomes included hemorrhagic stroke, intracranial hemorrhage (ICH), and major and minor bleeds. We excluded studies that evaluated patients exclusively from Asia, Africa, or the Middle East. We also sought to identify studies which used the same patients and linked these as companion papers to an individual study.
Data Extraction and Quality Assessment of Individual Studies
Pairs of investigators screened all citations and abstracts for eligibility, and those considered relevant by either investigator advanced to full-text review. Paired investigators then reviewed all full-text articles and resolved disagreements through discussion or adjudication by a third investigator. Paired investigators independently abstracted data and assessed study quality. Disagreements were resolved by consensus or arbitration by a third investigator. Articles that represented evidence from the same overall study were linked to avoid duplication of patient cohorts.
We assessed methodological quality, or risk of bias, for each individual study using tools specific to the study’s characteristics. For studies assessing diagnostic accuracy, we used the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.13 Our outcome-specific quality assessment classified study outcomes as containing low, medium, or high risk of bias as defined by QUADAS-2.
Data Synthesis and Analysis
We summarized key features of the included studies for each key question, including information on study design; patient characteristics; clinical settings; diagnostic tools; and intermediate, final, and adverse event outcomes. We ordered our findings by diagnostic comparison, and then within these comparisons by outcome, with long-term final outcomes emphasized.
Grouping interventions by prediction tool, we determined the feasibility of completing a quantitative synthesis (i.e., meta-analysis) based on the volume of relevant literature (at least three appropriate studies), conceptual homogeneity of the studies in terms of study population and outcomes, and completeness of the reporting of results. When at least three comparable studies reported the same outcome, we used the R statistical package (version 3.1.2) (The R Foundation) with the “metafor” meta-analysis library (version 1.9-7) to synthesize available c-statistics, which quantify the discrimination ability of studied tools, for each appropriate thromboembolic or bleeding risk prediction tool. We used the random-effects DerSimonian and Laird estimator14 to generate summary values. In addition, we used the Knapp–Hartung approach to adjust the standard errors of the estimated coefficients. Since the diagnostic tools considered are not binary, it was not possible to consider summary receiver operating characteristic (ROC) curves. When possible, the c-statistics were pooled by considering their estimated values (point estimates) and confidence intervals, and the “generic point estimates” effect specification option in the Comprehensive Meta-Analysis software. For a clinical prediction rule, we assumed that a c-statistic <0.6 had no clinical value, 0.6–0.7 had limited value, 0.7–0.8 had modest value, and >0.8 had discrimination adequate for genuine clinical utility.15
Strength of Evidence
We assigned strength of evidence scores for each diagnostic tool using the approach described in the AHRQ’s Methods Guide.11,16 We assessed five domains: study limitations; consistency; directness; precision; and reporting bias, which includes publication bias, outcome reporting, and analysis reporting bias. These domains were considered qualitatively, and a summary rating of high, moderate, or low strength of evidence was assigned for each outcome after independent assessment and discussion by two reviewers. In cases where ratings were impossible or imprudent to make, a grade of “insufficient” was assigned.
Role of the Funding Source
This topic was nominated and funded by PCORI for systematic review by an EPC in partnership with AHRQ. A representative from AHRQ served as a Contracting Officer’s Representative (COR) and provided technical assistance during the conduct of the full evidence report. The AHRQ COR and PCORI program officers provided comments on draft versions of the protocol and full evidence report. PCORI and AHRQ did not directly participate in the literature search; determination of study eligibility criteria; data analysis or interpretation; or preparation, review, or approval of the manuscript for publication.
RESULTS
We screened 11,274 publications and found 45 articles (25 studies) for KQ1 and 34 articles (18 studies) for KQ2 that investigated our included tools for determining stroke or bleeding risk in patients with nonvalvular AF and that met the other inclusion criteria. We combined these newly identified studies with those included in the 2013 review, yielding total of 83 articles (61 studies) for KQ1 and 57 articles (38 studies) for KQ2 included in this updated review (Figure 1). Complete results of the review, including long-term stroke and bleeding risk summaries, are in the full report.
Figure 1. Literature Flow Diagram.
Abbreviation: KQ=Key QuestionFigure 2A.
Predicting Thromboembolic Risk in Patients with AF
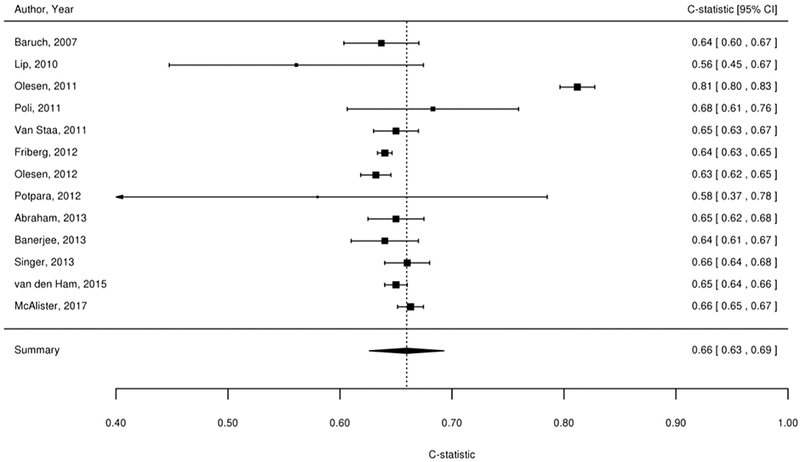
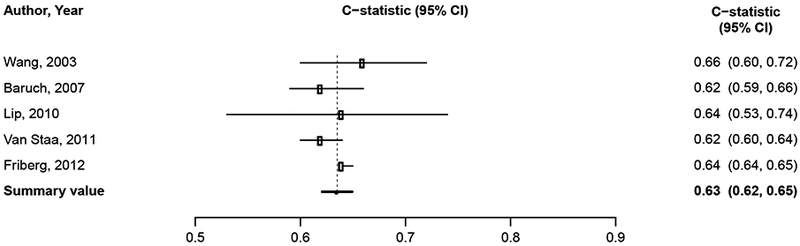
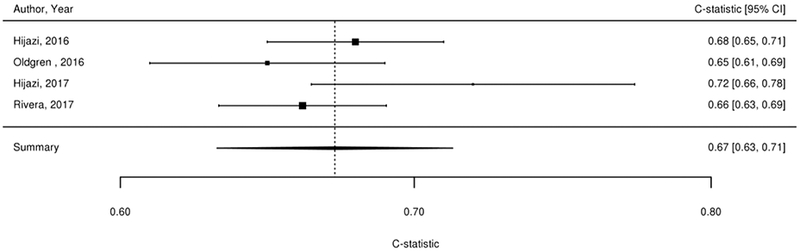
We considered findings from the 61 studies reporting the predictive value of the CHADS2, CHA2DS2-VASc, Framingham, and ABC stroke clinical tools for thromboembolic risk (Table 1). Twenty-nine studies directly compared the predictive ability for thromboembolic events of the CHADS2 risk score with other risk scores,17-45 24 compared CHA2DS2-VASc,18-21,23,24,26,37,39-54 6 compared Framingham,18,24,33,34,37,55 and 4 compared ABC stroke.54,56-58 C-statistics for predicting thromboembolic risk, when available, are reported in Appendix Table 3. Sufficient data existed to permit meta-analysis of studies evaluating c-statistics for the CHADS2 score using a continuous score (Figure 2A) and categorical score (Figure 2B), the CHA2DS2-VASc continuous score (Figure 2C) and categorical score (Figure 2D), the Framingham categorical score (Figure 2E), and the ABC stroke categorical score (Figure 2F). For both the continuous and categorical CHADS2 scores (continuous: 14 studies with 489,335 patients; categorical: 16 studies, 548,464 patients; Table 2A), there was moderate strength of evidence that the scores provide limited prediction of stroke events (continuous: c-statistic of 0.69; 95% CI 0.66 to 0.73; categorical: c-statistic of 0.66; 95% CI 0.63 to 0.69). There was also moderate strength of evidence (16 studies, 511,481 patients) that the continuous CHA2DS2-VASc score provides limited prediction of stroke events (c-statistic of 0.66; 95% CI 0.63 to 0.69). For the categorical CHA2DS2-VASc score (13 studies, 496,683 patients), there was low strength evidence of its ability to predict stroke risk (c-statistic of 0.64; 95% CI 0.58 to 0.70. Based on a meta-analysis of 6 studies (282,572 patients), we found moderate strength of evidence that the categorical Framingham score provides limited prediction of stroke events (c-statistic of 0.63; 95% CI 0.62 to 0.65). For the categorical ABC score (4 studies, 25,614 patients), we found a moderate strength of evidence of limited prediction of stroke events (c-statistic of 0.67; 95% CI 0.63 to 0.71) (Table 2A).
Table 1.
Description and Interpretation of Included Risk Scores
| Thromboembolic Risk Score |
Reference | Risk Factors Included | Interpretation |
|---|---|---|---|
| CHADS2 | Gage, 200135 | Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, prior Stroke/transient ischemic attack [2 points] | Low (0), moderate (1-2), high (3-6) |
| CHA2DS2-VASc | Lip, 201037 | Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥75 [2 points], Diabetes mellitus, prior Stroke/transient ischemic attack/thromboembolism [2 points], Vascular disease, Age 65–74, Sex category female | Low (0), moderate (1), high (2-9) |
| Framingham | Advancing age, female sex, increasing systolic blood pressure, prior stroke or transient ischemic attack, and diabetes | ||
| ABC | Hijazi, 201693 | Age, Biomarkers (cTnI-hs and NT-proBNP), and Clinical history (prior stroke/TIA) | Low <1%, moderate 1%-2%, high >2% |
| Bleeding Risk Score |
Reference | Risk Factors Included | Interpretation |
| ABC | Hijazi, 201693 | Age, biomarkers [GDF-15, cTnT-hs, and haemoglobin], and clinical history [previous bleeding] | Low <1%, medium 1-2%, high >2% |
| ATRIA | Fang, 201176 | Anemia, renal disease (CrCl <30) (3 points each); age ≥75 (2 points); any prior bleeding, hypertension (1 point each) | Low (0-3), moderate (4), high (5-10) |
| BRI | Beyth, 1998109 | Age ≥65, GI bleed in past 2 weeks, previous stroke, comorbidities (recent MI, hematocrit <30%,diabetes, creatinine >1.5), with 1 point for presence of each condition and 0 if absent | Low (0), moderate (1-2), high (3-4) |
| HAS-BLED | Pisters, 20109 | Hypertension, abnormal renal (CrCl <50) or liver function (1 point each); stroke, bleeding history or predisposition, labile INR (TTR <60%), age >65, drugs of interest/alcohol (1 point each) | Low (0), moderate (1-2), high (≥3) |
| HEMORR2HAGES | Gage, 200679 | Liver/renal disease, ethanol abuse, malignancy, age >75, low platelet count or function, re-bleeding risk, uncontrolled hypertension, anemia, genetic factors (CYP2C9), risk of fall or stroke (1 point for each risk factor present with 2 points for previous bleed) | Low (0-1), moderate (2-3), high (≥4) |
ABC=age, biomarkers, clinical history; ATRIA=Anticoagulation and Risk Factors in Atrial Fibrillation; BRI=Bleeding Risk Index; cTnT-hs=high-sensitivity cardiac troponin T; CrCl=creatinine clearance; GDF=growth differentiation factor-15; GI=gastrointestinal; HAS-BLED=Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly; HEMORR2HAGES=Hepatic or renal disease, Ethanol abuse, Malignancy, Older (age >75 years), Reduced platelet count or function, Re-bleeding risk (2 points), Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; INR=international normalized ratio; MI=myocardial infarction; TTR=time in therapeutic range
Figure 2A-F. Summary Estimate of C-Statistics for Prediction Ability of Clinical Tools For Thromboembolic Risk.
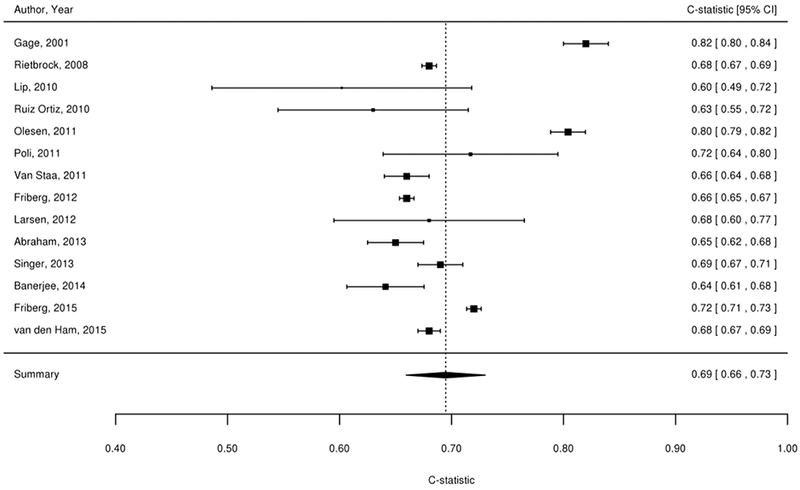
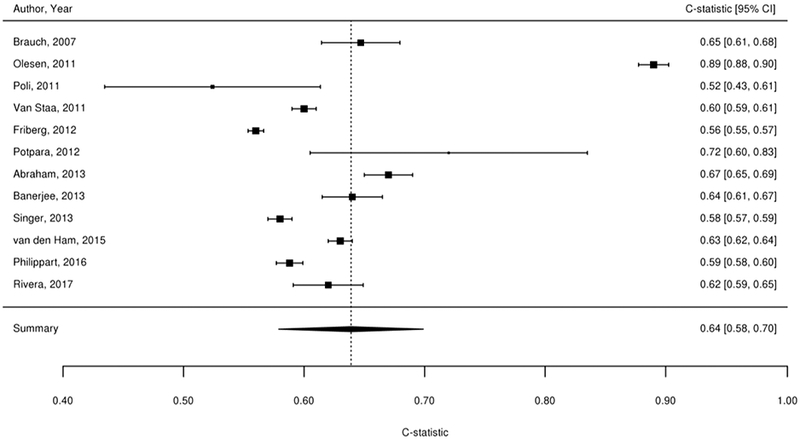
2A: CHADS2 continuous score
2B: CHADS2 categorical score
2C: CHA2DS2-VASc continuous score
2D: CHA2DS2-VASc categorical score
2E: Framingham categorical score
2F: ABC stroke categorical score
Table 2A.
Strength of Evidence Domains For Prediction of Thromboembolic Risk
| Outcome | Number of Studies (Subjects) |
Risk of Bias | Consistency | Directness | Precision | SOE and Effect (95% CI) |
|---|---|---|---|---|---|---|
| CHADS2 (Categorical) | 16 (548,464) | Observational/Moderate | Inconsistent | Direct | Precise |
SOE=Moderate Limited risk prediction ability (c-statistics 0.66, 95% CI 0.63 to 0.69) |
| CHADS2 (Continuous) | 14 (489,335) | Observational/Moderate | Inconsistent | Direct | Precise |
SOE=Moderate Limited risk prediction ability (c-statistic=0.69; 95% CI 0.66 to 0.73) |
| CHA2DS2-VASc (Categorical) | 13 (496,683) | Observational/Moderate | Inconsistent | Direct | Imprecise |
SOE=Low Limited risk prediction ability (c-statistic=0.64; 95% CI 0.58 to 0.70) |
| CHA2DS2-VASc (Continuous) | 16 (511,481) | Observational/Moderate | Inconsistent | Direct | Precise |
SOE=Moderate Limited risk prediction ability (c-statistic=0.66; 95% CI 0.63 to 0.69) |
| Framingham (Categorical) | 6 (282,572) | Observational/Moderate | Consistent | Direct | Precise |
SOE=Moderate Limited risk prediction ability (c-statistic=0.63; 95% CI 0.62 to 0.65) |
| Framingham (Continuous) | 4 (274,538) | Observational/Moderate | Consistent | Direct | Imprecise |
SOE=Low Limited risk prediction ability (c-statistic ranges between 0.64 and 0.69 across studies) |
| ABC (Categorical) | 4 (25,614) | Observational/Moderate | Consistent | Direct | Imprecise |
SOE=Moderate Limited risk prediction ability (c-statistic=0.67; 95% CI 0.63 to 0.71) |
| Imaging Risk Tools | 7 (4,962) | Observational/Moderate | Inconsistent | Direct | Imprecise | SOE=Insufficient |
Abbreviations: CHADS2=Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, prior Stroke/transient ischemic attack (2 points); CHA2DS2-VASc=Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥75 (2 points), Diabetes mellitus, prior Stroke/transient ischemic attack/thromboembolism (2 points), Vascular disease, Age 65–74, Sex category female; CI=confidence interval; INR=international normalized ratio; NA=not applicable; SOE=strength of evidence
Seven imaging studies examined specific anatomical findings and their association with stroke risk in patients with AF.59-65 Imaging studies included magnetic resonance imaging, magnetic resonance angiography quantification of left atrial appendage dimensions, transesophageal echocardiography, and transthoracic echocardiography. There was insufficient evidence for the relationship between findings on echocardiography (transthoracic) and subsequent stroke based on 7 studies (4 low risk of bias, 3 medium risk of bias; 4,962 patients) that reported discrepant results.
We found 20 studies that evaluated either the predictive role of international normalized ratio (INR), pattern of atrial fibrillation, renal impairment, or other risk factors.31,43,48,57,66-75 There was insufficient evidence, however, for further meta-analysis of the results. These abstracted data are in the full report.
Predicting Bleeding Risk in Patients with AF
Of the 38 studies which explored bleeding risk in patients with AF, 26 studies evaluated various risk scores (BRI, HEMORR2HAGES, HAS-BLED, ATRIA, ABC) for estimating the outcome of major bleeding risk in patients with AF, including patients on warfarin, aspirin, and no antithrombotic therapy.9,18,21,22,46,54,76-97 Thirteen studies (10 low risk of bias, 2 medium risk of bias, 1 high risk of bias; 351,985 patients) compared different risk scores (BRI, HEMORR2HAGES, HAS-BLED, ATRIA, ABC) in predicting major bleeding events in AF patients on warfarin. These studies differed markedly in population, major bleeding rates, and statistics reported for evaluating risk prediction scores for major bleeding events.
Assessment of major bleeding events based on individual risk factors was reported by 17 studies (Appendix Table 4). Eight of these (7 low risk of bias, 1 medium risk of bias; 322,010 patients) evaluated the risk of major bleeding in patients with chronic kidney disease (CKD). All studies demonstrated increased risk of bleeding in patients with CKD (moderate strength of evidence). Other risk factors abstracted included the impact of INR, age, prior stroke, presence of heart disease, diabetes mellitus, sex, cancer, race/ethnicity, and cognitive impairment however evidence was insufficient to support findings (results in full report).
Most available studies for KQ2 included ICH within the outcome “major bleeding,” but three studies presented this outcome separately. One of these studies evaluated both HAS-BLED and HEMORR2HAGES,18 another study evaluated both HAS-BLED and ATRIA97 and a third study evaluated INR.66 The single included study comparing HAS-BLED and HEMORR2HAGES did not show a statistically significant difference between the risk scores in prediction abilities for ICH in any patient population. Better understanding ICH risk prediction will be particularly important, because this represents the most devastating variety of major bleeding event that patients on anticoagulation suffer.
The comparative risk discrimination abilities of each clinical tool was evaluated, when data were available, for (1) major bleeding risk in AF patients on warfarin, (2) AF patients on aspirin alone, (3) AF patients not on therapy, and (4) ICH risk in AF patients on warfarin (see Appendix Table 5 for c-statistics). For AF patients on warfarin, evidence favored HAS-BLED based on two studies demonstrating that it has significantly higher prediction (by c-statistic) for major bleeding events than other scores among patients on warfarin, but the majority of studies showed no statistically significant differences in prediction abilities, reducing the strength of evidence (moderate; Table 2B). For AF patients on aspirin alone, three studies (2 low risk of bias, 1 medium risk of bias; 177,538 patients) comparing different combinations of bleeding risk scores (BRI, HEMORR2HAGES, and HAS-BLED) in predicting major bleeding events showed no statistically significant differences (low strength of evidence). Among AF patients not on therapy, six studies (4 low risk of bias, 2 medium risk of bias; 310,607 patients) comparing different combinations of bleeding risk scores (BRI, HEMORR2HAGES, HAS-BLED, and ATRIA) in predicting major bleeding events showed no statistically significant differences (low strength of evidence). Evaluating ICH in AF patients on warfarin, one study (low risk of bias; 48,599 patients) compared HEMORR2HAGES and HAS-BLED in predicting ICH. This study showed no statistically significant difference in prediction abilities between the two scores (low strength of evidence).
Table 2B.
