Abstract
Clinician-rated toxicity data has been systematically collected within oncology clinical research using the National Cancer Institute’s CTCAE scale, providing estimates of the occurrence and severity of toxicity from cancer treatment. CTCAE is being supplemented by collection of patient-reported outcome (PRO) toxicity within clinical research and clinical practice, where PRO has demonstrable benefits. There is general agreement that PRO data is more sensitive and reliable than CTCAE data, particularly for subjective adverse effects. Based on this premise, researchers have begun to use PRO toxicity data collected within prospective clinical trials as the primary endpoint to discover pharmacogenetic and other predictive biomarkers of treatment-related toxicity. This perspective raises caution about the superiority of PRO data to CTCAE data for biomarker research, particularly in regards to chemotherapy-induced peripheral neuropathy (PN). The reader is provided an introduction to PRO and their integration into clinical research and practice, comparisons of PN data collected by PRO and CTCAE, examples of attempts to use PRO PN data for biomarker discovery, and evidence suggesting that PRO may not be superior to CTCAE for PN biomarker studies. The perspective concludes with a proposed approach for empirically testing whether PRO or CTCAE data is the better option for use in PN biomarker research, which can serve as a model for similar comparisons within other treatment-related toxicities.
Pharmacogenetic analyses require accurate treatment outcomes data to maximize the likelihood of successfully discovering a genetic predictor. Integration of patient-reported outcome (PRO) toxicity data into oncology clinical research and practice, where it has documented benefit, provides an alternative to clinician-graded toxicity data; however, it is unclear whether the benefits of PRO extend to pharmacogenetic discovery.
Clinician-Graded and Patient-Reported Toxicity Data in Clinical Research and Practice
Since the mid-1980s the National Cancer Institute (NCI) has mandated that physician-reported toxicity data be collected during oncology clinical trials using the NCI Common Terminology Criteria for Adverse Events (NCI CTCAE) at a minimum. The current version, CTCAE V4.0, enables collection of more than 1,000 distinct AEs on a clinician-graded 0–5 scale. There is concern that reliance on CTCAE grading has led to incomplete recognition and documentation of treatment-related AEs, particularly for subjective toxicities such as fatigue, nausea, and peripheral neuropathy (PN)1. Subjective toxicity information must be described by the patient to the clinician, who must recognize that it represents an AE, assess the severity grade based on the CTCAE, and document the toxicity in the study record. This complex process introduces several opportunities for information to be misinterpreted or lost, particularly in multi-center clinical trials in which toxicity assessment is conducted by many different clinicians with diverse approaches and varying levels of attentiveness to toxicity assessment and documentation (Figure 1).
Figure 1: Flow of Information from a Treated Patient to PRO or CTCAE PN Data.
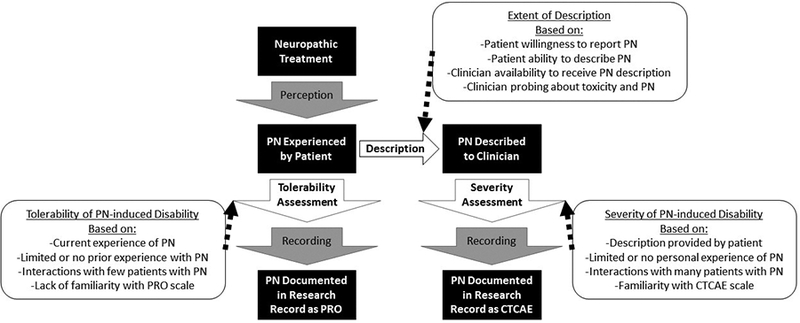
The flow of information from patient to the research record as either PRO or CTCAE includes several steps during which information can be lost or misinterpreted. Generation of CTCAE data requires a description by the patient to the clinician, which represents an additional opportunity for information loss. PRO and CTCAE each require an assessment, either the patient (PRO) assesses the tolerability of PN or the clinician (CTCAE) assesses the severity of PN; it is possible that clinicians have a more consistent scale for assessment than do patients.
Since the early 2000s, there has been a major push toward supplementing CTCAE with validated patient-reported outcome (PRO) information, which has several potential advantages. Incidence and severity of toxicity tend to be higher when using PRO than CTCAE, and matched comparisons generally report poor to modest agreement, which is lowest for subjective toxicities. PRO can also provide more detailed symptom information and use a larger dynamic range, potentially enabling earlier detection and identification of subtle changes in severity2. In parallel with the integration of PRO into clinical research, real-time collection and sharing of PRO with clinicians during treatment has been reported to improve patient survival3. One of the main benefits of using PRO in clinical practice is that they reflect the effect the AE is having on the patient’s quality of life, enabling clinicians and patients to make shared treatment decisions based on the individual patient’s personal preferences and treatment goals.
