Abstract
Context:
Palliative care interventions have shown promise in improving quality of life and reducing healthcare utilization among patients with chronic organ failure.
Objectives:
To evaluate the effect of a palliative care intervention for adults with end stage liver disease (ESLD).
Methods:
Randomized controlled trial of ESLD patients admitted to the hepatology service at a tertiary referral center whose attending hepatologist indicated they would not be surprised if the patient died in the following year on a standardized questionnaire. Control group patients received usual care. Intervention group patients received inpatient specialist palliative care consultations and outpatient phone follow-up by a palliative care nurse. The primary outcome was time until first readmission. Secondary outcomes included days alive outside the hospital, referral to hospice care, death, readmissions, patient quality of life, depression, anxiety, and quality of end of life care over 6 months.
Results:
The trial stopped early due to difficulties accruing patients. Of 293 eligible patients, only 63 patients enrolled, 31 in the intervention group and 32 in the control group. This pace of enrollment was only 25% of what the study had planned and so it was deemed infeasible to complete. Despite stopping early, intervention group patients had a lower hazard of readmission (HR 0.36, 95% CI 0.16-0.83, p=0.017) and greater odds of having more days alive outside of the hospital than control group patients (OR 3.97, 95% CI: 1.14-13.84, p=0.030). No other statistically significant differences were observed.
Conclusion:
Logistical obstacles hindered completion of the trial as originally designed. Nevertheless, a preemptive palliative care intervention resulted in increased time to first readmission and more days alive outside of the hospital in the first six months after study entry.
Keywords: cirrhosis, end stage liver disease, palliative care, randomized control trials, healthcare utilization, quality of life
Palliative care (PC) is specialized medical care focused on providing patients with relief from the symptoms, pain, and stress of serious or life-limiting illness, regardless of diagnosis, by anticipating, preventing, and treating suffering. In oncology settings, specialist palliative care interventions have demonstrated increased quality of life, decreased healthcare costs, and improved survival when initiated early in the course of cancer treatment.1-4 Trials in heart failure have shown similar promise for palliative care interventions.5-8
End-stage liver disease (ESLD), a progressive illness due to advanced liver disease, has been recognized as condition in which early palliative care may be beneficial.9,10 Patients with ESLD have high rates of severe symptoms, high levels of healthcare utilization, and a low rate of advance care planning.11,12 Moreover, for patients with decompensated cirrhosis, liver transplantation is the only cure. As of March 19, 2019, 13,355 candidates were registered on the waiting list for liver transplant in the US.13
Previous studies in ESLD patients have shown associations of palliative care interventions with improvements in symptom control, mood, and quality of life and reduction in healthcare utilization.9,10 These results suggest that the many patients who are not candidates for transplantation and those on the waitlist who may have considerable wait time would likely benefit from specialist palliative care. Yet, in one study of patients who were de-listed or were declined transplantation, only 11% of patients were referred for specialty palliative care.11
We designed and implemented a randomized, controlled trial of a specialist palliative care intervention for ESLD patients admitted as inpatients to our institution. Due to slower than expected enrollment, the trial was terminated before full enrollment. Here we report the results of the patients enrolled before the trial was halted.
Methods
After obtaining approval from our Institutional Review Board, we implemented the Creation of Models for Palliative Assessments to Support Severe Illness Trial (COMPASS Trial, ) to assess a palliative care intervention for patients requiring inpatient care for ESLD. The trial was designed with a pre-specified evaluation of feasibility at the times when 30 and 150 patients had enrolled and completed 6-month follow-up. If the trial showed feasibility at these times with study procedures conducted as intended and no signs of significant patient dropout for mortality or other reasons, the trial was planned for enrollment of 400 patients. Due to slow accrual, logistical problems with obtaining informed consent and conducting study procedures in a fast-moving acute hospitalization, and challenges with consistent follow-up, the study was stopped early. After the first interim analysis, enrollment was halted. However, study procedures were continued for all currently enrolled patients out to a full 6 months to maximize meaningful data contribution for an abridged analysis.
Setting and Patient Population
Patients admitted to the hepatology service at an urban, academic referral center met inclusion criteria if they had a diagnosis of ESLD and their attending hepatologist answered “no” to the question, “Would you be surprised if this patient died within 1 year?”.14 This question was administered to them via a secure online survey about the patients on their census sent every weekday during the study period. Exclusion criteria were the following: 1) age less than 18 years; 2) inability to give written, informed consent by either patient or surrogate; 3) inability to respond to questions in English; 4) refusal of permission to enroll by the treating hepatologist; 4) primary hepatology follow up outside our institution; and 5) post-transplant patient status.
