Abstract
Objectives
To quantify Gleason score (GS) heterogeneity within multiparametric magnetic resonance imaging-targeted prostate biopsies and to determine impact on NCCN risk stratification.
Methods
An IRB-approved retrospective study was performed on men who underwent Artemis (MRI-transrectal-ultrasound fusion) targeted biopsy (TB) for suspected prostate cancer between 2012–2015. Intra-target heterogeneity was defined as a difference in GS between two cores within a single target in patients with ≥ 2 positive cores. PSA, maximum tumor diameter, apparent diffusion coefficient (ADC), MRI suspicion score, prostate volume, systematic biopsy (SB) GS, and T-stage were analyzed for correlation with heterogeneity. Changes in NCCN risk based on high versus low GS on TB, SB alone, and SB+TB were compared.
Results
Fifty-three patients underwent targeted biopsy of 73 suspected lesions. Seventy-percent (51/73) had ≥ 2 positive cores, thus meeting inclusion criteria for heterogeneity analysis. Fifty-five percent (28/51) of qualifying targets showed GS heterogeneity. None of the evaluated factors showed a significant relationship with heterogeneity. NCCN low, intermediate, and high-risk groups were 30%, 49%, and 21% with SB alone. Adding low GS TB to SB resulted in 17%, 55%, 28% in each risk group, while using high GS+SB resulted in 4%, 54%, and 42%. Overall, the addition of TB resulted in higher NCCN risk groups in 38% of cases.
Conclusion
Over half of mpMRI-defined targets demonstrated GS heterogeneity. The addition of high GS from targeted biopsy leads to risk inflation compared to using systematic biopsy alone. Further research is needed on how to integrate these findings into current risk stratification models and clinical practice.
Keywords: Gleason score, heterogeneity, prostate cancer, prostate biopsy, targeted biopsy, upstaging
Introduction
The current standard of reference for prostate tissue sampling is transrectal ultrasound (TRUS)-guided 12-core systematic biopsy (SB). This approach, however, often fails to detect significant disease because of inadequate target definition by TRUS, as evidenced by the 20–30% Gleason score (GS) upgrading seen at the time of radical prostatectomy.(1–6)
The advent of multiparametric magnetic resonance imaging (mpMRI) has resulted in better visualization of intra-prostatic lesions that were not previously detectable with CT- imaging or with transrectal ultrasound.(7–9) The fusion of mpMRI with real-time TRUS during biopsies in the clinic has permitted the use of MRI data to target areas at greatest risk for having prostate cancer.(10)
As the evidence for using targeted biopsy mounts, the downstream implications for the treating radiation oncologist are far from understood. The goal is to be able to reliably determine the nature and the extent of disease before making a treatment decision. The identification of the highest GS is a critical piece of information in our current risk stratification model. With targeted biopsies we have the ability to more accurately sample areas at high risk of harboring prostate cancer, but there are many unanswered questions regarding the ideal approach to finding the highest GS within a particular target. If the MRI target is homogeneous, perhaps one targeted biopsy is sufficient to accurately classify the highest GS. However, if there is significant intraprostatic target heterogeneity, it may be necessary to take multiple targeted biopsies from a particular lesion in order to quantify the true disease pathology. The clinical implication of how to integrate targeted biopsy GS information into risk stratification schemes, which are currently based on systematic biopsy, also deserves further study.
To this end, we reviewed the pathology reports of patients who had undergone targeted biopsies and were referred to our radiation oncology clinic. We then analyzed targeted biopsy GS heterogeneity and determined the impact of changes in GS on NCCN risk group stratification.
Methods and Materials
Patient Selection
This was an IRB-approved retrospective pilot study on a consecutive series of patients with biopsy-proven prostate cancer who were referred to the University of California, Los Angeles (UCLA) Radiation Oncology Department. All patients had a 3T mpMRI followed by systematic and targeted biopsies with the Artemis (Eigen; Grass Valley, CA, USA) MRI-US fusion device between 2012 and 2015. Digital rectal exam (DRE) was performed for clinical staging and patients had at least one pre-treatment PSA blood test. Patients who had not undergone targeted biopsy and those with recurrent disease were excluded.
