Abstract
Background:
Hepato- and nephrotoxicity of fluoride have been demonstrated in animals, but few studies have examined potential effects in humans. This population-based study examines the relationship between chronic low-level fluoride exposure and kidney and liver function among United States (U.S.) adolescents. This study aimed to evaluate whether greater fluoride exposure is associated with altered kidney and liver parameters among U.S. youth.
Methods:
This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (2013-2016). We analyzed data from 1,983 and 1,742 adolescents who had plasma and water fluoride measures respectively and did not have kidney disease. Fluoride was measured in plasma and household tap water. Kidney parameters included estimated glomerular filtration rate (calculated by the original Schwartz formula), serum uric acid, and the urinary albumin creatinine ratio. Liver parameters were assessed in serum and included alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, blood urea nitrogen, gamma glutamate transferase, and albumin. Survey-weighted linear regression examined relationships between fluoride exposure and kidney and liver parameters after covariate adjustment. A Holm-Bonferroni correction accounted for multiple comparisons.
Results:
The average age of adolescents was 15.4 years. Median water and plasma fluoride concentrations were 0.48 mg/L and 0.33 μmol/L respectively. A 1 μmol/L increase in plasma fluoride was associated with a 10.36 mL/min/1.73 m2 lower estimated glomerular filtration rate (95% CI: −17.50, − 3.22; p =0.05), a 0.29 mg/dL higher serum uric acid concentration (95% CI: 0.09, 0.50; p =0.05), and a 1.29 mg/dL lower blood urea nitrogen concentration (95%CI: −1.87, − 0.70; p < 0.001). A 1 mg/L increase in water fluoride was associated with a 0.93 mg/dL lower blood urea nitrogen concentration (95% CI: −1.44, −0.42; p =0.007).
Conclusions:
Fluoride exposure may contribute to complex changes in kidney and liver related parameters among U.S. adolescents. As the study is cross-sectional, reverse causality cannot be ruled out; therefore, altered kidney and/or liver function may impact bodily fluoride absorption and metabolic processes.
Keywords: Fluoride, Kidney, Liver, United States, Adolescents
1.1. Introduction
Approximately 74% of the United States (U.S.) population that relies on public water distribution systems receives chemically fluoridated water for the purpose of preventing tooth decay 1 The most commonly used fluoridating chemical is hydrofluorosilicic acid, although sodium fluorosilicate and sodium fluoride are used in some water treatment processes 2 Until 2015, the recommended U.S. drinking water fluoride concentration range was 0.7 – 1.2 mg/L. However, this concentration was lowered in 2015 to 0.7 mg/L in part due to concerns about the rising prevalence of dental fluorosis – visually detectable changes in tooth enamel due to excess fluoride exposure, among U.S. youth 3,4.
Among healthy adults, approximately 60% of absorbed fluoride is excreted in urine by the kidneys, while the corresponding percentage among children is approximately 45% 5,6. The kidneys, followed by the liver, accumulate more fluoride than any other organ system in the body 7,8. Therefore, these organs and their intersectional processes may be especially vulnerable to effects of fluoride, even among healthy individuals. Additionally, fluoride is absorbed in calcified tissues – such as bones and teeth, as well as calcium-containing glands such as the pineal gland.
While fluoride exposure in adulthood has been associated with nephrotoxicity 9,10 few studies have examined associations between fluoride exposure and kidney or liver function in youth. Three prior studies conducted in India, Japan and/or China found potential evidence of both kidney and liver function decline in children and/or adolescents exposed to relatively high fluoride concentrations 11-14 Findings of a fourth study conducted in Mexico were inconsistent15,16. The few studies conducted among young animals also demonstrated adverse renal and hepatic effects of fluoride, even at low concentrations17-19. Taken together these findings suggest that fluoride may be developmentally nephrotoxic and hepatotoxic. However, whether these findings apply to low-level fluoride exposures relevant to U.S. youth has not been investigated.
Our study aimed to examine the relationship between fluoride exposure, measured in blood plasma and drinking water, and kidney and liver parameters among adolescents in the U.S.. We hypothesized that higher blood plasma and water fluoride concentrations would be associated with altered kidney and liver parameters in this population.
1.2. Materials and Methods
1.2.1. Participants
We utilized data from the National Health and Nutrition Examination Survey (NHANES) collected from 2013-2016, the years that publicly available fluoride biomonitoring data were collected and available at the time of analysis. NHANES is a program of studies conducted by the Centers for Disease Control and Prevention that is designed to assess health and nutrition status of a nationally representative, noninstitutionalized sample of people of all ages living in the U.S.. It employs questionnaires, in-home interviews and physical examinations at mobile examination centers where blood and urine are collected. 20 This study was exempted from review by the Icahn School of Medicine at Mount Sinai’s (ISMMS) Institutional Review Board (#1702145).
Plasma fluoride concentrations were measured among 4,470 participants aged 6-19 years and tap water fluoride concentrations were measured among 8,087 participants aged 0-19 years. Our analysis included adolescents aged 12-19 years because the renal and hepatic parameters examined herein were not measured in children under 12, except for the urinary albumin to creatinine ratio. Our sample included participants who had either plasma or water fluoride measurements and complete data for all covariates and outcomes. Missing data were less than 15 percent for all outcome measures, and less than 10 percent for covariates among participants who had all outcome measures. We excluded 2 participants with suggestive kidney disease, as indicated by estimated glomerular filtration rate ≤ 60 mL/min/1.73m2. Additionally, since protein intake can influence kidney and liver function test results, we excluded 1 participant with a reported daily protein intake of 0 grams, and 3 participants with reported daily protein intakes greater than 400 grams as these were considered likely to be erroneous values. There were 1,985 adolescents who met inclusion criteria for analyses. Of those, 1,983 participants had plasma fluoride levels and were included in analyses. For analyses of water fluoride, 1,942 participants had water fluoride levels and we excluded an additional 200 participants who reported that they did not drink tap water, resulting in a sample size of 1,742. Participant selection is depicted in Figure S1. Supplemental Table S1 compares demographic characteristics of the current overall study sample (n=1985) and all adolescents ages 12-19 over the same years (NHANES 2013-2016). We applied sampling weights to account for the complex NHANES survey design as recommended by the National Center for Health Statistics (NCHS) 21. The weighted samples for plasma and water fluoride analyses represented 25,930,302 and 23,287,332 adolescents in the U.S. respectively.
1.2.2. Fluoride measures
Fluoride concentrations were measured in blood plasma and household tap water samples. Tap water and blood collection times were not standardized. Plasma fluoride concentrations reflect fluoride intake as well as individual differences in fluoride metabolism 5. Plasma fluoride was measured via an ion-specific electrode and hexamethyldisiloxane (HMDS) method, and household water samples were measured via an ion-specific electrode. Both plasma and water fluoride concentrations were measured at the College of Dental Medicine, Georgia Regents University, Augusta, GA. They were measured in duplicate (using the same sample) and the average of these values was released. The lower limit of detection (LLOD) for plasma fluoride was 0.25 nmol, while the LLOD for water fluoride was 0.10 mg/L 22,23. Approximately 89% and 100% (all) of participants, had values above the LLOD for water fluoride and plasma fluoride respectively 24,25.