Potentially eligible patients were approached by study staff, who obtained informed consent from the patient or, if the patient lacked capacity, from a surrogate decision-maker. If surrogate consent was secured, attempts were made over time to obtain informed consent from the patient when capacity was regained. Patients were also asked to identify a primary caregiver to participate in the study - someone familiar with the patient’s diagnoses and treatment, having daily or near daily contact with the patient in a variety of settings (home, hospital, etc.), and someone the patient felt comfortable answering survey questions about his/her care.
Enrolled patients were randomized 1:1 to receive either usual care (control) or a palliative care intervention (intervention). Randomization was operationalized using the REDCap randomization module populated with a block randomization sequence generated using the blockrand R package with random block sizes of 2, 4, 6, or 8; group assignment was masked prior to randomization.15
Intervention
Intervention patients had a request placed for an inpatient consultation by a board-certified palliative care physician or palliative care certified nurse practitioner from our institution’s palliative care consultation service. The content of this consultation was left to the judgment of the palliative care provider, as was the need for follow-up during the index hospitalization. Intervention patients also received a binder of materials that included general information about palliative care services, specific information about the COMPASS Trial, and templates to document important information such as names of medicines, contact information, and upcoming appointments. The packet also contained palliative care contact information printed on a magnet, a wallet card, and a brochure (included in the online appendix).
After discharge from the index hospitalization, nurses from our institution’s palliative care unit conducted follow-up telephone conversations with the patient and/or their caregiver. The nurses used a semi-structured telephone guide (included in the online appendix), informed in part by the Re-Engineered Discharge toolkit supported by the Agency for Healthcare Research and Quality.16 They assessed the patient’s physical and mental functioning; reviewed medications, discharge instructions, advance care plans and goals of care; responded to patient questions; and identified needs for additional care. If the palliative care nurse identified a need for further care (e.g., referral to hospice, appointment with the outpatient palliative care office, appointment for symptom management, etc.), the nurse engaged the palliative care team, the hepatology team, or both, as indicated.
The initial telephone contact was attempted within 96 hours after discharge, with continued attempts up to seven days after discharge. If unsuccessful after 7 days, the nurses would try again at 4 weeks after discharge to align with the planned follow up schedule. After the initial contact, conversations were scheduled to occur at least monthly, and at a higher frequency if the patient wished. If intervention patients were readmitted to our institution, inperson palliative care consultations by the inpatient palliative care consult team were requested, and the telephone conversation scheduling resumed after discharge. Monthly conversations were expected to continue for one year, until study termination, or death, whichever occurred first.
Control (Usual Care)
Patients in the control group received usual care for their ESLD under the direction of their attending hepatologist. Control patients could be referred for either inpatient or outpatient palliative care consultation if their providers felt such consultations were clinically indicated. Control patients receiving palliative care services remained in the control group for purposes of the intention-to-treat analysis.
Outcomes
The primary outcome was the time to first hospital readmission after index hospitalization discharge. The secondary clinical outcomes were days alive outside the hospital, referral to hospice, death, or readmission. The original study design called for follow-up to 12 months, but, since the study was terminated for feasibility, observation of these outcomes only extended to 6 months. Quality of life, mood, quality of care, and stress were assessed at study enrollment, and then again by email, phone, or mailed hard-copy at 1, 3, 6, 9 and 12 months after randomization. The assessments were completed by the participant when able, otherwise by the participant’s preferred surrogate. Quality of life was assessed using the Chronic Liver Disease Questionnaire (CLDQ), a 29-item, liver-disease-specific quality of life instrument,17,18 and the EQ-5D-5L, a generic measure of health-related utility consisting of 5 items.19,20 The PROMIS short-form anxiety and depression scales were also administered.21 Finally, a modified version of the Quality of End-of-Life Care: Patient Questionnaire was administered.22 Caregivers were also administered a modified version of the Quality of End-of-Life Care: Patient Questionnaire, with wording changed to make it applicable to caregivers.