MRI Technique
The MRI technique has been described in our previous publication.(11) mpMRI was performed with a 3T Siemens SOMATOM® Trio™ Tim or 3T Skyra scanner (Siemens, Erlangen, Germany) using a multichannel external phased-array body coil. mpMRI sequence parameters included multiplanar (axial and coronal) T2-weighted, diffusion-weighted, and dynamic contrast-enhanced imaging. Morphological T2-weighted images were used for image interpretation. All images were interpreted by one of three experienced radiologists with expertise in prostate MRI. Each region of interest (ROI) within the prostate was identified and then assigned an institution-standardized image grade on a scale of 1–5 (1 = clinically significant disease highly unlikely to 5 = clinically significant disease is highly likely).
Biopsy Technique
All men had targeted and systematic biopsies performed by a single, experienced UCLA urologist using the Artemis device with a spring-loaded biopsy gun and 18-gauge needles, as described previously.(12) The ROIs identified on MRI were electronically loaded into Artemis before beginning a conventional TRUS biopsy session in the urology clinic. Fusion of the MRI and real-time US was performed by the device software. For targeted biopsy, one core was obtained every 3 mm along the longest axis of the lesion before systematic biopsy sampling. Systematic biopsy sampling with ≥10 cores was then performed as pre-selected by the Artemis device, independent of the MRI results. A minimum of 10 cores was selected to limit the number of possible targeted biopsy upgrades because of inadequate systematic sampling.
Heterogeneity and Risk Stratification Analyses
Patients were included in the heterogeneity portion of the study only if ≥ 2 positive cores were found on targeted biopsy so that a comparison within the target could be made. Analysis was performed based solely upon the results of TB by comparing the core with the lowest GS to the core with the highest GS. SB results were not included. Heterogeneity was defined as a difference of at least 1 GS between cores. Therefore, GS 3+4 vs. 4+3 was not considered heterogeneous for this analysis. Further analysis was performed to determine if heterogeneity was associated with any of the following factors: PSA, max tumor diameter on MRI, Apparent Diffusion Coefficient (ADC), MRI suspicion score, prostate volume, systematic biopsy GS, and T-stage. Student t-tests assuming unequal variance were used to compare the means of evaluated factors for correlation. A p-value of <0.05 was used to indicate statistical significance.
The effect of TB on NCCN risk group classification (“upstaging”) was determined by examining T-stage, PSA, and GS. The impact of heterogeneity on risk grouping was performed by staging patients based on the lowest GS within the target lesion, and then by the highest GS within the target lesion. Upstaging was defined as an upward change in risk group (low- to intermediate-, intermediate- to high-, or low- to high-risk). For targets with only 1 positive core, the same GS was used for both high and low, and the patient was not upstaged. For targets with no positive cores, the systematic GS was used and the patient was not upstaged. Similar metrics were used to determine the rate of upstaging between systematic and combined (targeted + systematic) biopsy.
Results
There were a total of 53 patients included in the study. The median age was 72 years (56–83) and the PSA mean ± SD was 9.3 ±5.8 ng/dl. Further patient characteristics are summarized by risk group in Table 1. The mean number of systematic biopsy (SB) cores obtained was 12 (10–16), and the mean number of positive SB cores per patient was 2.9 (24.1%).
