Abstract
Regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (Foxp3) play a requisite role in the maintenance of immunological homeostasis and prevention of peripheral self-tolerance breakdown. Although Foxp3 by itself is neither necessary nor sufficient to specify many aspects of the Treg cell phenotype, its sustained expression in Treg cells is indispensable for their phenotypic stability, metabolic fitness, and regulatory function. In this Review, we summarize recent advances in Treg cell biology, with a particular emphasis on the role of Foxp3 as a transcriptional modulator and metabolic gatekeeper essential to an effective immune regulatory response. We discuss these findings in the context of human inborn errors of immune dysregulation, with a focus on FOXP3 mutations, leading to Treg cell deficiency. We also highlight emerging concepts of therapeutic Treg cell reprogramming to restore tolerance in the settings of immune dysregulatory disorders.
Keywords: Autoimmunity, Foxp3, immune dysregulation, immune tolerance, immunometabolism, Interleukin 2, IPEX, Rapamycin, Regulatory T cells, Regulatory T cell Reprogramming
Introduction
Since its discovery close to two decades ago, Foxp3 has emerged as a key regulator of immune tolerance by virtue of its function as a master switch factor involved in the differentiation of regulatory T (Treg) cells. Its deficiency in humans gives rise to an autoimmune lymphoproliferative disease: immune dysregulation, polyendocrinopathy, enteropathy X-linked syndrome (IPEX), and to a homologous scurfy phenotype in mice. In the interim, much has been learnt about how Foxp3 orchestrates Treg cell responses, and how mutations subvert its functions to promote disease. In this review, we survey novel insights into mechanisms of Foxp3 action including its versatility in directing tissue and immune response-specific outcomes by co-opting different transcriptional programs, the vulnerability of such co-option to dysregulation leading to reprogramming of Treg cells towards T effector cell phenotypes, and the emerging role of Foxp3 as a metabolic gatekeeper that maintains the identity and regulatory functions of Treg cells. We also focus on the mechanisms by which gene mutations selectively impair distinct aspects of Foxp3 function, and therapeutic interventions aimed at restoring Treg cell function in the context of Foxp3 deficiency.
Historic perspective
Treg cells were originally described as a subpopulation of CD4+ T cells characterized by high expression of the IL-2 receptor (IL-2R) alpha chain (CD25) and ability to control autoimmunity in mice elicited by thymic manipulation or lymphopenic complementation [1-5]. In 2000, Chatila et al described mutations in the gene encoding the transcription factor forkhead (FKH) box (Fox) P3 (Foxp3), originally called JM2, as the cause of an autoimmune lymphoproliferative disorder in human subjects termed X-linked autoimmunity-allergic dysregulation syndrome (XLAAD) and later codified as IPEX [6]. IPEX and scurfy-causing mutations in FOXP3 and its orthologous mouse gene, respectively, were also described shortly thereafter [7, 8]. The identification of Foxp3 as essential for controlling Treg cell function was established by seminal studies demonstrating that the lymphoproliferative disease in scurfy mice results from lack of functional Treg cells [9, 10]. Enforced expression of Foxp3 in conventional murine CD4+CD25− T (Tconv) cells led to the acquisition of a regulatory phenotype, while adoptive transfer of CD4+CD25+Foxp3+ Treg cells into neonatal scurfy mice prevented autoimmune disease development [9]. Subsequently, studies in mice using Foxp3 reporter alleles demonstrated that thymic development of Treg cells proceeds uninterrupted in the absence of functional Foxp3 but leads to the generation of aberrant effector memory-like Treg cells that lack regulatory function [11, 12]. Similarly, CD4+CD25high Treg-like cells from human subjects with loss of function FOXP3 mutations failed to suppress autologous effector T cell responses despite being comparable in number and phenotype to those of healthy donors [13]. In addition to their essential role in the maintenance of peripheral tolerance to self-tissues, it is now appreciated that Treg cells play a critical role in enforcing tolerance to the “extended self’, including the commensal flora and innocuous environmental antigens, as well as mediating broad homeostatic and tissue repair functions [14].
Natural and induced Foxp3+ Treg cells
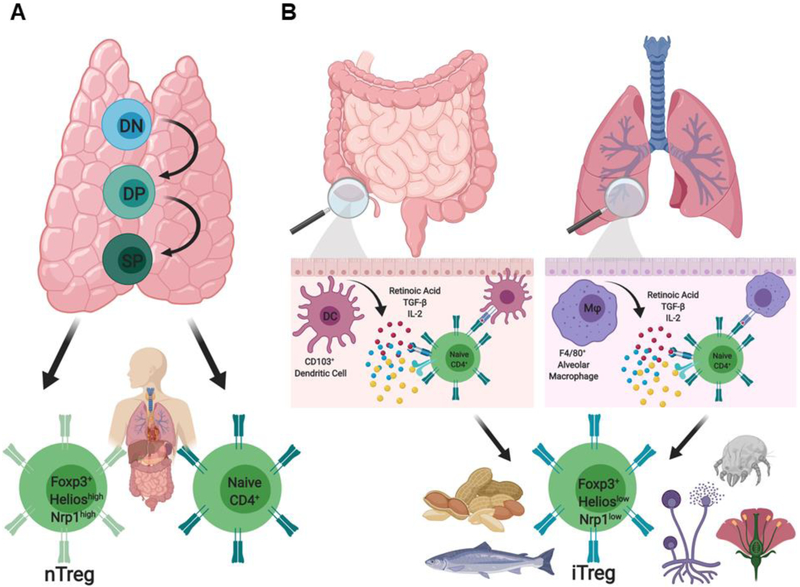
Treg cells represent 5 to 10 % of the total CD4+ T cell pool and express αβ T cell receptors (TCR) with a broad repertoire that is largely distinct from that of Tconv cells [15, 16]. Treg cells derive from two distinct populations that act synergistically to enforce peripheral tolerance (Fig. 1) [17]. CD4+CD25+Foxp3+ natural regulatory T (nTreg) cells differentiate in the thymus from immature precursors and play a critical role in enforcing tolerance to self-antigens (Fig. 1). Induced regulatory T (iTreg) cells are generated de novo extrathymically from naive Tconv cells in select niches, especially those at the mucosal interfaces including the gastrointestinal and respiratory tracts, that offer specialized antigen-presenting cells producing transforming growth factor beta (TGF-β) and retinoic acid, as well as the availability of conducive commensal metabolites (Fig. 1) [18]. The generation of iTreg cells in the gastrointestinal tract is facilitated by mucosal CD103+CD11c+ dendritic cells (DCs), while the same role is played by the alveolar macrophages in the lungs [19, 20] (Fig. 1). iTreg cells can also be generated in-vitro following TCR activation of naïve Tconv cells in the presence of IL-2 and TGF-β [21]. As a function of their distinct developmental ontology, the TCR repertoires of nTreg and iTreg cells are largely non-overlapping [22]. While the TCR repertoire of iTreg cells is directed towards commensal antigens and environmental allergens, nTreg cells express an anti-self-biased TCR repertoire [23, 24] (Fig. 1). This minimal TCR repertoire overlap enables the specification of complimentary antigen coverage in the maintenance of peripheral tolerance, with the presence of both cell types required for optimal tolerance induction [22].
Figure 1. Natural and induced Treg cell subsets.
The peripheral Treg cell pool is composed of 2 distinct populations, nTreg and iTreg cells, which express similar levels of the transcription factor Foxp3 but have non-overlapping TCR repertoires. (A) Foxp3+HelioshighNeuropilinhighnTreg cells differentiate in the thymus and play a critical role in enforcing tolerance to self-antigens. (B) Foxp3+HelioslowNeuropilinlow iTreg cells are generated de novo extrathymically in peripheral lymphoid tissue from naïve CD4+CD25− Tconv cells in the presence of retinoic acid, TGF-β, and IL-2 following TCR engagement by CD103+ dendritic cells or F4/80+ macrophages in the intestinal mucosa and alveola inerstitia, respectively. Unlike nTreg cells, iTreg cells are skewed in favor of recognizing non-self antigens, including the commensal flora and infectious agents, and innocuous antigens such as allergens and foods.
Epigenetic mechanisms regulate Treg cell lineage stability and phenotypic identity [25-28]. For example, the Treg-specific demethylation region (TSDR) within the conserved non-coding sequences 2 (CNS2) of the Foxp3 locus is mostly demethylated in nTreg cells, whereas it is only partially demethylated in iTreg cells or Tconv cells that transiently express Foxp3. nTreg cells also exhibit a specific hypomethylation pattern in genes such as Il2ra, Ctla4, Tnfrsf18, and Ikzf2 as well as histone modifications including trimethylation of histone H3 lysine 4 (H3K4me) [29]. In the context of an inflammatory environment, iTreg cells are particularly prone to destabilization, may lose Foxp3 expression and degenerate into effector cells (ex-Treg) that contribute to diseases pathogenesis [30, 31]. nTreg cells may also destabilize in the context of chronic inflammatory/autoimmune processes and acquire effector-like phenotypes [32, 33].
