Abstract
Context:
The optimal dose of fentanyl sublingual spray (FSS) for exertional dyspnea has not been determined.
Objective:
We examined the effect of two doses of prophylactic FSS on exertional dyspnea.
Methods:
In this parallel, dose-finding, double-blind randomized clinical trial, opioid-tolerant cancer patients completed a shuttle walk test at baseline. Patients completed a second shuttle walk test 10 minutes after a single dose of FSS equivalent to either 35-45% (high dose) or 15-25% (low dose) of the total daily opioid dose. The primary outcome was change in modified dyspnea Borg scale (0-10) between the first and second shuttle walk tests. Secondary outcomes included adverse events as well as changes in walk distance, vital signs, and neurocognitive function.
Results:
Thirty of the 50 enrolled patients completed the study. High dose FSS (n=13) resulted in significantly lower dyspnea (mean change −1.42; 95% CI −2.37, −0.48; P=0.007) and greater walk distance (mean change 44 m; P=0.001) compared to baseline. Low dose FSS (n=17) resulted in a non-significant reduction in dyspnea (mean change −0.47; 95% CI −1.26, 0.32; P=0.24) and significant increase in walk distance (mean change 24 m; P=0.01) compared to baseline. Global evaluation showed high dose group was more likely to report at least somewhat better improvement (64% vs. 24%; P=0.06). No significant adverse events or detriment to vital signs or neurocognitive function were detected.
Conclusions:
Prophylactic FSS was well tolerated and demonstrated a dose-response relationship in improving both dyspnea and walk distance. High dose FSS should be tested in confirmatory trials.
Keywords: dyspnea, fentanyl, neoplasms, opioid analgesics, physical exertion, randomized controlled trial
Introduction
Dyspnea, a common symptom among cancer patients, is highly distressing and debilitating.(1)Episodic dyspnea is defined as “a severe worsening of breathlessness intensity or unpleasantness beyond usual fluctuations in the patient’s perception”.(2) The most common presentation of episodic dyspnea is on exertion, which can significantly limit patients’ mobility and function.(1, 3, 4) There are currently few evidence-based treatment options available for dyspnea.(5, 6) Although several clinical practice guidelines recommend opioids as the first line option for palliation of dyspnea,(7, 8) much remains unknown regarding the optimal opioid, dose, and dosing schedule for management of dyspnea, particularly that which presents on exertion.
As exertional dyspnea predictably increases with activity, the use of opioids prior to activity could potentially reduce this distressing symptom and thereby maximize activity level. Rapid-onset fentanyl formulations are particularly attractive for this purpose given their fast onset of action and ease of administration.(9) Indeed, several small randomized controlled trials conducted by our group and others on subcutaneous fentanyl,(10) nebulized fentanyl,(11, 12) fentanyl pectin nasal spray,(13) and fentanyl buccal tablet(14) demonstrated prophylactic administration to be associated with improved dyspnea and/or exercise capacity, providing preliminary evidence to support the efficacy of rapid onset fentanyl.
Given the efficacy of these rapid-onset fentanyl formulations, fentanyl sublingual spray (FSS) would also be expected to provide effective prophylaxis of exertional dyspnea. A better understanding of the effect of FSS on exertional dyspnea may expand novel therapeutic options for this distressing symptom. In this pilot dose-finding randomized clinical trial, we estimated the within-arm effects of high and low doses of prophylactic FSS on dyspnea, walk distance, and incidence of adverse events. We hypothesized that both dyspnea and walk distance would improve with prophylactic FSS administration, particularly in the high dose group.
Methods
Patients
The Institutional Review Board at MD Anderson Cancer Center approved this study. Patients were recruited from the Supportive Care and Thoracic Medical Oncology outpatient clinics at MD Anderson Cancer Center. All patients provided written informed consent. Inclusion criteria were age ≥18; diagnosis of cancer with evidence of active disease; episodic dyspnea, defined as dyspnea with an average intensity level over the past 7 days of ≥3 on a 0-10 point numeric rating scale (NRS) upon significant exertion, or continuous dyspnea ≤7 with worsening upon significant exertion; on strong opioids with morphine equivalent daily dose (MEDD) of 80-500 mg/day for ≥1 week, with stable (i.e. ±30%) regular dose over the last 24 hours; Karnofsky performance status (KPS) ≥50%; and able to walk with or without walking aid. Pregnant patients and patients with dyspnea at rest ≥7, allergy to fentanyl, history of active opioid abuse within the past 12 months, supplemental oxygen requirement >6 L/minute, severe anemia (hemoglobin <7 g/dL), Memorial Delirium Assessment Scale score of >13/30, or any contraindication to completing a shuttle walk test (SWT) were excluded.
Study Design
This was an investigator-initiated, double-blind, randomized clinical trial examining two dose schedules of FSS for exertional dyspnea. All participants performed a baseline SWT without any pre-medications. This was followed by a rest period in which dyspnea intensity was assessed every 5 minutes. Once patients’ dyspnea intensity returned to baseline+1 or lower, they were given a single dose of FSS 10 minutes before performing a second SWT. The study protocol is available upon request.
Randomization and Blinding
Randomization was conducted by the study pharmacist in a 1:1 ratio using permuted blocks with a size of 6, stratified by baseline level of dyspnea Borg scale at rest (i.e. 0-3 vs. 4-6). Allocation was concealed by a secured website that was only accessible to the study pharmacist after patient enrollment. A research nurse not otherwise involved in the study administered the FSS. Patients and other research staff were blinded to randomization and group assignment until after statistical analyses were complete. Maintenance of blinding was assessed at the end of study by asking patients and research staff to guess the study assignment.