Table 1 –
Patient Characteristics
| NCCN Risk Group** | ||||
|---|---|---|---|---|
| Patient Characteristics | Total | Low | Intermediate | High |
| Number of Patients | 53 | 2 | 29 | 22 |
| Median Age (years (range)) | 72 (56–83) | 70.5 (69–72) | 72 (56–80) | 72.5 (57–83) |
| PSA (ng/dl) (mean ± SD) | 9.3 ± 5.8 | 3.7 ± 1.3 | 8.2 ± 4.0 | 11.2 ± 7.2 |
| PSA range | 2.3–24.7 | 2.7–4.6 | 3.8–19.9 | 2.3–24.7 |
| T-Stage | ||||
| T1 | 41 | 2 | 25 | 14 |
| T2A | 7 | 0 | 2 | 5 |
| T2B-C | 5 | 0 | 2 | 3 |
| Gleason Score* | ||||
| 6 | 3 | 2 | 0 | 0 |
| 7 | 30 | 0 | 29 | 1 |
| 8+ | 20 | 0 | 0 | 21 |
| MRI prostate volume (cc) (mean ± SD) | 51.7 ± 21.7 | 63.0 ± 9.9 | 51.6 ± 21.7 | 50.9 ± 22.9 |
NCCN risk group and Gleason score categories based on highest overall Gleason score from systematic or targeted biopsy
A total of 73 target lesions were identified on mpMRI and biopsied using Artemis ultrasound fusion. The results are summarized in Table 2. The mean number of target lesions biopsied per patient was 1.38 (range 1–3). A mean of 4.3 cores (range 3–7) were taken from each target; 2.7 (63%) cores were positive for cancer. All 53 patients had at least one positive target biopsy. Of the 73 targets, 10 (14%) were negative, 12 (16%) had a single positive core, and 51 (70%) had 2 or more cores positive for prostate cancer.
TABLE 2.
Biopsy Characteristics
| Biopsy Characteristics | |
|---|---|
| Total targeted biopsies | 73 |
| Mean targets per patient (range) | 1.38 (1–3) |
| Total biopsy cores | 313 |
| Mean cores per targeted biopsy | 4.3 |
| Total positive cores | 198 |
| % cores positive | 63% |
| Targeted biopsies with: | |
| 0 positive cores* | 10 |
| 1 positive core* | 12 |
| 2+ positive cores | 51 |
| Targeted Biopsy Gleason Score Heterogeneity** | |
| Yes | 28/51 (55%) |
| No | 23/51 (45%) |
Excluded from heterogeneity analysis
Defined as two cores from a single targeted biopsy having a different Gleason Score
Heterogeneity analysis was performed only on the 51 targets with 2 or more positive cores. There was no difference in the GS between cores in 23/51 (45%) of the qualifying target lesions. In the remaining 28/51 (55%), there was a difference of at least 1 GS between the cores. Of these 28 heterogeneous targets, 20/28 (71%) had a GS difference of 1 and 8/28 (29%) had a GS difference of 2–3 (two patients GS 6 to 8, four GS 6 to 9, and two GS 7 to 9).
Table 3 summarizes the comparison of PSA, ADC, maximum tumor diameter, MRI prostate volume, and MRI suspicion score in targets with and without heterogeneity. None of the evaluated factors demonstrated a significant correlation, but there was a trend for patients with heterogeneity to have a lower ADC (882 vs. 956 mm2/s, p = 0.081) and a higher MRI suspicion score.
Table 3 –
Comparative analysis for patients with and without GS heterogeneity
| Compared factors (mean) | GS Variation | No GS Variation | P-value |
|---|---|---|---|
| Number of targets | 28 | 23 | - |
| PSA (ng/dl) | 7.3 | 10.4 | 0.53 |
| Max Tumor Diameter (cm) | 1.3 | 1.4 | 0.53 |
| MRI prostate volume (cc) | 48.5 | 53.8 | 0.40 |
| ADC (mm2/s) | 882 | 956 | 0.081 |
| MRI suspicion score* | 4.2 | 3.9 | 0.27 |
Each region of interest (ROI) identified was assigned an institution-standardized image grade on a scale of 1 (clinically significant disease is highly unlikely to be present) to 5 (clinically significant disease is highly likely to be present).