Phenotypic classification of Foxp3+ Treg cells
In addition to their constitutively high CD25 cell surface expression, Treg cells express low levels of the IL-7 receptor alpha chain (CD127), which inversely correlates with Foxp3 expression [34]. Treg cells also express high levels of the cytotoxic T-lymphocyte antigen 4 (CTLA-4), as well as molecules that constitute a canonical Treg cell surface signature, including the glucocorticoid-induced tumor necrosis factor receptor (TNFR) family related protein (GITR) and inducible co-stimulator (ICOS) [reviewed in 35]. However, the phenotypic demarcation of Treg cells using these markers is complicated by their frequent upregulation in Tconv cells following TCR engagement. In human CD4+ Tconv cells, Foxp3 can be transiently induced at low levels in response to TCR stimulation, and activation inducible upregulation of other “canonical” Treg cell markers (e.g CTLA-4) in Tconv cells can further stymie the identification of bone fide Treg cell populations. With that in mind, careful gating on CD4+CD25hiCD127low T cells in human peripheral blood can identify a population that is > 90 % Treg cells as confirmed by high Foxp3 expression and demethylation at the Foxp3 locus (34, 36, 37).
Human peripheral blood Treg cells have been functionally subdivided into distinct subsets based on their expression of the common leukocyte antigen isoform RA (CD45RA) and Foxp3 [38]. While CD45RA+Foxp3low cells represent a resting population of Treg cells (rTreg), CD45RA− Foxp3high cells constitute an activated Treg cell (aTreg) fraction that is derived from rTregs in-vitro and in-vivo [38]. This fractionation approach has been leveraged clinically to demonstrate a decrease in the proportion of aTreg cells in human subjects with autoimmune conditions such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [38, 39]. In mice, resting and activated Treg cell populations are functionally delineated by expression of the markers Ly-6C, CD44 and CCR7 [40, 41]. Additional markers have been identified that help segregate nTreg from iTreg cells [42-45]. Helios, a member of the Ikaros transcription factor family, is highly expressed in nTreg cells, but not in iTreg cells (Fig. 1) [42, 43]. Similarly, neuropilin-1 (Nrp-1) may also serve as an additional marker that is expressed at high levels on nTreg cells, although its expression may be upregulated on iTreg cells in the context of inflammation (Fig. 1) [44, 45].
Treg cell lineage specification and Foxp3
The initial discovery of Foxp3 as a “master switch gene” of the Treg cell lineage being absolutely required for Treg cell phenotypic and functional specification has evolved into a view of Foxp3 as a molecular “orchestrator” of the Treg cell program (Fig. 2). Previously we demonstrated that development of nTreg cells in the thymi of mice with a mutant Foxp3 allele proceeds uninterrupted, and that both Foxp3-deficient and sufficient Treg cells share a core Treg cell transcriptome with intact transcriptional activity at the Foxp3 locus [11]. A study by Gavin et al similarly demonstrated that Foxp3 largely consolidates pre-existing features of Treg cell precursors to solidify rather than establish the Treg cell lineage [12]. Moreover, cross sectional analysis of the Treg cell signature revealed that Foxp3 plays a relatively minor role in specifying the transcriptional landscape of Treg cells [46, 47]. Nevertheless, Foxp3 activity was critical for conferring Treg cell suppressor function by stabilizing and amplifying Treg cell expression of genes such as Il2ra, Ctla4 and Tnfrsf18 [11, 12]. In addition, Foxp3 dependent repression of effector cytokine gene expression (e.g IL-4, IFN-γ, IL-17 and IL-21) and downregulation of the cyclic nucleotide phosphodiesterase 3B (PDE3B) activity is essential for the maintenance of Treg cell homeostasis [11]. Collectively these studies demonstrate that additional elements upstream of Foxp3 specify the Treg lineage as part of a higher order regulatory network and that Foxp3 is absolutely required for Treg cell suppressor function.
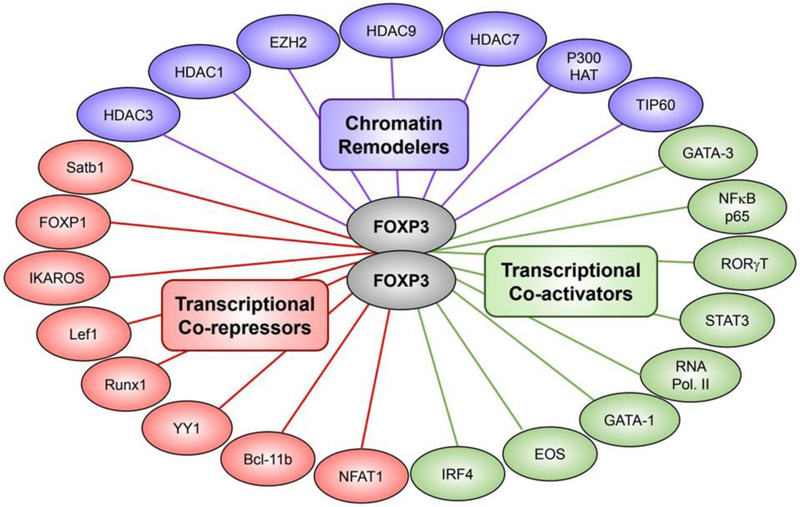
Figure 2. The Foxp3 interactome.
Foxp3 can modulate the transcriptome of Treg cells by distinct mechanisms depending on its interaction with diverse binding partners. Foxp3 can modulate the accessibility of genes to several transcription factors through its interaction with chromatin remodelers including the histone acetyltransferases P300 and TIP60. Additionally, Foxp3 may interact with various transcriptional co-activators or co-repressors, leading to the upregulation or downregulation of gene expression, respectively.
Other Fox transcription factors also contribute to Treg cell lineage specification. Foxp1 promotes Foxp3 occupancy of several Treg cell signature genes, and its deficiency results in diminished expression of key molecules implicated in Treg cell suppressor function including CTLA-4 and CD25 [48]. Similarly, Foxo transcription factors play a critical role Treg cell lineage specification through direct control of Foxp3 and Ctla4 activity [49, 50]. In particular, Foxo1 represses Treg cell IFN-γ expression, and its deficiency in Treg cells precipitates a fatal inflammatory disorder similar to that of Foxp3 deficiency [51]. In turn, repression of Foxo1 is critical for the differentiation of activated Treg cells as evidenced by dysregulated Treg cell homing to non-lymphoid tissues and impaired suppression of CD8+ T cell dependent inflammation in mice expressing a constitutively active Foxo1 in the Treg cell compartment [52]. Although Foxo3 deficient mice do not exhibit manifestations of spontaneous T cell activation, T cell specific deletion of both genes encoding the Foxo1 and Foxo3 transcription factors precipitates a multifocal inflammatory disease due to compromised Treg cell differentiation and function [50, 53]. Collectively, these studies illustrate the critical, non-redundant roles played by different Fox transcription factors in the Treg cell lineage.
The modular nature of Foxp3+ Treg cell suppressor functions
Treg cells target a broad array of innate and adaptive immune cells, including CD4+ and CD8+ T lymphocytes, B lymphocytes, monocytes/macrophages, DCs, mast cells and innate lymphoid cells (ILC) [reviewed in 54]. Although multiple immunoregulatory mechanisms are utilized by Treg cells to limit inflammatory immune responses, individual mechanisms may be operative in a dedicated modular fashion that is restricted to particular tissue sites or distinct inflammatory settings. Treg cells can enforce suppressor function by diverse mechanisms that target specific effector pathways and/or responses, including contact-dependent mechanisms, immunomodulatory cytokines such as IL-10, TGF-β and IL-35, as well as through metabolic perturbation of target cells (Fig. 3). Mutations in human subjects and mouse models have clarified the mechanisms by which these pathways contribute to peripheral tolerance maintenance and the consequences of their disruption in promoting disease.
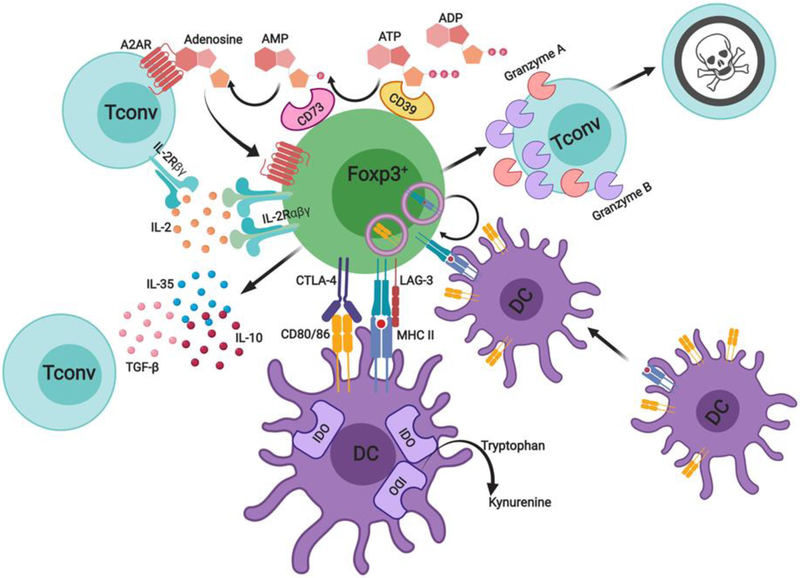
Figure 3. The modular nature of Treg cell suppression.