Statistical Analysis
The primary analysis proceeded using an intent-to-treat (ITT) approach. Kaplan-Meier curves and the log-rank test were used to compare the time to first readmission between groups unadjusted for baseline covariates. The time to readmission was then compared between groups using the cause-specific Cox proportional hazards model to account for the competing risk of death, with adjustment for pre-specified covariates. Days alive outside of the hospital were compared between groups with a Wilcoxon rank sum test, and a proportional odds model was then fit to adjust for baseline covariates. For comparing days alive and out of hospital, analysis was restricted to patients who had not withdrawn from the study at 6 months after randomization. The pre-specified baseline covariates included age, sex, model of end-stage liver disease (MELD) score, etiology of ESLD, and whether any palliative care services were received at baseline; this latter reflects that some patients in the control arm might have received palliative care as part of their usual care. Multiple imputation was used to replace missing covariate information. Due to the small sample size and concerns about overfitting, the list of covariates was reduced to those with the strongest association with the outcome using the general rule that there should be no more than m/10 variables included in the regression, where m is the number of events for time to event outcomes and total number of subjects for numeric outcomes.23 Sensitivity analyses were conducted using models incorporating the complete list of covariates, and these did not substantially change the magnitude or direction of the estimated effects of the intervention. All analyses were carried out using R (version 3.5.2).
Results
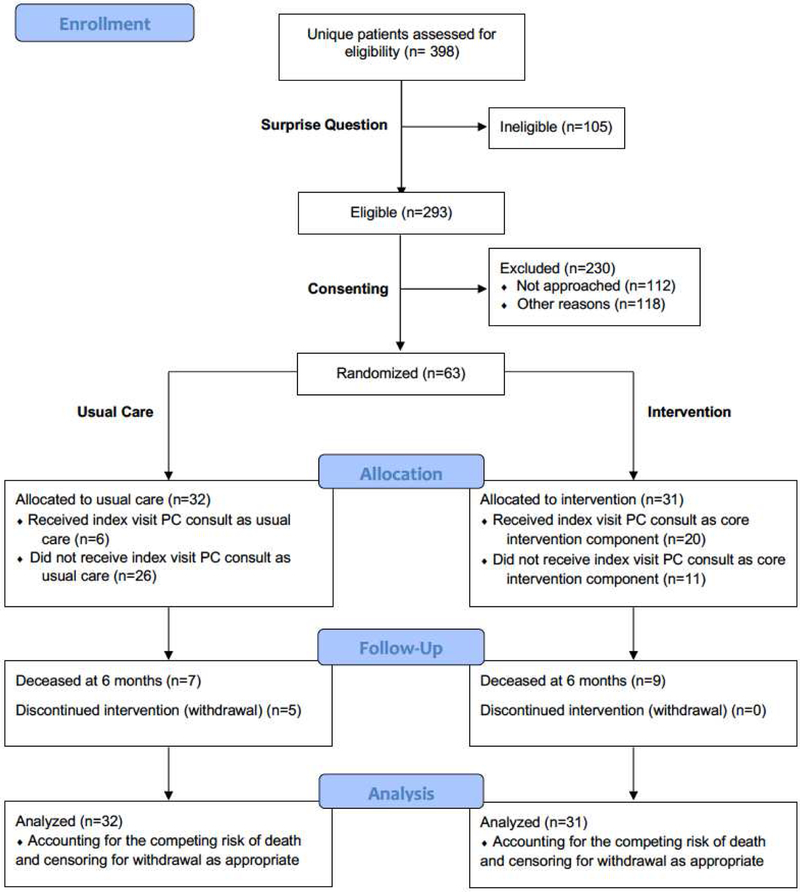
At the point of first interim analysis, early stopping rules were applied. Further enrollment was discontinued, but existent participants remained on study until all were followed out to at least 6 months. Of 470 surprise questions sent and 398 unique patients screened, 63 patients were enrolled. There were 32 patients in the control group and 31 in the intervention group (see Figure 1). Five patients withdrew from the study within 6 months of enrollment, all from the control arm—there were no significant differences in demographic or disease-related factors when the withdrawn patients were compared to the control patients who did not withdraw. Participants are characterized in Table 1. There were 32 males and 31 females; 90% were white. The most common etiology of ESLD was non-alcoholic steatohepatitis (35%), followed by alcoholic cirrhosis (25%). The median MELD score at enrollment was 24 (IQR 18-27).
Figure 1: CONSORT Diagram.