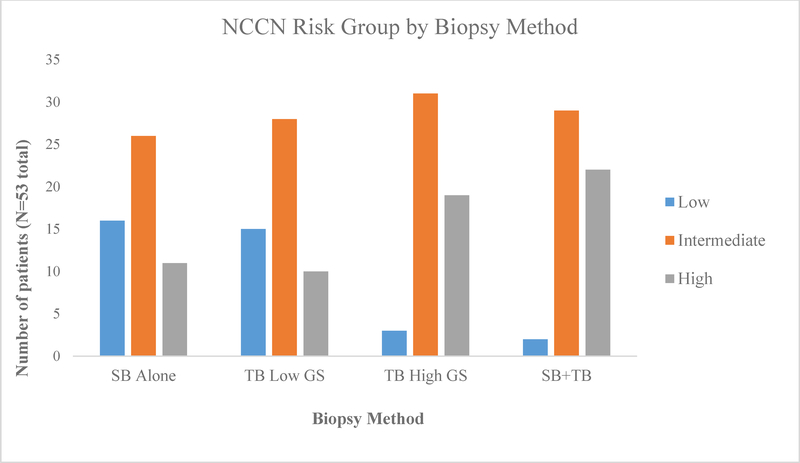
The effect of heterogeneity on NCCN risk group was analyzed using targeted biopsy low GS vs. high GS to stratify patients (Table 4). We also looked at the overall impact of targeted biopsy by comparing risk groups using the GS from systematic biopsy alone with the overall highest using a combination of the two (SB+TB). Using only systematic biopsy resulted in 30% low-, 49% intermediate-, and 21% high-risk patients. Combining the high GS from TB with the SB results resulted in 4% low-, 54% intermediate-, and 42% high-risk patients. The low GS on TB combined with SB resulted in 17%, 55%, and 28% in each risk group, respectively. Overall, 43% (n=23) of the patients had a higher GS detected on TB vs. SB. This led to 38% (n=20) of patients being placed into a higher risk group when adding information gleaned from the TB to the SB results, including 5 patients categorized as low-risk on SB moving to high-risk disease based on the TB. Figure 1 provides a graphical illustration of the risk group differences.
Table 4 –
Risk Grouping by Biopsy Type
| NCCN Sub-analysis (n=53 patients) | Low | Intermediate | High |
|---|---|---|---|
| By Systematic Biopsy only | 16 (30%) | 26 (49%) | 11 (21%) |
| By TB with the lowest GS* | 15 (28%) | 28 (53%) | 10 (19%) |
| By TB with the highest GS* | 3 (6%) | 31 (58%) | 19 (36%) |
| By Combined Systematic + TB** | 2 (4%) | 29 (54%) | 22 (42%) |
For patients with only 1 positive targeted biopsy the same GS for “lowest GS” and “highest GS” was used and for those with no positive targeted biopsies the highest systematic biopsy GS was used.
The highest targeted biopsy GS was used
TB = targeted biopsy
Figure 1:
Demonstrates the differences in NCCN risk groups for the 53 patients when stratified by biopsy method. Note that the proportion of low-risk patients sharply decreases (from 30% to 4%), while the high-risk group doubles (from 21% to 42%), when adding targeted biopsy (TB) to systematic biopsy (SB). “TB Low GS” refers to using the lowest GS found on targeted biopsy whereas “TB High GS” refers to using the highest GS found. The contrast of risk grouping between “TB Low GS” and “TB High GS” demonstrates that when using “TB Low GS” NCCN risk group distribution is similar to using SB alone whereas greater differences are seen with using “TB High GS”.
Discussion
Growing data demonstrates MRI-ultrasound fusion targeted biopsy improves prostate cancer detection compared to office-based 12-core systematic TRUS-guided biopsy. A recent prospective study on 1042 men undergoing this technique demonstrated improved detection rates for clinically significant prostate cancers (GS ≥ 7).(13) The role of targeted biopsies will likely increase as further evidence mounts. The primary goal of this technique has been to enhance cancer detection, but less consideration has been given to its impact on definitive treatment decisions. In this study we also evaluated the impact of targeted biopsy GS heterogeneity on NCCN risk stratification.