1.2.3. Kidney and liver parameters
Serum was analyzed for markers of kidney and liver function at the Collaborative Laboratory Services, Ottumwa, Iowa as part of a standard biochemistry profile. From 2013-2016 a Beckman Coulter UniCel DxC 800 Synchron chemistry analyzer was utilized; while from 2015-2016 a Beckman Coulter UniCel DxC 660i Synchron Access chemistry analyzer was utilized as well. Urine samples were analyzed for albumin and creatinine at the University of Minnesota via a Turner Digital Fluorometer, Model 450 and Roche Cobas 6000 Analyzer respectively. Urine sample collection time was not standardized. All analytical results were at or above the LLOD.
Estimated Glomerular Filtration Rate (eGFR).
Glomerular filtration rate is considered the gold standard index of kidney function26. We calculated eGFR with serum creatinine concentrations using the original Schwartz formula 27:
This formula is appropriate when serum creatinine concentrations are measured via a Jaffe rate method, as the larger coefficients account for the potentially higher serum creatinine levels associated with this method. In the revised formula the coefficient k = 0.413; whereas in the original formula k = 0.7 for adolescent boys and k = 0.55 for adolescent girls. Among children, adolescents and young adults, eGFR values < 75 mL/min/1.73 m2 are considered abnormal, and those ≤ 60 mL/min/1.73 m2 are reflective of chronic kidney disease28.
Serum Uric Acid (SUA).
Uric acid is a waste product of purine metabolism that is excreted in urine. Dysregulation of SUA levels are common in kidney and metabolic disorders. SUA was measured using a timed endpoint method. The LLOD was 0.5 mg/dL. The standard reference range for uric acid for children and adolescents aged 10-18 years is 3.5-7.3 mg/dL. For males and females over 18 years the reference ranges are 3.6-8.4 mg/dL and 2.9-7.5 mg/dL respectively 29
Albumin Creatinine Ratio (ACR).
Increased levels of urinary albumin are present with various renal diseases, including chronic kidney disease and end stage renal disease, as well as subclinical glomerular dysfunction. Urinary creatinine correlates with urinary volume and excretion rate. The albumin to creatinine ratio is used to detect kidney disease or dysfunction30. Urinary albumin was measured via a solid-phase fluorescent immunoassay 31and urinary creatinine was measured via an enzymatic endpoint method32. The LLOD for urinary albumin was 0.3 μg/mL, while the reportable lower limit for urinary creatinine was 5 mg/dL32,33. Among children and young adults, an ACR of < 10 mg/g is considered normal, an ACR of 20-30 mg/g is considered mildly increased, an ACR of 30 to 300 mg/g is moderately increased (termed “microalbuminuria”), and an ACR of > 300 mg/g is severely increased (termed “macroalbuminuria”)34
Blood Urea Nitrogen (BUN).
Urea is a waste product of nitrogen-containing compounds, such as amino acids, metabolized by the liver and excreted in urine. High BUN levels may reflect kidney dysfunction (e.g. reduced ability to excrete urea) whereas low BUN levels may reflect liver dysfunction (e.g., impaired protein metabolism) or malnutrition. BUN was measured using an enzymatic conductivity rate method35-36. The analytical measurement range measured via the Beckman UniCel DxC 800 Synchron was 1-150 mg/dL (or up to 300 mg/dL with ORDAC enabled). When measured with the Beckman Coulter UniCel DxC 660i Synchron it was 5-100 mg/dL (or up to 300 mg/dL with ORDAC enabled). The standard reference range for BUN for people aged 5-15 years is 7-18 mg/dL. For those over 15 years it is 6 – 23 mg/dL35,36.
Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT).
Serum aminotransferases are enzymes present in liver and cardiac tissue. Elevations can reflect hepatocyte and myocardial cell damage or disease states. AST and ALT were measured via enzymatic rate and kinetic rate methods respectively. The LLOD for both was 5.0 IU/L. The standard reference range for serum or plasma AST for people ages 10-20 years is 13-38 IU/L37. The standard reference ranges for serum or plasma ALT are 8-29 IU/L and 8-36 IU/L for 10-20 year-old females and males respectively 38.
Alkaline Phosphatase (ALP).
ALP is an enzyme present in bone and liver cells and can be used to diagnose liver, bone and parathyroid disease. ALP was measured via a kinetic rate method. The LLOD was 5.0 IU/L. The standard reference range for serum or plasma ALP for individuals ages 12-16 is 67-382 IU/L, while for those > 16 years of age it is 36-113 IU/L39.
Serum Albumin.
Albumin is synthesized in the liver and is a major component of plasma where it plays a key role in maintaining oncotic pressure. Serum concentrations can be used to assess kidney and/or liver disease or dysfunction. Serum albumin concentrations were measured via a timed endpoint method. The analytic range was 1.0-7.0 g/dL. The standard reference range for serum or plasma albumin for healthy children and adolescents aged 1-18 years is 3.1-4.8 g/dL. For individuals over 18 years it is 3.5-5.0 mg/dL when measured with the Beckman Coulter UniCel DxC 660i Synchron Access chemistry analyzer and 3.7 – 4.7 mg/dL when measured with the Beckman Coulter UniCel DxC 800 Synchron analyzer. 40,41.
Gamma-glutamyl Transaminase (GGT).
GGT is an enzyme present in hepatocytes and a sensitive indicator of liver disease and more specific to liver function than AST/ALT. Serum GGT was measured via an enzymatic rate method. The analytical range was 5-750 IU/L or up to 3000 IU/L with ORDAC enabled. The reference ranges for males and females aged 10-15 years are 7–26 IU/L and 8–23 IU/L respectively. The reference ranges for males and females >15 years are 10-65 IU/L and 8-36 IU/L respectively42.
1.2.4. Covariates
Covariates were selected a priori based on prior empirical evidence associated with fluoride exposure and kidney/liver function. They included: age, sex, body mass index, race, the ratio of family income to poverty, and daily protein intake 6,43-46. Additionally, we adjusted for serum cotinine level as a biomarker of tobacco smoke exposure in sensitivity analyses including only the 2013-2014 NHANES cycle, since cotinine was only assessed in the 2013-2014 cycle (see analysis section 1.2.5). The ratio of family income to poverty was calculated by dividing annual family income by the poverty guidelines specific to the survey year. Daily protein intake was obtained from a 24-hour food recall. Although, two 24-hour food recalls were conducted (one in person and one via telephone), we only used protein intake estimates from the in-person interview because most of the study sample did not complete the telephone interview. Only participants whose recall estimates were determined by the NCHS to be reliable were included in this study.
1.2.5. Statistical Analyses
All analyses applied survey weights from the mobile exam center visit (i.e. MEC weights) to account for the clustered sample design, survey non-response, over-sampling, poststratification, and sampling error, and to permit generalization to the United States population47. Given that we utilized dietary variables as covariates and/or exclusion criteria (i.e. protein intake; tap water consumption) we applied reweighted MEC weights to our dietary sample prior to analyses according to NCHS guidance. The MEC weights were recalculated based on our dietary subsample using an adjustment factor (See Appendix A). Descriptive statistics and regression analyses were performed using SAS (V.9.4) software. We used Pearson correlation to examine the relationship between plasma and water fluoride concentrations (both log2-transformed).