Legend: PC=palliative Care
Table 1:
Demographic Characteristics
| Characteristic | Control Group (n=32) | Intervention Group (n=31) |
|---|---|---|
| Median Age (IQR) | 57.95 (51.42, 63.63) | 58.28 (44.21, 64.54) |
| Sex | ||
| Male | 18 (56%) | 14 (45%) |
| Female | 14 (44%) | 17 (55%) |
| Race and Ethnicity | ||
| Non-Hispanic White | 31 (97%) | 26 (84%) |
| African-American | 1 (3%) | 5 (16%) |
| Highest Level of Education | ||
| Less than high school | 5 (16%) | 4 (13%) |
| High school | 7 (22%) | 12 (39%) |
| Some College | 12 (37%) | 6 (19%) |
| Completed College | 5 (16%) | 6 (19%) |
| Did not answer | 3 (9%) | 3 (10%) |
| Marital Status | ||
| Married | 15 (47%) | 15 (48%) |
| Never Married | 3 (9%) | 8 (26%) |
| Widowed | 2 (6%) | 1 (3%) |
| Divorced/Separated | 9 (28%) | 5 (16%) |
| Did not answer | 3 (9%) | 2 (6%) |
| Etiology of ESLD | ||
| Alcohol | 10 (31%) | 6 (19%) |
| NASH | 8 (25%) | 14 (45%) |
| Hepatitis B | 2 (6%) | 2 (6%) |
| Hepatitis C | 8 (25%) | 2 (6%) |
| Other | 4 (12%) | 7 (23%) |
| Median MELD Score at Enrollment (IQR) | 24 (18, 28) | 24 (20, 26) |
Legend: ESLD=end stage liver disease; NASH=non-alcoholic steatohepatitis; MELD=model of end-stage liver disease; IQR=interquartile range
Though there were some elements of COMPASS that worked well, the investigative team faced considerable challenges with regard to enrollment that led to the determination of nonfeasibility. At the stopping point, enrollment in the study had progressed only at approximately 25% of the planned rate. Among eligible patients, 112 of 293 were not physically able to be approached before discharge. When recruiters were able to meet with patients in attempt to consent, 55 of 181 declined. After conversation, some patients wanted more time to consider (39 of 181), or patients requested approach deferral (16 of 181). Others were excluded per protocol for confounding factors such as language barrier, incarceration, and outside follow-up intended.
A specialist palliative care visit during the index hospitalization occurred in 21 out of 31 (65%) of the intervention group patients; the other 35% were discharged from the hospital after enrollment but before a palliative care provider could see them. Among the 32 control patients, six (19%) received a specialist palliative care visit at the index hospitalization. For these control patients, need for specialty palliative care consultation was determined by the patient’s attending hepatologist. Part of the intervention also included patients receiving a palliative care consult upon being readmitted to the hospital. With 30 intervention patient readmissions, only 13 palliative care consults were ordered (43%). However, when consult orders were placed, delivery was achieved 92% of the time (12 of 13).
Additionally, as a further component of the intervention, nurses from our institution’s palliative care unit conducted follow-up telephone conversations with the patient and/or their caregiver generally within 96 hours of discharge and then monthly from there onward, unless increased frequency was requested (2 of 31 participants wanted calls every 2 weeks). At the 96-hour time point, 19 of 42 protocolized calls successfully connected with participants. For the duration of the study across the entire intervention arm, participants who were per-protocol prescribed had the opportunity to receive 155 calls with 68 of them being completed with 0-6 attempts made per time point. Thus, the global receipt of prescribed call contact was 44%. Four of 31 patients received 100% of the phone-call based intervention, and nine received 0%.
Patient- and caregiver-reported outcomes were also difficult to collect. We successfully completed the battery of tests with 48 participants at baseline, and 22, 14 and 10 participants at 1, 3 and 6 months, respectively. This equated to completions rates of: 75±4%, 46±8%, 34±3%, and 25±3%, accounting for withdrawals and deaths. These completion rates are extremely low, and future studies of palliative care interventions in this patient population will require strategies to improve completion of surveys to allow meaningful comparisons.