Our results demonstrated heterogeneity within mpMRI-targeted lesions, with 55% of foci showing a difference in Gleason score, including a modest number of GS 6 to 8–9 differences. We also calculated that the heterogeneity has the potential to impact NCCN risk grouping. This demonstrates that approaching targeted biopsy with a single core may be inadequate. Rather, taking 1 core every 3mm across the region of interest (ROI), as performed at our institution, may provide a more accurate assessment of the true significance of a designated target.(14) However, it is beyond the scope of our analysis to be able to determine the ideal number of targeted biopsies that need to be performed in order to optimize the chances of finding the highest GS within a target.
The addition of TB to SB reduced the low-risk group substantially (30% to 4%) and doubled the number of high-risk patients (21% to 42%). There was no apparent change in intermediate-risk because a similar number of cases moved from low to intermediate-risk as those moving from intermediate into high-risk. Our rate of upstaging (38%) when adding targeted biopsy results to systematic biopsy is fairly consistent with the literature and confirms the usefulness of using a combined approach.(11, 15, 16)
How then should this information be used? Should a patient determined to have high-risk prostate cancer using TB be treated the same way as when they are placed in this risk group based on SB? It may be that such cases would be over-treated with multiple modalities when they might equally well be treated with a single modality, such as brachytherapy alone.(17) This concept of risk inflation from targeted biopsies is not novel and has been discussed previously.(18) However, there is currently little data in the literature to help answer this question.
We also attempted to determine potential associated factors for GS heterogeneity but none of the outcome predictors we evaluated were statistically associated in our study. There was a trend with ADC and MRI suspicion score and as this was a pilot study with a low number of patients, a larger sample may have been significant. This conceptually appears reasonable, as the MRI measures are surrogates of disease aggressiveness, suggesting that more aggressive disease may be associated with higher GS heterogeneity. This is consistent with radical prostatectomy studies showing a greater continuum of GS with aggressive disease compared with a more homogenous distribution in lower risk disease.(19)
While we did not have a direct comparison to prostatectomy available in our population, there are several series that have examined this concept of grade heterogeneity on whole mount specimens. One such study found that 84% of multifocal cancers showed heterogeneity across foci.(20) However, they also showed that 58% of single foci contained just a single grade while 42% had 2 or more grades of cancer. Similar findings have been demonstrated in a more recent analysis that found that 50% of tumors harbored at least 3 different histological grades.(19) These findings are very similar to our 55% rate of heterogeneity within a target. Complicating these findings is the concept of multifocality, where as high as 60–90% of prostate cancers may harbor multiple foci.(21) With this in mind, it is possible that as tumor volume increases over time, the foci culminate together effectively fusing into a single lesion. Because of this, a “single” target on MRI may represent the confluence of several foci, thus contributing the finding of heterogeneity in this study. Prostatectomy specimens have indirectly supported this finding although the theory is still being debated.(20) It is more likely that heterogeneity may be a result of the evolution of low grade to high grade disease within a single foci. This theory is supported by data from Aihara et al. where prostatectomy specimens showed a high grade cancer within a larger, well-differentiated tumor 53% of the time, while 30% of cases showed a low grade cancer within a large, poorly differentiated tumor.(19) This underscores the unpredictability of the source and progression of heterogeneity, but highlights it’s significance across multiple modalities.
Limitations include that this was a pilot study representing a small subset of the total number of patients undergoing targeted biopsy at our institution, and that it was a single-institution and retrospective experience. Given the small sample size, we were unable to stratify patients further to determine subgroups that may have had variable clinical impact. Additionally, our cohort does not include men with negative biopsies who would not have been referred to our department. Another limitation is possible selection bias as we only studied cases with an mpMRI identifiable target. As it has been shown that mpMRI preferentially identifies more aggressive lesions and is less likely to detect low risk foci, there may be an inclusion bias towards patients with aggressive disease, thus inflating the rate of upstaging. Finally, there was no clinicopathological correlation from prostatectomy specimens for this subset. It is possible that we continue to underestimate the extent of disease with TB.(16)
Conclusion
Our study of MRI-ultrasound fusion guided biopsies demonstrated that more than half of the targeted biopsies (TB) are heterogeneous with respect to Gleason score. Additionally, 43% of patients have a higher-grade cancer detected on TB compared to standard TRUS systematic biopsy. Inclusion of the TB information into NCCN risk group stratification shifted 38% of cases into higher risk group categories. These findings have potentially significant implications for patient management. How to best integrate this information into clinical practice will need further study.