Treg cells suppress innate and adaptive immune responses through multiple mechanisms in order to enforce immunological tolerance. These include inhibitory cytokines such as IL-10, TGF-β1 and IL-35, suppression of antigen presentation by professional antigen presenting cells (CTLA-4, LAG-3), granzyme and perforin dependent target cell cytolysis. Additional mechanisms include the generation of immunosuppressive adenosine by Treg cell ectoenzymes CD39 and CD73, and competition for endogenous IL-2 through expression of the high affinity IL-2 receptor alpha chain (CD25). Importantly, individual modules (e.g IL-10, TGF-β1) operate in a non-redundant manner to prevent peripheral tolerance breakdown, while mutations affecting the respective modules (e.g. CTLA-4, IL-2Rα and β subunits, TGF-β1 etc), give rise to distinct immune dysregulatory human diseases.
A prime example of contact-dependent Treg cell-mediated suppression involves the CTLA-4/lipopolysaccharide-responsive beige-like anchor (LRBA) axis. CTLA-4 downregulates major costimulatory molecule expression on antigen presenting cells (APCs) and limits costimulatory signals by cell extrinsic depletion of its ligands CD80 and CD86 (Fig. 3) [55, 56]. Moreover, CTLA-4 may additionally stimulate indoleamine 2,3-dioxygenase (IDO) expression in human and murine DC subsets, which induces catabolism of the essential amino acid tryptophan to starve T cells [57]. In mice, Treg cell specific deletion of CTLA-4 results in a fatal autoimmune lymphoproliferative disease [58]. Human subjects with heterozygous mutations in CTLA-4 also present with an autosomal dominant autoimmune lymphoproliferative disease, while autosomal recessive mutations in LRBA, which regulates CTLA-4 cell surface expression, result in a phenotypically similar disease [59, 60]. CTLA-4 antagonism using monoclonal anti-CTLA-4 antibodies (mAbs) reverses Treg suppressor function in-vivo, while its germline deficiency in mice leads to a fatal lymphoproliferative disorder within the first month of life [61, 62].
Additional mechanisms by which Treg cells may act to control the immune response through cell contact involve the interaction of LAG-3 on Treg cells with MHC-II on immature DCs, leading to suppression of DC maturation (Fig. 3) [63]. Antigen specific Tregs may also physically remove cognate peptide MHC-II complexes from the surface of DC’s in-vivo in a CTLA-4 independent manner (Fig. 3) [64]. A different suppressive mechanism involves Treg cell-mediated cytolysis of target cells, which is dependent on the activity of granzyme B (Fig. 3) [65]. Similarly, human Treg cells expressing granzyme A have been shown to induce target cell killing in a perforin dependent manner in-vitro (Fig. 3) [66]. This mechanism may be relevant to the profound immune dysregulation in patients with hemophagocytic lymphohistiocytosis (HLH) disorders, the causative mutations of which, including those in the perforin gene, may result in defective Treg cell cytolytic function [67, 68].
Treg cells also exert regulation through soluble intermediates. The ecto-enzymes ATP apyrase (CD39) and ecto-5’-AMP-nucleotidase (CD73), which in tandem generate the immunosuppressive purine nucleoside adenosine (Fig. 3) [69]. Adenosine can signal via the high affinity G protein-coupled (GPRC) adenosine A2A receptor (A2AR) expressed on T cells to mediate inhibitory signaling in a cAMP dependent manner [70]. Moreover, Treg cells have been proposed to act as an IL-2 sink through their constitutive CD25 expression and IL-2 signals are required for optimal Treg suppressor function through STAT5b activation (Fig. 3) [71, 72].
The immunoregulatory cytokines IL-10 and TGF-β represent two key Treg cell effector modules with specialized functions. Treg cell derived IL-10 is particularly important in restraining immunological hyperreactivity at environmental interfaces including the colon, skin, and lung [73, 74]. Mice harboring IL-10 deficient Treg cells develop spontaneous colitis and exhibit heightened immune-mediated reactivity in the airways [73]. Similarly, ablation of the interleukin 10 receptor (IL-10R) in Treg cells impairs STAT3 phosphorylation and results in selective dysregulation of TH17 immune responses accompanied by development of severe immune-mediated colitis [74]. Moreover, IL-10 may promote the expansion and function of Foxp3+ iTreg cells by enhancing Foxo1 nuclear localization and augmenting STAT3 signaling [75]. In human subjects, IL-10/IL10R gene mutations are associated with chronic gastrointestinal inflammatory disorders such as ulcerative colitis and Crohn’s disease (reviewed in 76, 77].
TFG-β represents another dedicated module of Treg suppression that plays a privileged role at mucosal interfaces. For example, Treg cells require TFG-β signals to appropriately limit IL-17 production and dampen TH17 cell responses in the gastrointestinal tract [78]. Bi-allelic Treg cell-specific deletion of Tgfb1 in mice results in a scurfy mouse like phenotype, while monoallelic deletion results in allergic dysregulation, reflecting a non-redundant privileged role for Treg cell-derived TGF-β1 in controlling the immune response (manuscript in preparation). In humans, heterozygous loss of function mutations in TGFBR1 and TGFBR2 gives rise to the Loeys Dietz syndrome, associated with allergic dysregulation in addition to non-immune somatic phenotypes [79]. Biallelic TGFB1 loss of function mutations results in severe inflammatory bowel disease and encephalopathy [80]. Together these findings emphasize the critical role of the TGF-β module in regulating both allergic and autoimmune processes.
Tissue and functional adaptation of Foxp3+ Treg cells
The suppressive function of Treg cells is critical for the prevention of autoimmunity and collateral damage to healthy tissues and organs resulting from inflammatory immune responses. Treg cells also play an important role in enforcing homeostasis in non-lymphoid tissues including skeletal muscle, visceral adipose tissue (VAT), and the colonic lamina propria. The unique phenotype and function of Treg cells at these sites is largely dictated by the local environment as well as tissue specific physiological cues [reviewed in 81]. For example, Treg cells enriched in VAT express the peroxisome proliferator-activated receptor gamma (PPARγ) and are critically involved in the control of VAT inflammation and insulin sensitivity [82, 83]. Similarly, expression of the retinoid-related orphan receptor alpha (RORα) in skin resident Treg cells drives expression of the tumor necrosis factor receptor superfamily member 25 (TNFRSF25, also known as death receptor 3 or DR3) which promotes Treg cell mediated suppression of ILC-2 activation and leads to restraint of allergic skin inflammation [84]. More recently, skin resident Treg cells expressing high levels of the Notch ligand family member Jagged 1 (Jag1) were shown to facilitate the differentiation of hair follicle stem cells (HFSC) and promote hair follicle regeneration [85]. In skeletal muscle, a distinct population of Treg cells producing the epidermal growth factor amphiregulin promoted repair of damaged muscle following acute muscle injury [86]. Similarly, in a mouse model of muscular dystrophy, Treg cell depletion was associated with exacerbation of muscle injury [87].
Interestingly, selective deficiency of amphiregulin in Treg cells did not affect their suppressor function but lead to severe lung damage during influenza viral infection [88]. In the central nervous system, Treg cells were shown to exhibit regenerative properties, including the capacity to promote oligodendrocyte progenitor cell differentiation and myelination [89]. Collectively, these studies highlight the importance of phenotypic and functional adaptation of Treg cells in diverse tissue environments that shape distinct non-immunomodulatory functions of Treg cells.