Study Interventions
The single administration of FSS was dosed using a proportional versus titration approach as supported by multiple studies of rapid onset fentanyl for exertional dyspnea.(13, 14) Dose of FSS was calculated per group assignment (high or low dose) and MEDD, using the assumptions that 2.5 mg of oral morphine is equivalent to 1 mg of intravenous morphine and 10 mcg of intravenous fentanyl, and that FSS has a bioavailability of 70%. The high and low dose groups received FSS equivalent to 35-45% and 15-25% of MEDD, respectively (Table 1). These ranges were selected based on the proportional opioid doses used in previous dyspnea trials. (10, 13–16) Patients were asked to swallow any saliva, apply the entire medication spray unit sublingually, and hold the medication there for 30-60 seconds.
Table 1.
Proportional Dosing of Fentanyl Sublingual Spray
| Patient’s MEDD (mg/day) | FSS dose administered (mcg)* | MEDD Range (%)‡ | Number of patients who received each dose (%) |
|---|---|---|---|
| High dose group (target FSS 35-45% of MEDD) | |||
| 80-100 | 200 | 35.0-43.2 | 4 (31) |
| 101-150 | 300 | 35.0-52.0 | 1 (8) |
| 151-200 | 400 | 35.0-46.4 | 3 (23) |
| 201-250 | 500 | 35.0-43.5 | 2 (15) |
| 251-300 | 600 | 35.0-41.8 | 2 (15) |
| 301-400 | 800 | 35.0-46.5 | 1 (8) |
| 401-500 | 1000 | 35.0-43.6 | 0 |
| Low dose group (target FSS 15-25% of MEDD) | |||
| 80-130 | 100 | 13.5-21.6 | 7 (41) |
| 131-210 | 200 | 16.7-26.7 | 4 (24) |
| 211-280 | 300 | 18.8-24.9 | 1 (6) |
| 281-450 | 400 | 15.6-24.9 | 5 (29) |
| 451-500 | 600 | 21.0-23.3 | 0 |
Abbreviations: FSS, fentanyl sublingual spray; MEDD, morphine equivalent daily dose.
FSS is available in 100, 200, 400, 600, and 800 mcg dosage forms. If a particular dose was not readily available, a combination of two dosage forms was administered to achieve the desired dose.
The calculations were based on the assumptions that 1 mg of intravenous morphine is equivalent to 10 mcg of intravenous fentanyl, and that FSS has a bioavailability of 70%. Thus, 100 mcg of FSS is equivalent to 17.5 mg of oral morphine.
Shuttle Walk Tests
The SWTs were conducted according to published procedures.(17–19) Briefly, patients were asked to walk back and forth between two cones 10 m apart. Walking speed was dictated by an audio signal played on a tape cassette, starting at 30 m/min and increasing by 10 m/min after every minute until the patient either could not reach the cone before the next audio signal or became too dyspneic.
Study Assessments and Endpoints
Patient characteristics including age, sex, race, and dyspnea level were recorded at baseline. Vital capacity (VC), forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1 / FVC ratio, peak inspiratory flow, and peak expiratory flow were also assessed at baseline using a MicroLoop Spirometer (Micro Direct Inc., Lewiston, ME).
The primary outcome was dyspnea intensity “now” using the modified Borg scale, which ranges from 0 (“no shortness of breath”) to 10 (“worst possible shortness of breath”).(20–22) This scale has been validated in multiple studies, with a minimal clinically significant difference of 1 point.(23) Dyspnea unpleasantness was also measured using the same modified Borg scale. Dyspnea intensity and unpleasantness were both assessed prior to each SWT, every minute during the test, and at the end of each test. Total walk distance and walk time were recorded for each SWT.
Fatigue modified Borg scale (0=none, 10=worst) and vital signs (heart rate, respiratory rate, blood pressure, and oxygen saturation) were collected immediately before and after each SWT. Neurocognitive function and adverse effects were assessed immediately prior to medication administration as well as after the second SWT. Neurocognitive testing was conducted according to published procedures and included finger tapping, arithmetic, reverse memory of digits, and visual memory.(13, 24) Adverse effects including dizziness, drowsiness, nausea, and itchiness “now” were assessed using a 0-10 point NRS (0=absent, 10=worst possible).
After completion of the second SWT, patients’ overall impression of change was assessed by asking if his/her dyspnea was better, about the same, or worse after medication administration. If better or worse were chosen, patients were asked to further qualify if the improvement/deterioration was “hardly any,” “a little,” “somewhat,” “moderately,” “a good deal,” “a great deal,” or “a very great deal.”(25, 26)
Statistical Analysis
This study was designed to estimate the effect size of high and low dose FSS for powering future studies. Inclusion of 15 patients in each group allowed for 80% power to detect an effect size of 0.78 in difference of the modified Borg score dyspnea intensity change between the first and second SWT using a two-sided paired t-test with a significance level of 0.05. This study was not powered to test differential treatment effects between the two treatment groups.
Baseline demographics were summarized using descriptive statistics. The mean difference between the first and second SWT was calculated for key study outcomes. Given that dyspnea increased with distance walked, we examined two metrics in post-hoc analyses: the slope of modified Dyspnea Borg Scale per 100 meter walked, and the slope of modified Dyspnea Borg Scale per minute walked. Due to the small sample size, the non-parametric Wilcoxon signed rank test was used for within group (before and after) comparisons, and the Wilcoxon rank sum test was used for between-group analyses. Fisher’s exact test was used to test the difference between groups in proportion of patients that reported their dyspnea to be at least “somewhat better.” All available data was used for analysis without imputation for missing data (<1%).
The Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC) and S+ 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for statistical analysis.
Results
Patient Characteristics
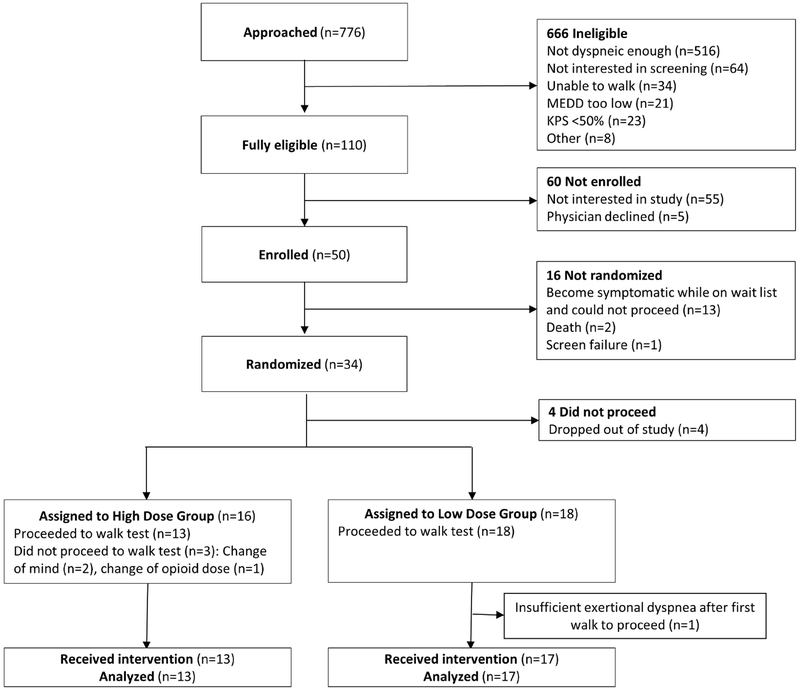
Fifty patients were enrolled between February 8, 2016 and October 19, 2018. Thirty (60%) completed all study assessments, of which 13 and 17 were assigned to the high and low dose groups, respectively (Figure 1). The average age was 52 (range 22-77) years, and 20 (67%) were female (Table 2).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Flowchart.
Abbreviations: KPS, Karnofsky performance scale; MEDD, morphine equivalent daily dose
Table 2.
Baseline Patient Characteristics
| High Dose n=13 (%)* | Low Dose n=17 (%)* | All patients N=30 (%)* | |
|---|---|---|---|
| Age, mean (SD) | 53 (16) | 51 (10) | 52 (13) |
| Female sex | 9 (69.2) | 11 (64.7) | 20 (66.7) |
| Race | |||
| Caucasian | 11 (84.6) | 12 (70.6) | 23 (76.7) |
| Black | 0 (0) | 1 (5.9) | 1 (3.3) |
| Hispanic | 2 (15.4) | 3 (17.6) | 5 (16.7) |
| Asian | 0 (0) | 1 (5.9) | 1 (3.3) |
| Education | |||
| High school or less | 7 (53.8) | 2 (11.8) | 9 (30.0) |
| College | 6 (46.2) | 12 (70.6) | 18 (60.0) |
| Advanced degree | 0 (0) | 3 (17.6) | 3 (10) |
| Cancer type | |||
| Breast | 2 (15.4) | 6 (35.3) | 8 (26.7) |
| Gastrointestinal | 2 (15.4) | 2 (11.8) | 4 (13.3) |
| Genitourinary | 3 (23.1) | 2 (11.8) | 5 (16.7) |
| Gynecological | 2 (15.4) | 1 (5.9) | 3 (10) |
| Head and neck | 0 (0) | 2 (11.8) | 2 (6.7) |
| Respiratory | 4 (30.8) | 3 (17.6) | 7 (23.3) |
| Others | 0 (0) | 1 (5.9) | 1 (3.3) |
| Cancer stage | |||
| Metastatic or recurrent | 10 (76.9) | 14 (82.4) | 24 (80) |
| Locally advanced | 3 (23.1) | 2 (11.8) | 5 (16.7) |
| Localized | 0 (0) | 1 (5.9) | 1 (3.3) |
| Average dyspnea NRS during breakthrough episodes over the last week, mean (SD) | 5.2 (1.9) | 5.7 (2.1) | 5.4 (2.0) |
| CAGE positivity (>=2) | 3 (23.1) | 2 (11.8) | 5 (16.7) |
| Chronic obstructive pulmonary disease | 1 (7.7) | 3 (17.6) | 4 (13.3) |
| Concurrent therapies (scheduled) | |||
| Opioids | 13 (100) | 17 (100) | 30 (100) |
| Bronchodilators | 1 (7.7) | 1 (5.9) | 2 (6.7) |
| Steroids | 1 (7.7) | 3 (17.6) | 4 (13.3) |
| Supplemental oxygen | 1 (7.7) | 0 (0) | 1 (3.3) |
| Concurrent therapies (as needed) | |||
| Opioids | 11 (84.6) | 14 (82.4) | 25 (83.3) |
| Bronchodilators | 4 (30.8) | 3 (17.6) | 7 (23.3) |
| Steroids | 1 (7.7) | 3 (17.6) | 4 (13.3) |
| Supplemental oxygen | 1 (7.7) | 2 (11.8) | 3 (10) |
| Bedside spirometry measures, mean (SD) | |||
| FEV1 | 2.4 (0.9) | 2.4 (0.6) | 2.4 (0.7) |
| FEV1 % predicted | 76.0 (21.6) | 79.0 (17.0) | 77.8 (18.6) |
| FVC | 2.97 (1.0) | 3.0 (0.8) | 2.9 (0.9) |
| FVC % predicted | 73.5 (21.3) | 79.2 (17.4) | 76.9 (18.8) |
| FEV1 / FVC ratio (%) | 84.0 (8.4) | 79.6 (7.8) | 81.4 (8.2) |
| Maximal inspiratory pressure, mean (SD), cm H2O | 90.8 (24.2) | 85.8 (22.5) | 88.1 (23.0) |
| Morphine equivalent daily dose in mg/day, median (IQR) | 179 (131, 227) | 193 (134, 252) | 186 (150, 224) |