Survey-weighted linear regression was used to model kidney and liver parameters as a function of plasma or water fluoride concentrations while adjusting for covariates. For regression analyses we included laboratory generated values for water fluoride values below the LLOD; however, we imputed water fluoride values below the LLOD as LLOD/ √2 in our calculation of descriptive statistics. We note that imputation (or lack thereof) did not appreciably change the results of regression analyses. We explored potentially influential values using a Cook’s Distance estimate; none were identified. Assumptions pertaining to normality, homogeneity of variance and linearity were satisfied for models testing the relationship between plasma fluoride and eGFR, SUA, BUN, serum albumin or GGT, as well as for models testing the relationship between water fluoride and BUN, serum albumin or SUA. For remaining models, linear regression assumptions were not satisfied. Therefore, a log2 transformation was applied to skewed fluoride variables, and skewed outcome variables, including: ACR, ALT, AST, ALP, and GGT, to satisfy assumptions. The relationship between plasma fluoride and ALP remained nonlinear after the transformation, and thus, we tested a quadratic relationship in the regression model. We also included a fluoride*sex interaction term in our models to test for sex-specific effects; however, it was not significant in any of the models, and therefore was removed. We also conducted sensitivity analyses to examine whether adjusting for cotinine exposure or removing participants with serum cotinine levels ≥10 ng/mL48 influenced the relationship between plasma fluoride concentrations and kidney/liver parameters for participants in NHANES 2013-2014 (the only years in which both plasma fluoride and cotinine were measured). A two-tailed alpha of 0.05 was the criteria for statistical significance for regression analyses. We applied a Holm-Bonferroni correction to account for multiple comparisons for each fluoride variable.
1.3. Results
Demographic characteristics are presented in Table 1. Table S1 compares demographics between current study participants and all adolescents in NHANES 2013-2016. The average age of participants was 15.4 years.
Table 1.
Demographic characteristics according to sample participating in NHANES 2013-2016
| Demographic Characteristic | Overall Sample | Plasma Fluoride Sample | Water Fluoride Sub- sample a |
|---|---|---|---|
| n = 1985 N= 25,942,026 |
n=1983 N= 25,930,302 |
n= 1742 N= 23,287,332 |
|
| Age (yrs.); Mean (SE) | 15.38 (0.07) | 15.37 (0.07) | 15.32 (0.07) |
| Sex; N (%) | |||
| Male | 13,672,321(52.7) | 13,665,854 (52.7) | 12,494,779 (53.7) |
| Female | 12,269,705 (47.3) | 12,264,448 (47.3) | 10,792,553 (46.3) |
| BMI; M (SE) | 24.34 (0.24) | 24.34 (0.24) | 24.21 (0.25) |
| BMI Categories b; N (%): | |||
| Underweight | 841,241 (3.3) | 834,774 (3.2) | 724,010 (3.1) |
| Normal Weight | 14,660,261 (56.8) | 14,660,261 (56.8) | 13,422,456 (57.9) |
| Overweight | 4,698,550 (18.2) | 4,698,550 (18.2) | 4,144,842 (17.9) |
| Obese | 5,608,569 (21.7) | 5,603,312 (21.7) | 4,901,026 (21.1) |
| Race/ethnicity | |||
| Mexican American; N (%) | 3,806,271 (14.7) | 3,801,014 (14.7) | 3,160,150 (13.6) |
| Other Hispanic | 1,953,725 (7.5) | 1,953,725 (7.5) | 1,635,251 (7.0) |
| Non-Hispanic White | 14,544,657 (56.1) | 14,544,657 (56.1) | 13,382,896 (57.5) |
| Non-Hispanic Black | 3,220,902 (12.4) | 3,220,902 (12.4) | 2,871,360 (12.3) |
| Non-Hispanic Asian | 1,069,372 (4.1) | 1,069,372 (4.1) | 967,296 (4.2) |
| Other Race-Including Multi-Racial | 1,347,100 (5.2) | 1,340,634 (5.2) | 1,270,379 (5.5) |
| Daily Protein Intake (gm) | 75.52 (1.21) | 75.53 (1.21) | 75.86 (1.30) |
| Ratio of Family Income to Poverty | 2.47 (0.10) | 2.47 (0.10) | 2.51 (0.10) |
Note. Sampling weights were applied for calculation of demographic descriptive statistics and therefore Ns for frequencies represent the weighted sample size. Re-weighting for the dietary sample was not applied for calculation of descriptive statistics above.
Participants who reported that they did not drink the tap water were excluded;
n = 1972 for entire sample, n = 1970 for plasma F sample and n = 1732 for water F subsample due to missing data for this variable
Descriptive statistics for fluoride and kidney and liver parameters are presented in Table 2. The mean household water fluoride concentration among participants who drank tap water fell below the recommended level (mean = 0.48 mg/L); however, values between the 75th and 95th percentiles were above this level ranging from 0.71 to 1.00 mg/L. Participants generally had normal kidney and liver function (i.e. eGFR ranged between 84-212 mL/min/1.73 m2). However, SUA and BUN measurements at the 5th percentile were below their respective reference ranges. Additionally, ACR values at the 95th percentile (98 participants) fell in the microalbuminuria range. Fluoride concentrations in plasma and tap water were moderately positively correlated (r = 0.42, p <0.001).
Table 2.
Descriptive statistics of fluoride exposure and kidney and liver measures
| Measure | Arithmetic Mean (Standard Error) |
Median | 5th percentile | 95th percentile |
|---|---|---|---|---|
| Plasma fluoride (μmol/L) a | 0.40 (0.01) | 0.33 | 0.16 | 0.81 |
| Tap water fluoride (mg/L) b | 0.48 (0.03) | 0.48 | 0.07 | 1.00 |
| eGFR (mL/min/1.73 m2) | 147.98 (1.21) | 143.55 | 106.25 | 203.66 |
| SUA (mg/dL) | 5.07 (0.04) | 4.92 | 3.07 | 7.21 |
| Albumin/creatinine ratio(mg/g) | 24.63 (1.93) | 7.49 | 3.03 | 67.08 |
| BUN (mg/dL) | 11.25 (0.17) | 10.41 | 5.80 | 16.59 |
| ALT (IU/L) | 19.57 (0.38) | 15.72 | 10.15 | 38.57 |
| ALP (IU/L) | 134.26 (2.91) | 96.70 | 48.41 | 323.58 |
| AST (IU/L) | 23.81 (0.37) | 21.62 | 15.25 | 35.01 |
| Serum albumin (g/dL) | 4.51 (0.01) | 4.46 | 3.96 | 4.96 |
| GGT (IU/L) | 14.35 (0.28) | 11.88 | 7.15 | 27.48 |
Note. Sampling weights were applied for calculation of all descriptive statistics. N=25,942,026 (unweighted n = 1985);
N = 25,930,302 (unweighted n = 1983);
N = 23,287,332 (unweighted n = 1742); Samples were reweighted to the dietary sample prior to calculating these descriptive statistics as these were the values utilized in regression analyses. Only standard errors changed following reweighting.