Strength of Evidence Domains For Prediction of Bleeding Riska
| Outcome | Number of Studies (Subjects) |
Risk of Bias | Consistency | Directness | Precision | SOE and Effect (95% CI) |
|---|---|---|---|---|---|---|
| Summary c-statistic (Patients on Warfarin) | ||||||
| BRI | 4 (11,939) | Observational/Moderate | Consistent | Direct | Precise |
SOE=Moderate Limited risk discrimination ability (c-statistic ranging from 0.56 to 0.65) |
| HEMORR2HAGES | 10 (115,348) | Observational/Moderate | Consistent | Direct | Imprecise |
SOE=Moderate Limited risk discrimination ability (c-statistic ranging from 0.53 to 0.78) |
| HAS-BLED | 11 (194,839) | Observational/Moderate | Consistent | Direct | Imprecise |
SOE=Moderate Modest risk discrimination ability (c-statistic ranging from 0.50 to 0.80) |
| ATRIA | 7 (76,163) | Observational/Moderate | Inconsistent | Direct | Imprecise | SOE=Insufficient |
| ABC | 1 (22,998) | Observational/Moderate | NA | Direct | Precise |
SOE=Low Limited risk discrimination (c-statistic of 0.65 in validation study) |
| Comparative Risk Discrimination Abilities | ||||||
| Major bleeding events among patients with AF on warfarin | 13 (351,985) | Observational/Moderate | Consistent | Direct | Imprecise |
SOE=Moderate Favors HAS-BLED |
| Intracranial hemorrhage among patients with AF on warfarin | 2 (71,597) | Observational/Moderate | NA | Direct | Precise |
SOE=Low No evidence of a difference |
| Major bleeding events among patients with AF on aspirin alone | 3 (177,538) | Observational/Moderate | Inconsistent | Direct | Imprecise |
SOE=Low No evidence of a difference |
| Major bleeding events among patients with AF not on antithrombotic therapy | 6 (310,607) | Observational/Moderate | Consistent | Direct | Imprecise |
SOE=Low No evidence of a difference |
C-statistics given are for categorical risk scores unless otherwise noted.
ABC=age, biomarkers, clinical history; AF=atrial fibrillation; ATRIA=Anticoagulation and Risk Factors in Atrial Fibrillation; BRI=Bleeding Risk Index; CHADS2=Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, prior Stroke/transient ischemic attack (2 points); CHA2DS2-VASc=Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥75 (2 points), Diabetes mellitus, prior Stroke/transient ischemic attack/thromboembolism (2 points), Vascular disease, Age 65–74, Sex category female; CI=confidence interval; HAS-BLED=Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly; HEMORR2HAGES=Hepatic or renal disease, Ethanol abuse, Malignancy, Older (age >75 years), Reduced platelet count or function, Re-bleeding risk (2 points), Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; INR=international normalized ratio; KQ=Key Question; NA=not applicable; SOE=strength of evidence
DISCUSSION
Our review included studies comparing the diagnostic accuracy and impact on clinical decisionmaking of available clinical tools, imaging tools, and associated risk factors for predicting thromboembolic and bleeding risk in patients with atrial fibrillation. For predicting thromboembolic risk, the CHADS2, CHA2DS2-VASc, and ABC scores appeared similar and had the best predictive abilities given the available evidence, but this advantage was not substantial on an absolute basis. Imaging risk tools, however, found conflicting results when the presence of a left atrial thrombus was assessed, and there was insufficient evidence to support conclusions regarding the predictive ability of the presence of a left atrial thrombus. Among the tools for predicting risk of major bleeding and ICH, there was a suggestion that HAS-BLED is the best score for predicting major bleeds in patients on warfarin, although it only has modest prediction abilities. However, the majority of studies for other patient scenarios showed no statistically significant differences in predictive accuracy among tools.
Findings in Relation to What is Already Known
ESC guidelines recommend using the CHA2DS2VASc score, and AHA guidelines recommend using the CHADS2 or CHA2DS2-VASc to categorize thromboembolic risk when making treatment decisions in patients with atrial fibrillation.98 Additionally, recent ACCP, ANZ, and APHRS guidelines endorse using the CHA2DS2-VASc score (excluding sex in the calculation under ACCP and ANZ guidelines) to identify low risk patients that can be excluded from anticoagulation.99-101 In the current CER, we found that of the available risk scores, the CHADS2 and CHA2DS2VASc scores are the most commonly studied and that the CHADS2, CHA2DS2-VASc, and ABC risk scores appeared to be similar and to have the highest predictive ability for stroke events. While some studies have explored the inclusion of biomarkers in stroke risk scores such as the ABC stroke risk score, and preliminary evidence supports the ABC score being comparable to CHADS2 and CHA2DS2-VASc, the experience with ABC is limited and more data are needed on the contribution of these and other biomarkers to the overall risk assessment. Further, few comparisons of the ABC score in predicting thromboembolic risk have been completed in “real world” populations, which may better clarify its predictive ability.102
In predicting bleeding risk, our review found limited evidence favoring the HAS-BLED risk score based on two studies demonstrating that it has a significantly higher predictive ability for major bleeding events than other scores among patients on warfarin. The majority of studies, however, showed no statistically significant differences in prediction, which reduced the SOE. Recent evidence suggests that inclusion of time to therapeutic range (TTR), included in the HAS-BLED score, might enhance the predictive ability of other bleeding scores.54,87 Bleeding risk scores are not included in the most recent AHA/ACC guideline recommendations on AF, and they are generally not used to decide whether to prescribe an oral anticoagulant to individual patients. However, bleeding risk scores may inform shared decision making discussions of the risks of stroke and bleeding incorporating patients’ values and preferences. As more data on stroke and bleeding risk scores emerge, it is possible that improvement in the tools and methods for risk stratification of both stroke and bleeding will be important to better individualize treatment using different oral anticoagulants in patients with AF.
Limitations of the Evidence Base and the Comparative Effectiveness Review Process
Comparisons across studies were difficult due to varying categorical arrangements of stroke risk scores, inter-study differences in approach to calculating some of the bleeding risk scores, limited comparison of bleeding risk scores across populations, heterogeneous patient populations, and the variability in treating patients with antiplatelets and oral anticoagulants. It is known that risk scores correlate to differing event rates based on patient setting and treatment, such as whether they are in a clinical trial or in the outpatient setting, which further added to between study event rate discrepancies.103 Additionally, there was inconsistency among individual studies in reporting measures of calibration, strength of association, and diagnostic accuracy. While the nature of a meta-analysis precludes the ability to directly account for individual study-level bias, we were able to carefully assess for risk of bias, consistency, directness, precision, and strength of evidence as outlined by best practice guidelines in systematic review methodology.
Further, our conclusions may be limited by the limitations in the development and validation of risk scores. Specifically, although many of the studies use clinical data sources to derive or validate these risk scores, some studies relied on billing data and institutional electronic medical records to identify patients with AF and comorbidity information, which could underestimate stroke risk due to lack of clinical adjudication of events. Likewise, lack of validated results or common event definitions for the endpoints of thromboembolism and bleeding could have underestimated the performance of these risk scores. Additionally, lack of standard definitions for comorbidities such as heart failure, diabetes mellitus, and hypertension could also lead to discrepancies across studies validating the various risk scores. Moreover, our review included both ambulatory and hospitalized patients, which inherently introduces bias in comparing studies and results give the heterogeneity with regards to stability of covariates, concomitant medications, stroke inducing procedures, etc.
Our review methods also had limitations. Our study was limited to English-language publications and excluded studies conducted exclusively in Asia, Africa, or the Middle East. We also limited our analysis to studies published since 2000 as the recent literature was considered the most relevant to today’s clinical and policy uncertainties. Lastly, we were unable to include systematic review of all available clinical risk score tools for stroke and bleeding risk. We are aware of other tools, such as QStroke and ORBIT scores, but our scope was focused on the scores used most frequently in clinical settings and prioritized through the stakeholder panel and topic refinement process with PCORI.
Research Recommendations
In our analyses, we have identified several areas for recommended future research. Given the aforementioned limitations of the currently available studies, further studies are needed that: (1) utilize complete data; (2) use validated clinical outcomes; and (3) compare all available risk scores using consistent and appropriate statistical evaluations.
Despite the availability and validation of numerous tools for both stroke and bleeding risk assessment in patients with nonvalvular AF, meaningful comparisons of the tools could not be performed in this CER. Although the 2014 AHA/ACC guideline recommends using the CHA2DS2-VASc score for stroke risk stratification and that all patients with a CHA2DS2-VASc score of ≥2 be considered for oral anticoagulant therapy, the guideline acknowledged the limitation of current risk tools, including the CHA2DS2-VASc score, to identify patients at high risk for thromboembolic risk. As a response to this poor predictive ability in high-risk patients, recently published ACCP, ANZ, and APHRS guidelines suggest using the CHA2DS2-VASc score to identify low-risk patients in the initial step of determining whether antithrombotic therapy should be offered.99-101 Whether biomarkers such as brain natriuretic peptide, C-reactive protein or troponin can enhance the CHA2DS2-VASc score and as a result be incorporated in guideline recommendations remains to be seen.
Also, the current ACC/AHA guidelines7 do not recommend use of bleeding risk scores, but rather focusing on modifiable bleeding risks. Our results found moderate strength of evidence for modest risk discrimination of the HAS-BLED score; how this modestly predictive score could potentially be utilized in clinical treatment decisions has yet to be investigated. Preliminary data in non-clinical-trial populations show that biomarkers may not enhance risk scores’ predictive ability of bleeding risk and further research is needed to conclusively determine whether biomarkers (e.g., brain natriuretic peptide, C-reactive protein or troponin) can enhance these scores.102,104
With the growing prevalence of digitized medical records, there is an opportunity to continue to evaluate and modify risk prediction tools to improve their accuracy in predicting stroke and bleeding risk, particularly with newer anticoagulants diffusing into clinical practice. These records might also facilitate research investigating risk as a non-static variable, observing changes in risk factors as predictive for stroke or bleeding events.105,106 Also, newer clinical markers (e.g., MRI to assess scar), comorbidities (i.e., renal failure, etc.) and biomarkers should be tested and validated with or alongside current risk tools to improve their prediction of both stroke and bleeding risks. Additionally, more specific guidelines on how to use risk scores and apply necessary therapies, possibly in the form of physician decision-support tools, will be important for clinical decisionmaking. Efforts to create computer-based clinical decision-making supporting tools are ongoing and may represent a way to better integrate clinical risk tools into practice.107,108 Preliminary evidence of such decision support systems is discussed within the full AHRQ report.
In addition, although we are able to identify patients at risk for stroke, many of these patients are also at a high risk for bleeding. Thus, there is a need for a score that could be used for decisionmaking about antithrombotic therapy in AF patients taking into account both thromboembolic and bleeding risks. Scores that identify only patients at risk for stroke or only those at risk for bleeding are not so helpful since the clinical factors in these scores are usually similar and treatments which reduce one or the other risk may increase the other for the same patient. Another challenge is that both stroke events and bleeding events are on a spectrum of severity and therefore predicting overall stroke might not align with outcomes that matter most to patients. For example, some strokes may have symptoms lasting <24 hours with complete resolution, whereas others can cause death. It may be good for future risk tools to account for differences in severity of outcomes. Another research need specific to bleeding risk is a prospective comparison of the standard deviation of transformed international normalized ratio (SDTINR) and time in therapeutic range (TTR) to establish which variable has better predictive accuracy for major bleeding including ICH.
Additionally, even assuming an optimal risk prediction score can be identified, further work is needed to clarify how scores should be used prospectively in clinical practice. Clinical risk scores must take into account the balance between simplicity and practicality versus accurate prediction, especially in a high capacity clinical environment. While clinical risk scores are necessarily reductionist and cannot feasibly consider all patient parameters, our results here show moderate predictive ability of risk scores that can be calculated relatively easily from patient history and demographics. Future research might explore this trade-off between ease of implementation and increasing the predictive value of clinical risk scores with more difficult-to-obtain parameters such as biomarkers.
Conclusions
Overall, we found that CHADS2, CHA2DS2-VASc, and ABC stroke scores have the best prediction for stroke events in patients with AF among the risk scores we reviewed, whereas HAS-BLED provides the best prediction for bleeding risk. Imaging tools require further evidence in regard to their appropriate use in clinical decisionmaking. Additionally, simple clinical decision tools are needed that incorporate both stroke risk and bleeding risk to assist providers treating patients with AF. Additional work will be required to develop risk tools for patients to discriminate those individuals with AF where the bleeding risk may be high enough to warrant more intensive follow-up and monitoring. These tools could be embedded into electronic medical record systems for point-of-care decisionmaking, developed into applications for smartphones and tablets, or be delivered via web-based interfaces. Additional evidence of the use of these stroke and bleeding risk scores (and clinical decision tools which balance these risks) among patients on therapy is also required.
ACKNOWLEDGMENTS
The authors thank Jamie Conklin, M.S.L.I.S., for help with the literature search and retrieval; Samantha E. Bowen, Ph.D., and Amanda J. McBroom, Ph.D., for assistance with project leadership; and Liz Wing, M.A., for editorial assistance.
Appendix
PubMed® Search Strategy (February 14, 2018)
KQ 1 & KQ 2: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic and patient outcome efficacy) of available clinical and imaging tools and associated risk factors for predicting thromboembolic risk? In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
Appendix Table 1.
Exact Search Strings
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR "Atrial Flutter"[Mesh] OR "atrial flutter"[tiab] |
| #2 | "Cerebrovascular Disorders"[Majr:NoExp] OR "Stroke"[Mesh] OR "Thromboembolism"[Mesh] OR "Hemorrhage"[Mesh:NoExp] OR "Intracranial Hemorrhages"[Mesh] OR "Brain Ischemia"[Mesh] OR "Prothrombin Time"[Mesh] OR stroke[tiab] OR strokes[tiab] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR thromboembolic[tiab] OR thromboses[tiab] OR hemorrhage[tiab] OR hemorrhages[tiab] OR hemorrhaging[tiab] OR hemorrhagic[tiab] OR haemorrhage[tiab] OR haemorrhages[tiab] OR haemorrhaging[tiab] OR haemorrhagic[tiab] OR (("bleeding"[tiab] OR bleed[tiab] OR bleeds[tiab]) AND (major[tiab] OR risk[tiab] OR event[tiab])) OR ((Systemic[tiab] OR paradoxical[tiab] OR crossed[tiab]) AND (embolism[tiab] OR embolisms[tiab])) OR ((brain[tiab] OR cerebral[tiab] OR brainstem[tiab] OR "brain stem"[tiab]) AND (ischemia[tiab] OR ischaemia[tiab] OR ischemias[tiab] OR ischaemias[tiab] OR infarction[tiab] OR infarctions[tiab])) OR (transient[tiab] AND (ischemic[tiab] OR ischaemic[tiab] OR ischaemia[tiab] OR ischemia[tiab]) AND (attack[tiab] OR attacks[tiab])) OR TIA[tiab] OR TIAs[tiab] OR "cerebrovascular accident"[tiab] OR "cerebrovascular accidents"[tiab] OR CVA[tiab] OR CVAs[tiab] OR "brain vascular accident"[tiab] OR "brain vascular accidents"[tiab] |
| #3 | "Risk"[Mesh] OR risk[tiab] OR risks[tiab] OR "Predictive Value of Tests"[Mesh] OR predict[tiab] OR predicts[tiab] OR predicting[tiab] OR predictor[tiab] OR predictors[tiab] OR predictive[tiab] |
| #4 | #1 AND #2 AND #3 |
| #5 | #4 NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #6 | #5 NOT ("Animals"[MeSH Terms] NOT "Humans"[MeSH Terms]) |
| #7 | #6 NOT (("Adolescent"[Mesh] OR "Child"[Mesh] OR "Infant"[Mesh]) NOT "Adult"[Mesh]) |
| #8 | "Randomized Controlled Trial"[Publication Type] OR "Controlled Clinical Trial"[Publication Type] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR "Clinical Trial"[Publication Type] OR "clinical trial"[tiab] OR "clinical trials"[tiab] OR "Evaluation Studies"[Publication Type] OR "Evaluation Studies as Topic"[MeSH Terms] OR "evaluation study"[tiab] OR "evaluation studies"[tiab] OR "intervention study"[tiab] OR "intervention studies"[tiab] OR "Case-control Studies"[MeSH Terms] OR "case-control"[tiab] OR "Cohort Studies"[Mesh Terms] OR cohort[tiab] OR "Longitudinal Studies"[MeSH Terms] OR longitudinal[tiab] OR longitudinally[tiab] OR "Prospective Studies"[Mesh Terms] OR "prospective"[tiab] OR prospectively[tiab] OR "Retrospective Studies"[MeSH Terms] OR "retrospective"[tiab] OR "Follow-Up Studies"[Mesh Terms] OR "follow up"[tiab] OR "Comparative Study"[Publication Type] OR "comparative study"[tiab] OR systematic[subset] OR "systematic review"[tiab] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[MeSH Terms] OR "meta-analysis"[tiab] OR "meta-analyses"[tiab] OR "meta synthesis"[tiab] OR "meta syntheses"[tiab] OR "Multicenter Study"[Publication Type] OR "Multicenter Study"[tiab] OR multicentre[tiab] OR "Registries"[Mesh Terms] OR registry[tiab] OR registries[tiab] OR "Sensitivity and Specificity"[Mesh] OR Sensitivity[tiab] OR specificity[tiab] OR valid[tiab] OR validity[tiab] OR validation[tiab] OR "validation studies"[publication type] |
| #9 | #7 AND #8 |
| #10 | #9 AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication]) |
KQ 3: What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
In patients with nonvalvular atrial fibrillation?