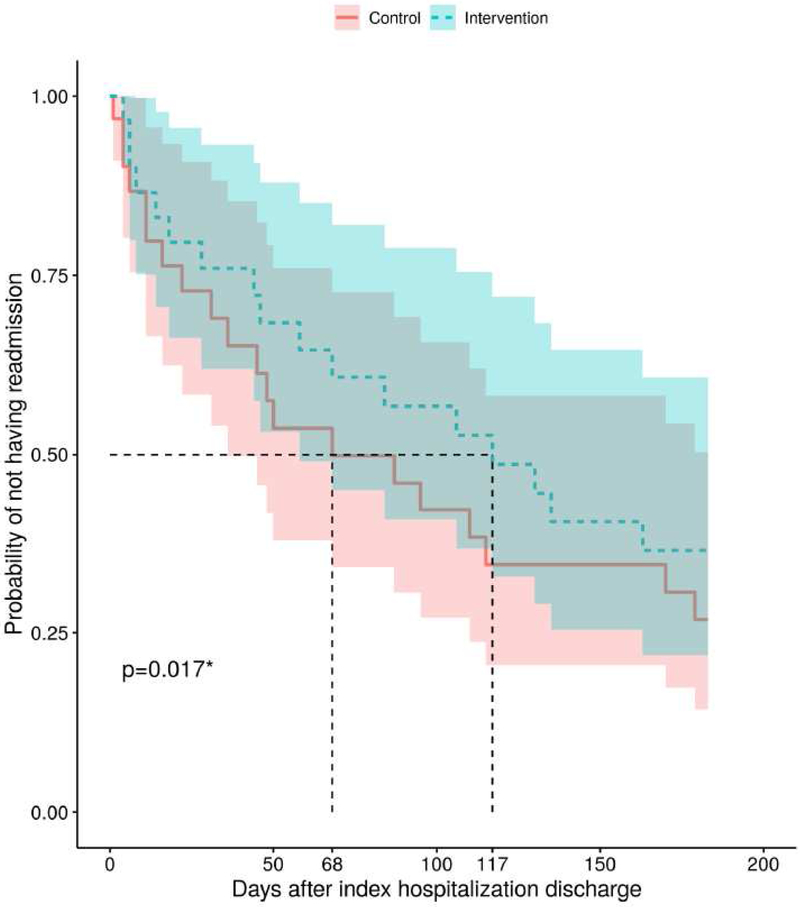
Unadjusted outcomes were not statistically different between study arms. The Kaplan-Meier curve for time to first readmission are shown in Figure 2. Median time to readmission was 68 days for the control group and 117 days for the intervention group, excluding patients who withdrew (interquartile ranges are unable to be calculated as neither group reached the 25% point on the Kaplan-Meir curve before follow-up concluded). The median number of days alive outside the hospital during the six months after randomization was 174 (IQR 59.5-183) for the intervention group and 178 (IQR 86.5-182.5) for the control group. Four patients in each group were referred to hospice, while 20 control participants (63%) and 17 intervention participants (55%) experienced at least one readmission within six months. Seven control participants (22%) and nine intervention participants (29%) had died by six months. Time to hospice referral and time to death did not significantly differ between the groups on unadjusted analysis, and there were not enough events to conduct meaningful adjusted analysis for these variables.
Figure 2: Kaplan-Meier Curve for Time to First Readmission.
*by the Cox proportional hazards model, controlling for etiology and palliative care consultation at index hospitalization
After adjusting for covariates, time to readmission was delayed in the intervention group compared to the control group: hazard ratio for readmission 0.36 (95% CI 0.16-0.83, p=0.017). Similarly, intervention patients had higher odds of having more days alive outside the hospital than control patients (OR 3.97, 95% CI: 1.14-13.84, p=0.030). (Full results of the regression models are given in the Supplement in eTables 1&2)
During the model selection process, we found that the presence or absence of a palliative care consultation during the index hospitalization was one of the more highly associated covariates with the outcomes. Presence or absence of a palliative care consultation was distinct from group assignment because some control group patients had palliative care consultations requested by the hepatology team and some intervention group patients did not receive their consultation as specified in the protocol. In the models holding group assignment constant, a palliative care consultation was associated with less favorable outcomes (i.e. higher hazard for readmission and lower odds for having more days alive outside the hospital) than not receiving a consultation (see eTables 1&2). When controlling for group assignment, palliative care consultation is likely a marker of severity of illness. Control patients who received palliative care consultations were likely sicker than control patients who did not. For intervention patients, palliative care consultations were delivered unless the patient was discharged prior to the palliative care team being able to see them, so intervention group patients without palliative care consultations were likely less sick than the intervention group patients who remained in the hospital long enough to receive the consultations. Palliative care consultation as a marker of disease severity would explain why assignment to the intervention group was associated with more favorable outcomes, but receiving a palliative care consultation was associated with less favorable outcomes in the adjusted models.