1.3.1. Plasma Fluoride Regression Results
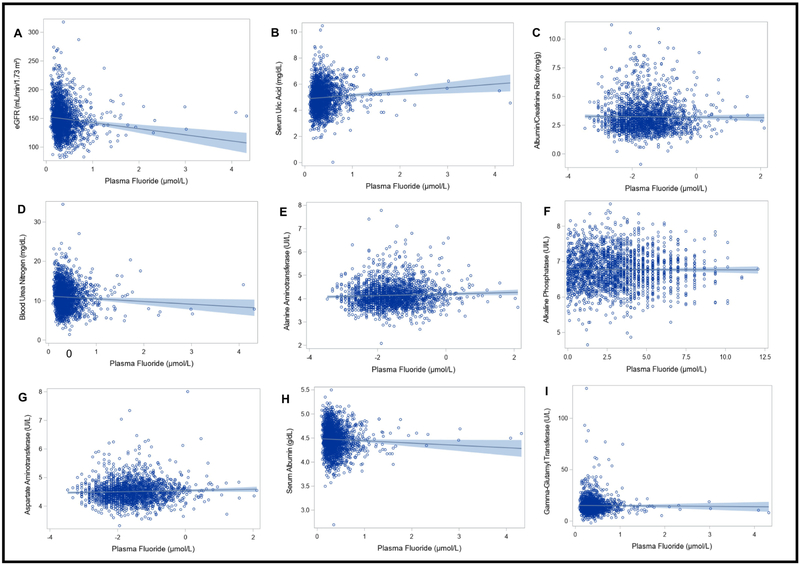
In linear regression models adjusted for covariates, higher plasma fluoride concentrations were associated with lower eGFR, higher SUA, and lower BUN (B: −10.36, 95%CI: −17.50, − 3.22, p = 0.05; B: 0.29, 95%CI: 0.09, 0.50, p = 0.05; and B: −1.29, 95%CI: −1.87, −0.70, p <0.001 respectively). Therefore, a 1 μmol/L increase in plasma fluoride was associated with a 10.36 mL/min/1.73 m2 lower eGFR, a 0.29 mg/dL higher SUA concentration, and a 1.29 mg/dL lower BUN concentration. Plasma fluoride concentrations were not associated with the remaining kidney or liver parameters examined herein (Table 3) (Figure 1).
Table 3.
Associations between plasma fluoride and kidney and liver measures
| Outcomes | Unstandardized Beta (95% Cl) |
Uncorrected p | Holm-Bonferroni Corrected p |
|---|---|---|---|
| eGFR | −10.36 (−17.50, −3.22) | 0.01 | 0.05* |
| SUA | 0.29 (0.09, 0.50) | 0.01 | 0.05* |
| ACRa | 0.08 (−0.04, 0.19) | 0.20 | >0.99 |
| BUN | −1.29 (−1.87, −0.70) | <0.001 | <0.001* |
| ALT a | 0.03 (−0.02, 0.08) | 0.27 | >0.99 |
| ALPa, b | 0.00 (−0.01, 0.01) | 0.95 | >0.99 |
| AST a | 0.00 (−0.04, 0.04) | >0.99 | >0.99 |
| Serum albumin | −0.03 (−0.09, 0.03) | 0.29 | >0.99 |
| GGT | −0.71 (−1.92, 0.50) | 0.24 | >0.99 |
Note. Regression analyses were adjusted for age, sex, race, body mass index, ratio of family income to poverty and daily protein intake. Sampling weights were applied to these regression analyses; N=25,930,302; unweighted n = 1983; MEC weights were re-weighted to our dietary sample for regression analyses;
Plasma fluoride exposure and outcome variables were log2 transformed;
Model included a quadratic term;
Significant at p ≤ 0.05 after Holm-Bonferroni correction; Regression results remained consistent regardless of whether MEC weights or re-weighted MEC weights were applied.
Figure 1. Associations between plasma fluoride and kidney and liver measures.
Each figure depicts a regression line with 95% confidence intervals; circles represent individual data points. Sample weighted regressions were adjusted for age, sex, race, body mass index, ratio of family income to poverty and daily protein intake (N=25,930,302; unweighted n = 1983). Plasma fluoride and outcome variables were log2 transformed for analyses with albumin/ creatinine ratio, alanine aminotransferase, alkaline phosphatase (ALP) and aspartate amino transferase. The model with ALP included a quadratic term. Cook’s distance estimates were used to test for influential data points; none were identified.
1.3.2. Water Fluoride Regression Results
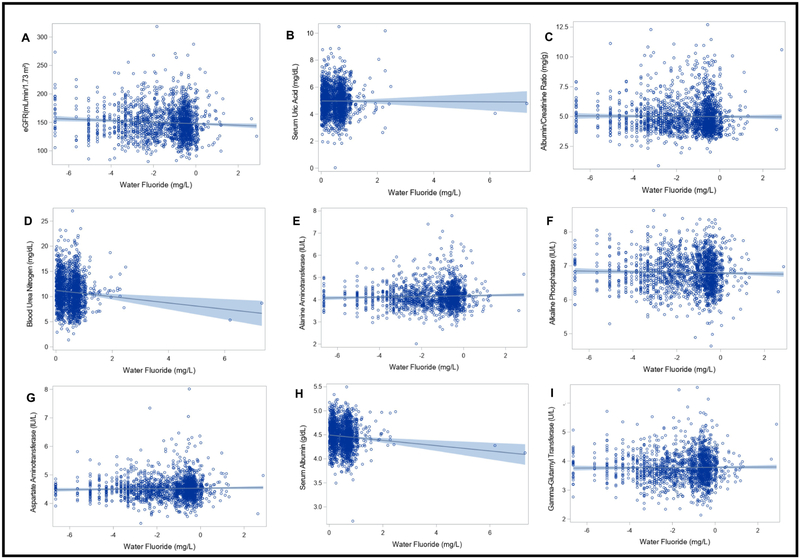
In linear regression models adjusted for covariates, higher water fluoride concentrations were associated with lower BUN (B = −0.93, 95%CI: −1.44, −0.42, p= 0.007). Therefore, a 1 mg/L increase in household tap water fluoride concentration was associated with a 0.93 mg/dL lower BUN concentration. Water fluoride concentrations were not significantly associated with the remaining kidney or liver parameters examined herein (Table 4) (Figure 2).
Table 4.
Associations between water fluoride and kidney and liver measuresa
| Outcomes | Unstandardized Beta (95% CI) |
Uncorrected p | Holm- Bonferroni Corrected p |
|---|---|---|---|
| eGFR b | −1.03 (−2.93, 0.87) | 0.28 | >0.99 |
| SUA | 0.05 (−0.07, 0.18) | 0.47 | > 0.99 |
| ACR c | −0.01 (−0.07, 0.06) | 0.79 | > 0.99 |
| BUN | −0.93 (−1.44, −0.42) | <0.001 | 0.007* |
| ALT c | 0.01 (−0.02, 0.03) | 0.62 | > 0.99 |
| ALP c | −0.02 (−0.04, 0.00) | 0.02 | 0.16 |
| AST c | −0.00 (−0.02, 0.01) | 0.68 | > 0.99 |
| Serum albumin | −0.06 (−0.12, 0.00) | 0.07 | 0.47 |
| GGT c | −0.01 (−0.04, 0.02) | 0.60 | > 0.99 |
Note. Regression analyses were adjusted for age, sex, race, body mass index, ratio of family income to poverty and daily protein intake. Sampling weights were applied to these regression analyses; N = 23,287,332; unweighted n = 1742; MEC weights were re-weighted to our dietary sample for regression analyses;
Participants who reported not drinking tap water were excluded from these analyses;
Water fluoride was log2 transformed in this model;
Water fluoride and outcome variables were log2 transformed
significant atp ≤ 0.05 after Holm-Bonfenoni correction; Regression results remained consistent regardless of whether MEC weights or re-weighted MEC weights were applied.