In specific subpopulations of patients with nonvalvular atrial fibrillation?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR "Atrial Flutter"[Mesh] OR "atrial flutter"[tiab] |
| #2 | "Cerebrovascular Disorders"[Majr:NoExp] OR "Stroke"[Mesh] OR "Thromboembolism"[Mesh] OR "Hemorrhage"[Mesh:NoExp] OR "Intracranial Hemorrhages"[Mesh] OR "Brain Ischemia"[Mesh] OR "Prothrombin Time"[Mesh] OR stroke[tiab] OR strokes[tiab] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR thromboembolic[tiab] OR thromboses[tiab] OR hemorrhage[tiab] OR hemorrhages[tiab] OR hemorrhaging[tiab] OR hemorrhagic[tiab] OR haemorrhage[tiab] OR haemorrhages[tiab] OR haemorrhaging[tiab] OR haemorrhagic[tiab] OR (("bleeding"[tiab] OR bleed[tiab] OR bleeds[tiab]) AND (major[tiab] OR risk[tiab] OR event[tiab])) OR ((Systemic[tiab] OR paradoxical[tiab] OR crossed[tiab]) AND (embolism[tiab] OR embolisms[tiab])) OR ((brain[tiab] OR cerebral[tiab] OR brainstem[tiab] OR "brain stem"[tiab]) AND (ischemia[tiab] OR ischaemia[tiab] OR ischemias[tiab] OR ischaemias[tiab] OR infarction[tiab] OR infarctions[tiab])) OR (transient[tiab] AND (ischemic[tiab] OR ischaemic[tiab] OR ischaemia[tiab] OR ischemia[tiab]) AND (attack[tiab] OR attacks[tiab])) OR TIA[tiab] OR TIAs[tiab] OR "cerebrovascular accident"[tiab] OR "cerebrovascular accidents"[tiab] OR CVA[tiab] OR CVAs[tiab] OR "brain vascular accident"[tiab] OR "brain vascular accidents"[tiab] |
| #3 | "Risk"[Mesh] OR risk[tiab] OR risks[tiab] OR "Safety"[Mesh] OR safety[tiab] OR safe[tiab] OR "Incidence"[Mesh] OR efficacy[tiab] OR efficacious[tiab] OR "prevention and control"[Subheading] OR prevent[tiab] OR prevents[tiab] OR preventing[tiab] OR prevention[tiab] OR "Treatment Outcome"[Mesh] OR "adverse effects"[Subheading] OR side effect*[tiab] OR (adverse[tiab] AND (interaction*[tiab] or response*[tiab] or effect*[tiab] or event*[tiab] or reaction*[tiab] or outcome*[tiab])) OR (unintended[tiab] AND (interaction*[tiab] or response*[tiab] or effect*[tiab] or event*[tiab] or reaction*[tiab] or outcome*[tiab])) OR (unintentional[tiab] AND (interaction*[tiab] or response*[tiab] or effect*[tiab] or event*[tiab] or reaction*[tiab] or outcome*[tiab])) OR (unwanted[tiab] AND (interaction*[tiab] or response*[tiab] or effect*[tiab] or event*[tiab] or reaction*[tiab] or outcome*[tiab])) OR (unexpected AND (interaction*[tiab] or response*[tiab] or effect*[tiab] or event*[tiab] or reaction*[tiab] or outcome*[tiab])) OR (undesirable AND (interaction*[tiab] or response*[tiab] or effect*[tiab] or event*[tiab] or reaction*[tiab] or outcome*[tiab])) OR "drug safety"[tiab] OR "drug toxicity"[tiab] OR tolerability[tiab] OR harm[tiab] OR harms[tiab] OR harmful[tiab] OR "treatment emergent"[tiab] OR complication*[tiab] OR toxicity[tiab] |
| #4 | #1 AND #2 AND #3 |
| #5 | "Anticoagulants"[Mesh] OR "Warfarin"[Mesh] OR "Heparin"[Mesh] OR "Vitamin K/antagonists and inhibitors"[Mesh] OR "Rivaroxaban"[Mesh] OR Antithrombins[Pharmacological Action] OR "Dabigatran"[Mesh] OR "Blood Coagulation Factor Inhibitors"[Mesh] OR "Anticoagulants"[Pharmacological Action] OR "Factor Xa Inhibitors"[Pharmacological Action] OR "apixaban"[Supplementary Concept] OR "edoxaban"[Supplementary Concept] OR warfarin[tiab] OR coumadin[tiab] OR "vitamin k"[tiab] OR enoxaparin[tiab] OR lovenox[tiab] OR rivaroxaban[tiab] OR xarelto[tiab] OR dabigatran[tiab] OR pradaxa[tiab] OR heparin[tiab] OR apixaban[tiab] OR eliquis[tiab] OR edoxaban[tiab] OR lixiana[tiab] OR anticoagulant[tiab] OR anticoagulants[tiab] OR anticoagulation[tiab] OR "thrombin inhibitor"[tiab] OR "thrombin inhibitors"[tiab] OR antithrombin[tiab] OR antithrombins[tiab] OR antithrombotic[tiab] OR "factor Xa inhibitor"[tiab] OR "factor Xa inhibitors"[tiab] OR "Blood clotting inhibitor"[tiab] OR "blood clotting inhibitors"[tiab] |
| #6 | #4 AND #5 |
| #7 | "Platelet Aggregation Inhibitors"[Mesh] OR "Aspirin"[Mesh] OR "Dipyridamole"[Mesh] OR "Platelet Aggregation Inhibitors"[Pharmacological Action] OR clopidogrel[Supplementary Concept] OR clopidogrel[tiab] OR plavix[tiab] OR aspirin[tiab] OR dipyridamole[tiab] OR aggrenox[tiab] OR persantine[tiab] OR curantil[tiab] OR antiplatelet[tiab] OR anti-platelet[tiab] OR antiplatelets[tiab] OR anti-platelets[tiab] OR "platelet aggregation inhibitors"[tiab] OR "platelet aggregation inhibitor"[tiab] OR "platelet inhibitors"[tiab] OR "platelet inhibitor"[tiab] OR "platelet antagonists"[tiab] OR "platelet antagonist"[tiab] |
| #8 | #4 AND #7 |
| #9 | "atrial appendage/surgery"[Mesh Terms] OR "Septal Occluder Device"[Mesh] OR "atrial appendage"[tiab] OR "atrial appendages"[tiab] OR "atrium appendage"[tiab] OR "auricular appendage"[tiab] OR "auricular appendages"[tiab] OR LAA[tiab] OR occluder[tiab] OR occluders[tiab] OR occlusion[tiab] OR AMPLATZER[tiab] OR AtriClip[tiab] OR PLAATO[tiab] OR Watchman[tiab] OR (atrial[tiab] AND modification[tiab]) OR lariat[tiab] OR atricure[tiab] |
| #10 | #4 AND #9 |
| #11 | #6 OR #8 OR #10 |
| #12 | #11 NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #13 | #12 NOT ("Animals"[MeSH Terms] NOT "Humans"[MeSH Terms]) |
| #14 | #13 NOT (("Adolescent"[Mesh] OR "Child"[Mesh] OR "Infant"[Mesh]) NOT "Adult"[Mesh]) |
| #15 | "Randomized Controlled Trial"[Publication Type] OR "Controlled Clinical Trial"[Publication Type] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR "Clinical Trial"[Publication Type] OR "clinical trial"[tiab] OR "clinical trials"[tiab] OR "Evaluation Studies"[Publication Type] OR "Evaluation Studies as Topic"[MeSH Terms] OR "evaluation study"[tiab] OR "evaluation studies"[tiab] OR "intervention study"[tiab] OR "intervention studies"[tiab] OR "Case-control Studies"[MeSH Terms] OR "case-control"[tiab] OR "Cohort Studies"[Mesh Terms] OR cohort[tiab] OR "Longitudinal Studies"[MeSH Terms] OR longitudinal[tiab] OR longitudinally[tiab] OR "Prospective Studies"[Mesh Terms] OR "prospective"[tiab] OR prospectively[tiab] OR "Retrospective Studies"[MeSH Terms] OR "retrospective"[tiab] OR "Follow-Up Studies"[Mesh Terms] OR "follow up"[tiab] OR "Comparative Study"[Publication Type] OR "comparative study"[tiab] OR systematic[subset] OR "systematic review"[tiab] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[MeSH Terms] OR "meta-analysis"[tiab] OR "meta-analyses"[tiab] OR "meta synthesis"[tiab] OR "meta syntheses"[tiab] OR "Multicenter Study"[Publication Type] OR "Multicenter Study"[tiab] OR multicentre[tiab] OR "Registries"[Mesh Terms] OR registry[tiab] OR registries[tiab] OR "Sensitivity and Specificity"[Mesh] OR Sensitivity[tiab] OR specificity[tiab] OR valid[tiab] OR validity[tiab] OR validation[tiab] OR "validation studies"[publication type] |
| #16 | #14 AND #15 |
| #17 | #16 AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication]) |
PubMed® Search Strategy (August 14, 2012)
KQ 1: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of available clinical and imaging tools for predicting thromboembolic risk?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR (atrial[tiab] AND fibrillation[tiab]) OR afib[tiab] OR "atrial flutter"[MeSH Terms] OR "atrial flutter"[tiab] |
| #2 | chads2[tw] OR chads2-vasc[tw] OR "Magnetic Resonance Imaging"[Mesh] OR MRI[tw] OR "Cardiac Imaging Techniques"[Mesh] OR "Tomography, X-Ray Computed"[Mesh] OR "Echocardiography"[Mesh] OR ((transthoracic[tw] OR transesophageal[tw]) AND echo[tw]) OR TTE[tw] OR TEE[tw] OR CT-scan[tw] |
| #3 | "Stroke"[Mesh] OR stroke[tw] OR thromboembolism[tw] OR "Thromboembolism"[Mesh] OR thromboembolic[tw] OR "brain ischemia"[MeSH Terms] OR (brain[tw] AND ischemia[tw]) OR (brain[tw] AND ischaemia[tw]) OR (transient[tw] AND (ischemic[tw] OR ischaemic[tw] OR ischaemia[tw] OR ischemia[tw]) AND attack[tw]) OR TIA[tw] |
| #4 | #1 AND #2 AND #3 |
| #5 | (("diagnosis"[Subheading] OR "diagnosis"[tiab] OR "diagnosis"[MeSH Terms]) OR "treatment outcome"[MeSH Terms] OR outcome[tiab] OR outcomes[tiab]) OR (reliability[tw] OR accuracy[tw] OR accurate[tw] OR Sensitivity[tw] OR specificity[tw] OR "Sensitivity and Specificity"[Mesh] OR valid[tw] OR validity[tw] OR validation[tw] OR decision[tw] OR decisions[tw] OR "decision making"[MeSH Terms] OR assessment[tw]) |
| #6 | #5 AND #4 |
| #7 | #6 NOT (animals[mh] NOT humans[mh]), Limits: English, Publication Date from 2000 to present |
KQ 2: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR (atrial[tiab] AND fibrillation[tiab]) OR afib[tiab] OR "atrial flutter"[MeSH Terms] OR "atrial flutter"[tiab] |
| #2 | "Age Factors"[Mesh] OR "Dementia"[Mesh] OR "Accidental Falls"[Mesh] OR "International Normalized Ratio"[Mesh] OR age[tiab] OR dementia[tiab] OR INR[tiab] OR fall[tiab] OR falls[tiab] OR "international normalized ratio"[tiab] OR paroxysmal[tiab] OR persistent[tiab] OR permanent[tiab] OR stratification[tiab] OR classification[tiab] OR schema[tiab] OR has-bled[tiab] OR (cognitive[tw] AND impairment[tw]) OR cognition[tw] OR ((prior[tiab] OR previous[tiab] OR first[tiab]) AND stroke[tiab]) |
| #3 | "Intracranial Hemorrhages"[Mesh] OR "Hemorrhage"[Mesh:noexp] OR hemorrhage[tw] OR hemorrhaging[tw] OR bleeding[tw] OR bleed[tw] OR hemorrhagic[tw] OR haemorrhage[tw] OR haemorrhaging[tw] OR haemorrhagic[tw] |
| #4 | #1 AND #2 AND #3 |
| #5 | (("diagnosis"[Subheading] OR "diagnosis"[tiab] OR "diagnosis"[MeSH Terms]) OR "treatment outcome"[MeSH Terms] OR outcome[tiab] OR outcomes[tiab]) OR (reliability[tw] OR accuracy[tw] OR accurate[tw] OR Sensitivity[tw] OR specificity[tw] OR "Sensitivity and Specificity"[Mesh] OR valid[tw] OR validity[tw] OR validation[tw] OR decision[tw] OR decisions[tw] OR "decision making"[MeSH Terms] OR assessment[tw]) |
| #6 | #5 AND #4 |
| #7 | #6 NOT (animals[mh] NOT humans[mh]), Limits: English, Publication Date from 2000 to present |
KQ 3: What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
In patients with nonvalvular atrial fibrillation?
In specific subpopulations of patients with nonvalvular atrial fibrillation?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR (atrial[tiab] AND fibrillation[tiab]) OR afib[tiab] OR "atrial flutter"[MeSH Terms] OR "atrial flutter"[tiab] |
| #2 | "Anticoagulants"[Mesh] OR "Anticoagulants"[Pharmacological Action] OR warfarin[tw] OR "Warfarin"[Mesh] OR coumadin[tw] OR "Vitamin K/antagonists and inhibitors"[Mesh] OR vitamin k[tw] OR "Heparin"[Mesh] OR "Enoxaparin"[Mesh] OR enoxaparin[tw] OR lovenox[tw] OR "rivaroxaban" [Supplementary Concept] OR rivaroxaban[tw] OR xarelto[tw] OR "dabigatran etexilate" [Supplementary Concept] OR dabigatran[tw] OR pradaxa[tw] OR heparin[tw] OR "apixaban" [Supplementary Concept] OR apixaban[tw] OR eliquis[tw] OR "edoxaban" [Supplementary Concept] OR edoxaban[tw] OR lixiana[tw] |
| #3 | "Platelet Aggregation Inhibitors"[Mesh] OR "Platelet Aggregation Inhibitors"[Pharmacological Action] OR "clopidogrel" [Supplementary Concept]OR clopidogrel[tw] OR plavix[tw] OR "Aspirin"[Mesh] OR aspirin[tw] OR "Dipyridamole"[Mesh] OR dipyridamole[tw] OR aggrenox[tw] OR persantine[tw] OR antiplatelet[tw] OR anti-platelet[tw] OR antiplatelets[tw] OR anti-platelets[tw] |
| #4 | Atrial Appendage/surgery[mesh] OR atrial appendage[tw] OR LAA[tw] OR occluder[tw] OR AMPLATZER[tw] OR AtriClip[tw] OR PLAATO[tw] OR Watchman[tw] OR (atrial[tw] AND modification[tw]) OR "atriacure isolator"[tw] |
| #5 | "Stroke"[Mesh] OR stroke[tw] OR thromboembolism[tw] OR "Thromboembolism"[Mesh] OR thromboembolic[tw] OR "brain ischemia"[MeSH Terms] OR (brain[tw] AND ischemia[tw]) OR (brain[tw] AND ischaemia[tw]) OR (transient[tw] AND (ischemic[tw] OR ischaemic[tw] OR ischaemia[tw] OR ischemia[tw]) AND attack[tw]) OR TIA[tw] |
| #6 | #1 AND (#2 OR #3 OR #4) AND #5 |
| #7 | "evaluation studies"[Publication Type] OR "evaluation studies as topic"[MeSH Terms] OR "evaluation study"[tw] OR evaluation studies[tw] OR "intervention studies"[MeSH Terms] OR "intervention study"[tw] OR "intervention studies"[tw] OR "case-control studies"[MeSH Terms] OR "case-control"[tw] OR "cohort studies"[MeSH Terms] OR cohort[tw] OR "longitudinal studies"[MeSH Terms] OR "longitudinal"[tw] OR longitudinally[tw] OR "prospective"[tw] OR prospectively[tw] OR "retrospective studies"[MeSH Terms] OR "retrospective"[tw] OR "follow up"[tw] OR "comparative study"[Publication Type] OR "comparative study"[tw] OR systematic[subset] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[MeSH Terms] OR "meta-analysis"[tw] OR "meta-analyses"[tw] OR randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tw] OR "clinical trials"[tw] NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #8 | #7 AND #6 |
| #9 | #8 NOT (animals[mh] NOT humans[mh]), Limits: English, Publication Date from 2000 to present |
KQ 4: What are the comparative safety and effectiveness of available strategies for anticoagulation in patients with nonvalvular atrial fibrillation who are undergoing invasive procedures?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR (atrial[tiab] AND fibrillation[tiab]) OR afib[tiab] OR "atrial flutter"[MeSH Terms] OR "atrial flutter"[tiab] |
| #2 | "Anticoagulants"[Mesh] OR "Anticoagulants"[Pharmacological Action] OR warfarin[tw] OR "Warfarin"[Mesh] OR coumadin[tw] OR "Vitamin K/antagonists and inhibitors"[Mesh] OR vitamin k[tw] OR "Heparin"[Mesh] OR "Enoxaparin"[Mesh] OR enoxaparin[tw] OR lovenox[tw] OR "rivaroxaban" [Supplementary Concept] OR rivaroxaban[tw] OR xarelto[tw] OR "dabigatran etexilate" [Supplementary Concept] OR dabigatran[tw] OR pradaxa[tw] OR heparin[tw] OR "apixaban" [Supplementary Concept] OR apixaban[tw] OR eliquis[tw] OR "edoxaban" [Supplementary Concept] OR edoxaban[tw] OR lixiana[tw] |
| #3 | "Surgical Procedures, Operative"[Mesh] OR /surgery[mesh] OR ((surgical[tw] OR invasive[tw]) AND (procedure[tw] OR procedures[tw])) OR "dental care"[MeSH Terms] OR (dental[tw] AND (procedure[tw] OR procedures[tw])) OR surgery[tw] OR procedures[tiab] OR procedure[tiab] |
| #4 | #1 AND #2 AND #3 |
| #5 | "evaluation studies"[Publication Type] OR "evaluation studies as topic"[MeSH Terms] OR "evaluation study"[tw] OR evaluation studies[tw] OR "intervention studies"[MeSH Terms] OR "intervention study"[tw] OR "intervention studies"[tw] OR "case-control studies"[MeSH Terms] OR "case-control"[tw] OR "cohort studies"[MeSH Terms] OR cohort[tw] OR "longitudinal studies"[MeSH Terms] OR "longitudinal"[tw] OR longitudinally[tw] OR "prospective"[tw] OR prospectively[tw] OR "retrospective studies"[MeSH Terms] OR "retrospective"[tw] OR "follow up"[tw] OR "comparative study"[Publication Type] OR "comparative study"[tw] OR systematic[subset] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[MeSH Terms] OR "meta-analysis"[tw] OR "meta-analyses"[tw] OR randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tw] OR "clinical trials"[tw] NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #6 | #5 AND #4 |
| #7 | #7 NOT (animals[mh] NOT humans[mh]), Limits: English, Publication Date from 2000 to present |
KQ 5: What are the comparative safety and effectiveness of available strategies for switching between warfarin and other novel oral anticoagulants, in patients with nonvalvular atrial fibrillation?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR (atrial[tiab] AND fibrillation[tiab]) OR afib[tiab] OR "atrial flutter"[MeSH Terms] OR "atrial flutter"[tiab] |
| #2 | "warfarin"[MeSH Terms] OR warfarin[tw] OR coumadin[tw] |
| #3 | "antithrombins"[MeSH Terms] OR "antithrombins"[tiab] OR ("direct"[tiab] AND "thrombin"[tiab] AND "inhibitors"[tiab]) OR "direct thrombin inhibitors"[tiab] OR "antithrombins"[Pharmacological Action] |
| #4 | "Anticoagulants"[Mesh] OR "Anticoagulants" [Pharmacological Action] OR anticoagulant[tiab] OR anticoagulants[tiab] |
| #5 | "evaluation studies"[Publication Type] OR "evaluation studies as topic"[MeSH Terms] OR "evaluation study"[tw] OR evaluation studies[tw] OR "intervention studies"[MeSH Terms] OR "intervention study"[tw] OR "intervention studies"[tw] OR "case-control studies"[MeSH Terms] OR "case-control"[tw] OR "cohort studies"[MeSH Terms] OR cohort[tw] OR "longitudinal studies"[MeSH Terms] OR "longitudinal"[tw] OR longitudinally[tw] OR "prospective"[tw] OR prospectively[tw] OR "retrospective studies"[MeSH Terms] OR "retrospective"[tw] OR "follow up"[tw] OR "comparative study"[Publication Type] OR "comparative study"[tw] OR systematic[subset] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[MeSH Terms] OR "meta-analysis"[tw] OR "meta-analyses"[tw] OR randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tw] OR "clinical trials"[tw] NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #6 | #1 AND #2 AND (#3 OR #4) AND #5 |
| #7 | #6 NOT (animals[mh] NOT humans[mh]), Limits: English, Publication Date from 2000 to present |
KQ 6: What are the comparative safety and effectiveness of available strategies for resuming anticoagulation therapy or performing a procedural intervention as a stroke prevention strategy following a hemorrhagic event (stroke, major bleed, or minor bleed) in patients with nonvalvular atrial fibrillation?