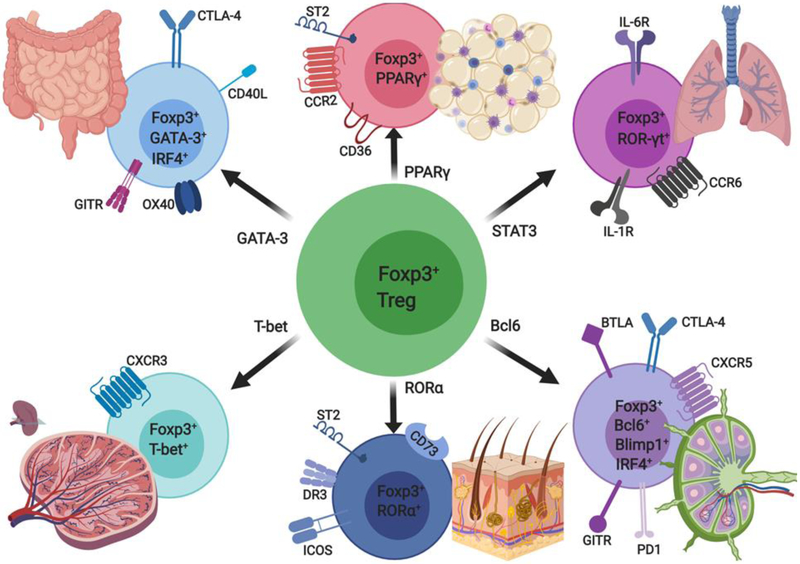
It is now appreciated that Treg cells show a functional plasticity that results in the acquisition of distinct helper T (TH) cell transcriptional machinery leading to superior control of the corresponding TH cell immune response (Fig. 4) [33, 90-94]. For example, STAT3 activation in Treg cells is required for optimal suppression of TH17 immune responses and results in the acquisition by Treg cells of TH17 specific molecules, such as the interleukin 6 receptor (IL-6R) [90] (Fig. 4). Similarly, expression of the TH1 transcriptional master regulator T-bet in Treg cells is critical for efficient control of TH1 immune responses and prevention of TH1 mediated autoimmunity [91]. Foxp3 dependent expression of the interferon regulatory factor-4 (IRF4) endows Treg cells with a preferential capacity to suppress TH2 immune responses while IRF4 deficiency in Treg cells promotes dysregulation of TH2 immune responses [92]. Similarly, the TH2 master regulator GATA-3 binds to the CNS2 of the Foxp3 locus to promote its activity [93]. Furthermore, follicular regulatory T (TFR) cells expressing the chemokine receptor CXCR5 are critically dependent on the transcriptional repressor Bcl6 for efficient control of the germinal center reaction as well as plasma cell differentiation [94]. Collectively these studies underscore the importance of molecular co-option by Treg cells of diverse TH cell transcriptional machinery as mechanism for optimal control of TH specific immune responses. The susceptibility of such co-option to pathological Treg cell reprogramming and dysregulation is discussed below.
Figure 4. Functional adaptation of Treg cells.
Treg cells regulate Tconv cell responses in an individually TH cell lineage dependent manner. To suppress TH1 responses Treg cells express the TH1 associated transcription factor T-bet and upregulate CXCR3 expression to transiently localize with expanded effector TH1 cells. Similarly, Treg cells partially acquire GATA-3 and ROR-γt transcriptional programs to enforce control of TH2 and TH17 cell responses respectively. Expression of RORα in skin resident Treg cells is critical for expression of death receptor 3 (DR3) which in turn promotes ILC2 activation and control of allergic skin inflammation. CXCR5+ follicular regulatory T (TFR) cells expressing the transcription factor Bcl6 control the germinal center reaction. In visceral adipose tissue (VAT), Treg cells express the peroxisome proliferator-activated receptor (PPAR)-γ, which plays an important role in restoration of insulin sensitivity and maintenance of VAT Treg cell function and phenotype.
Pathological reprogramming of Foxp3+ Treg cells
Treg cells exhibit a wide versatility in directing immune response-specific outcomes by their ability to hijack distinct molecular modules directing specialized TH cell responses (Fig. 4) [33, 90-94]. For example, Treg cells undergo “abortive” Th1 reprogramming by upregulating Tbx21 expression in an IFN-γ STAT1 dependent manner that results in their acquisition of a partial Th1 program characterized by CXCR3 expression (Fig. 4) [33]. However, in the context of this appropriation, Treg cells fail to upregulate expression of the interleukin receptor 12 beta 2 subunit (IL12Rβ2), which is required for completion of TH1 differentiation. Failure of such restraint in the context of aberrant inflammation may lead to redirection toward TH like phenotypes resulting in the dysfunctional reprogramming of Treg cells. For example, our laboratory recently demonstrated that constitutive Notch signaling destabilizes Treg cells by promoting nTreg cell TH1 like skewing [32].
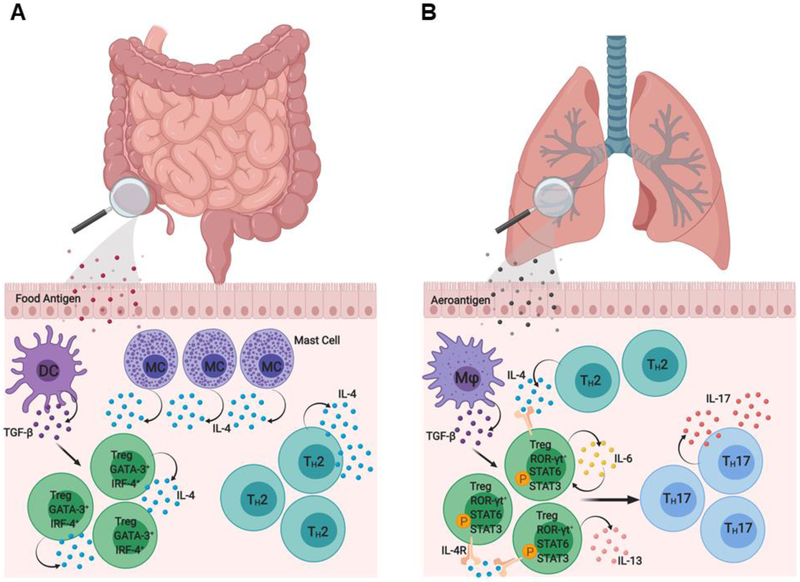
Recent studies have elucidated mechanisms by which Treg cells may acquire effector T cell attributes that contribute to their pathogenic reprogramming in disease states characterized by persistent inflammation (Fig. 5). Mice carrying a tyrosine (Y) to phenylalanine (F) substitution at position 709 of the murine IL-4Rα (Il4raF709), which inactivates the receptor immunotyrosine inhibitory motif (ITIM) [95, 96], model human polymorphisms in the interleukin-4 receptor alpha chain (IL-4Rα) that promote enhanced STAT6 activation. The Il4raF709 mice exhibit exaggerated TH2 polarization, augmented antigen induced IgE responses, and exacerbated airway inflammation and hyperreactivity. Critically, Treg cells from Il4raF709 mice acquired a TH2-like phenotype characterized by augmented STAT6 signaling and heightened expression of canonical TH2 transcription factors including IRF-4 and GATA-3 (Fig. 5) [97]. This TH2 reprogramming was also evident in peripheral antigen specific Treg cells of milk allergic children [97]. Importantly, Treg lineage specific ablation of Il13, Il14 or Stat6 in Il4raF709 mice reversed TH2 reprogramming and restored Treg cell regulatory function.
Figure 5. Pathogenic Treg cell reprogramming.
Two illustrative examples of pathogenic Treg cell reprogramming in human diseases. (A) In food allergy, allergen-specific Treg cells in the intestinal mucosa can acquire a pathogenic TH2 cell-like phenotype characterized by increased GATA-3 expression and enhanced IL-4 production. This pathological TH2 cell-like reprogramming results in the accumulation of dysfunctional antigen-specific Treg cells which fail to control effector TH2 and mast cell responses to promote allergic disease. (B) In asthma, a human IL-4Rα allele bearing a glutamine to arginine substitution at position 576 (IL-4Rα-Q576R) promotes asthma severity by driving mixed TH2-TH17 inflammation. In addition to activating STAT6, IL-4/IL-13 signaling via IL-4/IL-4R-R576 variant activates downstream MAPKs, which in turn drive an autocrine IL-6/ STAT3 activation loop. This activation results in a pathological TH17 cell-like reprogramming of nascent allergen-specific iTreg cells in the lung, characterized by increased ROR-γt expression and elevated IL-17 production.
Another example of pathological reprogramming of iTreg cells in the context of allergic airway inflammation was provided in studies on a human IL-4R variant with a glutamine (Q) to arginine (R) substitution at position 576. This variant acts as an allergic asthma susceptibility gene and strongly correlates with the prevalence and clinical severity of asthma [98]. Mice homozygous for the Il4raR576 allele exhibited exaggerated allergic airway inflammation following sensitization and challenge with allergens, driven by the destabilization of allergen specific iTreg cells towards a pathogenic TH17 like phenotype (Fig. 5) [99]. Treg specific deletion of Il6ra and Rorc or neutralization with an anti-IL-6 mAb prevented airway inflammation pursuant to TH17 Treg cell reprogramming.
In the gut, microbiota induced expression of the transcriptional factor ROR-γt in Treg cells is indispensable for regulation of TH17 colonic inflammation as well as restriction of TH2 immune mediated pathology [100, 101]. More recently, we demonstrated that dysbiosis promotes the breakdown of oral tolerance as a result of a failure to induce protective ROR-γt dependent iTreg responses in food allergic children and mice, allowing instead for the emergence of pathogenic Th2 [102].
Collectively, these studies reveal an important role for pathological Treg cell reprogramming in sustaining chronic inflammatory allergic and autoimmune diseases, and the potential for interventions aimed at resetting such reprogramming as a novel therapeutic approach.