| Karnofsky performance status, mean (SD) | 73.9 (10.4) | 70.6 (9.0) | 72 (9.6) |
| Edmonton Symptom Assessment System | |||
| Pain | 5.9 (2.7) | 4.8 (2.0) | 5.3 (2.4) |
| Fatigue | 5.2 (1.8) | 3.9 (2.5) | 4.4 (2.3) |
| Nausea | 2.8 (3.9) | 0.8 (1.6) | 1.7 (2.9) |
| Depression | 1.2 (1.5) | 1.9 (2.4) | 1.6 (2.1) |
| Anxiety | 2.1 (1.9) | 3.0 (2.5) | 2.6 (2.3) |
| Drowsiness | 4.5 (2.6) | 3.1 (2.8) | 3.7 (2.8) |
| Shortness of breath | 3.8 (2.1) | 3.8 (2.5) | 3.8 (2.3) |
| Appetite | 3.1 (2.7) | 2.8 (2.5) | 2.9 (2.5) |
| Sleep | 3.1 (2.2) | 4.2 (3.4) | 3.7 (2.9) |
| Feeling of well being | 2.6 (1.9) | 3.9 (2.3) | 3.4 (2.2) |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NRS, numeric rating scale; SD, standard deviation
Unless otherwise specified.
Dyspnea Intensity, Unpleasantness, and Walk Distance
Consistent with our study design, the median (IQR) proportional dose that patients actually received (i.e. FSS dose divided by total MEDD) was 40% (38%, 42%) for the high dose group and 20% (19%, 22%) for the low dose group. High dose FSS was associated with a significant within-arm reduction between the first and second SWT in modified Borg scale dyspnea intensity (mean change −1.4; 95% CI −2.4, −0.5; P=0.007; Table 3). In contrast, low dose FSS was associated with non-significant within-arm improvement (mean change −0.5; 95% CI −1.3, 0.3; P=0.24). Dyspnea unpleasantness showed similar, though non-significant (P=0.06 and 0.10 in high and low dose groups, respectively) trends.
Table 3.
Study Outcomes for High and Low Dose Fentanyl Sublingual Spray (FSS) Groups
| High Dose Group, mean (SD) [95% CI] | Low Dose Group, mean (SD) [95% CI] | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | First walk | Second walk | Difference | P-Value* | First walk | Second walk | Difference | P-Value* |
| Modified Borg Scale: Dyspnea Intensity (primary outcome) | ||||||||
| Beginning of walk | 0.6 (0.8) | 0.7 (1.0) | 0.1 (0.5) | 0.44 | 0.5 (0.7) | 0.4 (0.8) | −0.06 (0.6) | 0.84 |
| End of walk | 5.1 (1.1) | 3.8 (1.4) | −1.3 (1.6) | 5.1 (2.0) | 4.5 (2.1) | −0.5 (1.4) | ||
| Difference | 4.5 (1.7) | 3.1 (1.5) | −1.4 (1.6) [−2.4, −0.5] |
0.007 | 4.6 (1.7) | 4.1 (2.2) | −0.5 (1.6) [−1.3, 0.3] |
0.24 |
| Difference/distance walked (/100 m) | 1.5 (0.9) | 0.9 (0.7) | −0.6 (0.5) [−0.9, −0.3] |
<0.001 | 1.6 (0.9) | 1.3 (0.7) | −0.3 (0.5) [−0.6, −0.1] |
0.03 |
| Difference/min walked (/min) | 0.8 (0.3) | 0.5 (0.3) | −0.3 (0.3) [−0.4, −0.1] |
<0.001 | 0.8 (0.3) | 0.7 (0.4) | −0.1 (0.2) [−0.3, −0.008] |
0.05 |
| Modified Borg Scale: Dyspnea Unpleasantness | ||||||||
| Beginning of walk | 0.6 (0.8) | 0.7 (1.3) | 0.1 (0.7) | 0.94 | 0.4 (1.0) | 0.4 (1.0) | −0.03 (0.3) | >0.99 |
| End of walk | 3.9 (1.7) | 2.9 (1.9) | −1.0 (1.9) | 4.1 (2.4) | 3.4 (2.8) | −0.7 (1.3) | ||
| Difference | 3.3 (2.0) | 2.2 (1.8) | −1.0 (1.8) | 0.06 | 3.7 (2.1) | 3.0 (2.5) | −0.6 (1.4) | 0.10 |
| Walk distance (m) | 354.6 (155.8) | 398.3 (148.7) | 43.7 (30.0) [25.6, 61.8] |
0.001 | 343.2 (148.4) | 367.4 (159.6) | 24.2 (35.7) [5.8, 42.6 ] |
0.01 |