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR (atrial[tiab] AND fibrillation[tiab]) OR afib[tiab] OR "atrial flutter"[MeSH Terms] OR "atrial flutter"[tiab] |
| #2 | "Anticoagulants"[Mesh] OR "Anticoagulants"[Pharmacological Action] OR warfarin[tw] OR "Warfarin"[Mesh] OR coumadin[tw] OR "Vitamin K/antagonists and inhibitors"[Mesh] OR vitamin k[tw] OR "Heparin"[Mesh] OR "Enoxaparin"[Mesh] OR enoxaparin[tw] OR lovenox[tw] OR "rivaroxaban" [Supplementary Concept] OR rivaroxaban[tw] OR xarelto[tw] OR "dabigatran etexilate" [Supplementary Concept] OR dabigatran[tw] OR pradaxa[tw] OR heparin[tw] OR "apixaban" [Supplementary Concept] OR apixaban[tw] OR eliquis[tw] OR "edoxaban" [Supplementary Concept] OR edoxaban[tw] OR lixiana[tw] |
| #3 | "Intracranial Hemorrhages"[Mesh] OR "Hemorrhage"[Mesh:noexp] OR hemorrhage[tw] OR hemorrhaging[tw] OR bleeding[tw] OR bleed[tw] OR hemorrhagic[tw] OR haemorrhage[tw] OR haemorrhaging[tw] OR haemorrhagic[tw] |
| #4 | Resume[tiab] OR resumed[tiab] OR restart[tiab] OR restarted[tiab] OR restarting[tiab] OR re-initiate[tiab] OR reinitiate[tiab] OR continue[tiab] OR continued[tiab] OR start[tiab] OR "time factors"[MeSH Terms] OR resumption[tiab] OR reinitiating[tiab] OR resuming[tiab] OR continuing[tiab] |
| #5 | #1 AND #2 AND #3 AND #4 |
| #6 | "evaluation studies"[Publication Type] OR "evaluation studies as topic"[MeSH Terms] OR "evaluation study"[tw] OR evaluation studies[tw] OR "intervention studies"[MeSH Terms] OR "intervention study"[tw] OR "intervention studies"[tw] OR "case-control studies"[MeSH Terms] OR "case-control"[tw] OR "cohort studies"[MeSH Terms] OR cohort[tw] OR "longitudinal studies"[MeSH Terms] OR "longitudinal"[tw] OR longitudinally[tw] OR "prospective"[tw] OR prospectively[tw] OR "retrospective studies"[MeSH Terms] OR "retrospective"[tw] OR "follow up"[tw] OR "comparative study"[Publication Type] OR "comparative study"[tw] OR systematic[subset] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[MeSH Terms] OR "meta-analysis"[tw] OR "meta-analyses"[tw] OR randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tw] OR "clinical trials"[tw] NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #7 | #5 AND #6 |
| #8 | #7 NOT (animals[mh] NOT humans[mh]), Limits: English, Publication Date from 2000 to present |
Embase® Search Strategy (February 14, 2018)
Platform: Embase.com
KQ 1 & KQ 2: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic and patient outcome efficacy) of available clinical and imaging tools and associated risk factors for predicting thromboembolic risk? & In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
| #1 | 'atrial fibrillation'/exp OR 'heart atrium flutter'/exp OR 'atrial fibrillation':ab,ti OR 'atrial flutter':ab,ti |
| #2 | 'cerebrovascular disease'/de OR 'cerebrovascular accident'/exp OR 'thromboembolism'/exp OR 'bleeding'/de OR 'brain hemorrhage'/exp OR 'brain ischemia'/exp OR 'prothrombin time'/exp OR stroke:ab,ti OR strokes:ab,ti OR thromboembolism:ab,ti OR thromboembolisms:ab,ti OR thromboembolic:ab,ti OR thromboses:ab,ti OR hemorrhage:ab,ti OR hemorrhages:ab,ti OR hemorrhaging:ab,ti OR hemorrhagic:ab,ti OR haemorrhage:ab,ti OR haemorrhages:ab,ti OR haemorrhaging:ab,ti OR haemorrhagic:ab,ti OR ((bleeding OR bleed OR bleeds) NEAR/2 (major OR risk OR event)):ab,ti OR ((systemic OR paradoxical OR crossed) NEXT/2 (embolism OR embolisms)):ab,ti OR ((brain OR cerebral OR brainstem OR 'brain stem') NEXT/2 (ischemia OR ischaemia OR ischemias OR ischaemias OR infarction OR infarctions)):ab,ti OR (transient NEXT/2 (ischemic OR ischaemic OR ischaemia OR ischemia) NEXT/2 (attack OR attacks)):ab,ti OR tia:ab,ti OR tias:ab,ti OR 'cerebrovascular accident':ab,ti OR 'cerebrovascular accidents':ab,ti OR cva:ab,ti OR cvas:ab,ti OR 'brain vascular accident':ab,ti OR 'brain vascular accidents':ab,ti |
| #3 | 'risk'/exp OR risk:ab,ti OR risks:ab,ti OR 'prediction and forecasting'/exp OR predict:ab,ti OR predicts:ab,ti OR predicting:ab,ti OR predictor:ab,ti OR predictors:ab,ti OR predictive:ab,ti |
| #4 | #1 AND #2 AND #3 |
| #5 | #4 NOT ('case report'/exp OR 'case study'/exp OR 'a case report':ti OR ': case report':ti OR 'editorial'/exp OR 'letter'/exp OR 'note'/exp OR [conference abstract]/lim) |
| #6 | #5 AND [humans]/lim |
| #7 | #6 NOT (('adolescent'/exp OR 'child'/exp) NOT 'adult'/exp) |
| #8 | 'clinical trial'/exp OR 'clinical study'/exp OR 'controlled study'/exp OR randomized:ab,ti OR randomised:ab,ti OR randomization:ab,ti OR randomisation:ab,ti OR randomly:ab,ti OR placebo:ab,ti OR trial:ab,ti OR groups:ab,ti OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR crossover*:ab,ti OR (cross NEXT/1 over*):ab,ti OR 'clinical trial':ab,ti OR 'clinical trials':ab,ti OR 'clinical study':ab,ti OR 'clinical studies':ab,ti OR 'evaluation study'/exp OR 'evaluation study':ab,ti OR 'evaluation studies':ab,ti OR 'intervention study':ab,ti OR 'intervention studies':ab,ti OR 'case-control':ab,ti OR 'cohort analysis'/exp OR cohort:ab,ti OR longitudinal:ab,ti OR longitudinally:ab,ti OR 'prospective':ab,ti OR prospectively:ab,ti OR 'retrospective':ab,ti OR 'follow up'/exp OR 'follow up':ab,ti OR 'comparative study'/exp OR 'comparative study':ab,ti OR 'comparative studies':ab,ti OR 'systematic review':ab,ti OR 'meta-analysis':ab,ti OR 'meta-analyses':ab,ti OR 'meta synthesis':ab,ti OR 'meta syntheses':ab,ti OR 'survival analysis'/exp OR 'multicenter study'/exp OR 'multicenter study':ab,ti OR multicentre:ab,ti OR 'register'/exp OR registry:ab,ti OR registries:ab,ti OR 'sensitivity and specificity'/exp OR sensitivity:ab,ti OR specificity:ab,ti OR valid:ab,ti OR validity:ab,ti OR validation:ab,ti OR 'validation study'/exp |
| #9 | #7 AND #8 |
| #10 | #9 AND [1-8-2011]/sd |
| #11 | #10 AND [embase]/lim NOT [medline]/lim |
KQ 3: What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
In patients with nonvalvular atrial fibrillation?
In specific subpopulations of patients with nonvalvular atrial fibrillation?
| #1 | 'atrial fibrillation'/exp OR 'heart atrium flutter'/exp OR 'atrial fibrillation':ab,ti OR 'atrial flutter':ab,ti |
| #2 | 'cerebrovascular disease'/de OR 'cerebrovascular accident'/exp OR 'thromboembolism'/exp OR 'bleeding'/de OR 'brain hemorrhage'/exp OR 'brain ischemia'/exp OR 'prothrombin time'/exp OR stroke:ab,ti OR strokes:ab,ti OR thromboembolism:ab,ti OR thromboembolisms:ab,ti OR thromboembolic:ab,ti OR thromboses:ab,ti OR hemorrhage:ab,ti OR hemorrhages:ab,ti OR hemorrhaging:ab,ti OR hemorrhagic:ab,ti OR haemorrhage:ab,ti OR haemorrhages:ab,ti OR haemorrhaging:ab,ti OR haemorrhagic:ab,ti OR ((bleeding OR bleed OR bleeds) NEAR/2 (major OR risk OR event)):ab,ti OR ((systemic OR paradoxical OR crossed) NEXT/2 (embolism OR embolisms)):ab,ti OR ((brain OR cerebral OR brainstem OR 'brain stem') NEXT/2 (ischemia OR ischaemia OR ischemias OR ischaemias OR infarction OR infarctions)):ab,ti OR (transient NEXT/2 (ischemic OR ischaemic OR ischaemia OR ischemia) NEXT/2 (attack OR attacks)):ab,ti OR tia:ab,ti OR tias:ab,ti OR 'cerebrovascular accident':ab,ti OR 'cerebrovascular accidents':ab,ti OR cva:ab,ti OR cvas:ab,ti OR 'brain vascular accident':ab,ti OR 'brain vascular accidents':ab,ti |
| #3 | 'risk'/exp OR risk:ab,ti OR risks:ab,ti OR 'safety'/exp OR safety:ab,ti OR safe:ab,ti OR 'incidence'/exp OR efficacy:ab,ti OR efficacious:ab,ti OR 'prevention':lnk OR prevent:ab,ti OR prevents:ab,ti OR preventing:ab,ti OR prevention:ab,ti OR 'treatment outcome'/exp OR 'adverse drug reaction':lnk OR (side NEXT/1 effect*):ab,ti OR (adverse NEXT/3 (interaction* OR response* OR effect* OR event* OR reaction* OR outcome*)):ab,ti OR (unintended NEXT/3 (interaction* OR response* OR effect* OR event* OR reaction* OR outcome*)):ab,ti OR (unintentional NEXT/3 (interaction* OR response* OR effect* OR event* OR reaction* OR outcome*)):ab,ti OR (unwanted NEXT/3 (interaction* OR response* OR effect* OR event* OR reaction* OR outcome*)):ab,ti OR (unexpected NEXT/3 (interaction* OR response* OR effect* OR event* OR reaction* OR outcome*)):ab,ti OR (undesirable NEXT/3 (interaction* OR response* OR effect* OR event* OR reaction* OR outcome*)):ab,ti OR 'drug safety':ab,ti OR 'drug toxicity':ab,ti OR tolerability:ab,ti OR harm:ab,ti OR harms:ab,ti OR harmful:ab,ti OR 'treatment emergent':ab,ti OR complication*:ab,ti OR toxicity:ab,ti |
| #4 | #1 AND #2 AND #3 |
| #5 | 'anticoagulant agent'/exp OR warfarin:ab,ti OR coumadin:ab,ti OR 'vitamin k':ab,ti OR enoxaparin:ab,ti OR lovenox:ab,ti OR rivaroxaban:ab,ti OR xarelto:ab,ti OR dabigatran:ab,ti OR pradaxa:ab,ti OR heparin:ab,ti OR apixaban:ab,ti OR eliquis:ab,ti OR edoxaban:ab,ti OR lixiana:ab,ti OR anticoagulant:ab,ti OR anticoagulants:ab,ti OR anticoagulation:ab,ti OR 'thrombin inhibitor':ab,ti OR 'thrombin inhibitors':ab,ti OR antithrombin:ab,ti OR antithrombins:ab,ti OR antithrombotic:ab,ti OR 'factor Xa inhibitor':ab,ti OR 'factor Xa inhibitors':ab,ti OR 'Blood clotting inhibitor':ab,ti OR 'blood clotting inhibitors':ab,ti OR clopidogrel:ab,ti OR plavix:ab,ti OR aspirin:ab,ti OR dipyridamole:ab,ti OR aggrenox:ab,ti OR persantine:ab,ti OR curantil:ab,ti OR antiplatelet:ab,ti OR anti-platelet:ab,ti OR antiplatelets:ab,ti OR anti-platelets:ab,ti OR 'platelet aggregation inhibitors':ab,ti OR 'platelet aggregation inhibitor':ab,ti OR 'platelet inhibitors':ab,ti OR 'platelet inhibitor':ab,ti OR 'platelet antagonists':ab,ti OR 'platelet antagonist':ab,ti |
| #6 | ('heart atrium appendage'/de AND 'surgery':lnk) OR 'septal occluder'/exp OR 'atrial appendage':ab,ti OR 'atrial appendages':ab,ti OR 'atrium appendage':ab,ti OR 'auricular appendage':ab,ti OR 'auricular appendages':ab,ti OR LAA:ab,ti OR occluder:ab,ti OR occluders:ab,ti OR occlusion:ab,ti OR AMPLATZER:ab,ti OR AtriClip:ab,ti OR PLAATO:ab,ti OR Watchman:ab,ti OR (atrial:ab,ti AND modification:ab,ti) OR lariat:ab,ti OR atricure:ab,ti |
| #7 | #4 AND (#5 OR #6) |
| #8 | #7 NOT ('case report'/exp OR 'case study'/exp OR 'editorial'/exp OR 'letter'/exp OR 'note'/exp OR [conference abstract]/lim) |
| #9 | #8 AND [humans]/lim |
| #10 | #9 NOT (('adolescent'/exp OR 'child'/exp) NOT 'adult'/exp) |
| #11 | 'clinical trial'/exp OR 'clinical study'/exp OR 'controlled study'/exp OR randomized:ab,ti OR randomised:ab,ti OR randomization:ab,ti OR randomisation:ab,ti OR randomly:ab,ti OR placebo:ab,ti OR trial:ab,ti OR groups:ab,ti OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR crossover*:ab,ti OR (cross NEXT/1 over*):ab,ti OR 'clinical trial':ab,ti OR 'clinical trials':ab,ti OR 'clinical study':ab,ti OR 'clinical studies':ab,ti OR 'evaluation study'/exp OR 'evaluation study':ab,ti OR 'evaluation studies':ab,ti OR 'intervention study':ab,ti OR 'intervention studies':ab,ti OR 'case-control':ab,ti OR 'cohort analysis'/exp OR cohort:ab,ti OR longitudinal:ab,ti OR longitudinally:ab,ti OR 'prospective':ab,ti OR prospectively:ab,ti OR 'retrospective':ab,ti OR 'follow up'/exp OR 'follow up':ab,ti OR 'comparative study'/exp OR 'comparative study':ab,ti OR 'comparative studies':ab,ti OR 'systematic review':ab,ti OR 'meta-analysis':ab,ti OR 'meta-analyses':ab,ti OR 'meta synthesis':ab,ti OR 'meta syntheses':ab,ti OR 'survival analysis'/exp OR 'multicenter study'/exp OR 'multicenter study':ab,ti OR multicentre:ab,ti OR 'register'/exp OR registry:ab,ti OR registries:ab,ti OR 'sensitivity and specificity'/exp OR sensitivity:ab,ti OR specificity:ab,ti OR valid:ab,ti OR validity:ab,ti OR validation:ab,ti OR 'validation study'/exp |
| #12 | #10 AND #11 |
| #13 | #12 AND [1-8-2011]/sd |
| #14 | #13 AND [embase]/lim NOT [medline]/lim |
Embase® Search Strategy (August 14, 2012)
Platform: Embase.com
KQ 1: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of available clinical and imaging tools for predicting thromboembolic risk?
| #1 | 'heart atrium fibrillation'/exp OR 'heart atrium flutter'/exp OR “atrial fibrillation”:ab,ti OR (atrial:ab,ti AND fibrillation:ab,ti) OR afib:ab,ti OR “atrial flutter”:ab,ti |
| #2 | 'nuclear magnetic resonance imaging'/exp OR 'cardiac imaging'/exp OR 'computer assisted tomography'/exp OR 'echocardiography'/exp OR chads2:ab,ti OR 'chads2 vasc':ab,ti OR (transthoracic:ab,ti AND echo:ab,ti) OR (transesophageal:ab,ti AND echo:ab,ti) OR tte:ab,ti OR tee:ab,ti OR 'ct scan':ab,ti |
| #3 | 'stroke'/exp OR 'thromboembolism'/exp OR 'brain ischemia'/exp OR stroke:ab,ti OR thromboembolism:ab,ti OR thromboembolic:ab,ti OR (brain:ab,ti AND ischemia:ab,ti) OR (brain:ab,ti AND ischaemia:ab,ti) OR (transient:ab,ti AND (ischemic:ab,ti OR ischaemic:ab,ti OR ischaemia:ab,ti OR ischemia:ab,ti) AND attack:ab,ti) OR TIA:ab,ti |
| #4 | #1 AND #2 AND #3 |
| #5 | 'diagnosis'/exp OR 'treatment outcome'/exp OR 'sensitivity and specificity'/exp OR 'clinical decision making'/exp OR 'decision making'/exp OR diagnosis:ab,ti OR outcome:ab,ti OR outcomes:ab,ti OR reliability:ab,ti OR accuracy:ab,ti OR accurate:ab,ti OR Sensitivity:ab,ti OR specificity:ab,ti OR valid:ab,ti OR validity:ab,ti OR validation:ab,ti OR decision:ab,ti OR decisions:ab,ti OR assessment:ab,ti |
| #6 | #5 AND #4 |
| #7 | #6 Limits: Humans, English, 2000 - present |
| #8 | #7 AND [embase]/lim NOT [medline]/lim |
KQ 2: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
| #1 | 'heart atrium fibrillation'/exp OR 'heart atrium flutter'/exp OR “atrial fibrillation”:ab,ti OR (atrial:ab,ti AND fibrillation:ab,ti) OR afib:ab,ti OR “atrial flutter”:ab,ti |
| #2 | 'age'/exp OR 'dementia'/exp OR 'falling'/exp OR 'international normalized ratio'/exp OR “age factors”:ab,ti OR “age factor”:ab,ti OR age:ab,ti OR dementia:ab,ti OR INR:ab,ti OR fall:ab,ti OR falls:ab,ti OR "international normalized ratio":ab,ti OR paroxysmal:ab,ti OR persistent:ab,ti OR permanent:ab,ti OR stratification:ab,ti OR classification:ab,ti OR schema:ab,ti OR has-bled:ab,ti OR (cognitive:ab,ti AND impairment:ab,ti) OR cognition:ab,ti OR ((prior:ab,ti OR previous:ab,ti OR first:ab,ti) AND stroke:ab,ti) |
| #3 | 'brain hemorrhage'/exp OR 'bleeding'/exp OR hemorrhage:ab,ti OR hemorrhaging:ab,ti OR bleeding:ab,ti OR bleed:ab,ti OR hemorrhagic:ab,ti OR haemorrhage:ab,ti OR haemorrhaging:ab,ti OR haemorrhagic:ab,ti |
| #4 | #1 AND #2 AND #3 |
| #5 | 'diagnosis'/exp OR 'treatment outcome'/exp OR 'sensitivity and specificity'/exp OR 'clinical decision making'/exp OR 'decision making'/exp OR diagnosis:ab,ti OR outcome:ab,ti OR outcomes:ab,ti OR reliability:ab,ti OR accuracy:ab,ti OR accurate:ab,ti OR Sensitivity:ab,ti OR specificity:ab,ti OR valid:ab,ti OR validity:ab,ti OR validation:ab,ti OR decision:ab,ti OR decisions:ab,ti OR assessment:ab,ti |
| #6 | #5 AND #4 |
| #7 | #6 Limits: Humans, English, 2000 - present |
| #8 | #7 AND [embase]/lim NOT [medline]/lim |
KQ 3: What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
In patients with nonvalvular atrial fibrillation?
In specific subpopulations of patients with nonvalvular atrial fibrillation?
| #1 | 'heart atrium fibrillation'/exp OR 'heart atrium flutter'/exp OR “atrial fibrillation”:ab,ti OR (atrial:ab,ti AND fibrillation:ab,ti) OR afib:ab,ti OR “atrial flutter”:ab,ti |
| #2 | 'anticoagulant agent'/exp OR 'warfarin'/exp OR 'vitamin K group'/exp OR 'heparin'/exp OR 'enoxaparin'/exp OR 'rivaroxaban'/exp OR 'dabigatran etexilate'/exp OR 'apixaban'/exp OR 'edoxaban'/exp |
| #3 | warfarin:ab,ti OR coumadin:ab,ti OR vitamin k:ab,ti OR enoxaparin:ab,ti OR lovenox:ab,ti OR rivaroxaban:ab,ti OR xarelto:ab,ti OR dabigatran:ab,ti OR pradaxa:ab,ti OR heparin:ab,ti OR apixaban:ab,ti OR eliquis:ab,ti OR edoxaban:ab,ti OR lixiana:ab,ti |
| #4 | #2 OR #3 |
| #5 | 'antithrombocytic agent'/exp OR 'clopidogrel'/exp OR 'acetylsalicylic acid'/exp OR 'dipyridamole'/exp OR clopidogrel:ab,ti OR plavix:ab,ti OR aspirin:ab,ti OR dipyridamole:ab,ti OR aggrenox:ab,ti OR persantine:ab,ti OR antiplatelet:ab,ti OR anti-platelet:ab,ti OR antiplatelets:ab,ti OR anti-platelets:ab,ti |
| #6 | 'heart atrium appendage'/exp OR atrial appendage:ab,ti OR LAA:ab,ti OR occluder:ab,ti OR AMPLATZER:ab,ti OR AtriClip:ab,ti OR PLAATO:ab,ti OR Watchman:ab,ti OR (atrial:ab,ti AND modification:ab,ti) OR “atriacure isolator”:ab,ti |
| #7 | 'stroke'/exp OR 'thromboembolism'/exp OR 'brain ischemia'/exp OR stroke:ab,ti OR thromboembolism:ab,ti OR thromboembolic:ab,ti OR (brain:ab,ti AND ischemia:ab,ti) OR (brain:ab,ti AND ischaemia:ab,ti) OR (transient:ab,ti AND (ischemic:ab,ti OR ischaemic:ab,ti OR ischaemia:ab,ti OR ischemia:ab,ti) AND attack:ab,ti) OR TIA:ab,ti |
| #8 | #1 AND (#4 OR #5 OR #6) AND #7 |
| #9 | ('randomized controlled trial'/exp OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEAR/1 over*):ab,ti OR placebo*:ab,ti OR (doubl* NEAR/1 blind*):ab,ti OR (singl* NEAR/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR 'clinical study'/exp OR “clinical trial”:ti,ab OR “clinical trials”:ti,ab OR 'controlled study'/exp OR 'evaluation'/exp OR “evaluation study”:ab,ti OR “evaluation studies”:ab,ti OR “intervention study”:ab,ti OR “intervention studies”:ab,ti OR “case control”:ab,ti OR 'cohort analysis'/exp OR cohort:ab,ti OR longitudinal*:ab,ti OR prospective:ab,ti OR prospectively:ab,ti OR retrospective:ab,ti OR 'follow up'/exp OR “follow up”:ab,ti OR 'comparative effectiveness'/exp OR 'comparative study'/exp OR “comparative study”:ab,ti OR “comparative studies”:ab,ti OR 'evidence based medicine'/exp OR “systematic review”:ab,ti OR “meta-analysis”:ab,ti OR “meta-analyses”:ab,ti) NOT ('editorial'/exp OR 'letter'/exp OR 'case report'/exp) |
| #10 | #8 AND #9 |
| #11 | #10 Limits: Humans, English, 2000 - present |
| #12 | #11 AND [embase]/lim NOT [medline]/lim |
KQ 4: What are the comparative safety and effectiveness of available strategies for anticoagulation in patients with nonvalvular atrial fibrillation who are undergoing invasive procedures?