Foxp3 as a metabolic gatekeeper
Treg cells exhibit a unique metabolic signature in comparison to other TH cell subsets, which sustains their proliferative and regulatory functions while constraining the development of effector programs normally associated with Th cells [103, 104] (Fig. 6). While TH1, TH2, and TH17 cells express high levels of the glucose transporter 1 (GLUT1) and display a heightened glycolytic rate, Treg cells at steady state preferentially utilize fatty acid oxidation (FAO) and pyruvate dependent oxidative phosphorylation (OXPHOS) to satisfy their energetic demands [reviewed in 105] (Fig. 6). Foxp3 confers a metabolic advantage on Treg cells in low-glucose lactate rich environments by suppressing Myc gene expression and Treg cell glycolysis in order to maintain regulatory function [103]. In contrast, inflammatory triggers acting via toll like receptors (TLR) promote Treg cell proliferation by engaging the PI(3)K-AKT-mTORC1 pathway, which leads to GLUT1 upregulation and augmentation of glycolysis [106] (Fig. 6). Although upregulation of GLUT1 promotes Treg cell expansion, it dampens Treg cell regulatory function and reduces expression levels of Foxp3. Furthermore, expression of Foxp3 results in upregulation of several mitochondrial protein-encoding genes, including components of the electron transport chain [107]. This in turn drives an increase in the respiratory capacity of Treg cells and augments their ability to utilize fatty acids in order to fuel OXPHOS. More recently, it was shown that loss of the mitochondrial complex III in Treg cells promotes systemic autoimmunity by impairing Treg cell suppressor function without altering expression of Foxp3 [108]. Collectively, these studies suggest that Treg cell metabolic pathways can be therapeutically exploited to enhance or brake peripheral tolerance.
Figure 6. Metabolic states of Treg cells in health and disease.
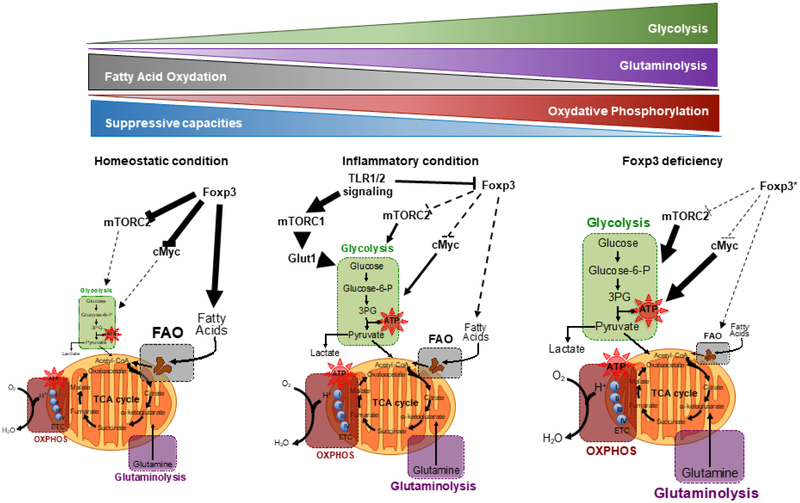
Under homeostatic condition, Foxp3 controls Treg cell metabolism by promoting fatty acid oxidation (FAO) and by limiting glycolysis through the inhibition of c-Myc and mTORC2 pathways. Under inflammatory condition, signals such as those via TLR1/2 promote glycolysis by inducing Glut1 expression in an mTORC1-dependent manner and by modulating Foxp3 expression in Treg cells. In IPEX patients and Foxp3-deficient mice, the Foxp3-deficient Treg cells undergo metabolic reprogramming characteristic of an effector memory cell-like phenotype, involving heightened aerobic glycolysis, tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation (OXPHOS), driven to large extent by mTORC2 dysregulation. Other changes include loss of fatty acid oxidation and increased glutaminolysis.
We have further explored the role of Foxp3 as a metabolic regulator in Foxp3-deficient mice in which a mutant Foxp3 allele directs the expression of a humanized Cre recombinase (iCre) fused with EGFP (Foxp3ΔEGFPiCre) [109]. Foxp3 deficiency dysregulated mTORC2 signaling and induced metabolic reprogramming of the mutant Treg cells characterized by augmented glycolysis and OXPHOS (Fig. 6). Mutant Treg cell specific deletion of the mTORC2 adaptor gene rictor ameliorated disease in Foxp3ΔEGFPiCre mice, reversed augmentation of glycolytic and respiratory activity, and partially restored mutant Treg cell suppressor function [109]. Similarly, Foxp3-deficient Treg cells of IPEX patients exhibited dysregulated mTORC2 signaling and augmented glycolysis, both of which were reversed upon treatment with mTOR inhibitors such as rapamycin to reestablish regulatory function [109]. These data demonstrate that Foxp3 plays a critical role in orchestrating Treg cell metabolism and suggests that therapeutic targeting of metabolic pathways of Foxp3-deficeint Treg cells may provide an important conceptual advance in the restoration of immune tolerance.
Foxp3 and Treg cell defects in human autoimmune disease
A number of mendelian disorders of immune dysregulation have been identified as a consequence of impaired Treg cell function and development [110, 111]. Loss of function mutations affecting FOXP3 lead to the generation of defective Treg cells and give rise to the IPEX syndrome in human subjects. This syndrome exclusively affects males and is frequently lethal in the absence of Treg cell reconstitution by hematopoietic stem cell (HSC) transplantation. Consistent with its X-linked mode of inheritance, female carriers are asymptomatic. The immunopathology results from unrestrained T cell activation, with an additional contribution by autoantibodies. The hallmarks of this syndrome include autoimmune enteropathy, autoimmune endocrinopathy, eczematous dermatitis, and immune mediated cytopenia’s. The autoimmune endocrinopathy is characterized by the early onset (1st year of life) of insulin-dependent type 1 diabetes (T1D) with islet cell destruction. Additionally, patients present with allergic dysregulation that manifests as food allergy, increased serum IgE levels, peripheral eosinophilia, and prominent Th2 skewing that collectively indicate the breakdown of oral tolerance.
FOXP3 is comprised of 12 exons encoding a 431 amino acid Foxp3 protein that consists of a C-terminal FKH domain, a proline rich N-terminal (PRR) domain, a C2H2 zinc finger (ZF), and a central leucine zipper (LZ) domain (Fig. 7). The FHK domain is required for DNA binding activity, nuclear localization, and interacts with several transcription factors including the nuclear factor of activated T cells (NFAT), leading to expression of canonical Treg cell components such as CD25, GITR and CTLA-4 [112]. The LZ and ZF domains are involved in protein to protein interactions with the latter implicated in assembly of higher order complexes of Foxp3 [reviewed in 111]. Several proteins interact with FOXP3 at its N-terminus, including the transcription factor hypoxia inducible factor 1 alpha (HIF1α) and the histone acetyltransferase p300 [reviewed in 111].
Figure 7. The spectrum of Human Foxp3 mutations.
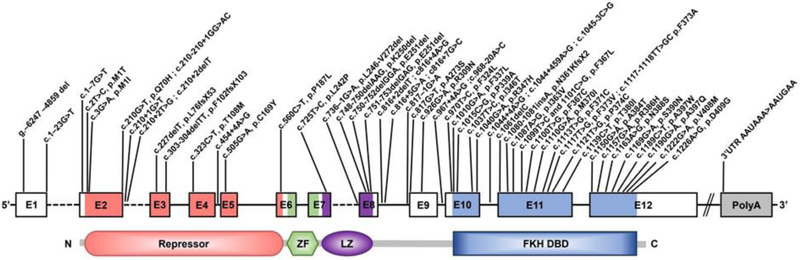
Schematic representation of FOXP3 illustrating the exons, the protein domains and mapped mutations of described IPEX patients. Amino acid changes are referred to by their single letter code. The N-terminal proline rich repressor domain (Repressor), zinc finger (ZF) motif, leucine zipper domain (LZ) and the forkhead DNA-binding domain (FKH DBD) are indicated.
Studies in mice revealed that Foxp3 can bind to thousands of genomic sites and interact with diverse binding partners to orchestrate the development and function of Treg cells (Fig. 2) [113-115]. It is thought that Foxp3 interacts with its binding partners in a modular fashion, where distinct functional domains of Foxp3 complexes act as simple repressors or activators in a dominant manner to drive the Foxp3 program [113-115]. Conversely, evidence for a “collaborative” view for distinct Foxp3 domains operating as a flexible molecular machine is now emerging [116]. For example, to elucidate function-structure relationships within Foxp3 and assess its ability to bind DNA and regulate transcriptional activity, Kwon et al performed a systematic alanine scan mutagenesis of Foxp3 [116]. Interestingly, the mutations affected transcriptional activity in a largely integrative manner, often implicating the entire molecule rather than ascribing distinct functions to individual Foxp3 domains. Moreover, missense mutations in Foxp3 with no overt autoimmune manifestations revealed Treg cell defects only in the context of systemic stress, implicating such mutations in non-classical IPEX like pathologies.