| Walk time (min) | 6.2 (1.9) | 6.7 (1.7) | 0.5 (0.4) [0.3, 0.7] |
<0.001 | 6.0 (1.9) | 6.3 (1.8) | 0.3 (0.4) [0.18, 0.5] |
0.009 |
| Fatigue Borg Scale | ||||||||
| Beginning of walk | 2.8 (2.4) | 2.3 (1.7) | −0.5 (1.5) | 0.31 | 2.4 (2.6) | 1.9 (2.3) | −0.5 (1.1) | 0.10 |
| End of walk | 4.8 (1.9) | 3.3 (1.9) | −1.5 (2.7) | 4.1 (1.8) | 3.5 (1.9) | −0.4 (1.2) | ||
| Difference | 2.0 (3.0) | 1.0 (1.0) | −1.0 (2.8) | 0.13 | 1.4 (2.5) | 1.5 (2.3) | 0.2 (1.6) | 0.64 |
| Heart Rate (beats/min) | ||||||||
| Beginning of walk | 90.5 (16.6) | 87.7 (13.5) | −2.9 (10.2) | 0.38 | 77.6 (13.9) | 79.5 (15.4) | 1.9 (6.3) | 0.19 |
| End of walk | 100.6 (19.2) | 101.2 (22.1) | 0.5 (16.5) | 87.5 (26.8) | 88.3 (29.6) | 0.8 (8.8) | ||
| Difference | 10.1 (16.0) | 13.5 (16.7) | 3.4 (12.5) | 0.15 | 9.9 (23.25) | 8.8 (22.9) | −1.1 (8.3) | 0.64 |
| Respiratory Rate (breaths/min) | ||||||||
| Beginning of walk | 17.4 (3.5) | 16.0 (3.7) | −1.4 (3.3) | 0.17 | 15.5 (3.4) | 15.4 (4.0) | −0.1 (2.5) | 0.95 |
| End of walk | 21.1 (3.0) | 20.9 (4.9) | −0.2 (4.1) | 20.4 (6.2) | 18.8 (5.2) | −1.6 (4.0) | ||
| Difference | 3.7 (4.1) | 4.9 (5.3) | 1.2 (6.4) | 0.72 | 4.9 (4.4) | 3.5 (4.1) | −1.5 (3.8) | 0.15 |
| Systolic Blood pressure (mm Hg) | ||||||||
| Beginning of walk | 130.9 (19.4) | 127.2 (18.3) | −3.6 (15.0) | 0.48 | 121.8 (18.7) | 117.5 (16.9) | −4.4 (12.7) | 0.20 |
| End of walk | 142.4 (15.9) | 145.7 (14.6) | 3.3 (10.1) | 131.4 (13.7) | 136.3 (14.1) | 4.9 (11.4) | ||
| Difference | 11.5 (12.7) | 18.5 (9.3) | 6.9 (13.7) | 0.10 | 9.5 (18.2) | 18.8 (14.1) | 9.3 (20.0) | 0.10 |
| Diastolic Blood pressure (mm Hg) | ||||||||
| Beginning of walk | 76.6 (11.7) | 77.7 (6.8) | 1.1 (7.0) | 0.62 | 71.8 (10.6) | 73.9 (11.0) | 2.2 (6.0) | 0.17 |
| End of walk | 81.5 (8.6) | 85.9 (6.5) | 4.4 (6.1) | 75.4 (10.7) | 78.2 (13.3) | 2.8 (9.0) | ||
| Difference | 4.9 (7.1) | 8.2 (7.1) | 3.3 (6.7) | 0.11 | 3.6 (8.8) | 4.2 (7.4) | 0.7 (10.1) | 0.44 |
| Oxygen Saturation (%) | ||||||||
| Beginning of walk | 97.7 (1.4) | 96.7 (2.1) | −1 (1.2) | 0.02 | 97.2 (2.0) | 97.2 (2.2) | 0.1 (1.5) | 0.95 |
| End of walk | 97.9 (2.0) | 97.6 (1.9) | −0.3 (1.0) | 97.9 (2.2) | 97.9 (2.8) | 0 (1.4) | ||
| Difference | 0.2 (1.2) | 0.9 (1.0) | 0.7 (1.7) | 0.21 | 0.7 (2.0) | 0.7 (1.5) | −0.1 (1.7) | 0.87 |
| Neurocognitive Testing | ||||||||
| Before medication | Post second walk test | Difference | P-value* | Before medication | Post second walk test | Difference | P-value* | |
| Tapping | 42.1 (9.9) | 48.9 (11.2) | 6.9 (8.6) | 0.003 | 46.4 (8.4) | 49.8 (9.1) | 3.4 (6.3) | 0.01 |
| Arithmetic | 88.7 (42.5) | 84.8 (47.4) | −3.9 (27.2) | 0.56 | 52.8 (31.4) | 58.1 (38.3) | 5.2 (19.6) | 0.73 |
| Reverse digits | 4.5 (2.1) | 3.9 (2.4) | −0.5 (1.6) | 0.36 | 4.1 (2.1) | 4.9 (2.6) | 0.8 (1.7) | 0.10 |
| Visual | 5.4 (1.2) | 5.4 (1.3) | 0 (1.4) | >0.99 | 5.7 (0.8) | 5.5 (0.9) | −0.1 (0.9) | >0.99 |
Abbreviations: CI, confidence interval; FSS, fentanyl sublingual spray; SD, standard deviation
Wilcoxon signed rank test was used for all comparisons between the first and second shuttle walk test.