Comparison of CTCAE and PRO as an Endpoint for Biomarker Studies of Peripheral Neuropathy (PN)
PN data collected via CTCAE has substantial inter-observer variability and a known floor effect4. Several PRO tools have been developed to collect chemotherapy-induced PN including the Patient Neurotoxicity Questionnaire (PNQ), Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx), and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy Induced Peripheral Neuropathy (EORTC CIPN20)5. More than a dozen studies have directly compared one of these PRO instruments, scored according to published guidelines, with CTCAE (Table 1). Across these studies, the incidence and severity of PN is consistently higher when collected via PRO, and the correlations between CTCAE and PRO PN are poor to moderate (correlation coefficients: 0.2–0.7). The limited correlation is often used as evidence to support the conclusion that PRO PN data are more sensitive and reliable than CTCAE data, however, this conclusion is difficult to empirically test without an objective benchmark to anchor comparisons.
Table 1:
Comparisons of Patient and Clinician Reported Peripheral Neuropathy
| PRO and Subscale | n | Collection Time Point | Objective Assessmentsb | Findings | Journal Reference |
|---|---|---|---|---|---|
| FACT/GOG-Ntx | 99 | BL (controls) or Post-Tx (cases) | Yes | Ntx significantly worse in cases than controls (p<0.001) | Calhoun, Welshman et al. 2003 |
| 27 | BL, each cycle, 3M Post-Tx | Yes | Ntx and CTCAE Spearman’s ρ=−0.59 (p=0.001) | Moore, Donnelly et al. 2003 | |
| 134 | Each cycle | Ntx sensory ROC curve AUC=0.90 | Huang, Brady et al. 2007 | ||
| 300 | BL, C3, C5, C7, 7M and 12M | Ntx and CTCAE sensory Spearman’s ρ=0.45 | Shimozuma, Ohashi et al. 2009 | ||
| 422 | BL, within 4W of Tx end, 6M Post-Tx | Ntx and CTCAE sensory Pearson r=0.69 | Cella, Huang et al. 2010 | ||
| 29 | Each cycle | Yes | NTx sensory worse in patients with sensory CTCAE≥1 (p<0.001) | Griffith, Couture et al. 2014 | |
| EORTC QLQ-CIPN20 | 230 | BL | CIPN20 sensory and CTCAE sensory Pearson r=0.20 (p≤0.01) | Lavoie Smith, Barton et al. 2013 | |
| 281 | Post-Tx | Yes | CIPN20 and CTCAE sensory Spearman’s ρ=0.47 (p≤0.007) | Cavaletti, Cornblath et al. 2013 | |
| 281 | Post-Tx | Yes | CIPN20 sensory and CTCAE sensory linear correlation β=13.3 (p<0.001) | Alberti, Rossi et al. 2014 | |
| 414 | Each cycle | CIPN20 sensory and CTCAE linear association (p<0.0001) | Le-Rademacher, Kanwar et al. 2017 | ||
| PNQ | 300 | BL, C3, C5, C7, 7M and 12M | PNQ sensory and CTCAE sensory Spearman’s ρ=0.44 | Shimozuma, Ohashi et al. 2009 | |
| 35 | BL, 8W, 16W | PNQ sensory and CTCAE sensory Spearman’s ρ=0.58 | Kuroi, Shimozuma et al. 2009 | ||
| 20 | Post-Tx | Yes | PNQ and CTCAE sensory statistical comparison NR | Bennett, Park et al. 2012 |
Used NCI-Sanofi grading scale, which is similar to NCI CTCAE
Objective assessments included: Thermal discrimination, Vibration perception, Nerve conduction, Quantitative sensory testing, Mechanical stimulation with a monofilament, Grip strength, Pinprick sensibility, Deep tendon reflexes
Acronyms: NR: Not reported, ROC: receiver operating curve, BL: baseline, Post-Tx: after treatment ended, C: cycle, W: week, M: month
It is critically important that pharmacogenomics studies use accurate treatment outcomes data. The first wave of genome-wide association studies in taxane-induced PN, conducted in clinical trials designed prior to the introduction of PRO, relied on CTCAE data as the endpoint of interest and frequently noted the limitations of CTCAE graded PN6–8. As PN collection within clinical research has transitioned from CTCAE to PRO, the biomarker research community has followed. Several recent PN biomarker studies have elected to use PRO data as the primary endpoint or conducted parallel analyses of PRO and CTCAE data (Table 2). Two of these studies were secondary analyses of PRO data collected within prospective interventional clinical trials, within which systematically collected CTCAE data was also available9, 10, whereas in the remaining three cases investigators decided to collect PRO instead of CTCAE within prospective registries or case-control studies of PN11–13. These studies also used a variety of strategies for translating PRO data into an outcome for analysis, ranging from using the continuous PRO score to arbitrarily or empirically selecting thresholds to dichotomize patients into PN “cases” and no-PN “controls,” as described in Table 2. The decision to use PRO instead of CTCAE PN data in these biomarker discovery studies was likely based on the assumption that the benefits of PRO for clinical research and practice extend to biomarker research. Indeed, the ability to collect PRO data more frequently, identification of a greater number of PN cases via PRO, enhanced sensitivity of PRO to detect subtle changes in severity, and improved specificity of PN symptom collection via PRO should substantially improve analytical power for PN biomarker discovery.