The spectrum of Foxp3 mutations in IPEX have been extensively documented and reviewed and are summarized in Fig. 7 [117-119]. Although IPEX is associated with both missense and nonsense mutations of FOXP3, the majority fall within the former group and affect all domains, but disproportionately the FKH DNA binding domain (Fig. 7). Several of these mutations have been studied at great detail, notably A384T [120,121]. Mouse models of these mutations have also clarified the mechanisms by which they act to promote disease. Emerging genotype/phenotype relationships in IPEX suggest that similar genotypes do not necessarily result in similar phenotypes with respect to disease severity and clinical presentation [122]. However, mutations within the functional domains of FOXP3, particularly those affecting the FKH and leucine zipper domains, are generally associated with more severe clinical phenotypes while missense mutations, small in-frame deletions, and mutations within the promoter and 5’ untranslated region of FOXP3 are associated with a milder clinical phenotype [reviewed in 111].
A common IPEX missense mutation (A384T) (Fig. 7) in the FKH domain of Foxp3 disrupts its binding to the histone acetyltransferase Tat-interaction protein 60 (TIP60) and abolishes the suppressor function of Treg cells [120]. The same mutation was reported in another study to broaden the DNA binding specificity of Foxp3 and results in tissue-specific autoimmunity and impaired Treg cell fitness through enhanced repression of BATF activity [121]. A mutation (M370I) in the domain swap interface of Foxp3, which is required for dimerization through the FKH domain, resulted in deregulation of the TH2 locus and generation of TH2-like Treg cells expressing the transcription factor GATA-3 [123]. Moreover, expression of this Foxp3 variant in mice promoted the development of unrestrained TH2 immune responses independent of IL4/IL-13 production by Teff cells [123]. A recent report demonstrated that expression of an alternatively spliced isoform of FOXP3, which excludes exon 2 (FOXP3Δ2), was sufficient to support Treg cell development and mitigate some distinct features of classical IPEX, even in the absence of full length FOXP3 [124]. Overall, these findings emphasize distinct actions by which FOXP3 mutations may act to subvert Treg cell regulatory function in order to promote immune dysregulation and autoimmunity.
IPEX-like and other Treg cell-related disorders with functional Foxp3
A number of inborn errors of immune dysregulation that affect Treg cell development have been described in patients who lack detectable mutations in FOXP3 but have overlapping features with IPEX syndrome [111]. Loss of function mutations in genes along the IL-2 receptor axis, including IL2RA/B and STAT5B, are well studied examples [reviewed in 111]. CD25 deficiency due to IL2RA mutations mimics classical IPEX in presenting with eczema, enteropathy, and autoimmunity and lymphoproliferation, reflecting both the failure of Treg cells to regulate effector cells by means of IL-2 deprivation (the IL-2 sink effect) and also the role of IL-2 in mediating Treg cell fitness [125-128]. However, patients with CD25 deficiency are distinguished by a susceptibility to chronic infections, particularly to herpes family viruses [125-128]. More recently, homozygous mutations in IL2RB were shown to result in severe early onset multi-system autoimmunity, characterized by diminished frequencies of Treg cells with similar features to IL2RA deficiency, including a susceptibility to viral infections [129, 130]. Consistent with an absolute requirement for STAT5b in IL-2R signaling, several features of STAT5B deficiency are shared with IL2RA deficiency and include decreased numbers of Treg cells characterized by reduced Foxp3 expression and impaired regulatory function [131, 132]. More recently, dominant negative heterozygous mutations in STAT5B have been described that give rise to growth hormone insensitivity and immune dysregulation with eczema and elevated IgE but in the absence of frank immunodeficiency [133].
A prominent cause of IPEX-like disorders are mutations affecting the CTLA4-LRBA axis [134-135]. Homozygous loss of function mutations in LRBA, which result in the near total loss of CTLA4 expression, result in profound Treg cell dysregulation characterized by impaired Treg cell-mediated suppression, marked T follicular helper (TFH) cell expansion, contraction of the Treg cell compartment in general and the T follicular regulatory compartment in particular and increased autoantibody production [60, 134]. Mutations in CTLA4, the overwhelming majority of which are heterozygous in nature, largely phenocopy the presentation of LRBA deficiency, albeit with variable penetrance and more indolent disease onset and course [135].
Many primary immunodeficiencies, some of which are listed below, are associated with Treg cell dysfunction, reflecting the role of those pathways impacted by the respective deficiency in Treg cell development and differentiated functions. Hypomorphic mutations affecting the recombination-activating gene 1 (RAG1) and RAG2 give rise to severe restrictions on the Treg cell repertoire, leading to a phenotype of immune dysregulation and autoimmunity [136, 137]. Examples ranging across a spectrum of decreasing disease severity include Omenn’s syndrome, atypical SCID, and delayed onset combined immunodeficiency with granulomas and/or autoimmunity [138]. All of these disorders manifest immune dysregulation reflective of Treg cell deficiency, characterized by restricted repertoire diversity [138]. Mutations in other genes such as those involved in the store-operated calcium entry, including STIM1 and ORAI 1, encoding stromal interaction molecule 1 and calcium release activated modulator 1 respectively, give rise to a disorder of immunodeficiency and autoimmunity with the latter related to impaired T follicular and tissue resident Treg cell differentiation [139]. Other immunodeficiency-causing gene defects, including those affecting the actin cytoskeleton such as the dedicator of cytokinesis 8 (DOCK8) and Wiskott-Aldrich protein (WASP) deficiencies, may also present with an IPEX-like phenotype or more typically immune dysregulation due to the profound impairment of Treg cell function [140, 141]. Similarly, homozygous loss of function mutations in the CARMIL2 (RLTPR) gene, encoding the capping protein regulator and myosin 1 linker 2, which is critical for CD28 co-stimulatory signaling and cytoskeletal organization, results in the profound depletion of Treg cells and severe Th2 cell skewing [142-144]. Gain of function mutations in STAT1 may result in an IPEX like phenotype despite normal Treg cell frequencies, possibly reflecting altered transcriptional programs in these cells [145].
Therapeutic manipulation of Foxp3 Treg cells
The critical role of Foxp3+ Treg cells in the maintenance of immunological homeostasis makes them an attractive immunotherapeutic target for the induction or restoration of tolerance. Decreased Treg cell function as well as numerical Treg cell deficiencies have been described in several autoimmune diseases in human subjects and experimental models in mice [reviewed in 146]. Several clinical studies using already approved drugs (e.g IL-2, rapamycin) have shown that Treg cells can be successfully expanded in-vivo with clinically meaningful benefits [reviewed in 147]. Low dose IL-2 therapy, which preferentially activates Treg cells as a consequence of their constitutive CD25 expression, was effective in the treatment of graft versus host disease (GvHD) as well as hepatitis C virus- induced vasculitis [148-150]. Robust expansion of Treg cells following low dose IL-2 therapy has also been observed in patients with type 1 diabetes (T1D) and alopecia areata, where Treg cell recruitment in lesional skin was accompanied by partial regrowth of hair scalp and reduced effector CD8+ T cell infiltration [151-153]. Promising results have also emerged from clinical trials in systemic lupus erythematosus (SLE)[154]. Moreover, low dose IL-2 was shown to selectively increase the function and abundance of Treg cells in patients with SLE without affecting the frequencies of TH1 and TH2 cells [155]. Remarkably, all patients in this trial exhibited decreased disease activity at the end of the study, accompanied by decreased TH17 and TFH cell numbers [155].
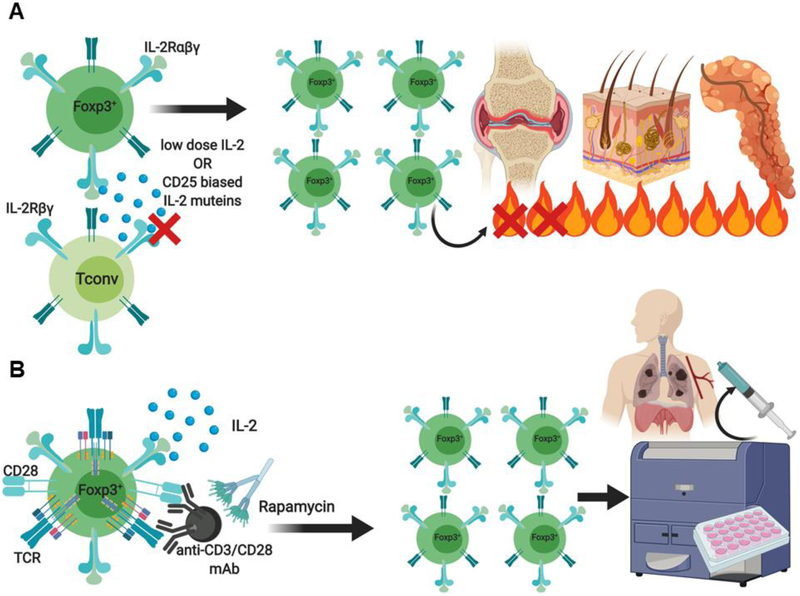
In addition to low dose IL-2, other therapeutic approaches include the development of IL-2 Fc fusion proteins or biochemically coupling IL-2 to molecular carriers such as polyethylene glycol (PEG) for the generation of novel IL-2 muteins that preferentially stimulate Treg cell activity [156-158] (Fig. 8). Furthermore, the generation of orthogonal IL-2 cytokine receptor complexes as well as anti-IL-2 antibodies, which when complexed with IL-2 can preferentially stimulate the expansion of Treg or Tconv cells, is also underway [156-158]. This concept is based on mouse studies demonstrating that IL-2/antibody complexes elicit preferential expansion of Treg cells in-vivo [159], leading to protection from experimental autoimmune encephalomyelitis (EAE) and prevention of pancreatic islet allograft rejection [160]. Alternatively, massive ex-vivo expansion of patient derived polyclonal nTreg cells for the purposes of adoptive cell transfer (ACT) therapy in autoimmune disease settings is already under exploration (Fig. 8). The first clinical studies utilizing ex-vivo expanded polyclonal nTreg cells for ACT in GvHD and T1D demonstrated the feasibility and safety of this approach [161, 162]. A more recent escalation phase 1 clinical trial in T1D with polyclonal Treg cells was also shown to be well tolerated [163]. Interestingly, these studies revealed long-term retention of a subset of the ex-vivo transferred polyclonal Treg cells with a broad regulatory phenotype. ex-vivo transferred polyclonal Treg cells with a broad regulatory phenotype.