The SWT distance significantly increased in both the high (43.7 m; 95% CI 25.6 m, 61.8 m; P=0.001) and low (24.2 m; 95% CI 5.8 m, 42.6 m; P=0.009) dose groups (Table 3). In post-hoc analyses, using the metric dyspnea intensity/min walked, significant improvement was noted with both high (−0.3/min; 95% CI −0.4/min, −0.1/min; P<0.001) and low (−0.1/min; 95% CI −0.3/min, −0.008/min; P=0.05) dose FSS.
There was no significant difference in the second SWT between the high and low dose groups in either change in modified Borg scale dyspnea intensity (3.1 vs. 4.1; P=0.21) or in walk distance (398.3 vs. 367.4 m; P=0.65).
Fatigue and Physiologic Function
Comparing between the first and second SWT, no significant within-group differences in fatigue or vital signs were detected in either treatment group (Table 3).
Neurocognitive Function and Adverse Effects
Compared to baseline, finger tapping improved significantly after study medication administration in both high (+6.9; P=0.003) and low (+3.4; P=0.01) dose FSS groups (Table 3). No changes were detected in the other three neurocognitive tests (Table 3).
One patient in the high dose group and five patients in low dose group reported ≥1 point increase in dizziness after the second SWT. The average change in the four side effects (dizziness, drowsiness, nausea, and itchiness) ranged from −0.4 points (nausea in high dose group) and 0.24 points (dizziness in low dose group; Table 4).
Table 4.
Adverse Effects in High and Low Dose Fentanyl Sublingual Spray (FSS) Groups*
| Mean Change (SD) | Number of patients with worse scores after drug administration (%) | |||
|---|---|---|---|---|
| Variable | High Dose (n=13) | Low Dose (n=17) | High Dose (n=13) | Low Dose (n=17) |
| Dizziness | −0.2 (0.7) | 0.24 (0.9) | 1 (7.7) | 5 (29.4) |
| Drowsiness | 0 (2.4) | −0.3 (0.7) | 2 (15.4) | 1 (5.9) |
| Nausea | −0.4 (1.0) | −0.1 (0.2) | 0 (0) | 0 (0) |
| Itchiness | 0 (0) | 0.1 (0.2) | 0 (0) | 1 (5.9) |
Abbreviations: SD, standard deviation.
Each adverse effect was measured using a 0-10 point numeric rating scale (0=none, 10=worst) immediately before drug administration and immediately after the second walk test (approximately 20 min later).
Global Symptom Evaluation and Blinding
A greater proportion of patients in the high versus low dose group reported that their dyspnea was at least “somewhat better” (8/13 [64%] vs. 4/17 [24%]; P=0.06).
Regarding blinding, treatment assignment was guessed correctly by 1/13 (7%) patients and 6/13 (46%) research staff among the high dose group, and 8/17 (47%) patients and 6/17 (35%) research staff among the low dose group.
Discussion
In this dose-finding randomized trial, both high and low dose prophylactic FSS improved exertional dyspnea. Improvement in dyspnea intensity and walk distance was consistent with our hypothesis, and demonstrated a promising dose-response relationship. One-time FSS administration was well tolerated even at high dose (proportional to 35-52% of MEDD). These preliminary data strongly support further examination of high dose FSS in a larger confirmatory trial.
In a prior pilot double-blind, placebo controlled randomized trial, we reported subcutaneous fentanyl given at 15-25% of MEDD 15 minutes before a structured walk test was associated with significant within-arm improvement in exertional dyspnea intensity at the end of the walk (NRS −1.8; 95% CI −3.2, −0.4). Subcutaneous fentanyl at 15-25% of MEDD was also associated with greater walk distance (+37.2 m; 95% CI 5.8 m, 68.6 m) as compared to baseline.(10) In another randomized trial, we demonstrated fentanyl pectin nasal spray administered at 15-25% of MEDD 20 minutes prior to exertion to be associated with significant reduction in dyspnea intensity at the end of a 6 minute walk test (−2.0; 95% −3.5, −0.6) and improved walk distance (+23.8 m; 95% CI 1.3 m, 46.2 m).(13) A third trial with fentanyl buccal tablet dosed at 20-50% of MEDD given 30 minutes before 6 minute walk test revealed significant within-arm reduction in dyspnea NRS between 0 and 6 minutes (−2.4; 95% CI −3.5, −1.3), and non-significant improvement in walk distance (−1 m; 95% CI −22.3 m, 20.3 m).(14) The present study builds on these results by investigating the one of the fastest acting opioids (i.e. FSS) on exertional dyspnea. Instead of a placebo-controlled trial, this dose-finding study allowed us to examine the impact of high and low proportional dose.
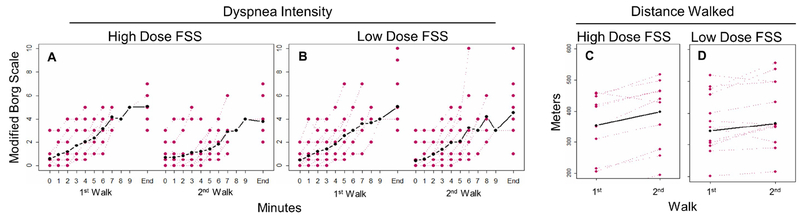
Consistent with our hypothesis, high dose FSS significantly improved both SWT walk distance and dyspnea at the end of the SWT (Figure 2). As dyspnea reliably increases with exertion, it is particularly important to account for the amount of activity when examining exertional dyspnea. Thus, we propose a metric of dyspnea change per 100 m, which allows for better adjustment based on the extent of exertion, with significant change observed with both high (−0.6; 95% CI −0.9, −0.3) and low (−0.3; 95% CI −0.6, −0.1) dose FSS. Future studies are needed to validate this outcome and determine its minimal clinically important difference.