Figure 2. Associations between water fluoride and kidney and liver measures.
Each figure depicts a regression line with 95% confidence intervals; circles represent individual data points. Sample weighted regression analyses were adjusted for age, sex, race, body mass index, ratio of family income to poverty and daily protein intake (Participants who reported not drinking tap water were excluded; N = 23,287,332; unweighted n = 1742). Water fluoride and outcome variables were log2 transformed for analyses with albumin/creatinine ratio, alanine aminotransferase, alkaline phosphatase, aspartate amino transferase and gamma-glutamyl transferase. Water fluoride was log2 transformed for the analysis with eGFR. Cook’s distance estimates were used to test for influential data points; none were identified.
1.3.3. Sensitivity Analysis
Associations between plasma fluoride and kidney and liver measures separated by NHANES cycle are presented in Table S2. Cotinine-adjusted associations between plasma fluoride and kidney and liver measures for NHANES 2013-2014 are presented in Table S3 (Note: 2013-2014 was the only cycle in our study with available serum cotinine data). Compared to the 2013-2014 results without cotinine adjustment (Table S2), our findings did not change appreciably when cotinine was included as a covariate in the survey-weighted covariate-adjusted regression model (Table S3). When participants with serum cotinine levels ≥10 ng/mL were excluded from the regression analysis (n=949), the association between plasma fluoride and eGFR had a greater magnitude of effect, but did not reach statistical significance (B:−5.50, 95%, Cl: −13.77, 2.77, uncorrectedp = 0.18) (Table S3. In the association between plasma fluoride and BUN, the magnitude of association was attenuated and marginally statistically significant (uncorrectedp = 0.06). The association between plasma fluoride and SUA was relatively unchanged in magnitude or significance level (Table S3).
1.4. Discussion
To our knowledge, this study represents the first population-based study in the U.S. to examine the relationship between chronic low-level fluoride exposure and kidney and liver related parameters among adolescents. We included a breadth of kidney and liver measures to examine these relationships. Furthermore, we adjusted for factors that can influence fluoride exposure or absorption, kidney and liver function, or access to healthcare, such as socioeconomic status as well as multiple comparisons. We utilized plasma fluoride concentrations as they account for both fluoride intake and individual differences in fluoride absorption and metabolism 5. Conversely, household tap water fluoride concentrations are unaffected by individual differences in fluoride metabolism; yet, water fluoride constitutes the primary source of U.S. fluoride exposure 49
Higher plasma fluoride concentrations were associated with changes in kidney and liver related parameters. Most notably, a 1 μmol/L increase in plasma fluoride was associated with a 10.36 mL/min/1.73 m2 lower eGFR. This is consistent with previous studies in which higher urinary fluoride and dental fluorosis were associated with lower eGFR among youth in China and India 12,13,15. However, it is inconsistent with a recent cross-sectional study in Mexico that found an association between higher urinary fluoride and increased eGFR among 374 children 15. Differing results could reflect eGFR measurement, participant age, and/or fluoride biomarkers utilized (i.e. urine vs. blood fluoride assessment). Specifically, in the study conducted in Mexico eGFR was determined from a single serum measure with the creatinine-cystatin C-based CKiD equation50, children were 5-2 years old, and fluoride was assessed in urine adjusted for specific gravity. We also found that adolescents with higher plasma fluoride tended to have higher SUA and lower BUN which can reflect altered kidney and liver function respectively; although, lower BUN levels can also reflect nutritional deficiencies51. Consistently, among adolescents who consumed tap water, those with higher household tap water fluoride concentrations tended to have lower BUN, which may indicate impaired protein metabolism.
Given the cross-sectional nature of this study, there are several possible interpretations for the findings. First, fluoride exposure may contribute to complex changes in kidney and liver parameters among U.S. adolescents. This possibility is supported by the consistency of our findings with research demonstrating a dose-response relationship between water fluoride levels above 2 mg/L and enzyme markers of liver and kidney dysfunction14. Although in the current study, tap water fluoride concentrations were generally below 1 mg/L. There are several mechanisms by which fluoride exposure may contribute to kidney dysfunction. First, studies with adult rats have shown that chronic low-level fluoride exposure can lead to glomerular hypercellularity and mesangial cell proliferation52, reduced kidney enzyme activity53, substrate inhibition of kidney arginase54, interstitial nephritis, and renal tubule hypertrophy and hyperplasia55. Increased apoptosis and tubular epithelial damage, including necrosis, have also been observed among children with high fluoride exposures 56. Chronic low-level fluoride exposure is also associated with decreased thyroid gland activity among children57-59 and adults60,61. Moreover, reduced thyroid gland function, within the clinically normal range, is associated with decreased eGFR62,63. Thus, fluoride exposure could potentially compromise kidney function via glomerular damage, or indirectly via suppression of the thyroid gland. However, this study did not aim to determine whether fluoride exposure is associated with clinical decrements in kidney function among US adolescents. Rather, this study aimed to examine subclinical changes in kidney or liver parameters associated with fluoride exposure among a generally healthy population. For example, the lowest GFR estimated in this study was 84 mL/min/1.73 m2, and therefore none were below the < 75 mL/min/1.73 m2 value considered reflective of abnormal kidney function. Future prospective studies including participants with and without kidney disease are needed to assess clinical changes in kidney of liver function. Additionally, if fluoride exposure does contribute to changes in kidney or liver parameters, future prospective studies are needed to examine critical windows of vulnerability for these effects; in particular, it is unknown whether these changes may result from early life exposures during vital stages of kidney and liver development, from cumulative exposure, or both.
An alternative interpretation for our findings is that poorer kidney function may contribute to increased plasma fluoride levels rather than resulting from them. This possibility is supported by our finding that water fluoride concentrations were not associated with kidney parameters. Furthermore, animals and humans with impaired renal function tend to have higher levels of bone and plasma/serum fluoride because they do not excrete fluoride as readily64-66. However, plasma fluoride, rather than water fluoride, may have been associated with kidney function parameters in this study because it may better reflect individual fluoride exposure.
A third possibility is that the relationship between fluoride exposure and kidney function is bidirectional or cyclical in nature; whereby fluoride hinders kidney function which contributes to decreased fluoride excretion, increased bodily fluoride absorption and further decrements in kidney function. Indeed, soluble fluoride that is not excreted in urine is ultimately absorbed in hard and soft tissues, such as bones or organ systems (including the kidneys) respectively5. Moreover, fluoride urinary excretion rates tend to be lower among children 5,6 because more fluoride is absorbed in bone in the growing skeletal system67. Therefore, increases in plasma fluoride could render children more vulnerable to other health effects of fluoride exposure. Indeed, adults and children with kidney disease have been shown to be at an increased risk of bone disease and severe dental fluorosis respectively, due to increased skeletal fluoride absorption 68-70.