Figure 8. Therapeutic manipulation of Foxp3+ Treg cells.
Examples of Treg cell-based therapy approaches. (A) Treg cells can be selectively expanded in-vivo with low dose IL-2 therapy or by IL-2 muteins engineered to preferentially interact with the high affinity IL-2 receptor alpha chain (CD25) expressed by Treg cells. (B) Alternatively, autologous CD4+CD25+CD127−Treg cells can be massively expanded ex vivo following stimulation with anti-CD3/CD28 mAbs in the presence of IL-2, rapamycin, or Treg cell-biased IL-2 muteins to generate a clinical grade adoptive Treg cell transfer therapy product.
A conceptually novel approach to restoring immune tolerance involves the metabolic reprogramming of Treg cells, including the inhibition of dysregulated mTORC2 activity in Foxp3-deficient Treg cells, including those of IPEX subjects, by using the dual mTOR inhibitor rapamycin [109]. Such an approach is consistent with the observation that rapamycin provides superior immunosuppression in patients with IPEX [164]. Combinatorial metabolic reprogramming approaches in which mTORC2 inhibition is allied with other interventions that suppress the exuberant oxidative phosphorylation and glutaminolysis found in Foxp3-deficient Treg cells and restores their defective fatty acid oxidation may further potentiate the regulatory functions of mutant Treg cells in IPEX subjects [109]. Finally, emerging genetic approaches including gene editing of mutant FOXP3 and related genes involved in monogenic disorders of immune dysregulation as well as engineering antigen-specific Treg cells for the purpose of treating autoimmune diseases promise novel approaches to therapy in the forthcoming years.
Conclusions
Since the discovery of Foxp3, a veritable revolution in our understanding of immune regulation and its disruption in a variety of human genetic and acquired disorders has emerged. Future research promises to deliver selective manipulation of the Treg cell responses as means of delivering precision immunotherapy. There is also the emerging role of Treg cells in the maintenance of tissue homeostasis, relevant to a variety of non-immune disorders, including metabolic and degenerative disorders.
Acknowledgements.
This work was supported by a National Institutes of Health grants 5R01AI085090 (to T.A.C.)
Footnotes
Competing Financial Interests Statement. The authors declare no competing financial interests.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: Peter Georgiev is a current employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
- 1.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156(6):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. [PubMed] [Google Scholar]
- 3.Bonomo A, Kehn PJ, Payer E, Rizzo L, Cheever AW, Shevach EM. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J Immunol. 1995;154(12):6602–11. [PubMed] [Google Scholar]
- 4.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184(2):387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J Exp Med. 1996;184(6):2393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106(12):R75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. [DOI] [PubMed] [Google Scholar]
- 11.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8(4):359–68. [DOI] [PubMed] [Google Scholar]
- 12.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445(7129):771–5. [DOI] [PubMed] [Google Scholar]
- 13.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116(6):1713–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harb Perspect Biol. 2012;4(9):a007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7(4):401–10. [DOI] [PubMed] [Google Scholar]
- 16.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25(2):249–59. [DOI] [PubMed] [Google Scholar]
- 17.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–35. [DOI] [PubMed] [Google Scholar]
- 18.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. [DOI] [PubMed] [Google Scholar]
- 19.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210(4):775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esterhazy D, Loschko J, London M, Jove V, Oliveira TY, Mucida D. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat Immunol. 2016;17(5):545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178(7):4022–6. [DOI] [PubMed] [Google Scholar]
- 22.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35(1):109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21(2):267–77. [DOI] [PubMed] [Google Scholar]
- 25.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38(6):1654–63. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158(4):749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Nguyen P, Liu W, Cheng C, Steeves M, Obenauer JC, et al. T cell receptor CDR3 sequence but not recognition characteristics distinguish autoreactive effector and Foxp3(+) regulatory T cells. Immunity. 2009;31(6):909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158(4):734–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37(5):785–99. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62–8. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol. 2015;16(11):1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37(3):501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A, Rudra D. Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Front Immunol. 2018;9:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother. 2015;64(10):1271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. [DOI] [PubMed] [Google Scholar]
- 39.Matsuki F, Saegusa J, Miyamoto Y, Misaki K, Kumagai S, Morinobu A. CD45RA-Foxp3(high) activated/effector regulatory T cells in the CCR7 + CD45RA-CD27 + CD28+central memory subset are decreased in peripheral blood from patients with rheumatoid arthritis. Biochem Biophys Res Commun. 2013;438(4):778–83. [DOI] [PubMed] [Google Scholar]
- 40.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211(1):121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delpoux A, Yakonowsky P, Durand A, Charvet C, Valente M, Pommier A, et al. TCR signaling events are required for maintaining CD4 regulatory T cell numbers and suppressive capacities in the periphery. J Immunol. 2014;193(12):5914–23. [DOI] [PubMed] [Google Scholar]
- 42.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, et al. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119(12):2810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):1713–22, S1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209(10):1723–42, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27(5):786–800. [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18(8):1197–209. [DOI] [PubMed] [Google Scholar]
- 48.Konopacki C, Pritykin Y, Rubtsov Y, Leslie CS, Rudensky AY. Transcription factor Foxp1 regulates Foxp3 chromatin binding and coordinates regulatory T cell function. Nat Immunol. 2019;20(2):232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11(7):618–27. [DOI] [PubMed] [Google Scholar]
- 50.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33(6):890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491(7425):554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529(7587):532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosaka T, Biggs WH 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101(9):2975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45. [DOI] [PubMed] [Google Scholar]
- 55.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105(29):10113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Z, Garg SK, Banerjee R. Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J Biol Chem. 2010;285(53):41525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. [DOI] [PubMed] [Google Scholar]
- 59.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135(1):217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. [DOI] [PubMed] [Google Scholar]
- 63.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180(9):5916–26. [DOI] [PubMed] [Google Scholar]
- 64.Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol. 2019;20(2):218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174(4):1783–6. [DOI] [PubMed] [Google Scholar]
- 66.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21(4):589–601. [DOI] [PubMed] [Google Scholar]
- 67.Voskoboinik I, Thia MC, De Bono A, Browne K, Cretney E, Jackson JT, et al. The functional basis for hemophagocytic lymphohistiocytosis in a patient with co-inherited missense mutations in the perforin (PFN1) gene. J Exp Med. 2004;200(6):811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voskoboinik I, Thia MC, Trapani JA. A functional analysis of the putative polymorphisms A91V and N252S and 22 missense perforin mutations associated with familial hemophagocytic lymphohistiocytosis. Blood. 2005;105(12):4700–6. [DOI] [PubMed] [Google Scholar]
- 69.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–20. [DOI] [PubMed] [Google Scholar]
- 71.Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol. 2007;179(6):3578–87. [DOI] [PubMed] [Google Scholar]
- 72.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17(11):1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58. [DOI] [PubMed] [Google Scholar]
- 74.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, et al. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J Immunol. 2015;195(8):3665–74. [DOI] [PubMed] [Google Scholar]
- 76.Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. IL-10 and IL-10 receptor defects in humans. Ann N Y Acad Sci. 2011;1246:102–7. [DOI] [PubMed] [Google Scholar]
- 77.Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X. IL-10 and IL-10 Receptor Mutations in Very Early Onset Inflammatory Bowel Disease. Gastroenterology Res. 2017;10(2):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, et al. Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity. 2017;46(4):660–74. [DOI] [PubMed] [Google Scholar]
- 79.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5(195):195ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kotlarz D, Marquardt B, Baroy T, Lee WS, Konnikova L, Hollizeck S, et al. Human TGF-beta1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat Genet. 2018;50(3):344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol. 2016;34:609–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404):549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malhotra N, Leyva-Castillo JM, Jadhav U, Barreiro O, Kam C, O'Neill NK, et al. RORalpha-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol. 2018;3(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell. 2017;169(6):1119–29 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6(258):258ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162(5):1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dombrowski Y, O'Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546(7658):421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458(7236):351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35(3):337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tachdjian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol. 2010;125(5):1128–36 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127(3):795–805 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42(3):512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104(5):1008–14. [DOI] [PubMed] [Google Scholar]