The extensive amount of missing data prevented meaningful comparisons of the caregiver- and patient-reported outcomes, which are shown in the Supplement in eFigure 1. We conducted an exploratory analysis on all patients with any survey data using linear mixed effects models and did not observe statistically significant differences between the treatment groups.
Discussion
This randomized, controlled trial of a palliative care intervention for patients with ESLD faced numerous difficulties as planned and therefore was stopped early. Difficulties with enrollment were the most prominent factors in the decision to stop early. Enrolling these patients proved to be a challenge, as indicated by the enrollment of only 34% of patients approached for study participation at a rate much slower than anticipated. We saw a higher immediate refusal rate than anticipated in planning the study.
Many patients indicated to staff that they were overwhelmed by the events occurring in the index hospitalization and were not ready to consider study participation by the time they were discharged. For a significant number of potential participants, concerns about insurance coverage for the palliative care consultation was reported as a barrier to participation. Given that the intervention was integrated within the context of routine delivery of care, the consult would have been a routinely billable event with no guarantee of insurance agreement to pay. Compounding this low rate of consent among patients was an even lower willingness of patients to identify a caregiver to participate in the study. However, most caregivers approached provided consent and were enthusiastic about participating, although the caregivers had low rates of response to the survey instruments.
Logistical challenges in enrolling patients also hindered the study. An array of complications contributed, including fundamentally not being able to connect with the potential participants before discharge. Once patients were identified as potentially eligible, there was often not enough time for study personnel to approach them before discharge. Identifying potentially eligible patients and having hepatologists answer the surprise question proved very efficient, but approaching and consenting patients was extremely time-consuming for patients whose hospital time was already occupied by management of their illness and diagnostic and therapeutic interventions. In fact, a missed opportunity was faced with almost 40% of those eligible with study personnel often having to approach each patient numerous times before finding an appropriate opportunity to discuss the study and obtain consent.
In addition to problems with enrollment, the study also faced significant challenges in the follow-up phase. For intervention patients, the index inpatient palliative care consultation was delivered fairly consistently. However, readmission-related consults were much more challenging to effect. These consults were placed by the study coordinator upon reviewing the medical records of the patients to monitor potential readmissions. As many of these readmissions occurred at night and on the weekends, orders were often not placed in time to allow the consult prior to patient discharge. Likewise, the outpatient phone calls were often unsuccessful. Nurses had to make multiple calls in attempt to reach the patients, who were commonly unavailable or would ask for the nurse to call back. Unfortunately, this resulted in considerable deviation from protocol. Additionally, as patients were readmitted, they then needed to restart their phone call component anchored to the most recent readmission discharge date, and the process for notifying the nurses of the new phone call schedule was admittedly somewhat convoluted. Similarly, complete participation in the follow-up survey assessments (whether by phone, hard copy, or electronically) was inconsistent for both groups, and there was extensive missingness in these caregiver- and patient-reported outcomes. For some of these patients, follow-up assessments appear to be burdensome, and future studies should carefully consider this burden as the intervention and assessments are designed.
Our results suggest potential opportunities for methods refinement in future trials of palliative care interventions in ESLD patients. Since enrollment and phone follow-up were so challenging, a pragmatic trial of inpatient palliative care consultation using existing electronic medical record data may be more feasible. If a traditional clinical trial with ESLD patients is conducted in the inpatient setting, staffing will need to be adequate to enroll these patients, which potentially would require an enroller to be present in the inpatient unit most of the day. Alternatively, the difficulty enrolling patients during the busy schedule of an inpatient stay suggests that enrolling at outpatient hepatologist visits might be a more fruitful strategy. Ideally, a partnership could be established to allow integrated consenting during care by the provider team.
The outcomes data we collected in the COMPASS Trial can be used by future trialists in making power calculations to choose the optimal sample size. On adjusted analysis, the intervention group of the COMPASS Trial showed a statistically significant advantage on the primary outcome, days until first readmission. On one secondary outcome, days alive outside of the hospital, intervention patients also had an advantage relative to controls on adjusted analysis. The lack of significant difference on unadjusted analysis is not surprising given that the trial was halted early and therefore had a small number of subjects with significant clinical heterogeneity.