| #1 | 'heart atrium fibrillation'/exp OR 'heart atrium flutter'/exp OR “atrial fibrillation”:ab,ti OR (atrial:ab,ti AND fibrillation:ab,ti) OR afib:ab,ti OR “atrial flutter”:ab,ti |
| #2 | 'anticoagulant agent'/exp OR 'warfarin'/exp OR 'vitamin K group'/exp OR 'heparin'/exp OR 'enoxaparin'/exp OR 'rivaroxaban'/exp OR 'dabigatran etexilate'/exp OR 'apixaban'/exp OR 'edoxaban'/exp |
| #3 | warfarin:ab,ti OR coumadin:ab,ti OR vitamin k:ab,ti OR enoxaparin:ab,ti OR lovenox:ab,ti OR rivaroxaban:ab,ti OR xarelto:ab,ti OR dabigatran:ab,ti OR pradaxa:ab,ti OR heparin:ab,ti OR apixaban:ab,ti OR eliquis:ab,ti OR edoxaban:ab,ti OR lixiana:ab,ti |
| #4 | #2 OR #3 |
| #5 | 'surgery'/exp OR 'dental care'/exp OR ((surgical:ab,ti OR invasive:ab,ti) AND (procedure:ab,ti OR procedures:ab,ti)) OR (dental:ab,ti AND (procedure:ab,ti OR procedures:ab,ti)) OR surgery:ab,ti OR procedures:ab,ti OR procedure:ab,ti |
| #6 | #1 AND #4 AND #5 |
| #7 | ('randomized controlled trial'/exp OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEAR/1 over*):ab,ti OR placebo*:ab,ti OR (doubl* NEAR/1 blind*):ab,ti OR (singl* NEAR/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR 'clinical study'/exp OR “clinical trial”:ti,ab OR “clinical trials”:ti,ab OR 'controlled study'/exp OR 'evaluation'/exp OR “evaluation study”:ab,ti OR “evaluation studies”:ab,ti OR “intervention study”:ab,ti OR “intervention studies”:ab,ti OR “case control”:ab,ti OR 'cohort analysis'/exp OR cohort:ab,ti OR longitudinal*:ab,ti OR prospective:ab,ti OR prospectively:ab,ti OR retrospective:ab,ti OR 'follow up'/exp OR “follow up”:ab,ti OR 'comparative effectiveness'/exp OR 'comparative study'/exp OR “comparative study”:ab,ti OR “comparative studies”:ab,ti OR 'evidence based medicine'/exp OR “systematic review”:ab,ti OR “meta-analysis”:ab,ti OR “meta-analyses”:ab,ti) NOT ('editorial'/exp OR 'letter'/exp OR 'case report'/exp) |
| #8 | #6 AND #7 |
| #9 | #8 Limits: Humans, English, 2000 - present |
| #10 | #9 AND [embase]/lim NOT [medline]/lim |
KQ 5: What are the comparative safety and effectiveness of available strategies for switching between warfarin and other novel oral anticoagulants, in patients with nonvalvular atrial fibrillation?
| #1 | 'heart atrium fibrillation'/exp OR 'heart atrium flutter'/exp OR “atrial fibrillation”:ab,ti OR (atrial:ab,ti AND fibrillation:ab,ti) OR afib:ab,ti OR “atrial flutter”:ab,ti |
| #2 | 'warfarin'/exp OR warfarin:ab,ti OR coumadin:ab,ti |
| #3 | antithrombins:ab,ti OR (direct:ab,ti AND thrombin:ab,ti AND inhibitors:ab,ti) OR (direct:ab,ti AND thrombin:ab,ti AND inhibitor:ab,ti) OR "direct thrombin inhibitors":ab,ti OR “Antithrombin III":ab,ti OR “Antithrombin Proteins":ab,ti OR argatroban:ab,ti OR bivalirudin:ab,ti OR “Heparin Cofactor II":ab,ti OR Hirudins:ab,ti OR inogatran:ab,ti OR lepirudin:ab,ti OR melagatran:ab,ti OR “SDZ MTH 958":ab,ti OR ximelagatran:ab,ti |
| #4 | 'anticoagulant agent'/exp OR anticoagulant:ab,ti OR anticoagulants:ab,ti |
| #5 | ('randomized controlled trial'/exp OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEAR/1 over*):ab,ti OR placebo*:ab,ti OR (doubl* NEAR/1 blind*):ab,ti OR (singl* NEAR/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR 'clinical study'/exp OR “clinical trial”:ti,ab OR “clinical trials”:ti,ab OR 'controlled study'/exp OR 'evaluation'/exp OR “evaluation study”:ab,ti OR “evaluation studies”:ab,ti OR “intervention study”:ab,ti OR “intervention studies”:ab,ti OR “case control”:ab,ti OR 'cohort analysis'/exp OR cohort:ab,ti OR longitudinal*:ab,ti OR prospective:ab,ti OR prospectively:ab,ti OR retrospective:ab,ti OR 'follow up'/exp OR “follow up”:ab,ti OR 'comparative effectiveness'/exp OR 'comparative study'/exp OR “comparative study”:ab,ti OR “comparative studies”:ab,ti OR 'evidence based medicine'/exp OR “systematic review”:ab,ti OR “meta-analysis”:ab,ti OR “meta-analyses”:ab,ti) NOT ('editorial'/exp OR 'letter'/exp OR 'case report'/exp) |
| #6 | #1 AND #2 AND (#3 OR #4) AND #5 |
| #7 | #6 Limits: Humans, English, 2000 - present |
| #8 | #7 AND [embase]/lim NOT [medline]/lim |
KQ 6: What are the comparative safety and effectiveness of available strategies for resuming anticoagulation therapy or performing a procedural intervention as a stroke prevention strategy following a hemorrhagic event (stroke, major bleed, or minor bleed) in patients with nonvalvular atrial fibrillation?
| #1 | 'heart atrium fibrillation'/exp OR 'heart atrium flutter'/exp OR “atrial fibrillation”:ab,ti OR (atrial:ab,ti AND fibrillation:ab,ti) OR afib:ab,ti OR “atrial flutter”:ab,ti |
| #2 | 'anticoagulant agent'/exp OR 'warfarin'/exp OR 'vitamin K group'/exp OR 'heparin'/exp OR 'enoxaparin'/exp OR 'rivaroxaban'/exp OR 'dabigatran etexilate'/exp OR 'apixaban'/exp OR 'edoxaban'/exp |
| #3 | warfarin:ab,ti OR coumadin:ab,ti OR vitamin k:ab,ti OR enoxaparin:ab,ti OR lovenox:ab,ti OR rivaroxaban:ab,ti OR xarelto:ab,ti OR dabigatran:ab,ti OR pradaxa:ab,ti OR heparin:ab,ti OR apixaban:ab,ti OR eliquis:ab,ti OR edoxaban:ab,ti OR lixiana:ab,ti |
| #4 | #2 OR #3 |
| #5 | 'brain hemorrhage'/exp OR 'bleeding'/exp OR hemorrhage:ab,ti OR hemorrhaging:ab,ti OR bleeding:ab,ti OR bleed:ab,ti OR hemorrhagic:ab,ti OR haemorrhage:ab,ti OR haemorrhaging:ab,ti OR haemorrhagic:ab,ti |
| #6 | 'time'/exp OR resume:ab,ti OR resumed:ab,ti OR restart:ab,ti OR restarted:ab,ti OR restarting:ab,ti OR re-initiate:ab,ti OR reinitiate:ab,ti OR continue:ab,ti OR continued:ab,ti OR start:ab,ti OR resumption:ab,ti OR reinitiating:ab,ti OR resuming:ab,ti OR continuing:ab,ti |
| #7 | #1 AND #4 AND #5 AND #6 |
| #8 | ('randomized controlled trial'/exp OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEAR/1 over*):ab,ti OR placebo*:ab,ti OR (doubl* NEAR/1 blind*):ab,ti OR (singl* NEAR/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR 'clinical study'/exp OR “clinical trial”:ti,ab OR “clinical trials”:ti,ab OR 'controlled study'/exp OR 'evaluation'/exp OR “evaluation study”:ab,ti OR “evaluation studies”:ab,ti OR “intervention study”:ab,ti OR “intervention studies”:ab,ti OR “case control”:ab,ti OR 'cohort analysis'/exp OR cohort:ab,ti OR longitudinal*:ab,ti OR prospective:ab,ti OR prospectively:ab,ti OR retrospective:ab,ti OR 'follow up'/exp OR “follow up”:ab,ti OR 'comparative effectiveness'/exp OR 'comparative study'/exp OR “comparative study”:ab,ti OR “comparative studies”:ab,ti OR 'evidence based medicine'/exp OR “systematic review”:ab,ti OR “meta-analysis”:ab,ti OR “meta-analyses”:ab,ti) NOT ('editorial'/exp OR 'letter'/exp OR 'case report'/exp) |
| #9 | #7 AND #8 |
| #10 | #9 Limits: Humans, English, 2000 - present |
| #11 | #10 AND [embase]/lim NOT [medline]/lim |
Cochrane Search Strategy (February 14, 2018)
Platform: Wiley
Database searched: Cochrane Database of Systematic Reviews
KQ 1 & KQ 2: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic and patient outcome efficacy) of available clinical and imaging tools and associated risk factors for predicting thromboembolic risk? & In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
| #1 | [mh "Atrial Fibrillation"] OR "atrial fibrillation":ab,ti OR [mh "Atrial Flutter"] OR "atrial flutter":ab,ti |
| #2 | [mh ^"Cerebrovascular Disorders"[mj]] or [mh Stroke] or [mh Thromboembolism] or [mh ^emorrhage] or [mh "Intracranial Hemorrhages"] or [mh "Brain Ischemia"] or [mh "Prothrombin Time"] or stroke:ab,ti or strokes:ab,ti or thromboembolism:ab,ti or thromboembolisms:ab,ti or thromboembolic:ab,ti or thromboses:ab,ti or hemorrhage:ab,ti or hemorrhages:ab,ti or hemorrhaging:ab,ti or hemorrhagic:ab,ti or haemorrhage:ab,ti or haemorrhages:ab,ti or haemorrhaging:ab,ti or haemorrhagic:ab,ti or ((bleeding or bleed or bleeds) near/2 (major or risk or event)):ab,ti or ((systemic or paradoxical or crossed) next/2 (embolism or embolisms)):ab,ti or ((brain or cerebral or brainstem or 'brain stem') next/2 (ischemia or ischaemia or ischemias or ischaemias or infarction or infarctions)):ab,ti or (transient next/2 (ischemic or ischaemic or ischaemia or ischemia) next/2 (attack or attacks)):ab,ti or TIA:ab,ti or TIAs:ab,ti or "cerebrovascular accident":ab,ti or "cerebrovascular accidents":ab,ti or CVA:ab,ti or CVAs:ab,ti or "brain vascular accident":ab,ti or "brain vascular accidents":ab,ti |
| #3 | [mh Risk] or risk:ab,ti or risks:ab,ti or [mh "Predictive Value of Tests"] or predict:ab,ti or predicts:ab,ti or predicting:ab,ti or predictor:ab,ti or predictors:ab,ti or predictive:ab,ti |
| #4 | {and #1-#3} |
| #5 | #4 Publication Year from 2011 |
KQ 3: What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
In patients with nonvalvular atrial fibrillation?
In specific subpopulations of patients with nonvalvular atrial fibrillation?
| #1 | [mh "Atrial Fibrillation"] OR "atrial fibrillation":ab,ti OR [mh "Atrial Flutter"] OR "atrial flutter":ab,ti |
| #2 | [mh ^"Cerebrovascular Disorders"[mj]] or [mh Stroke] or [mh Thromboembolism] or [mh ^emorrhage] or [mh "Intracranial Hemorrhages"] or [mh "Brain Ischemia"] or [mh "Prothrombin Time"] or stroke:ab,ti or strokes:ab,ti or thromboembolism:ab,ti or thromboembolisms:ab,ti or thromboembolic:ab,ti or thromboses:ab,ti or hemorrhage:ab,ti or hemorrhages:ab,ti or hemorrhaging:ab,ti or hemorrhagic:ab,ti or haemorrhage:ab,ti or haemorrhages:ab,ti or haemorrhaging:ab,ti or haemorrhagic:ab,ti or ((bleeding or bleed or bleeds) near/2 (major or risk or event)):ab,ti or ((systemic or paradoxical or crossed) next/2 (embolism or embolisms)):ab,ti or ((brain or cerebral or brainstem or 'brain stem') next/2 (ischemia or ischaemia or ischemias or ischaemias or infarction or infarctions)):ab,ti or (transient next/2 (ischemic or ischaemic or ischaemia or ischemia) next/2 (attack or attacks)):ab,ti or TIA:ab,ti or TIAs:ab,ti or "cerebrovascular accident":ab,ti or "cerebrovascular accidents":ab,ti or CVA:ab,ti or CVAs:ab,ti or "brain vascular accident":ab,ti or "brain vascular accidents":ab,ti |
| #3 | [mh Risk] or risk:ab,ti or risks:ab,ti or [mh Safety] or safety:ab,ti or safe:ab,ti or [mh Incidence] or efficacy:ab,ti or efficacious:ab,ti or [mh /PC] or prevent:ab,ti or prevents:ab,ti or preventing:ab,ti or prevention:ab,ti or [mh "Treatment Outcome"] or [mh /AE] or (side next/1 effect*):ab,ti or (adverse next/3 (interaction* or response* or effect* or event* or reaction* or outcome*)):ab,ti or (unintended next/3 (interaction* or response* or effect* or event* or reaction* or outcome*)):ab,ti or (unintentional next/3 (interaction* or response* or effect* or event* or reaction* or outcome*)):ab,ti or (unwanted next/3 (interaction* or response* or effect* or event* or reaction* or outcome*)):ab,ti or (unexpected next/3 (interaction* or response* or effect* or event* or reaction* or outcome*)):ab,ti or (undesirable next/3 (interaction* or response* or effect* or event* or reaction* or outcome*)):ab,ti or "drug safety":ab,ti or "drug toxicity":ab,ti or tolerability:ab,ti or harm:ab,ti or harms:ab,ti or harmful:ab,ti or "treatment emergent":ab,ti or complication*:ab,ti or toxicity:ab,ti |
| #4 | {and #1-#3} |
| #5 | [mh Anticoagulants] or [mh Warfarin] or [mh Heparin] or [mh "Vitamin K"/AI] or [mh Rivaroxaban] or [mh Antithrombins] or [mh Dabigatran] or [mh "Blood Coagulation Factor Inhibitors"] or [mh "Factor Xa Inhibitors"] or warfarin:ab,ti or coumadin:ab,ti or "vitamin k":ab,ti or enoxaparin:ab,ti or lovenox:ab,ti or rivaroxaban:ab,ti or xarelto:ab,ti or dabigatran:ab,ti or pradaxa:ab,ti or heparin:ab,ti or apixaban:ab,ti or eliquis:ab,ti or edoxaban:ab,ti or lixiana:ab,ti or anticoagulant:ab,ti or anticoagulants:ab,ti or anticoagulation:ab,ti or "thrombin inhibitor":ab,ti or "thrombin inhibitors":ab,ti or antithrombin:ab,ti or antithrombins:ab,ti or antithrombotic:ab,ti or "factor Xa inhibitor":ab,ti or "factor Xa inhibitors":ab,ti or "Blood clotting inhibitor":ab,ti or "blood clotting inhibitors":ab,ti |
| #6 | [mh "Platelet Aggregation Inhibitors"] or [mh Aspirin] or [mh Dipyridamole] or clopidogrel:ab,ti or plavix:ab,ti or aspirin:ab,ti or dipyridamole:ab,ti or aggrenox:ab,ti or persantine:ab,ti or curantil:ab,ti or antiplatelet:ab,ti or anti-platelet:ab,ti or antiplatelets:ab,ti or anti-platelets:ab,ti or "platelet aggregation inhibitors":ab,ti or "platelet aggregation inhibitor":ab,ti or "platelet inhibitors":ab,ti or "platelet inhibitor":ab,ti or "platelet antagonists":ab,ti or "platelet antagonist":ab,ti |
| #7 | [mh "atrial appendage"/SU] or [mh "Septal Occluder Device"] or "atrial appendage":ab,ti or "atrial appendages":ab,ti or "atrium appendage":ab,ti or "auricular appendage":ab,ti or "auricular appendages":ab,ti or LAA:ab,ti or occluder:ab,ti or occluders:ab,ti or occlusion:ab,ti or AMPLATZER:ab,ti or AtriClip:ab,ti or PLAATO:ab,ti or Watchman:ab,ti or (atrial:ab,ti and modification:ab,ti) or lariat:ab,ti or atricure:ab,ti |
| #8 | #4 and {or #5-#7} |
| #9 | #8 Publication Year from 2011 |
Cochrane Search Strategy (August 14, 2012)
Platform: Wiley
Database searched: Cochrane Database of Systematic Reviews
KQ 1: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of available clinical and imaging tools for predicting thromboembolic risk?
| #1 | (atrial fibrillation OR atrial flutter):ti,ab,kw |
| #2 | Magnetic Resonance Imaging explode all trees OR MeSH descriptor Cardiac Imaging Techniques explode all trees OR MeSH descriptor Tomography, X-Ray Computed explode all trees OR MeSH descriptor Echocardiography explode all trees OR (chads2 OR chads2-vasc OR TEE OR TTE OR ct-scan OR transthoracic echo OR transesophageal echo):ti,ab,kw |
| #3 | MeSH descriptor Stroke explode all trees OR MeSH descriptor Thromboembolism explode all trees OR MeSH descriptor Brain Ischemia explode all trees OR (thromboembolism OR thromboembolic OR brain ischemia OR brain ischaemia OR tia):ti,ab,kw OR (transient ischemic attack):ti,ab,kw or (transient ischaemic attack):ti,ab,kw or (transient ischemia attack):ti,ab,kw or (transient ischaemic attack):ti,ab,kw |
| #4 | #1 AND #2 AND #3 |
| #5 | #4, Limits: Cochrane Reviews, 2000 to 2012 |
KQ 2: In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
| #1 | (atrial fibrillation OR atrial flutter):ti,ab,kw |
| #2 | MeSH descriptor Age Factors explode all trees OR MeSH descriptor Dementia explode all trees OR MeSH descriptor Accidental Falls explode all trees OR MeSH descriptor International Normalized Ratio explode all trees OR age:ti,ab,kw OR dementia:ti,ab,kw OR INR:ti,ab,kw OR fall:ti,ab,kw OR falls:ti,ab,kw OR "international normalized ratio":ti,ab,kw OR paroxysmal:ti,ab,kw OR persistent:ti,ab,kw OR permanent:ti,ab,kw OR stratification:ti,ab,kw OR classification:ti,ab,kw OR schema:ti,ab,kw OR has-bled:ti,ab,kw OR cognitive impairment:ti,ab,kw OR cognition:ti,ab,kw OR ((prior:ti,ab,kw OR previous:ti,ab,kw OR first:ti,ab,kw) AND stroke:ti,ab,kw) |
| #3 | MeSH descriptor Intracranial Hemorrhages explode all trees OR MeSH descriptor Hemorrhage explode all trees OR hemorrhage:ti,ab,kw OR hemorrhaging:ti,ab,kw OR bleeding:ti,ab,kw OR bleed:ti,ab,kw OR hemorrhagic:ti,ab,kw OR haemorrhage:ti,ab,kw OR haemorrhaging:ti,ab,kw OR haemorrhagic:ti,ab,kw |
| #4 | #1 AND #2 AND #3 |
| #5 | MeSH descriptor Diagnosis explode all trees OR MeSH descriptor Treatment Outcome explode all trees OR MeSH descriptor Sensitivity and Specificity explode all trees OR MeSH descriptor Decision Making explode all trees OR diagnosis:ti,ab,kw OR outcome:ti,ab,kw OR outcomes:ti,ab,kw OR reliability:ti,ab,kw OR accuracy:ti,ab,kw OR accurate:ti,ab,kw OR Sensitivity:ti,ab,kw OR specificity:ti,ab,kw OR valid:ti,ab,kw OR validity:ti,ab,kw OR validation:ti,ab,kw OR decision:ti,ab,kw OR decisions:ti,ab,kw OR assessment:ti,ab,kw |
| #6 | #4 AND #5 |
| #7 | #6, Limits: Cochrane Reviews, 2000 to 2012 |
KQ 3: What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
In patients with nonvalvular atrial fibrillation?