Figure 2. Change in Dyspnea Intensity and Walk Distance With and Without Study Medications.
(A) In the high dose fentanyl sublingual spray (FSS) group, significantly lower modified dyspnea Borg scale (mean change −1.4; 95% CI −2.4, −0.5; P=0.007) was observed between the first (no medication) and second (10 minutes after FSS administration) walk. (B) In the low dose FSS group, there was also a non-significant reduction in modified dyspnea Borg scale (mean change −0.5; 95% CI −1.3, 0.3; P=0.24). Walk distance was increased after both high ([C]; mean change 44 m; P=0.001) and low ([D]; mean change 24 m; P=0.01) dose FSS administration as compared to baseline.
In this study, the SWT, rather than the 6 minute walk test, was used to induce exertional dyspnea. The SWT has been validated in cancer patients and has several potential advantages.(17, 19) It is externally paced, only requires a 10 m walkway, and has been found to be equivalent to or better than the 6 minute walk test in detecting a clinical response.(27–29) However, the SWT mandates rapid escalation in walking speed, which becomes extremely challenging after 10 minutes. In addition, the SWT is proprietary and thus is associated with financial cost and significant time for administrative processing.
This study supports the pharmacologic effect of prophylactic opioids for palliation of dyspnea. Consistent with previous clinical trials with fentanyl pectin nasal spray and fentanyl buccal tablet,(13, 14, 30, 31) FSS dose was estimated with a proportional versus titration approach. Higher proportional dose appeared to have a greater effect on exertional dyspnea. In contrast, several studies involving rapid onset fentanyl agents reported a lack of association between effective fentanyl dose and MEDD.(32, 33) All patients on these opioids for pain currently need to go through a titration phase. A proportional approach could make it easier to start patients on rapid onset fentanyl agents. Larger studies are needed to confirm these findings.
We did not detect any major adverse effects with administration of FSS. The average change in adverse effects was small (between −0.4 and 0.24 points on a 0-10 point NRS), and high dose FSS did not appear to result in more adverse effects relative to low dose FSS. No deterioration in neurocognitive function was observed despite the high dose, consistent with our previous exertional dyspnea trials.(10, 13, 14) This is in contrast to other non-exercise studies documenting a decline in neurocognitive function after opioid administration.(24) Further studies are needed to determine the potential protective effect of exercise.
This study has several limitations. First, patients were recruited mostly from palliative care and thoracic oncology clinics at a single tertiary cancer center and had a relatively high performance status. Our findings may not be generalizable to other settings and patient populations. Second, use of a rapid-onset opioid necessitated enrollment of only opioid-tolerant individuals. Further studies are needed to examine treatment strategies for opioid-naïve patients.(34) Third, the study was powered only to estimate within-group differences. Fourth, multiple exploratory outcomes were examined and should be considered hypothesis-generating. Fifth, because of the pilot nature of the study, it did not include a placebo group. However, previous double-blind placebo-controlled trials by our group enrolling similar patients suggested the placebo/training effect was small.(10, 13, 15) Sixth, this study was conducted in a highly controlled setting, and patients only received a single dose of FSS. Larger confirmatory trials are needed with more pragmatic designs and repeated dosing.
One of the key principles of palliative care is the preference for proactive, rather than reactive, approaches to symptom management. High dose FSS appears to be safe and efficacious in this preliminary study, and its rapid onset makes it particularly appealing for prophylactic applications. Based on the encouraging findings from this study, the next step is to conduct an adequately powered placebo-controlled trial to assess the prophylactic use of high dose FSS for exertional dyspnea. Future pragmatic studies should also examine repeated dosing, use in the home setting, FSS’s impact on daily activities and quality of life, and its adverse effects and addictive potential with longer term administration.
Acknowledgements:
The study personnel and study medications were supported by Insys Therapeutics, Inc. through an investigator-initiated research award to D.H. D.H. was also supported in part by grants from the National Cancer Institute (1R01CA214960-01A1; 1R01CA225701-01A1), the National Institute of Nursing Research (1R21NR016736-01), and the American Cancer Society (MRSG-14-1418-01-CCE). D.L. was supported in part by a National Institutes of Health Cancer Center Support Grant (P30CA016672).