Fluoride’s effects on the liver are less well-characterized; however, animal studies have shown that low-level fluoride exposure can increase fatty deposits in the liver 71 and affect liver protein expression72. High fluoride exposures can cause vacuolization of hepatocytes, cellular necrosis, dilated and hypertrophic liver tissue 18, substrate inhibition of liver arginase54, and increased oxidative stress and oxidative damage73,74. In this study, fluoride exposure was not associated with liver enzyme levels; however, higher concentrations of both water and plasma fluoride were associated with lower BUN. Taken together, these findings suggest that fluoride exposure may contribute to subclinical decrements in liver function. We speculate that this could potentially occur via interference by fluoride with liver amino acid metabolism or protein synthesis75. Additionally, since lower BUN levels may indicate protein malnutrition51, we also speculate that our findings may reflect subclinical interference of gastrointestinal processes by fluoride, although protein intake in our sample was within ‘normal’ ranges for adolescents on average. While high fluoride exposures have been shown to damage gastric mucosa76, no studies have examined gastrointestinal effects of low fluoride exposures. Mechanistic studies are needed to understand underlying mechanisms of potential hepatotoxic and/or gastrointestinal effects of fluoride.
This study had several limitations. First, since this study is cross-sectional, the directionality of relationships cannot be determined, particularly for associations of plasma fluoride and kidney/liver parameters. Therefore, additional longitudinal studies are needed to better understand the developmental nephro- and hepatotoxicological impacts of fluoride, and to parse directionality of these associations. Regardless, this study contributes important information regarding how plasma fluoride levels change in association with subclinical changes in kidney and liver parameters (or vice versa) in the U.S. population which was previously unreported. Second, blood sample collection time was not standardized; however, exposure misclassification based on collection time is more likely to bias estimates toward the null. Therefore, we consider it unlikely that lack of standardization for blood collection led to ‘false positive’ findings. Third, we did not have data on smoke exposure for participants in NHANES cycle 2015-2016 and therefore could not adjust for this in our main analyses. Still, we conducted sensitivity analyses adjusting for serum cotinine, a biomarker of nicotine exposure, and this did not change the findings. Therefore, even though smoking status may influence plasma fluoride levels44 and kidney/liver function, it was likely not a confounder in this study. Still, we had a limited dataset with which to examine this possibility so we cannot rule it out completely. Fourth, we did not control for physical activity level or alcohol consumption in our analyses as data for these variables were not available for the majority of our sample. Lastly, we could not examine whether associations between fluoride exposure and kidney and liver parameters differed geographically as geographic locations of participants are not publicly available.
While the dental benefits of fluoride are widely established77, recent concerns have been raised3,4,78 regarding the appropriateness of its widespread addition to drinking water or salt in North America. The current study suggests that there may be potential nephro- and hepatological health concerns to consider when evaluating fluoride use and appropriate levels in public health interventions. However, we emphasize that future studies are required to overcome the limitations of a single cross-sectional study.
1.4.1. Conclusion
Fluoride exposure may contribute to complex changes in kidney and liver related parameters among adolescents in the United States. However, as the study is cross-sectional, reverse causality is possible and altered kidney and liver function may impact bodily fluoride absorption and metabolic processes. Further studies are needed to examine the mechanisms by which chronic low-level fluoride exposure may impact kidney and liver related parameters during development and adolescent life stages, as well as the ways in which kidney and liver function influence bodily fluoride absorption.
Supplementary Material
Highlights.
Higher plasma fluoride concentrations are associated with changes in kidney and liver parameters among adolescents living in the United States (U.S.)
Higher water fluoride concentrations are associated with changes in blood urea nitrogen among U.S. adolescents
Fluoride exposure may contribute to complex changes in kidney and liver related parameters among U.S. adolescents
Altered kidney and/or liver function may impact bodily fluoride absorption and metabolic processes
Acknowledgments
We would like to thank the Centers for Disease Control and Prevention (CDC) for conducting NHANES as well as the NCHS employees who provided us with consultation regarding the application of survey weights. We would also like to thank the participants of the 2013-2014 and 2015-2016 NHANES cycles, without whom this research would not have been possible.
Sources of Funding
This work was supported in part by funding from the Mount Sinai Children’s Center Foundation and NIH/NEHS: R00ES027508; R01ES014930, R01ES013744; R24ES028522; P30ES023515.
Abbreviations:
- eGFR
estimated glomerular filtration rate
- ACR
albumin creatinine ratio
- BUN
blood urea nitrogen
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- AST
aspartate amino transferase
- GGT
gamma-glutamyl transferase
- SUA
Serum Uric Acid
Appendix A
To better account for the reduced sample size of the dietary recall dataset used in analyses herein, Mobile Exam Center (MEC) weights were re-weighted using and adjustment factor, as detailed below, according to NCHS guidance:
1. Sum the MEC weights of the domain =Σ domain, where ‘domain ’ refers to the gender, race and or age group of participants who meet inclusion criteria prior to reducing to the dietary sample.
2. Sum the MEC weights for study participants (SPs) in dietary sample = Σ SP
3. Calculated adjustment factor = Σ Domain/Σ SPs
4. For SPs in the dietary sample, the new derived weights are equal to the MEC weight multiplied by the adjustment factor of the domain. For SPs not in the dietary sample, the new derived weight was set to missing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention. Community Water Fluoridation: Fluoridation Statistics. 2014; https://www.cdc.gov/fluoridation/statistics/2014stats.htm Accessed September 25, 2018.
- 2.Centers for Diseaese Control and Prevention. Community water fluoridation: Water Fluoridation Additives. 2018; https://www.cdc.gov/fluoridation/engineering/wfadditives.htm Accessed Sept 25, 2018.