In summary, we found that a randomized, controlled trial of palliative care for patients with ESLD was very difficult to complete as planned. Enrolling these patients in the inpatient setting may not be a feasible approach for future clinical trials. Moreover, trials in this patient population will need to factor in the extensive effort required of study staff to obtain consent and consistent follow-up. Despite the problems resulting in the trial’s early closure, early palliative care intervention increased time to readmission and hospital free days compared to usual care with no effect on mortality, though our study was underpowered for the latter endpoint. These encouraging outcomes in this small trial indicate a potential signal for the effectiveness of specialty palliative care’s impact on time to readmission and days alive outside of the hospital in ESLD patients that could be investigated in further studies. The experience and knowledge we gained from this work has value for the palliative care research community as palliative care interventions begin to be explored in many other life-limiting diseases.
Supplementary Material
Acknowledgements
The authors would like to thank the following members of the VICTR team for their support in coordinating and conducting the research: Somsundaram Chettiar, Lynn Seabolt, Alyssa Powlus, and Emily Bruer. The authors would like to acknowledge the VUMC nurses, Megan Palmer, Rebecca Hixson, and Carrie Todd who helped support research participants of the Vanderbilt Palliative Care Program. The authors would also like to express their gratitude to the study participants and caregivers without whom this study would not have been possible.
The project described was supported by the VICTR Learning Healthcare System Platform under CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosures: The project described was supported by the VICTR Learning Healthcare System Platform under CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Dr. Shinall was funded by the National Cancer Institute (K12CA090625). The funding agencies had no role in the design or analysis of the trial. No other potential conflicts of interest are reported.
Appendix
The appendix contains the IRB-approved COMPASS participant facing materials. We include the generalized study brochure as well as the intervention component binder and magnet language template. Additionally, we present the standardized script followed by our palliative care nursing partners.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. 2016;316(20):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 3.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2015;33(13):1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015; 18(2): 134–142. [DOI] [PubMed] [Google Scholar]
- 6.Wong FKY, Ng AYM, Lee PH, et al. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart (British Cardiac Society). 2016;102(14):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brannstrom M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail. 2014;16(10):1142–1151. [DOI] [PubMed] [Google Scholar]
- 8.Bekelman DB, Plomondon ME, Carey EP, et al. Primary Results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: A Randomized Clinical Trial. JAMA Intern Med. 2015;175(5):725–732. [DOI] [PubMed] [Google Scholar]
- 9.Baumann AJ, Wheeler DS, James M, Turner R, Siegel A, Navarro VJ. Benefit of Early Palliative Care Intervention in End-Stage Liver Disease Patients Awaiting Liver Transplantation. J Pain Symptom Manage. 2015;50(6):882–886 e882. [DOI] [PubMed] [Google Scholar]
- 10.Lamba S, Murphy P, McVicker S, Harris Smith J, Mosenthal AC. Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012;44(4): 508–519. [DOI] [PubMed] [Google Scholar]
- 11.Poonja Z, Brisebois A, van Zanten SV, Tandon P, Meeberg G, Karvellas CJ. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol. 2014;12(4):692–698. [DOI] [PubMed] [Google Scholar]
- 12.Rossaro L, Troppmann C, McVicar JP, Sturges M, Fisher K, Meyers FJ. A strategy for the simultaneous provision of pre-operative palliative care for patients awaiting liver transplantation. Transpl Int. 2004;17(8):473–475. [DOI] [PubMed] [Google Scholar]
- 13.Sharing UNfO. Waiting List Candidates by Organ Type. https://unos.org/data/transplant-trends/waiting-list-candidates-by-organ-type/. Accessed March 19, 2019.
- 14.Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quality AfHRa. Re-Engineered Discahrge (RED) Toolkit. https://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html Accessed March 25, 2019.
- 17.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirivatanauksorn Y, Dumronggittigule W, Limsrichamrern S, et al. Quality of life among liver transplantation patients. Transplant Proc. 2012;44(2):532–538. [DOI] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell RT, Feurer ID, Wisawatapnimit P, Pinson CW. The validity of EQ-5D US preference weights in liver transplant candidates and recipients. Liver Transpl. 2009;15(1):88–95. [DOI] [PubMed] [Google Scholar]
- 21.Broderick JE, DeWitt EM, Rothrock N, Crane PK, Forrest CB. Advances in Patient-Reported Outcomes: The NIH PROMIS((R)) Measures. EGEMS (Wash DC). 2013; 1(1):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelberg RA, Downey L, Wenrich MD, et al. Measuring the quality of end-of-life care. J Pain Symptom Manage. 2010;39(6):951–971. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.