- 99.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349(6251):993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349(6251):989–93. [DOI] [PubMed] [Google Scholar]
- 102.Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat Med. 2019;25(7):1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017;25(6):1282–93 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Procaccini C, Carbone F, Di Silvestre D, Brambilla F, De Rosa V, Galgani M, et al. The Proteomic Landscape of Human Ex Vivo Regulatory and Conventional T Cells Reveals Specific Metabolic Requirements. Immunity. 2016;44(2):406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Newton R, Priyadharshini B, Turka LA. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17(6):618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17(12):1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Howie D, Cobbold SP, Adams E, Ten Bokum A, Necula AS, Zhang W, et al. Foxp3 drives oxidative phosphorylation and protection from lipotoxicity. JCI Insight. 2017;2(3):e89160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martinez-Reyes I, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565(7740):495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Charbonnier LM, Cui Y, Stephen-Victor E, Harb H, Lopez D, Bleesing JJ, et al. Functional Reprogramming of Regulatory T cells in the absence of Foxp3. Nat Immunol. 2019;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cepika AM, Sato Y, Liu JM, Uyeda MJ, Bacchetta R, Roncarolo MG. Tregopathies: Monogenic diseases resulting in regulatory T-cell deficiency. J Allergy Clin Immunol. 2018;142(6):1679– [DOI] [PubMed] [Google Scholar]
- 111.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25(6):708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–87. [DOI] [PubMed] [Google Scholar]
- 113.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151(1):153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13(10):1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15(6):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwon HK, Chen HM, Mathis D, Benoist C. FoxP3 scanning mutagenesis reveals functional variegation and mild mutations with atypical autoimmune phenotypes. Proc Natl Acad Sci U S A. 2018;115(2):E253–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.d'Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo CA. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2012;49(5):291–302. [DOI] [PubMed] [Google Scholar]
- 118.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bacchetta R, Barzaghi F, Roncarolo MG. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci. 2018;1417(1):5–22 [DOI] [PubMed] [Google Scholar]
- 120.Bin Dhuban K, d'Hennezel E, Nagai Y, Xiao Y, Shao S, Istomine R, et al. Suppression by human FOXP3(+) regulatory T cells requires FOXP3-TIP60 interactions. Sci Immunol. 2017;2(12). [DOI] [PubMed] [Google Scholar]
- 121.Hayatsu N, Miyao T, Tachibana M, Murakami R, Kimura A, Kato T, et al. Analyses of a Mutant Foxp3 Allele Reveal BATF as a Critical Transcription Factor in the Differentiation and Accumulation of Tissue Regulatory T Cells. Immunity. 2017;47(2):268–83 e9. [DOI] [PubMed] [Google Scholar]
- 122.Gambineri E, Perroni L, Passerini L, Bianchi L, Doglioni C, Meschi F, et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122(6):1105–12 e1. [DOI] [PubMed] [Google Scholar]
- 123.Van Gool F, Nguyen MLT, Mumbach MR, Satpathy AT, Rosenthal WL, Giacometti S, et al. A Mutation in the Transcription Factor Foxp3 Drives T Helper 2 Effector Function in Regulatory T Cells. Immunity. 2019;50(2):362–77 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frith K, Joly AL, Ma CS, Tangye SG, Lohse Z, Seitz C, et al. The FOXP3Delta2 isoform supports Treg cell development and protects against severe IPEX syndrome. J Allergy Clin Immunol. 2019;144(1):317–20 e8. [DOI] [PubMed] [Google Scholar]
- 125.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A. 1997;94(7):3168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr Res. 2000;48(1):6–11. [DOI] [PubMed] [Google Scholar]
- 127.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119(2):482–7. [DOI] [PubMed] [Google Scholar]
- 128.Goudy K, Aydin D, Barzaghi F, Gambineri E, Vignoli M, Ciullini Mannurita S, et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol. 2013;146(3):248–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Z, Gothe F, Pennamen P, James JR, McDonald D, Mata CP, et al. Human interleukin-2 receptor beta mutations associated with defects in immunity and peripheral tolerance. J Exp Med. 2019;216(6):1311–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fernandez IZ, Baxter RM, Garcia-Perez JE, Vendrame E, Ranganath T, Kong DS, et al. A novel human IL2RB mutation results in T and NK cell-driven immune dysregulation. J Exp Med. 2019;216(6):1255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol. 2006;177(5):2770–41 [DOI] [PubMed] [Google Scholar]
- 132.Jenks JA, Seki S, Kanai T, Huang J, Morgan AA, Scalco RC, et al. Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells. Clin Immunol. 2013;148(2):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klammt J, Neumann D, Gevers EF, Andrew SF, Schwartz ID, Rockstroh D, et al. Dominant-negative STAT5B mutations cause growth hormone insensitivity with short stature and mild immune dysregulation. Nat Commun. 2018;9(1):2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alroqi FJ, Charbonnier LM, Baris S, Kiykim A, Chou J, Platt CD, et al. Exaggerated follicular helper T-cell responses in patients with LRBA deficiency caused by failure of CTLA4-mediated regulation. J Allergy Clin Immunol. 2018;141(3):1050–9 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schwab C, Gabrysch A, Olbrich P, Patino V, Warnatz K, Wolff D, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol. 2018;142(6):1932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rowe JH, Delmonte OM, Keles S, Stadinski BD, Dobbs AK, Henderson LA, et al. Patients with CD3G mutations reveal a role for human CD3gamma in Treg diversity and suppressive function. Blood. 2018;131(21):2335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Villa A, Notarangelo LD. RAG gene defects at the verge of immunodeficiency and immune dysregulation. Immunol Rev. 2019;287(1):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Delmonte OM, Schuetz C, Notarangelo LD. RAG Deficiency: Two Genes, Many Diseases. J Clin Immunol. 2018;38(6):646–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vaeth M, Wang YH, Eckstein M, Yang J, Silverman GJ, Lacruz RS, et al. Tissue resident and follicular Treg cell differentiation is regulated by CRAC channels. Nat Commun. 2019;10(1):1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alroqi FJ, Charbonnier LM, Keles S, Ghandour F, Mouawad P, Sabouneh R, et al. DOCK8 Deficiency Presenting as an IPEX-Like Disorder. J Clin Immunol. 2017;37(8):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lexmond WS, Goettel JA, Lyons JJ, Jacobse J, Deken MM, Lawrence MG, et al. FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest. 2016;126(10):4030–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schober T, Magg T, Laschinger M, Rohlfs M, Linhares ND, Puchalka J, et al. A human immunodeficiency syndrome caused by mutations in CARMIL2. Nat Commun. 2017;8:14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sorte HS, Osnes LT, Fevang B, Aukrust P, Erichsen HC, Backe PH, et al. A potential founder variant in CARMIL2/RLTPR in three Norwegian families with warts, molluscum contagiosum, and T-cell dysfunction. Mol Genet Genomic Med. 2016;4(6):604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y, Ma CS, Ling Y, Bousfiha A, Camcioglu Y, Jacquot S, et al. Dual T cell- and B cell-intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med. 2016;213(11):2413–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131(6):1611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abbas AK, Trotta E, D RS, Marson A, Bluestone JA. Revisiting IL-2: Biology and therapeutic prospects. Sci Immunol. 2018;3(25). [DOI] [PubMed] [Google Scholar]
- 148.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365(22):2067–77. [DOI] [PubMed] [Google Scholar]
- 151.Rosenzwajg M, Churlaud G, Mallone R, Six A, Derian N, Chaara W, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1(4):295–305. [DOI] [PubMed] [Google Scholar]
- 153.Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150(7):748–51. [DOI] [PubMed] [Google Scholar]
- 154.Humrich JY, von Spee-Mayer C, Siegert E, Alexander T, Hiepe F, Radbruch A, et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann Rheum Dis. 2015;74(4):791–2. [DOI] [PubMed] [Google Scholar]
- 155.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–3. [DOI] [PubMed] [Google Scholar]
- 156.Trotta E, Bessette PH, Silveria SL, Ely LK, Jude KM, Le DT, et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med. 2018;24(7):1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sockolosky JT, Trotta E, Parisi G, Picton L, Su LL, Le AC, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science. 2018;359(6379):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spangler JB, Trotta E, Tomala J, Peck A, Young TA, Savvides CS, et al. Engineering a Single-Agent Cytokine/Antibody Fusion That Selectively Expands Regulatory T Cells for Autoimmune Disease Therapy. J Immunol. 2018;201(7):2094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–7. [DOI] [PubMed] [Google Scholar]
- 160.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol. 2009;133(1):22–6. [DOI] [PubMed] [Google Scholar]
- 162.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I,Juscinska J, et al. Administration of CD4+CD25highCD127− regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35(9):1817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barzaghi F, Amaya Hernandez LC, Neven B, Ricci S, Kucuk ZY, Bleesing JJ, et al. Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: An international multicenter retrospective study. J Allergy Clin Immunol. 2018;141(3):1036–49 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]