In specific subpopulations of patients with nonvalvular atrial fibrillation?
| #1 | (atrial fibrillation OR atrial flutter):ti,ab,kw |
| #2 | MeSH descriptor Anticoagulants explode all trees OR warfarin:ti,ab,kw OR coumadin:ti,ab,kw OR vitamin k:ti,ab,kw OR enoxaparin:ti,ab,kw OR lovenox:ti,ab,kw OR rivaroxaban:ti,ab,kw OR xarelto:ti,ab,kw OR dabigatran:ti,ab,kw OR pradaxa:ti,ab,kw OR heparin:ti,ab,kw OR apixaban:ti,ab,kw OR eliquis:ti,ab,kw OR edoxaban:ti,ab,kw OR lixiana:ti,ab,kw OR anticoagulants:ti,ab,kw OR OR anticoagulant:ti,ab,kw |
| #3 | MeSH descriptor Platelet Aggregation Inhibitors explode all trees OR clopidogrel:ti,ab,kw OR plavix:ti,ab,kw OR aspirin:ti,ab,kw OR dipyridamole:ti,ab,kw OR aggrenox:ti,ab,kw OR persantine:ti,ab,kw OR antiplatelet:ti,ab,kw OR anti-platelet:ti,ab,kw OR antiplatelets:ti,ab,kw OR anti-platelets:ti,ab,kw |
| #4 | MeSH descriptor Atrial Appendage explode all trees OR atrial appendage:ti,ab,kw OR LAA:ti,ab,kw OR occluder:ti,ab,kw OR AMPLATZER:ti,ab,kw OR AtriClip:ti,ab,kw OR PLAATO:ti,ab,kw OR Watchman:ti,ab,kw OR (atrial:ti,ab,kw AND modification:ti,ab,kw) OR "atriacure isolator":ti,ab,kw |
| #5 | MeSH descriptor Stroke explode all trees OR MeSH descriptor Thromboembolism explode all trees OR MeSH descriptor Brain Ischemia explode all trees OR (thromboembolism OR thromboembolic OR brain ischemia OR brain ischaemia OR tia):ti,ab,kw OR (transient ischemic attack):ti,ab,kw or (transient ischaemic attack):ti,ab,kw or (transient ischemia attack):ti,ab,kw or (transient ischaemic attack):ti,ab,kw |
| #6 | #1 AND (#2 OR #3 OR #4) AND #5 |
| #7 | #6, Limits: Cochrane Reviews, 2000 to 2012 |
KQ 4: What are the comparative safety and effectiveness of available strategies for anticoagulation in patients with nonvalvular atrial fibrillation who are undergoing invasive procedures?
| #1 | (atrial fibrillation OR atrial flutter):ti,ab,kw |
| #2 | MeSH descriptor Anticoagulants explode all trees OR warfarin:ti,ab,kw OR coumadin:ti,ab,kw OR vitamin k:ti,ab,kw OR enoxaparin:ti,ab,kw OR lovenox:ti,ab,kw OR rivaroxaban:ti,ab,kw OR xarelto:ti,ab,kw OR dabigatran:ti,ab,kw OR pradaxa:ti,ab,kw OR heparin:ti,ab,kw OR apixaban:ti,ab,kw OR eliquis:ti,ab,kw OR edoxaban:ti,ab,kw OR lixiana:ti,ab,kw OR anticoagulants:ti,ab,kw OR OR anticoagulant:ti,ab,kw |
| #3 | MeSH descriptor Surgical Procedures, Operative explode all trees OR MeSH descriptor Dental Care explode all trees OR surgical:ti,ab,kw OR invasive:ti,ab,kw OR procedures:ti,ab,kw OR surgery:ti,ab,kw OR procedure:ti,ab,kw |
| #4 | #1 AND #2 AND #3 |
| #5 | #4, Limits: Cochrane Reviews, 2000 to 2012 |
KQ 5: What are the comparative safety and effectiveness of available strategies for switching between warfarin and other novel oral anticoagulants, in patients with nonvalvular atrial fibrillation?
| #1 | (atrial fibrillation OR atrial flutter):ti,ab,kw |
| #2 | warfarin:ti,ab,kw OR coumadin:ti,ab,kw |
| #3 | MeSH descriptor Antithrombins explode all trees OR antithrombins:ti,ab,kw OR (direct:ti,ab,kw AND thrombin:ti,ab,kw AND inhibitors:ti,ab,kw) OR "direct thrombin inhibitors":ti,ab,kw OR "direct thrombin inhibitor":ti,ab,kw |
| #4 | MeSH descriptor Anticoagulants explode all trees OR anticoagulant:ti,ab,kw OR anticoagulants:ti,ab,kw OR vitamin k:ti,ab,kw OR enoxaparin:ti,ab,kw OR lovenox:ti,ab,kw OR rivaroxaban:ti,ab,kw OR xarelto:ti,ab,kw OR dabigatran:ti,ab,kw OR pradaxa:ti,ab,kw OR heparin:ti,ab,kw OR apixaban:ti,ab,kw OR eliquis:ti,ab,kw OR edoxaban:ti,ab,kw OR lixiana:ti,ab,kw |
| #5 | #1 AND #2 AND (#3 OR #4) |
| #6 | #5, Limits: Cochrane Reviews, 2000 to 2012 |
KQ 6: What are the comparative safety and effectiveness of available strategies for resuming anticoagulation therapy or performing a procedural intervention as a stroke prevention strategy following a hemorrhagic event (stroke, major bleed, or minor bleed) in patients with nonvalvular atrial fibrillation?
| #1 | (atrial fibrillation OR atrial flutter):ti,ab,kw |
| #2 | MeSH descriptor Anticoagulants explode all trees OR warfarin:ti,ab,kw OR coumadin:ti,ab,kw OR vitamin k:ti,ab,kw OR enoxaparin:ti,ab,kw OR lovenox:ti,ab,kw OR rivaroxaban:ti,ab,kw OR xarelto:ti,ab,kw OR dabigatran:ti,ab,kw OR pradaxa:ti,ab,kw OR heparin:ti,ab,kw OR apixaban:ti,ab,kw OR eliquis:ti,ab,kw OR edoxaban:ti,ab,kw OR lixiana:ti,ab,kw OR anticoagulant:ti,ab,kw OR anticoagulants:ti,ab,kw |
| #3 | MeSH descriptor Intracranial Hemorrhages explode all trees OR MeSH descriptor Hemorrhage explode all trees OR hemorrhage:ti,ab,kw OR hemorrhaging:ti,ab,kw OR bleeding:ti,ab,kw OR bleed:ti,ab,kw OR hemorrhagic:ti,ab,kw OR haemorrhage:ti,ab,kw OR haemorrhaging:ti,ab,kw OR haemorrhagic:ti,ab,kw |
| #4 | MeSH descriptor Time Factors explode all trees OR Resume:ti,ab,kw OR resumed:ti,ab,kw OR restart:ti,ab,kw OR restarted:ti,ab,kw OR restarting:ti,ab,kw OR re-initiate:ti,ab,kw OR reinitiate:ti,ab,kw OR continue:ti,ab,kw OR continued:ti,ab,kw OR start:ti,ab,kw OR resumption:ti,ab,kw OR reinitiating:ti,ab,kw OR resuming:ti,ab,kw OR continuing:ti,ab,kw |
| #5 | #1 AND #2 AND #3 AND #4 |
| #6 | #5, Limits: Cochrane Reviews, 2000 to 2012 |
PubMed® Search Strategy (February 12, 2018)
Contextual Question: What are currently available shared decision-making tools for patient and provider use for stroke prophylaxis in atrial fibrillation, and what are their relative strengths and weaknesses?
| ((("Atrial Fibrillation"[Mesh] OR "Atrial Flutter"[Mesh] OR "atrial fibrillation"[tiab] OR afib[tiab] OR "atrial flutter"[tiab])) AND ("Stroke"[Mesh] OR "Thromboembolism"[Mesh] OR "brain ischemia"[Mesh] OR stroke[tiab] OR strokes[tiab] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR thromboembolic[tiab] OR ((brain[tiab] OR cerebral[tiab]) AND (ischemia[tiab] OR ischaemia[tiab] OR ischemias[tiab] OR ischaemias[tiab])) OR (transient[tiab] AND (ischemic[tiab] OR ischaemic[tiab] OR ischaemia[tiab] OR ischemia[tiab]) AND (attack[tiab] OR attacks[tiab])) OR TIA[tiab] OR TIAs[tiab] OR “cerebrovascular accident”[tiab] OR “cerebrovascular accidents”[tiab] OR CVA[tiab] OR CVAs[tiab] OR “brain vascular accident”[tiab] OR “brain vascular accidents”[tiab])) AND ("Clinical Decision-Making"[Mesh] OR "Decision Support Systems, Clinical"[Mesh] OR "Decision Making, Computer-Assisted"[Mesh] OR "Decision Support Techniques"[Mesh] OR "Decision Making"[Mesh] OR "Decision Theory"[Mesh] OR "Medical Order Entry Systems"[Mesh] OR "Point-of-Care Systems"[Mesh] OR “decision”[tiab] OR "decision-making"[tiab]) AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication]) |
Grey Literature Searches
ClinicalTrials.gov (February 9, 2018)
| KQ1, KQ2, KQ3 | |
| Condition | atrial fibrillation OR afib OR atrial flutter |
| Outcome | stroke OR thromboembolism OR thromboembolic OR "brain ischemia" OR “brain ischaemia” OR (transient AND ischemic AND attack) OR TIA OR hemorrhage OR hemorrhaging OR bleeding OR bleed OR hemorrhagic OR haemorrhage OR haemorrhaging OR haemorrhagic |
Total number of results: 343
ClinicalTrials.gov (August 22, 2012)
| KQ1, KQ2, KQ3, KQ6 | |
| Condition | atrial fibrillation OR afib OR atrial flutter |
| Outcome | stroke OR thromboembolism OR thromboembolic OR "brain ischemia" OR “brain ischaemia” OR (transient AND ischemic AND attack) OR TIA OR hemorrhage OR hemorrhaging OR bleeding OR bleed OR hemorrhagic OR haemorrhage OR haemorrhaging OR haemorrhagic |
| KQ4 | |
| Condition | atrial fibrillation OR afib OR atrial flutter |
| Intervention | Anticoagulants OR anticoagulation OR warfarin OR coumadin OR vitamin k OR Heparin OR enoxaparin OR lovenox OR rivaroxaban OR xarelto OR dabigatran OR pradaxa OR apixaban OR eliquis OR edoxaban OR lixiana |
| Search Terms | Surgery OR procedures OR procedure |
| KQ5 | |
| Condition | atrial fibrillation OR afib OR atrial flutter |
| Intervention | (warfarin OR Coumadin) AND (Antithrombins OR antithrombin OR (direct AND thrombin AND (inhibitors OR inhibitor)) OR anticoagulant OR anticoagulants) |
Total number of results: 186
WHO: International Clinical Trials Registry Platform Search Portal (August 17, 2012)
| KQs 1-6 | |
| Condition | atrial fibrillation OR afib OR atrial flutter |
| Recruiting status | ALL |
Total number of results: 858
ProQuest COS Conference Papers Index (August 14, 2012)
| KQ1, KQ2, KQ3, KQ6 | |
| #1 | All (atrial fibrillation OR afib OR atrial flutter) |
| #3 | All (stroke OR thromboembolism OR thromboembolic OR "brain ischemia" OR “brain ischaemia” OR (transient AND (ischemic OR ischaemic) AND attack) OR TIA OR hemorrhage OR hemorrhaging OR bleeding OR bleed OR hemorrhagic OR haemorrhage OR haemorrhaging OR haemorrhagic) |
| #4 | #1 AND #2 AND #3 |
| KQ4 | |
| #1 | All (atrial fibrillation OR afib OR atrial flutter) |
| #2 | All (Anticoagulants OR anticoagulation OR warfarin OR coumadin OR vitamin k OR Heparin OR enoxaparin OR lovenox OR rivaroxaban OR xarelto OR dabigatran OR pradaxa OR apixaban OR eliquis OR edoxaban OR lixiana) |
| #3 | All (Surgery OR procedures OR procedure) |
| #4 | #1 AND #2 AND #3 |
| KQ5 | |
| #1 | All (atrial fibrillation OR afib OR atrial flutter) |
| #2 | All (warfarin OR Coumadin) |
| #3 | All (Antithrombins OR antithrombin OR (direct AND thrombin AND (inhibitors OR inhibitor)) OR anticoagulant OR anticoagulants) |
| #6 | #1 AND #2 AND #3 |
Total number of results: 352
Appendix Table 2.
Inclusion and Exclusion Criteria
| PICOTS Element |
Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Populations |
|
|
| Interventions | KQ 1: Clinical and imaging tools and associated risk factors for assessment/evaluation of thromboembolic risk:
KQ 2: Clinical tools and individual risk factors for assessment/evaluation of intracranial hemorrhage bleeding risk:
KQ 3: Anticoagulation, antiplatelet, and procedural interventions:
|
None |
| Comparators |
|
For KQ 3, studies that did not include an active comparator |
| Outcomes |
Patient-centered outcomes for KQ 3 (and for KQ 1 [thromboembolic outcomes] and KQ 2 [bleeding outcomes] under “Patient outcome efficacy”):
|
Study does not include any outcomes of interest |
| Timing |
|
None |
| Setting |
|
Studies which were conducted exclusively in Asia, Africa, or the Middle Eastb |
| Study design |
|
|
| Publications |
|
|
Appendix Table 3.
C-statistics from Studies Comparing Stroke Risk Scores of Interest
| Study | CHADS2 | Framingham | CHA2DS2-VASc | ABC-Stroke |
|---|---|---|---|---|
| Abraham, 201341 |
Continuous: 0.65 (95% CI 0.62 to 0.67) Categorical: 0.65 (95% CI 0.62 to 0.67) |
Continuous: 0.67 (95% CI 0.65 to 0.69) Categorical: 0.67 (95% CI 0.65 to 0.69) |
||
| Abumuaileq, 2015112 |
Continuous: Non-anticoagulated cohort: 0.69 (95 % CI 0.53 to 0.85) Anticoagulated cohort: 0.72 (95% CI 0.63 to 0.82) |
|||
| Banerjee, 201372 |
Categorical: 0.64 (95% CI 0.61 to 0.67) |
Categorical: 0.64 (95% CI 0.62 to 0.67) |
||
| Banerjee, 2014113 |
Continuous: 0.641 (95% CI 0.607 to 0.676) |
Continuous: 0.621 (95% CI 0.616 to 0.683) |
||
| Baruch, 200734 |
Categorical (Classic): 0.64 (95% CI 0.61 to 0.67) Categorical (Revised): 0.64 (95% CI 0.61 to 0.67) |
Categorical: 0.62 (95% CI 0.59 to 0.66) |
Categorical: 0.65 (95% CI 0.61 to 0.68) |
|
| Fang, 200833 |
Continuous: All patients: 0.60 Categorical: All patients: 0.58 Off therapy: 0.67 |
Continuous: All patients: 0.64 Categorical: All patients: 0.62 Off therapy: 0.69 |
||
| Friberg, 201218 |
Continuous: 0.66 (95% CI 0.65 to 0.66) Categorical (Revised): 0.61 (95% CI 0.61 to 0.62) Categorical (Classic): 0.64 (95% CI 0.64 to 0.65) |
Continuous: 0.67 (95% CI 0.66 to 0.67) Categorical: 0.64 (95% CI 0.64 to 0.65) |
Continuous: 0.67 (95% CI 0.67 to 0.68) Categorical: 0.56 (95% CI 0.56 to 0.57) |
|
| Friberg, 201571 |
Continuous: 0.72 (95% CI 0.72 to 0.73) |
Continuous: 0.71 (95% CI 0.71 to 0.72) |
||
| Gage, 200135 |
Continuous: 0.82 (95% CI 0.80 to 0.84) |
|||
| Hijazi, 201656 |
Continuous: Derivation cohort: 0.62 (95% CI 0.60 to 0.65) Validation cohort: 0.58 (95% CI 0.49 to 0.67) |
Categorical: Derivation cohort, TnI: 0.68 (95% CI 0.65 to 0.71) Derivation cohort, TnT: 0.67 (95% CI 0.65 to 0.70) Validation cohort, TnT: 0.66 (95% CI 0.58 to 0.74) |
||
| Primary paper: Granger, 201157 Relevant companion: Hijazi, 2017114 |
Categorical: Baseline data: TnI: 0.71 (95% CI 0.66 to 0.76) TnT: 0.70 (95% CI 0.65 to 0.75) 2 months: TnI: 0.72 (95% CI 0.66 to 0.77) TnT: 0.70 (95% CI 0.65 to 0.76) |
|||
| Primary paper: O’Brien, 201590 Relevant companion: Inohara, 201795 |
Continuous: 0.679 (95% CI 0.651 to 0.707) |
|||
| Larsen, 201244 |
Continuous: 0.68 (95% CI 0.59 to 0.76) |
Continuous: 0.69 (95% CI 0.60 to 0.77) |
||
| Lip, 201037 |
Continuous: 0.60 (95% CI 0.49 to 0.72) Categorical (Classic): 0.56 (95% CI 0.44 to 0.66) Categorical (Revised): 0.59 (95% CI 0.48 to 0.70) |
Continuous: 0.69 (95% CI 0.60 to 0.78) Categorical: 0.64 (95% CI 0.53 to 0.74) |
||
| McAlister, 2017115 |
Categorical: 0.663 (95% CI 0.652 to 0.675) |
Categorical: 0.661 (95% CI 0.649 to 0.672) |
||
| Oldgren, 201658 |
Continuous: 0.60 (95% CI 0.57 to 0.64) |
Categorical: 0.65 (95% CI 0.61to 0.69) |
||
| Olesen, 201123 |
Covariates analyzed as categorical variables: Continuous: 0.78 (95% CI 0.76 to 0.80) Categorical: 0.81 (95% CI 0.80 to 0.83) Covariates analyzed as continuous variables: Continuous: 0.80 (95% CI 0.79 to 0.82) Categorical: 0.81 (95% CI 0.80 to 0.83) |
Covariates analyzed as categorical variables: Continuous: 0.78 (95% CI 0.76 to 0.79) Categorical: 0.89 (95% CI 0.88 to 0.90) Covariates analyzed as continuous variables: Continuous: 0.79 (95% CI 0.78 to 0.81) Categorical: 0.89 (95% CI 0.88 to 0.90) |
||
| Olesen, 201239 |
Categorical: 0.63 (95% CI 0.62 to 0.65) |
Continuous: 0.66 (95% CI 0.65 to 0.68) |
||
| Philippart 2016116 |
Categorical: 0.588 (95% CI 0.577-0.599) Continuous: 0.641 (95% CI 0.631-0.652) |
|||
| Poli, 200930 |
Categorical: All patients: 0.68 On therapy: 0.52 |
|||
| Poli, 201126 |
Continuous (Revised): 0.72 (95% CI 0.64 to 0.80) Categorical (Classic): 0.68 (95% CI 0.61 to 0.76) Categorical (Revised): 0.60 (95% CI 0.51 to 0.67) |
Continuous: 0.72 (95% CI 0.65 to 0.80) Categorical: 0.52 (95% CI 0.44 to 0.61) |
||
| Potpara, 201220 |
Categorical: 0.58 (95% CI 0.38 to 0.79) |
Categorical: 0.72 (95% CI 0.61 to 0.84) |
||
| Rietbrock, 200832 |
Continuous (Classic): 0.68 (95% CI 0.68 to 0.69) Continuous (Revised): 0.72 (95% CI 0.72 to 0.73) |
|||
| Primary paper: Rivera-Caravaca, 201754 Relevant companion: Rivera-Caravaca, 2017102 |
Categorical (3.5 years): 0.600 (95% CI 0.567 to 0.625) Categorical (6.5 years): 0.620 (95% CI 0.590 to 0.648) |
Categorical (3.5 years): 0.663 (95% CI 0.634 to 0.690) Categorical (6.5 years): 0.662 (95% CI 0.633 to 0.690) |
||
| Ruff, 201648 |
Continuous: 0.586 (95% CI 0.565 to 0.607) |
|||
| Ruiz Ortiz, 201027 |
Continuous: 0.63 (95% CI 0.55 to 0.72) |
|||
| Singer, 201342 |
Continuous: 0.69 (95% CI 0.67 to 0.71) Categorical: 0.66 (95% CI 0.64 to 0.68) |
Continuous: 0.70 (95% CI 0.68 to 0.72) Categorical: 0.58 (95% CI 0.57 to 0.59) |
||
| van den Ham, 201545 |
Continuous: 0.68 (95% CI 0.67 to 0.69) Categorical (published low/moderate/high) 0.65 (95% CI 0.64 to 0.66) Categorical (optimized) 0.65 (95% CI 0.64 to 0.66) |
Continuous: 0.68 (95% CI 0.67 to 0.69) Categorical (published low/moderate/high) 0.59 (95% CI 0.59 to 0.60) Categorical (optimized) 0.63 (95% CI 0.62 to 0.64) |
||
| Van Staa, 201124 |
Continuous: 0.66 (95% CI 0.64 to 0.68) Categorical: 0.65 (95% CI 0.63 to 0.67) |
Continuous: 0.65 (95% CI 0.63 to 0.68) Categorical: 0.62 (95% CI 0.60 to 0.64) |
Continuous: 0.67 (95% CI 0.65 to 0.69) Categorical: 0.60 (95% CI 0.59 to 0.61) |
|
| Wang, 200355 |
Categorical: 0.62 |
Categorical: 0.66 (SD 0.03) |
Abbreviations: CHADS2=Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, prior Stroke/transient ischemic attack (2 points); CHA2DS2-VASc=Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥75 (2 points), Diabetes mellitus, prior Stroke/transient ischemic attack/thromboembolism (2 points), Vascular disease, Age 65–74, Sex category female; CI=confidence interval; SD=standard deviation
Appendix Table 4.