- 3.U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015;130(4):318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevalence and Severity of Dental Fluorosis in the United States, 1999-2004. NCHS Data Brief No. 53 2010; https://www.cdc.gov/nchs/products/databriefs/db53.htm Accessed October 27, 2018. [PubMed]
- 5.Buzalaf MA, Whitford GM. Fluoride metabolism. Monogr Oral Sci. 2011;22:20–36. [DOI] [PubMed] [Google Scholar]
- 6.Villa A, Anabalon M, Zohouri V, Maguire A, Franco AM, Rugg-Gunn A. Relationships between fluoride intake, urinary fluoride excretion and fluoride retention in children and adults: an analysis of available data. Caries Res. 2010;44(1):60–68. [DOI] [PubMed] [Google Scholar]
- 7.National Research Council. Fluoride in Drinking Water: A Scientific Review of EPAs Standards Washington, DC:2006. [Google Scholar]
- 8.Whitford GM, Pashley DH, Reynolds KE. Fluoride tissue distribution: short-term kinetics. Am J Physiol. 1979;236(2):F141–148. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Cordova MI, Cardenas-Gonzalez M, Aguilar-Madrid G, et al. Evaluation of kidney injury biomarkers in an adult Mexican population environmentally exposed to fluoride and low arsenic levels. Toxicol Appl Pharmacol. 2018;352:97–106. [DOI] [PubMed] [Google Scholar]
- 10.Dharmaratne RW. Exploring the role of excess fluoride in chronic kidney disease: A review. Hum Exp Toxicol. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Liu JL, Xia T, Yu YY, et al. [The dose-effect relationship of water fluoride levels and renal damage in children]. Wei sheng yan jiu = Journal of hygiene research. 2005;34(3):287–288. [PubMed] [Google Scholar]
- 12.Ando M, Tadano M, Yamamoto S, et al. Health effects of fluoride pollution caused by coal burning. Sci Total Environ. 2001;271(1-3):107–116. [DOI] [PubMed] [Google Scholar]
- 13.Khandare AL, Gourineni SR, Validandi V. Dental fluorosis, nutritional status, kidney damage, and thyroid function along with bone metabolic indicators in school-going children living in fluoride-affected hilly areas of Doda district, Jammu and Kashmir, India. Environ Monit Assess. 2017;189(11):579. [DOI] [PubMed] [Google Scholar]
- 14.Xiong X, Liu J, He W, et al. Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res. 2007;103(1):112–116. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Cordova MI, Gonzalez-Horta C, Ayllon-Vergara JC, et al. Evaluation of vascular and kidney injury biomarkers in Mexican children exposed to inorganic fluoride. Environ Res. 2018;169:220–228. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez-Cordova MI, Gonzalez-Horta C, Ayllon-Vergara JC, et al. Evaluation of vascular and kidney injury biomarkers in Mexican children exposed to inorganic fluoride. Environmental research. 2019;169:220–228. [DOI] [PubMed] [Google Scholar]
- 17.Shashi A SJ, Thapar SP. Toxic effects of fluoride on rabbit kidney. Fluoride. 2002;35(1):38–50. [Google Scholar]
- 18.Shashi A TS. Histopathology of fluoride-induced hepatotoxicity in rabbits. Fluoride. 2001;34(1):34–42. [Google Scholar]
- 19.Cardenas-Gonzalez MC, Del Razo LM, Barrera-Chimal J, et al. Proximal renal tubular injury in rats sub-chronically exposed to low fluoride concentrations. Toxicol Appl Pharmacol. 2013;272(3):888–894. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2018; https://www.cdc.gov/nchs/nhanes/index.htm Accessed October 17, 2018.
- 21.Centers for Disease Control and Prevention. Specifying Weighting Parameters. 2013; https://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/intro_i.htm Accessed November 20, 2018.
- 22.National Health and Nutrition Examination Survey. 2015-2016 Data Documentation, Codebook, and Frequencies: Fluoride - Plasma (FLDEP_I) 2017.
- 23.National Health and Nutrition Examination Survey. 2013-2014 Data Documentation, Codebook, and Frequencies: Fluoride - Plasma (FLDEP_H). 2016.
- 24.National Health and Nutrition Examination Survey. 2013-2014 Data Documentation, Codebook, and Frequencies: Fluoride - Water (FLDEW_H) 2016.
- 25.National Health and Nutrition Examination Survey. 2015-2016 Data Documentation, Codebook, and Frequencies: Fluoride - Water (FLDEW_I) 2017.
- 26.Levey AS, Inker LA. GFR as the Gold Standard: Estimated, Measured, and True. Am J Kidney Dis. 2016;67(1):9–12. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. [DOI] [PubMed] [Google Scholar]
- 28.Pottel H, Hoste L, Delanaye P. Abnormal glomerular filtration rate in children, adolescents and young adults starts below 75 mL/min/1.73 m(2). Pediatr Nephrol. 2015;30(5):821–828. [DOI] [PubMed] [Google Scholar]
- 29.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Uric Acid Refridgerated Serum: Beckman UniCel® DxC 800 Synchron & Beckman UniCel® DxC 660i Synchron Access Clinical Systems (Identical Method) NHANES 2015-2016. Ottumwa, IA: 2017. [Google Scholar]
- 30.Fuhrman DY, Schneider MF, Dell KM, et al. Albuminuria, Proteinuria, and Renal Disease Progression in Children with CKD. Clin J Am Soc Nephrol. 2017;12(6):912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chavers BM, Simonson J, Michael AF. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984;25(3):576–578. [DOI] [PubMed] [Google Scholar]
- 32.University of Minnesota. Urine Creatinine: Enzymatic Roche Cobas 6000 Analyzer. 2014.
- 33.University of Minnesota. Urine Albumin: Fluorescein Immunoassay by Sequoia-Turner Digital Fluorometer, Model 450. 2014.
- 34.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1). [DOI] [PubMed] [Google Scholar]
- 35.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Blood Urea Nitrogen (BUN); Refrigerated Serum; Beckman UniCel DxC 660i Synchron Access NHANES 2015-2016. Ottumwa, IA: 2017. [Google Scholar]
- 36.Collaborative Laboratory Services LLC. Laboratory Procedure Manual; Blood Urea Nitrogen (BUN); Refridgerated Serum; Beckman UniCel DxC 800 Synchron,. NHANES 2015-2016. Ottumwa, IA: 2017. [Google Scholar]
- 37.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Aspartate Aminotransferase (AST), Refrigerated Serum, Beckman UniCel® DxC 800 Synchron & Beckman UniCel® DxC 660i Synchron Access Clinical Systems (Identical Method) NHANES 2015-2016 Ottumwa, IA: 2017. [Google Scholar]
- 38.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Alanine Amino Transferase (ALT), Refrigerated Serum, Beckman UniCel® DxC 800 Synchron & Beckman UniCel® DxC 660i Synchron Access Clinical Systems (Identical Method) NHANES 2015-2016 Ottumwa, IA: 2017. [Google Scholar]
- 39.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Alkaline Phosphatase (ALP), Refrigerated Serum, Beckman UniCel® DxC 800 Synchron & Beckman UniCel® DxC 660i Synchron Access Clinical Systems (Identical Method) NHANES 2015-2016 Ottumwa, IA: 2017. [Google Scholar]
- 40.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Albumin, Refrigerated Serum, Beckman UniCel® DxC 800 Synchron NHANES 2015-2016 Ottumwa, IA: 2017. [Google Scholar]
- 41.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Albumin Refrigerate Serum: Beckman UniCel® DxC 660i Synchron Access NHANES 2015-2016 Ottumwa, IA: 2017. [Google Scholar]
- 42.Collaborative Laboratory Services LLC. Laboratory Procedure Manual: Gamma-Glutamyl Transferase (GGT), Refrigerated Serum, Beckman UniCel® DxC 800 Synchron & Beckman UniCel® DxC 660i Synchron Access Clinical Systems (Identical Method) NHANES 2015-2016 Ottumwa, IA: 2017. [Google Scholar]
- 43.Martinez-Mier EA, Soto-Rojas AE. Differences in exposure and biological markers of fluoride among White and African American children. J Public Health Dent. 2010;70(3):234–240. [DOI] [PubMed] [Google Scholar]
- 44.Jain RB. Concentrations of fluoride in water and plasma for US children and adolescents: Data from NHANES 2013-2014. Environ Toxicol Pharmacol. 2017;50:20–31. [DOI] [PubMed] [Google Scholar]
- 45.Boyde CD, Cerklewski FL. Influence of type and level of dietary protein on fluoride bioavailability in the rat. J Nutr. 1987;117(12):2086–2090. [DOI] [PubMed] [Google Scholar]
- 46.Moxey-Mims M Kidney Disease in African American Children: Biological and Nonbiological Disparities. Am J Kidney Dis. 2018;72(5s1):S17–s21. [DOI] [PubMed] [Google Scholar]
- 47.National Center for Health Statistics. Overview of NHANES Survey Design and Weights. 2013; https://www.cdc.gov/Nchs/tutorials/environmental/orientation/sample_design/index.htm Accessed November 21, 2018.