Footnotes
Disclosures: Speaking honorarium from Elekta for Dr. Mitchell Kamrava. Other authors have no conflicts of interest to report.
References
- 1.Bjurlin MA, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D, et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. The Journal of urology. 2013;189(6):2039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washington SL, Bonham M, Whitson JM, Cowan JE, Carroll PR. Transrectal ultrasonography-guided biopsy does not reliably identify dominant cancer location in men with low-risk prostate cancer. BJU international. 2012;110(1):50–5. [DOI] [PubMed] [Google Scholar]
- 3.D’Elia C, Cerruto MA, Cioffi A, Novella G, Cavalleri S, Artibani W. Upgrading and upstaging in prostate cancer: From prostate biopsy to radical prostatectomy. Molecular and clinical oncology. 2014;2(6):1145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes ET, Sundaram CP, Long R, Soltani M, Ercole CJ. Biopsy Gleason score: how does it correlate with the final pathological diagnosis in prostate cancer? British journal of urology. 1997;79(4):615–7. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, Presti JC Jr. Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69(3):495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg DM, Sauvageot J, Piantadosi S, Epstein JI. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. The American journal of surgical pathology. 1997;21(5):566–76. [DOI] [PubMed] [Google Scholar]
- 7.Schnall MD, Pollack HM. Magnetic resonance imaging of the prostate gland. Urologic radiology. 1990;12(2):109–14. [DOI] [PubMed] [Google Scholar]
- 8.Tarcan T, Turkeri L, Biren T, Kullu S, Gurmen N, Akdas A. The effectiveness of imaging modalities in clinical staging of localized prostatic carcinoma. International urology and nephrology. 1996;28(6):773–9. [DOI] [PubMed] [Google Scholar]
- 9.Ikonen S, Karkkainen P, Kivisaari L, Salo JO, Taari K, Vehmas T, et al. Magnetic resonance imaging of clinically localized prostatic cancer. The Journal of urology. 1998;159(3):915–9. [PubMed] [Google Scholar]
- 10.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313(4):390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamrava M, Hegde JV, Abgaryan N, Chang E, Le JD, Wang J, et al. Does the addition of targeted prostate biopsies to standard systemic biopsies influence treatment management for radiation oncologists? BJU international. 2015. [DOI] [PubMed] [Google Scholar]
- 12.Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. The Journal of urology. 2013;189(1):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, Lieu P, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urologic oncology. 2011;29(3):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu JC, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? The Journal of urology. 2014;192(2):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le JD, Stephenson S, Brugger M, Lu DY, Lieu P, Sonn GA, et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. The Journal of urology. 2014;192(5):1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka Y, Suzuki O, Isohashi F, Seo Y, Okubo H, Yamaguchi H, et al. High-Dose-Rate Brachytherapy as Monotherapy for Intermediate- and High-Risk Prostate Cancer: Clinical Results for a Median 8-Year Follow-Up. International journal of radiation oncology, biology, physics. 2015. [DOI] [PubMed] [Google Scholar]
- 18.Robertson NL, Hu Y, Ahmed HU, Freeman A, Barratt D, Emberton M. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. European urology. 2014;65(3):628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aihara M, Wheeler TM, Ohori M, Scardino PT. Heterogeneity of prostate cancer in radical prostatectomy specimens. Urology. 1994;43(1):60–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 20.Ruijter ET, van de Kaa CA, Schalken JA, Debruyne FM, Ruiter DJ. Histological grade heterogeneity in multifocal prostate cancer. Biological and clinical implications. The Journal of pathology. 1996;180(3):295–9. [DOI] [PubMed] [Google Scholar]
- 21.Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Human pathology. 2010;41(6):781–93. [DOI] [PubMed] [Google Scholar]