Summary of Results Evaluating Individual Risk Factors
| Study Design |
No. of Patients |
Followup | Bleeding Risk | Risk of Bias |
|---|---|---|---|---|
| Presence and severity of CKD | ||||
| Apostolakis, 201350 Observational |
2293 | NR | Major Bleeding Patients with more than mild CKD (CrCl 60 mL/min) had higher risk of major bleeding compare with patients with CrCl ≥60 mL/min: HR 1.58 (95% CI 1.05 to 2.39)* |
Low |
| Bassand, 2018117 | 28,628 | 2 years | Major Bleeding HR 1.74 (95% CI 1.34 to 2.26) |
Low |
| Friberg, 201571 Observational |
283,969 | Total: Median 2.1 years | Intracranial Bleeding Presence and severity of CKD: HR 1.50 (95% CI 1.28 to 1.74)* Any Bleeding Presence and severity of CKD: HR 2.24 (95% CI 2.14 to 2.35)* |
Low |
| Jun, 201770 Observational |
14,892 | 1 year | Compared to nonuse, warfarin therapy was not associated with higher risk for major bleeding except for those with eGFRs of 60 to 89 mL/min/1.73 m2 (HR 1.36; 95% CI 1.13 to 1.64). | Low |
| McAlister, 2017115 Observational |
58,451 | Median 31 months | eGFR, mL/min/1.732 ≥60 = 1.00 45-59 = 1.13 (95% CI 1.04 to 1.22) 30-44 = 1.25 (95% CI 1.14 to 1.37) <30 = 1.50 (95% CI 1.33 to 1.68) |
Low |
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 year (IQR 1.8) | Multivariable Analysis Major Bleeding HR 1.59 (95% CI 1.41 to 1.79)* |
Low |
| Pisters, 20109 Observational |
3456 | 1 year | Major Bleeding OR 2.86 (95% CI 1.33 to 6.18)* |
Low |
| Sherwood, 2015118 Observational |
14,263 | NR | Creatinine clearance (for each 5-U decrease to <60 ml/min) HR 1.06 (95% CI 1.01 to 1.12)* |
Low |
| Cognitive impairment | ||||
| Orkaby, 2017119 Observational |
2,572 | Mean 2.2 person-years following diagnosis of dementia | After diagnosis of dementia no statistically significant decrease in risk of major bleeding (HR 0.78, 95% CI 0.61 to 1.01, P = .06). |
Medium |
| INR | ||||
| An, 2017120 Observational |
32,074 | Total: 5 years Median 3.8 years |
Patients whose TTRs were < 65%, had a 2 times higher risk of major bleeding (HR 2.10, 95% CI 1.96 to 2.24) compared with patients with the highest TTR quartile (≥ 73%) | Low |
| Haas, 201647 Observational |
9,934 | 1 year | TTR <65% vs. ≥65% bleeding risk HR 1.54 (95% CI 1.04 to 2.26) | Low |
| Lind, 201267 Observational |
19,179 | 34718.9 patient-years | The bleeding risk HR for the SDTINR variable was 1.27 (95% CI 1.20 to 1.35), and the HR for TTR was 1.07 (95% CI 1.01 to 1.14) | High |
| Phelps, 2018121 Observational |
8,405 | 1 year | Major Bleeding OR 0.62 (95% CI 0.43 to 0.89) |
Moderate |
| Rivera-Caravaca, 2018122 Observational |
1,361 | 6 months Median follow-up 214 days | Major bleeding rates per year: TTR <20% = 1.47 and ≥20% = 2.93; TTR <65% = 3.03 and ≥65% = 2.10 |
Low |
| Age | ||||
| Bassand, 2018117 | 28,628 | 2 years | Major Bleeding (HRs) <65 = referent 65-69 = 1.30 (95% CI 0.86 to 1.96) 70-74=1.88 (95% CI 1.30 to 2.74) 75+=2.49 (95% CI 1.81 to 3.42) |
Low |
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 year (IQR 1.8) | Multivariable Analysis Major Bleeding Age: 65-74 yr HR 2.33 (95% CI 1.96 to 2.77)* >75yr HR 3.28 (95% CI 2.80 to 3.83)* |
Low |
| Goodman, 2014123 Observational |
14,264 | NR | Multivariable analysis Major Bleeding Age (per 5y increase) HR 1.17 (95% CI 1.12 to 1.23)* |
Low |
| Hankey, 2014124 Observational |
14,264 | NR | Intracranial Bleeding Age: HR for 10 years increase 1.35 (95% CI 1.13 to 1.63)* |
Medium |
| Olesen, 201217 Observational |
6348 | NR | Major Bleeding Age <65: Event Rate 0.39 (0.16 to 0.94) Age 65-74y: Event Rate 1.34 (0.60 to 2.97) Age>75: Event Rate 1.98 (1.10 to 3.58) |
Medium |
| Pisters, 20109 Observational |
3456 | 1 year | Major Bleeding Age >65: OR 2.66 (1.33-5.32)* |
Low |
| Renoux, 2017125 Observational |
147,622 | Mean follow up period 2.9 years | Female vs. Male for Major Bleeding <75 = HR 0.91 (95% CI 0.88 to 0.95) ≥75 = HR 0.96 (95% CI 0.89 to 1.02) |
Low |
| Sherwood, 2015118 Observational |
14,263 | NR | Major Bleeding (Gastrointestinal) Age (for each 5-yr increase): HR 1.11 (1.06 to 1.17)* |
Low |
| Prior stroke | ||||
| Bassand, 2018117 | 28,628 | 2-years | Major Bleeding HR 1.36 (95% CI 1.04 to 1.78) |
Low |
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 year (IQR 1.8) | Multivariable Analysis Major Bleeding HR 1.14 (95% CI 1.04 to 1.24)* |
Low |
| Hankey, 2014124 Observational |
14,264 | NR | Intracranial Bleeding HR 1.42 (95% CI 1.02 to 1.96)* |
Medium |
| Pisters, 20109 Observational |
3456 | 1 year | Major Bleeding Prior stroke: OR 0.94 (95% CI 0.32 to 2.86) |
Low |
| Hilkens, 2017126 Observational |
3623 | 2 years | C-statistic (95% CI) of risk scores for major bleeding in patients with a TIA or stroke on oral anticoagulants at 2 years HEMORR2 HAGES 0.63 (0.59 to 0.66) HAS-BLED 0.62 (0.58 to 0.65) ATRIA 0.66 (0.62 to 0.69) |
Low |
| Presence of heart disease | ||||
| Bassand, 2018117 | 28,628 | 2-years | Major Bleeding HR 1.07 (95% CI 0.84 to 1.36) |
Low |
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 year (IQR 1.8) | Multivariable Analysis Major Bleeding Presence of heart disease (Heart Failure): HR 1.15 (95% CI 1.07 to 1.24)* (Hypertension) HR 1.25 (95% CI 1.16 to 1.33)* |
Low |
| Goodman, 2014123 Observational |
14,264 | NR | Multivariable Model Major Bleeding Presence of heart disease (Hypertension): DBP >90 mm Hg (per 5-mm Hg increase) HR 1.28 (1.11 to 1.47)* |
Low |
| Hankey, 2014124 Observational |
14,264 | NR | Intracranial Bleeding HR 0.65 (95% CI 0.47 to 0.89)* |
Medium |
| Pisters, 20109 Observational |
3456 | 1 year | Major Bleeding Presence of heart disease (PA>160mmHg): OR 0.60 (95% CI 0.21 to 1.72) |
Low |
| Diabetes | ||||
| Bassand, 2018117 | 28,628 | 2-years | Major Bleeding HR 0.92 (95% CI 0.71 to 1.18) |
Low |
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 yr (IQR 1.8) | Multivariable Analysis Major Bleeding HR 1.01 (95% CI 0.92 to 1.11) |
Low |
| Sex | ||||
| Bassand, 2018117 | 28,628 | 2 years | Major Bleeding HR (Women) 1.14 (95% CI 0.90 to 1.45) |
Low |
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 yr (IQR 1.8) | Multivariable Analysis Major Bleeding Female HR 0.79 (95% CI 0.73 to 0.85)* |
Low |
| Goodman, 2014123 Observational |
14,264 | NR | Multivariable Model Major Bleeding Female vs. Male HR 0.82 (95% CI 0.70 to 0.95)* |
Low |
| Renoux, 2017125 Observational |
147,622 | Mean follow up period 2.9 years | Female vs. Male for Major Bleeding <75 = HR 0.91 (95% CI 0.88 to 0.95) ≥75 = HR 0.96 (95% CI 0.89 to 1.02) |
Low |
| Sherwood, 2015118 Observational |
14,263 | NR | Major Bleeding (Gastrointestinal) Male HR 1.21 (95% CI 1.01 to 1.44)* |
Low |
| Cancer | ||||
| Friberg, 201218 Observational |
182,678 | Total: Median 1.4 yr (IQR 1.8) | Multivariable Analysis Major Bleeding Cancer <3 years: HR 1.15 (95% CI 1.04 to 1.27)* |
Low |
| Race/Ethnicity | ||||
| Bassand, 2018117 | 28,628 | 2-years | Major Bleeding (HRs) Caucasian / Hispanic / Latino (referent) Asian = 0.61 (95% CI 0.44 to 0.84) Other = 0.51 (95% CI 0.16 to 1.61) |
Low |
| Hankey, 2014124 Observational |
14,264 | NR | Intracranial Bleeding Asian HR 2.02 (95% CI1.39 to 2.94) Black HR 3.25 (95% CI 1.43 to 7.41)* |
Medium |
p value<0.05
Abbreviations: ATRIA=Anticoagulation and Risk Factors in Atrial Fibrillation; BRI=Bleeding Risk Index; CI=confidence interval; CKD=chronic kidney disease; HAS-BLED=Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly; HR=hazard ratio; HEMORR2HAGES=Hepatic or renal disease, Ethanol abuse, Malignancy, Older (age >75 years), Reduced platelet count or function, Re-bleeding risk (2 points), Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; SE=standard error; INR=international normalized ratio; TIA=transient ischemic attack; TTR=time in therapeutic range; SDTinr=standardized deviation of transformed INF
Appendix Table 5.
C-statistics from Studies Comparing Scores of Interest for Prediction of Major Bleeding Riska
| Study | BRI | HEMORR2HAGES | HAS-BLED | ATRIA | ABC |
|---|---|---|---|---|---|
| Patients on warfarin | |||||
| Apostolakis, 201282d | – | 0.60 (95% CI 0.51 to 0.69) |
0.65 (95% CI 0.56 to 0.73) |
0.61 (95% CI 0.51 to 0.70) |
– |
| Barnes, 201486 | – | 0.66 (95% CI 0.61-0.74) |
0.69 (95% CI 0.63-0.75) |
0.67 (95% CI 0.61 to 0.74) |
– |
| Fang, 201176d,f | Categorical: 0.59 (95% CI 0.58 to 0.61) Continuous: 0.68 (95% CI 0.65 to 0.70) |
Categorical: 0.67 (95% CI 0.65 to 0.70) Continuous: 0.71 (95% CI 0.69 to 0.73) |
– | Categorical: 0.69 (95% CI 0.66 to 0.71) Continuous: 0.74 (95% CI 0.72 to 0.76) |
– |
| Friberg, 201218d | – | 0.63 (95% CI 0.61 to 0.64) |
0.61 (95% CI 0.59 to 0.62) |
– | – |
| Gage, 200679b,c | 0.65 (SE 0.03) | 0.67 (SE 0.04) | – | – | – |
| Hijazi, 201693 | – | – | ARISTOTLE: 0.61 (0.58 to 0.63) RE-LY: 0.60 (0.56 to 0.64) |
– | ARISTOTLE: 0.68 (0.65 to 0.70) RE-LY: 0.65 (0.61 to 0.70) |
| Jaspers Focks, 201688 | – | Major bleeding = 0.57 (95% CI 0.50 to 0.63) Clinically relevant bleeding = 0.53 (95% CI 0.50 to 0.57) Any bleeding = 0.53 (95% CI 0.50 to 0.57) |
Major Bleeding = 0.57 (95% CI 0.50 to 0.63) Clinically relevant bleeding = 0.50 (95% CI 0.47 to 0.54) Any bleeding = 0.51 (95% CI 0.47 to 0.54) |
Major Bleeding = 0.58 (95% CI 0.51 to 0.64) Clinically relevant bleeding = 0.52 (95% CI 0.49 to 0.56) Any bleeding= 0.53 (95% CI 0.50 to 0.57) |
– |
| Lip, 201177d | 0.56 (95% CI 0.51 to 0.60) |
0.61 (95% CI 0.56 to 0.65) |
0.66 (95% CI 0.61 to 0.70) |
– | – |
| Lip, 201285g | Categorical: 0.56 (95% CI 0.53 to 0.59) Continuous: 0.60 (95% CI 0.56 to 0.63) |
Categorical: 0.53 (95% CI 0.50 to 0.57) Continuous: 0.59 (95% CI 0.56 to 0.62) |
Categorical: 0.58 (95% CI 0.55 to 0.61) Continuous: 0.61 (95% CI 0.58 to 0.65) |
Categorical: 0.55 (95% CI 0.52 to 0.59) Continuous: 0.60 (95% CI 0.56 to 0.63) |
– |
| Lip, 201794 | – | – | 0.58 (95% CI 0.57-0.59) | 0.59 (95% CI 0.57-0.60) |
– |
| Olesen, 201181d | – | Categorical: 0.78 (95% CI 0.75 to 0.82) Continuous: 0.77 (95% CI 0.73 to 0.81) |
Categorical: 0.80 (95% CI 0.76 to 0.83) Continuous: 0.80 (95% CI 0.76 to 0.83) |
– | – |
| Pisters, 20109d,e | – | 0.64 (95% CI 0.53 to 0.75) |
0.69 (95% CI 0.59 to 0.80) |
– | – |
| Roldan, 201284h | – | – | Categorical: 0.68 (95% CI 0.65 to 0.71) Continuous: 0.71 (95% CI 0.68 to 0.74) |
Categorical: 0.59 (95% CI 0.55 to 0.62) Continuous: 0.68 (95% CI 0.65 to 0.71) |
– |
| Senoo, 201691 | – | – | 0.65 (95% CI 0.56 to 0.73) |
0.61 (95% CI 0.51 to 0.70) |
– |
| Patients on aspirin alone | |||||
| Friberg, 201218e | – | 0.60 (95% CI 0.59 to 0.61) |
0.59 (95% CI 0.58 to 0.60) |
– | – |
| Gage, 200679b,c | 0.69 (SE 0.05) | 0.72 (SE 0.05)b | – | – | – |
| Pisters, 20109d,e | – | 0.83 (95% CI 0.68 to 0.98) |
0.91 (95% CI 0.83 to 1.00) |
– | – |
| Patients off antithrombotic therapy | |||||
| Friberg, 201218e | – | 0.69 (95% CI 0.67 to 0.70) |
0.66 (95% CI 0.65 to 0.68) |
– | – |
| Gage, 200679b,c | 0.65 (SE 0.03) | 0.66 (SE 0.04) | – | – | – |
| Lip, 201177d | 0.50 (95% CI 0.44 to 0.57) |
0.62 (95% CI 0.52 to 0.72) |
0.66 (95% CI 0.55 to 0.74) |
– | – |
| Lip, 201285f | Categorical: 0.58 (95% CI 0.54 to 0.62) Continuous: 0.60 (95% CI 0.56 to 0.64) |
Categorical: 0.55 (95% CI 0.50 to 0.59) Continuous: 0.59 (95% CI 0.54 to 0.63) |
Categorical: 0.60 (95% CI 0.54 to 0.64) Continuous: 0.60 (95% CI 0.56 to 0.64) |
Categorical: 0.47 (95% CI 0.42 to 0.51) Continuous: 0.59 (95% CI 0.55 to 0.64) |
– |
| Olesen, 201181d | – | Categorical: 0.77 (95% CI 0.74 to 0.80) Continuous: 0.79 (95% CI 0.73 to 0.79) |
Categorical: 0.82 (95% CI 0.79 to 0.84) Continuous: 0.81 (95% CI 0.78 to 0.83) |
– | – |
| Pisters, 20109d,e | – | 0.81 (95% CI 0.00 to 1.00) |
0.85 (95% CI 0.00 to 1.00) |
– | – |
C-statistics given are for categorical risk scores unless otherwise noted.
Derivation study for HEMORR2HAGES.
P-value for 2-way between-score comparison not provided.
P-value for between-score comparison not provided.
Derivation study for HAS-BLED.
Derivation study for ATRIA.
P-values for all between-score comparisons >0.05 (not specified as <0.05 in source article).
P=0.035 for comparison of between-score categorical c-statistics and p=0.356 for comparison of between-score continuous c-statistics.