Disclosure statement: Insys Therapeutics, Inc. provided funding for research staff and study medications and participated in study design, but was not involved in data collection, statistical analyses, data interpretation, or manuscript preparation. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med 2009;12:29–36. [DOI] [PubMed] [Google Scholar]
- 2.Simon ST, Weingartner V, Higginson IJ, Voltz R, Bausewein C. Definition, categorization, and terminology of episodic breathlessness: consensus by an international Delphi survey. J Pain Symptom Manage 2014;47:828–838. [DOI] [PubMed] [Google Scholar]
- 3.Simon ST, Bausewein C, Schildmann E, et al. Episodic breathlessness in patients with advanced disease: a systematic review. J Pain Symptom Manage 2013;45:561–578. [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S, Aielli F, Adile C, et al. Epidemiology and Characteristics of Episodic Breathlessness in Advanced Cancer Patients: An Observational Study. J Pain Symptom Manage 2016;51:17–24. [DOI] [PubMed] [Google Scholar]
- 5.Hui D, Kilgore K, Frisbee-Hume S, et al. Dexamethasone for Dyspnea in Cancer Patients: A Pilot Double-Blind, Randomized, Controlled Trial. J Pain Symptom Manage 2016;52:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui D, Morgado M, Chisholm G, et al. High-flow oxygen and bilevel positive airway pressure for persistent dyspnea in patients with advanced cancer: a phase II randomized trial. J Pain Symptom Manage 2013;46:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MH, Smith T, Alvarez-Perez A, et al. : NCCN Clinical Practice Guidelines in Oncology. Palliative Care. 2017; http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed April 19, 2017, 2017. [Google Scholar]
- 8.Kloke M, Cherny N. Treatment of dyspnoea in advanced cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2015;26 Suppl 5:v169–173. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MJ, Hui D, Currow DC. Opioids, Exertion, and Dyspnea: A Review of the Evidence. Am J Hosp Palliat Care 2016;33:194–200. [DOI] [PubMed] [Google Scholar]
- 10.Hui D, Xu A, Frisbee-Hume S, et al. Effects of prophylactic subcutaneous fentanyl on exercise-induced breakthrough dyspnea in cancer patients: a preliminary double-blind, randomized, controlled trial. J Pain Symptom Manage 2014;47:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen D, Alsuhail A, Viola R, Dudgeon DJ, Webb KA, O’Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J Pain Symptom Manage 2012;43:706–719. [DOI] [PubMed] [Google Scholar]
- 12.Kotrach HG, Bourbeau J, Jensen D. Does nebulized fentanyl relieve dyspnea during exercise in healthy man? J Appl Physiol (1985) 2015;118:1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui D, Kilgore K, Park M, Williams J, Liu D, Bruera E. Impact of Prophylactic Fentanyl Pectin Nasal Spray on Exercise-Induced Episodic Dyspnea in Cancer Patients: A Double-Blind, Randomized Controlled Trial. J Pain Symptom Manage 2016;52:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui D, Kilgore K, Frisbee-Hume S, et al. Effect of Prophylactic Fentanyl Buccal Tablet on Episodic Exertional Dyspnea: A Pilot Double-Blind Randomized Controlled Trial. J Pain Symptom Manage 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon ST, Kloke M, Alt-Epping B, et al. EffenDys-Fentanyl Buccal Tablet for the Relief of Episodic Breathlessness in Patients With Advanced Cancer: A Multicenter, Open-Label, Randomized, Morphine-Controlled, Crossover, Phase II Trial. J Pain Symptom Manage 2016;52:617–625. [DOI] [PubMed] [Google Scholar]
- 16.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med 1993;119:906–907. [DOI] [PubMed] [Google Scholar]
- 17.Booth S, Adams L. The shuttle walking test: a reproducible method for evaluating the impact of shortness of breath on functional capacity in patients with advanced cancer. Thorax 2001;56:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revill SM, Morgan MD, Singh SJ, Williams J, Hardman AE. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax 1999;54:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med 2007;21:177–191. [DOI] [PubMed] [Google Scholar]
- 21.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care 1998;7:200–204. [PubMed] [Google Scholar]
- 22.Hui D, Morgado M, Vidal M, et al. Dyspnea in Hospitalized Advanced Cancer Patients: Subjective and Physiologic Correlates. J Palliat Med 2013;16:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD 2005;2:105–110. [DOI] [PubMed] [Google Scholar]
- 24.Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992;48:163–166. [DOI] [PubMed] [Google Scholar]
- 25.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996;49:1215–1219. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med 1993;118:622–629. [DOI] [PubMed] [Google Scholar]
- 27.Pepin V, Brodeur J, Lacasse Y, et al. Six-minute walking versus shuttle walking: responsiveness to bronchodilation in chronic obstructive pulmonary disease. Thorax 2007;62:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepin V, Laviolette L, Brouillard C, et al. Significance of changes in endurance shuttle walking performance. Thorax 2011;66:115–120. [DOI] [PubMed] [Google Scholar]
- 29.Pulz C, Diniz RV, Alves AN, et al. Incremental shuttle and six-minute walking tests in the assessment of functional capacity in chronic heart failure. Can J Cardiol 2008;24:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercadante S, Adile C, Cuomo A, et al. Fentanyl Buccal Tablet vs. Oral Morphine in Doses Proportional to the Basal Opioid Regimen for the Management of Breakthrough Cancer Pain: A Randomized, Crossover, Comparison Study. J Pain Symptom Manage 2015;50:579–586. [DOI] [PubMed] [Google Scholar]
- 31.Mercadante S, Aielli F, Adile C, Costanzi A, Casuccio A. Fentanyl Pectin Nasal Spray Versus Oral Morphine in Doses Proportional to the Basal Opioid Regimen for the Management of Breakthrough Cancer Pain: A Comparative Study. J Pain Symptom Manage 2016;52:27–34. [DOI] [PubMed] [Google Scholar]
- 32.Parikh N, Goskonda V, Chavan A, Dillaha L. Pharmacokinetics and dose proportionality of fentanyl sublingual spray: a single-dose 5-way crossover study. Clin Drug Investig 2013;33:391–400. [DOI] [PubMed] [Google Scholar]
- 33.Nalamachu SR, Parikh N, Dillaha L, Rauck R. Lack of correlation between the effective dose of fentanyl sublingual spray for breakthrough cancer pain and the around-the-clock opioid dose. Journal of opioid management 2014;10:247–254. [DOI] [PubMed] [Google Scholar]
- 34.Rauck RL, Oh DA, Singla N, et al. Pharmacokinetics of Fentanyl Sublingual Spray in Opioid-Naive Participants: Results of a Phase 1, Multiple Ascending Dose Study. Clin Drug Investig 2018;38:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]