- 48.Kim S Overview of Cotinine Cutoff Values for Smoking Status Classification. Int J Environ Res Public Health. 2016;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Health and Ecological Criteria Division. Office of Water. Fluoride: relative source contribution analysis. . United States Environmental Protection Agency;2010. [Google Scholar]
- 50.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar V, Chase P, Hammond K, O'Brien D. Alterations in blood biochemical tests in progressive protein malnutrition. Pediatrics. 1972;49(5):736–743. [PubMed] [Google Scholar]
- 52.Varner JA, Jensen KF, Horvath W, Isaacson RL. Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Res. 1998;784(1-2):284–298. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan WD. The in vitro and in vivo effects of fluoride on succinic dehydrogenase activity. Fluoride 1969;2:168–175. [Google Scholar]
- 54.Tormanen CD. Substrate inhibition of rat liver and kidney arginase with fluoride. J Inorg Biochem. 2003;93(3-4):243–246. [DOI] [PubMed] [Google Scholar]
- 55.McCay CM, Ramseyer WF, Smith CA. Effect of sodium fluoride administration on body changes in old rats. J Gerontol. 1957;12(1):14–19. [DOI] [PubMed] [Google Scholar]
- 56.Quadri J, Sarwar S, Sinha A, et al. Fluoride-associated ultrastructural changes and apoptosis in human renal tubule: a pilot study. 2018;37(11):1199–1206. [DOI] [PubMed] [Google Scholar]
- 57.Lin FF, Aihaiti, Zhao HX, Lin J, Jiang JY, Maimaiti, and Aiken. The relationship of a low-iodine and high-fluoride environment to subclinical cretinism in Xinjiang. IDD Newsletter. 1991;7(3):24–25. [Google Scholar]
- 58.Singh N, Verma KG, Verma P, Sidhu GK, Sachdeva S. A comparative study of fluoride ingestion levels, serum thyroid hormone & TSH level derangements, dental fluorosis status among school children from endemic and non-endemic fluorosis areas. Springerplus. 2014;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khandare AL, Validandi V, Gourineni SR, Gopalan V, Nagalla B. Dose-dependent effect of fluoride on clinical and subclinical indices of fluorosis in school going children and its mitigation by supply of safe drinking water for 5 years: an Indian study. Environ Monit Assess. 2018;190(3):110. [DOI] [PubMed] [Google Scholar]
- 60.Kheradpisheh Z, Mirzaei M, Mahvi AH, et al. Impact of Drinking Water Fluoride on Human Thyroid Hormones: A Case- Control Study. Sci Rep. 2018;8(1):2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malin AJ, Riddell J, McCague H, Till C. Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ Int. 2018;121(Pt 1):667–674. [DOI] [PubMed] [Google Scholar]
- 62.Anderson JLC, Gruppen EG, van Tienhoven-Wind L, et al. Glomerular filtration rate is associated with free triiodothyronine in euthyroid subjects: Comparison between various equations to estimate renal function and creatinine clearance. Eur J Intern Med. 2018;48:94–99. [DOI] [PubMed] [Google Scholar]
- 63.Asvold BO, Bjoro T, Vatten LJ. Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. Eur J Endocrinol. 2011;164(1):101–105. [DOI] [PubMed] [Google Scholar]
- 64.Turner CH, Owan I, Brizendine EJ, Zhang W, Wilson ME, Dunipace AJ. High fluoride intakes cause osteomalacia and diminished bone strength in rats with renal deficiency. Bone. 1996;19(6):595–601. [DOI] [PubMed] [Google Scholar]
- 65.Waterhouse C, Taves D, Munzer A. Serum inorganic fluoride: changes related to previous fluoride intake, renal function and bone resorption. Clin Sci (Lond). 1980;58(2):145–152. [DOI] [PubMed] [Google Scholar]
- 66.Rao TK, Friedman EA. Editorial: Fluoride and bone disease in uremia. Kidney Int. 1975;7(3):125–129. [DOI] [PubMed] [Google Scholar]
- 67.Committee on Fluoride in Drinking Water; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies. Fluoride in drinking water, a scientific review of EPA's standards: Chapter 3 - Pharmacokinetics of Fluoride 2006.
- 68.Ibarra-Santana C, Ruiz-Rodriguez Mdel S, Fonseca-Leal Mdel P, Gutierrez-Cantu FJ, Pozos-Guillen Ade J. Enamel hypoplasia in children with renal disease in a fluoridated area. J Clin Pediatr Dent. 2007;31(4):274–278. [DOI] [PubMed] [Google Scholar]
- 69.Lucas VS, Roberts GJ. Oro-dental health in children with chronic renal failure and after renal transplantation: a clinical review. Pediatr Nephrol. 2005;20(10):1388–1394. [DOI] [PubMed] [Google Scholar]
- 70.Johnson WJ TD, Jowsey J. Fluoridation and Bone Disease in Renal Patients In: Johansen E TD, Olsen TO,, ed. Continuing Evaluation of the Uses of Fluorides. AAAS Selected Symposium. Boulder, Colorado: Westview Press; 1979:275–293. [Google Scholar]
- 71.de Camargo AM, Merzel J. Histological and histochemical appearance of livers and kidneys of rats after long-term treatment with different concentrations of sodium fluoride in drinking water. Acta Anat (Basel). 1980;108(3):288–294. [DOI] [PubMed] [Google Scholar]
- 72.Pereira HA, Leite Ade L, Charone S, et al. Proteomic analysis of liver in rats chronically exposed to fluoride. PLoS One. 2013;8(9):e75343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atmaca N, Atmaca HT, Kanici A, Anteplioglu T. Protective effect of resveratrol on sodium fluoride-induced oxidative stress, hepatotoxicity and neurotoxicity in rats. Food Chem Toxicol. 2014;70:191–197. [DOI] [PubMed] [Google Scholar]
- 74.Xiao-ying Guo G-fS, Ying-chun Sun. Oxidative stress from fluoride-induced hepatotoxicity in rats. Fluoride. 2003;36(1):25–29. [Google Scholar]
- 75.Chattopadhyay A, Podder S, Agarwal S, Bhattacharya S. Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice. Arch Toxicol. 2011;85(4):327–335. [DOI] [PubMed] [Google Scholar]
- 76.Spak CJ, Sjostedt S, Eleborg L, Veress B, Perbeck L, Ekstrand J. Studies of human gastric mucosa after application of 0.42% fluoride gel. J Dent Res. 1990;69(2):426–429. [DOI] [PubMed] [Google Scholar]
- 77.O'Mullane DM, Baez RJ, Jones S, et al. Fluoride and Oral Health. Community Dent Health. 2016;33(2):69–99. [PubMed] [Google Scholar]
- 78.Aguilar-Diaz FDC, Morales-Corona F, Cintra-Viveiro AC, Fuente-Hernandez J. Prevalence of dental fluorosis in Mexico 2005-2015: a literature review. Salud Publica Mex. 2017;59(3):306–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.