Abbreviations: ABC=Age, biomarkers, clinical history; ATRIA=Anticoagulation and Risk Factors in Atrial Fibrillation; BRI=Bleeding Risk Index; CI=confidence interval; HAS-BLED=Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly; HEMORR2HAGES=Hepatic or renal disease, Ethanol abuse, Malignancy, Older (age >75 years), Reduced platelet count or function, Re-bleeding risk (2 points), Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; SE=standard error
Footnotes
DISCLAIMER
This project was funded under Contract No. 290-2015-00004-I from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Patient-Centered Outcomes Research Institute (PCORI), AHRQ, and the U.S. Department of Health and Human Services. AHRQ retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- 3.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 1994;74(3):236–241. [DOI] [PubMed] [Google Scholar]
- 4.Lee WC, Lamas GA, Balu S, Spalding J, Wang Q, Pashos CL. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ 2008;11(2):281–298. [DOI] [PubMed] [Google Scholar]
- 5.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119(5):448 e441–419. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113(5):359–364. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, Nozawa T, Hirai T, et al. Accumulation of risk factors increases risk of thromboembolic events in patients with nonvalvular atrial fibrillation. Circ J 2006;70(6):651–656. [DOI] [PubMed] [Google Scholar]
- 9.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138(5):1093–1100. [DOI] [PubMed] [Google Scholar]
- 10.Lopes RD, Crowley MJ, Shah BR, et al. Stroke Prevention in Atrial Fibrillation. AHRQ Comparative Effectiveness Reviews. 2013. [Google Scholar]
- 11.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Available at: https://www.effectivehealthcare.ahrq.gov/topics/cer-methods-guide/overview. Accessed November 27, 2017. [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Methods Guide for Medical Test Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Available at: https://www.effectivehealthcare.ahrq.gov/topics/methods-guidance-tests/overview-2012/ Accessed November 27, 2017. [Google Scholar]
- 13.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 15.Ohman EM, Granger CB, Harrington RA, Lee KL. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA 2000;284(7):876–878. [DOI] [PubMed] [Google Scholar]
- 16.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions-Agency for Healthcare Research and Quality and the Effective Health Care Program. J Clin Epidemiol 2010;63(5):513–523. [DOI] [PubMed] [Google Scholar]
- 17.Olesen JB, Fauchier L, Lane DA, Taillandier S, Lip GY. Risk factors for stroke and thromboembolism in relation to age among patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest 2012;141(1):147–153. [DOI] [PubMed] [Google Scholar]
- 18.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33(12):1500–1510. [DOI] [PubMed] [Google Scholar]
- 19.Olesen JB, Lip GY, Lane DA, et al. Vascular disease and stroke risk in atrial fibrillation: a nationwide cohort study. Am J Med 2012;125(8):826 e813–823. [DOI] [PubMed] [Google Scholar]
- 20.Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Prostran MS, Lip GY. Reliable identification of "truly low" thromboembolic risk in patients initially diagnosed with "lone" atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol 2012;5(2):319–326. [DOI] [PubMed] [Google Scholar]
- 21.Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: A net clinical benefit analysis using a 'real world' nationwide cohort study. Thromb Haemost 2011;106(4):739–749. [DOI] [PubMed] [Google Scholar]
- 22.Poli D, Testa S, Antonucci E, Grifoni E, Paoletti O, Lip GY. Bleeding and stroke risk in a real-world prospective primary prevention cohort of patients with atrial fibrillation. Chest 2011;140(4):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GY. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost 2011;9(1):39–48. [DOI] [PubMed] [Google Scholar]
- 25.Ad N, Henry L, Schlauch K, Holmes SD, Hunt S. The CHADS score role in managing anticoagulation after surgical ablation for atrial fibrillation. Ann Thorac Surg 2010;90(4):1257–1262. [DOI] [PubMed] [Google Scholar]
- 26.Poli D, Lip GY, Antonucci E, Grifoni E, Lane D. Stroke risk stratification in a "real-world" elderly anticoagulated atrial fibrillation population. J Cardiovasc Electrophysiol 2011;22(1):25–30. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz Ortiz M, Romo E, Mesa D, et al. Oral anticoagulation in nonvalvular atrial fibrillation in clinical practice: impact of CHADS(2) score on outcome. Cardiology 2010;115(3):200–204. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361(12):1139–1151. [DOI] [PubMed] [Google Scholar]
- 29.Crandall MA, Horne BD, Day JD, et al. Atrial fibrillation significantly increases total mortality and stroke risk beyond that conveyed by the CHADS2 risk factors. Pacing Clin Electrophysiol 2009;32(8):981–986. [DOI] [PubMed] [Google Scholar]
- 30.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Stroke risk in atrial fibrillation patients on warfarin. Predictive ability of risk stratification schemes for primary and secondary prevention. Thromb Haemost 2009;101(2):367–372. [PubMed] [Google Scholar]
- 31.Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009;124(1):37–41. [DOI] [PubMed] [Google Scholar]
- 32.Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J 2008;156(1):57–64. [DOI] [PubMed] [Google Scholar]
- 33.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol 2008;51(8):810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baruch L, Gage BF, Horrow J, et al. Can patients at elevated risk of stroke treated with anticoagulants be further risk stratified? Stroke 2007;38(9):2459–2463. [DOI] [PubMed] [Google Scholar]
- 35.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285(22):2864–2870. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Nodar JM, Marin F, Manzano-Fernandez S, et al. An evaluation of the CHADS2 stroke risk score in patients with atrial fibrillation who undergo percutaneous coronary revascularization. Chest 2011;139(6):1402–1409. [DOI] [PubMed] [Google Scholar]
- 37.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz Ortiz M, Romo E, Mesa D, et al. [Predicting embolic events in patients with nonvalvular atrial fibrillation: evaluation of the CHADS2 score in a Mediterranean population]. Rev Esp Cardiol 2008;61(1):29–35. [PubMed] [Google Scholar]
- 39.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: A nationwide cohort study. Thromb Haemost 2012;107(6):1172–1179. [DOI] [PubMed] [Google Scholar]
- 40.Fanola CL, Giugliano RP, Ruff CT, et al. A novel risk prediction score in atrial fibrillation for a net clinical outcome from the ENGAGE AF-TIMI 48 randomized clinical trial. Eur Heart J 2017;38(12):888–896. [DOI] [PubMed] [Google Scholar]
- 41.Abraham JM, Larson J, Chung MK, et al. Does CHA2DS2-VASc improve stroke risk stratification in postmenopausal women with atrial fibrillation? Am J Med 2013;126(12):1143.e1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2(3):e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lip GY, Connolly S, Yusuf S, et al. Modification of outcomes with aspirin or apixaban in relation to CHADS(2) and CHA(2)DS(2)-VASc scores in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Circ Arrhythm Electrophysiol 2013;6(1):31–38. [DOI] [PubMed] [Google Scholar]
- 44.Larsen TB, Lip GY, Skjoth F, Due KM, Overvad K, Hvilsted Rasmussen L. Added predictive ability of the CHA2DS2VASc risk score for stroke and death in patients with atrial fibrillation: the prospective Danish Diet, Cancer, and Health cohort study. Circ Cardiovasc Qual Outcomes 2012;5(3):335–342. [DOI] [PubMed] [Google Scholar]
- 45.van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative Performance of ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores Predicting Stroke in Patients With Atrial Fibrillation: Results From a National Primary Care Database. J Am Coll Cardiol 2015;66(17):1851–1859. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Nodar JM, Marin F, Roldan V, et al. Should We Recommend Oral Anticoagulation Therapy in Patients With Atrial Fibrillation Undergoing Coronary Artery Stenting With a High HAS-BLED Bleeding Risk Score? Circ Cardiovasc Interv 2012;5(4):459–466. [DOI] [PubMed] [Google Scholar]
- 47.Haas S, Ten Cate H, Accetta G, et al. Quality of Vitamin K Antagonist Control and 1-Year Outcomes in Patients with Atrial Fibrillation: A Global Perspective from the GARFIELD-AF Registry. PLoS One 2016;11(10):e0164076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruff CT, Giugliano RP, Braunwald E, et al. Cardiovascular Biomarker Score and Clinical Outcomes in Patients With Atrial Fibrillation: A Subanalysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial. JAMA Cardiol 2016;1(9):999–1006. [DOI] [PubMed] [Google Scholar]
- 49.Fauchier L, Clementy N, Bisson A, et al. Should Atrial Fibrillation Patients With Only 1 Nongender-Related CHA2DS2-VASc Risk Factor Be Anticoagulated? Stroke 2016;47(7):1831–1836. [DOI] [PubMed] [Google Scholar]
- 50.Apostolakis S, Guo Y, Lane DA, Buller H, Lip GY. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J 2013;34(46):3572–3579. [DOI] [PubMed] [Google Scholar]
- 51.Allan V, Banerjee A, Shah AD, et al. Net clinical benefit of warfarin in individuals with atrial fibrillation across stroke risk and across primary and secondary care. Heart 2017;103(3):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonde AN, Lip GY, Kamper AL, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014;64(23):2471–2482. [DOI] [PubMed] [Google Scholar]
- 53.Forslund T, Wettermark B, Wandell P, von Euler M, Hasselstrom J, Hjemdahl P. Risks for stroke and bleeding with warfarin or aspirin treatment in patients with atrial fibrillation at different CHA(2)DS(2)VASc scores: experience from the Stockholm region. Eur J Clin Pharmacol 2014;70(12):1477–1485. [DOI] [PubMed] [Google Scholar]
- 54.Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, et al. Importance of time in therapeutic range on bleeding risk prediction using clinical risk scores in patients with atrial fibrillation. Sci Rep 2017;7(1):12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 2003;290(8):1049–1056. [DOI] [PubMed] [Google Scholar]
- 56.Hijazi Z, Lindback J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37(20):1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365(11):981–992. [DOI] [PubMed] [Google Scholar]
- 58.Oldgren J, Hijazi Z, Lindback J, et al. Performance and Validation of a Novel Biomarker-Based Stroke Risk Score for Atrial Fibrillation. Circulation 2016;134(22):1697–1707. [DOI] [PubMed] [Google Scholar]
- 59.Beinart R, Heist EK, Newell JB, Holmvang G, Ruskin JN, Mansour M. Left atrial appendage dimensions predict the risk of stroke/TIA in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2011;22(1):10–15. [DOI] [PubMed] [Google Scholar]
- 60.Nair CK, Holmberg MJ, Aronow WS, Shen X, Li H, Lakkireddy D. Thromboembolism in patients with atrial fibrillation with and without left atrial thrombus documented by transesophageal echocardiography. Am J Ther 2009;16(5):385–392. [DOI] [PubMed] [Google Scholar]
- 61.Stollberger C, Chnupa P, Abzieher C, et al. Mortality and rate of stroke or embolism in atrial fibrillation during long-term follow-up in the embolism in left atrial thrombi (ELAT) study. Clin Cardiol 2004;27(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoddard MF, Singh P, Dawn B, Longaker RA. Left atrial thrombus predicts transient ischemic attack in patients with atrial fibrillation. Am Heart J 2003;145(4):676–682. [DOI] [PubMed] [Google Scholar]
- 63.Thambidorai SK, Murray RD, Parakh K, et al. Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study). Am J Cardiol 2005;96(7):935–941. [DOI] [PubMed] [Google Scholar]
- 64.Gupta DK, Giugliano RP, Ruff CT, et al. The Prognostic Significance of Cardiac Structure and Function in Atrial Fibrillation: The ENGAGE AF-TIMI 48 Echocardiographic Substudy. J Am Soc Echocardiogr 2016;29(6):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yarmohammadi H, Klosterman T, Grewal G, et al. Efficacy of the CHADS(2) scoring system to assess left atrial thrombogenic milieu risk before cardioversion of non-valvular atrial fibrillation. Am J Cardiol 2013;112(5):678–683. [DOI] [PubMed] [Google Scholar]
- 66.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003;349(11):1019–1026. [DOI] [PubMed] [Google Scholar]
- 67.Lind M, Fahlen M, Kosiborod M, Eliasson B, Oden A. Variability of INR and its relationship with mortality, stroke, bleeding and hospitalisations in patients with atrial fibrillation. Thromb Res 2012;129(1):32–35. [DOI] [PubMed] [Google Scholar]
- 68.Link MS, Giugliano RP, Ruff CT, et al. Stroke and Mortality Risk in Patients With Various Patterns of Atrial Fibrillation: Results From the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol 2017;10(1). [DOI] [PubMed] [Google Scholar]
- 69.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364(9):806–817. [DOI] [PubMed] [Google Scholar]
- 70.Jun M, James MT, Ma Z, et al. Warfarin Initiation, Atrial Fibrillation, and Kidney Function: Comparative Effectiveness and Safety of Warfarin in Older Adults With Newly Diagnosed Atrial Fibrillation. Am J Kidney Dis 2017;69(6):734–743. [DOI] [PubMed] [Google Scholar]
- 71.Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015;36(5):297–306. [DOI] [PubMed] [Google Scholar]
- 72.Banerjee A, Fauchier L, Vourc'h P, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol 2013;61(20):2079–2087. [DOI] [PubMed] [Google Scholar]
- 73.Flaker GC, Pogue J, Yusuf S, et al. Cognitive function and anticoagulation control in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2010;3(3):277–283. [DOI] [PubMed] [Google Scholar]
- 74.Ashburner JM, Go AS, Chang Y, et al. Effect of Diabetes and Glycemic Control on Ischemic Stroke Risk in AF Patients: ATRIA Study. J Am Coll Cardiol 2016;67(3):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McMurray JJ, Ezekowitz JA, Lewis BS, et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail 2013;6(3):451–460. [DOI] [PubMed] [Google Scholar]
- 76.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58(4):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57(2):173–180. [DOI] [PubMed] [Google Scholar]
- 78.Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest 2006;130(5):1390–1396. [DOI] [PubMed] [Google Scholar]
- 79.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151(3):713–719. [DOI] [PubMed] [Google Scholar]
- 80.Aspinall SL, DeSanzo BE, Trilli LE, Good CB. Bleeding Risk Index in an anticoagulation clinic. Assessment by indication and implications for care. J Gen Intern Med 2005;20(11):1008–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olesen JB, Lip GYH, Hansen PR, et al. Bleeding risk in 'real world' patients with atrial fibrillation: Comparison of two established bleeding prediction schemes in a nationwide cohort. Journal of Thrombosis and Haemostasis 2011;9(8):1460–1467. [DOI] [PubMed] [Google Scholar]
- 82.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED Bleeding Risk-Prediction Scores in Patients With Atrial Fibrillation Undergoing Anticoagulation: The AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) Study. J Am Coll Cardiol 2012. [DOI] [PubMed] [Google Scholar]
- 83.Gallego P, Roldan V, Torregrosa JM, et al. Relation of the HAS-BLED bleeding risk score to major bleeding, cardiovascular events, and mortality in anticoagulated patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5(2):312–318. [DOI] [PubMed] [Google Scholar]
- 84.Roldan V, Marin F, Fernandez H, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a 'real world' anticoagulated atrial fibrillation population. Chest 2012. [DOI] [PubMed] [Google Scholar]
- 85.Lip GY, Banerjee A, Lagrenade I, Lane DA, Taillandier S, Fauchier L. Assessing the Risk of Bleeding in Patients with Atrial Fibrillation: The Loire Valley Atrial Fibrillation Project. Circ Arrhythm Electrophysiol 2012. [DOI] [PubMed] [Google Scholar]
- 86.Barnes GD, Gu X, Haymart B, et al. The predictive ability of the CHADS2 and CHA2DS2-VASc scores for bleeding risk in atrial fibrillation: the MAQI(2) experience. Thromb Res 2014;134(2):294–299. [DOI] [PubMed] [Google Scholar]
- 87.Proietti M, Senoo K, Lane DA, Lip GY. Major Bleeding in Patients with Non-Valvular Atrial Fibrillation: Impact of Time in Therapeutic Range on Contemporary Bleeding Risk Scores. Sci Rep 2016;6:24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaspers Focks J, van Vugt SP, Albers-Akkers MT, et al. Low performance of bleeding risk models in the very elderly with atrial fibrillation using vitamin K antagonists. J Thromb Haemost 2016;14(9):1715–1724. [DOI] [PubMed] [Google Scholar]
- 89.Apostolakis S, Lane DA, Buller H, Lip GY. Comparison of the CHADS2, CHA2DS2-VASc and HAS-BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb Haemost 2013;110(5):1074–1079. [DOI] [PubMed] [Google Scholar]
- 90.O'Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J 2015;36(46):3258–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Senoo K, Proietti M, Lane DA, Lip GY. Evaluation of the HAS-BLED, ATRIA, and ORBIT Bleeding Risk Scores in Patients with Atrial Fibrillation Taking Warfarin. Am J Med 2016;129(6):600–607. [DOI] [PubMed] [Google Scholar]
- 92.Esteve-Pastor MA, Garcia-Fernandez A, Macias M, et al. Is the ORBIT Bleeding Risk Score Superior to the HAS-BLED Score in Anticoagulated Atrial Fibrillation Patients? Circ J 2016;80(10):2102–2108. [DOI] [PubMed] [Google Scholar]
- 93.Hijazi Z, Oldgren J, Lindback J, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387(10035):2302–2311. [DOI] [PubMed] [Google Scholar]
- 94.Lip GYH, Skjoth F, Nielsen PB, Kjaeldgaard JN, Larsen TB. The HAS-BLED, ATRIA and ORBIT Bleeding Scores in Atrial Fibrillation Patients Using Non-Vitamin K Antagonist Oral Anticoagulants. Am J Med 2017. [DOI] [PubMed] [Google Scholar]
- 95.Inohara T, Shrader P, Pieper K, et al. Association of Atrial Fibrillation Clinical Phenotypes with Treatment Patterns and Outcomes: A Multicenter Registry Study. JAMA Cardiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Proietti M, Hijazi Z, Andersson U, et al. Comparison of bleeding risk scores in patients with atrial fibrillation: insights from the RE-LY trial. J Intern Med 2017. [DOI] [PubMed] [Google Scholar]
- 97.Yao X, Gersh BJ, Sangaralingham LR, et al. Comparison of the CHA2DS2-VASc, CHADS2, HAS-BLED, ORBIT, and ATRIA Risk Scores in Predicting Non-Vitamin K Antagonist Oral Anticoagulants-Associated Bleeding in Patients With Atrial Fibrillation. Am J Cardiol 2017;120(9):1549–1556. [DOI] [PubMed] [Google Scholar]
- 98.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114(7):e257–354. [DOI] [PubMed] [Google Scholar]
- 99.Brieger D, Amerena J, Attia J, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ 2018;27(10):1209–1266. [DOI] [PubMed] [Google Scholar]
- 100.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018. [DOI] [PubMed] [Google Scholar]
- 101.Chiang CE, Okumura K, Zhang S, et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm 2017;33(4):345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, et al. Long-Term Stroke Risk Prediction in Patients With Atrial Fibrillation: Comparison of the ABC-Stroke and CHA2DS2-VASc Scores. J Am Heart Assoc 2017;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rivera-Caravaca JM, Esteve-Pastor MA, Marin F, et al. A Propensity Score Matched Comparison of Clinical Outcomes in Atrial Fibrillation Patients Taking Vitamin K Antagonists: Comparing the "Real-World" vs Clinical Trials. Mayo Clin Proc 2018;93(8):1065–1073. [DOI] [PubMed] [Google Scholar]
- 104.Proietti M, Rivera-Caravaca JM, Esteve-Pastor MA, Romiti GF, Marin F, Lip Gregory YH. Predicting bleeding events in anticoagulated patients with atrial fibrillation: a comparison between the HAS-BLED and GARFIELD-AF bleeding scores. JAHA 2018;7(18):[Online at 10.1161/JAHA.1118.009766]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao TF, Lip GYH, Lin YJ, et al. Incident Risk Factors and Major Bleeding in Patients with Atrial Fibrillation Treated with Oral Anticoagulants: A Comparison of Baseline, Follow-up and Delta HAS-BLED Scores with an Approach Focused on Modifiable Bleeding Risk Factors. Thromb Haemost 2018;118(4):768–777. [DOI] [PubMed] [Google Scholar]
- 106.Chao TF, Lip GYH, Liu CJ, et al. Relationship of Aging and Incident Comorbidities to Stroke Risk in Patients With Atrial Fibrillation. J Am Coll Cardiol 2018;71(2):122–132. [DOI] [PubMed] [Google Scholar]
- 107.Esteve-Pastor MA, Marin F, Bertomeu-Martinez V, et al. Do physicians correctly calculate thromboembolic risk scores? A comparison of concordance between manual and computer-based calculation of CHADS2 and CHA2 DS2 -VASc scores. Intern Med J 2016;46(5):583–589. [DOI] [PubMed] [Google Scholar]
- 108.Silbernagel G, Spirk D, Hager A, Baumgartner I, Kucher N. Electronic Alert System for Improving Stroke Prevention Among Hospitalized Oral-Anticoagulation-Naive Patients With Atrial Fibrillation: A Randomized Trial. J Am Heart Assoc 2016;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998;105(2):91–99. [DOI] [PubMed] [Google Scholar]
- 110.Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 2014;312(6):623–630. [DOI] [PubMed] [Google Scholar]
- 112.Abumuaileq RR, Abu-Assi E, Lopez-Lopez A, et al. Comparison between CHA2DS2-VASc and the new R2CHADS2 and ATRIA scores at predicting thromboembolic event in non-anticoagulated and anticoagulated patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord 2015;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banerjee A, Fauchier L, Bernard-Brunet A, Clementy N, Lip GY. Composite risk scores and composite endpoints in the risk prediction of outcomes in anticoagulated patients with atrial fibrillation. The Loire Valley Atrial Fibrillation Project. Thromb Haemost 2014;111(3):549–556. [DOI] [PubMed] [Google Scholar]
- 114.Hijazi Z, Lindahl B, Oldgren J, et al. Repeated Measurements of Cardiac Biomarkers in Atrial Fibrillation and Validation of the ABC Stroke Score Over Time. J Am Heart Assoc 2017;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McAlister FA, Wiebe N, Jun M, et al. Are Existing Risk Scores for Nonvalvular Atrial Fibrillation Useful for Prediction or Risk Adjustment in Patients With Chronic Kidney Disease? Can J Cardiol 2017;33(2):243–252. [DOI] [PubMed] [Google Scholar]
- 116.Philippart R, Brunet-Bernard A, Clementy N, et al. Oral anticoagulation, stroke and thromboembolism in patients with atrial fibrillation and valve bioprosthesis. The Loire Valley Atrial Fibrillation Project. Thromb Haemost 2016;115(5):1056–1063. [DOI] [PubMed] [Google Scholar]
- 117.Bassand JP, Accetta G, Al Mahmeed W, et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation. PLoS One 2018;13(1):e0191592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal Bleeding in Patients With Atrial Fibrillation Treated With Rivaroxaban or Warfarin: ROCKET AF Trial. J Am Coll Cardiol 2015;66(21):2271–2281. [DOI] [PubMed] [Google Scholar]
- 119.Orkaby AR, Ozonoff A, Reisman JI, Miller DR, Zhao S, Rose AJ. Continued Use of Warfarin in Veterans with Atrial Fibrillation After Dementia Diagnosis. J Am Geriatr Soc 2017;65(2):249–256. [DOI] [PubMed] [Google Scholar]
- 120.An J, Niu F, Zheng C, et al. Warfarin Management and Outcomes in Patients with Nonvalvular Atrial Fibrillation Within an Integrated Health Care System. J Manag Care Spec Pharm 2017;23(6):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Phelps E, Delate T, Witt DM, Shaw PB, McCool KH, Clark NP. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res 2018;163:54–59. [DOI] [PubMed] [Google Scholar]
- 122.Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, et al. Reduced Time in Therapeutic Range and Higher Mortality in Atrial Fibrillation Patients Taking Acenocoumarol. Clin Ther 2018;40(1):114–122. [DOI] [PubMed] [Google Scholar]
- 123.Goodman SG, Wojdyla DM, Piccini JP, et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2014;63(9):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke 2014;45(5):1304–1312. [DOI] [PubMed] [Google Scholar]
- 125.Renoux C, Coulombe J, Suissa S. Revisiting sex differences in outcomes in non-valvular atrial fibrillation: a population-based cohort study. Eur Heart J 2017;38(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 126.Hilkens NA, Algra A, Greving JP. Predicting Major Bleeding in Ischemic Stroke Patients With Atrial Fibrillation. Stroke 2017;48(11):3142–3144. [DOI] [PubMed] [Google Scholar]