Table 2:
Selected Biomarker Studies that have used Patient-Reported Outcomes as the Peripheral Neuropathy Endpoint
| PRO Instrument | PRO Scale | Outcome used in analysis | Parallel analysis of CTCAE | Biomarker | n | Findings | Ref |
|---|---|---|---|---|---|---|---|
| FACT-NTx | NTx score | Case defined as ≥ 20% worsening of cumulative score from baseline | No | Proteomics | 17 | Hypothesis-generating association with a protein signature | 11 |
| EORTC QLQ-CIPN20 | CIPN8* | Cumulative score at each week of treatment | No, but used PN-induced treatment disruption | Paclitaxel PK and clinical variables | 60 | PRO associated with cumulative dose, age, and alcohol intake. Treatment disruption associated with cumulative dose and paclitaxel pharmacokinetics | 12 |
| CIPN8* | Ordinal groups empirically derived from cumulative score | No | GWAS and clinical variables | 680 | PRO associated with age, smoking, and drinking. Hypothesis-generating associations with genetics | 13 | |
| CIPN20 | Cases and controls defined by selecting phenotypic extremes from distribution of increase in cumulative score throughout treatment | No | Candidate gene sequencing | 119 | PRO-derived cases carried more deleterious variants in genes associated with hereditary neuropathy | 9 | |
| EORTC QLQ-OV28 | Two neuropathy questions | Cases defined as response of “Quite a Bit” or “Very Much” on either question | Yes | Candidate genotypes and clinical variables | 454 | PRO associated with age, residual disease. CTCAE associated with age, bevacizumab, and bowel resection. Hypothesis-generating associations with genetics | 10 |
CIPN9 Sensory subscale without question 18 (difficulty hearing)
Abbreviations: PK: pharmacokinetics, GWAS: Genome-wide association study
While the benefits of PRO for clinical research and practice are established, I recommend the pharmacogenomics community pause to consider whether these benefits extend to biomarker research, specifically regarding PN. Although PN is grouped with other subjective toxicities for which PRO are thought to be superior to CTCAE, there is evidence that CTCAE data for PN are more reliable than CTCAE data for other subjective toxicities (i.e., anxiety, pain). Comparisons of seven subjective symptoms assessed via CTCAE by multiple clinicians found that PN had the highest inter-observer correlation coefficient (ICC=0.71) and the lowest incidence of CTCAE assessed by two observers differing by more than 1 grade (<1%)1. Furthermore, the floor effect noted in CTCAE PN data may not reflect a limitation of the scale; clinicians delay or discontinue treatment when they identify mild to moderate PN, therefore, it would be expected that grade 4 PN would rarely be encountered in CTCAE data.
More generally, the greater incidence of “severe” toxicity detected using PRO instruments may not actually indicate that CTCAE fails to detect clinically meaningful events, as has been generally assumed. Matched comparisons of CTCAE and PRO data for several toxicities, which did not include PN, found that despite detecting fewer toxicity events, CTCAE data was more strongly associated with future rehospitalization and survival than PRO data14. This suggests an alternative, but seldom considered, explanation. The discrepancy between PRO and CTCAE may reflect differences in the standard of “severe” between patients, who have likely never experienced the toxicity, and clinicians, who regularly interact with patients experiencing treatment-related toxicity and have seen cases of truly severe toxicity. The FACT/GOG-NTx and EORTC CIPN20 ask patients to rate a series of PN-related symptoms on a numerical scale (0 or 1 thru 4), corresponding to textual descriptors of severity. There is likely substantial variability between patients in their definition of “A Little” and “Quite a Bit,” as most patients have no prior experience or knowledge of PN and wouldn’t know the upper limit of PN severity corresponding to a score of “Very Much.” This has been empirically demonstrated for several subjective symptoms, which again did not include PN, by modeling matched PRO and clinician-documented data to confirm that patients have lower and more variable thresholds for classifying symptoms as severe15. This finding is consistent with the previously described evidence that there is strong agreement between clinicians when grading PN (ICC=0.71). However, this estimate was based on a small group of clinicians at a single institution and agreement would likely decrease in a larger, more diverse group of clinicians, particularly if those clinicians were not carefully assessing and documenting PN.
One of the primary advantages to using PRO data for clinical practice is the integration of a patient’s perception about the effect of toxicity on their life. In clinical practice, whether a patient is experiencing an objectively “severe” adverse effect is less relevant than whether the patient considers this adverse effect intolerable. Patients may have limited basis to accurately judge PN severity, but they are the only relevant assessor of whether the toxicity is tolerable, given their lifestyle and treatment priorities. There is undoubtedly a subset of patients for whom fine motor skills and tactile sensitivity are critical. For example a blind patient who reads Braille or a patient who loves to sew would consider an objectively minor amount of PN intolerable, and likely rate it as “Very Much” on a PRO. While this information is extremely useful for making treatment decisions for this patient in clinical care, classifying this patient as a case of “severe” PN in a pharmacogenetic analysis would be inappropriate and decrease the likelihood of identifying a genetic PN biomarker (Figure 2).
Figure 2: Assessment of PN Using PRO and CTCAE.
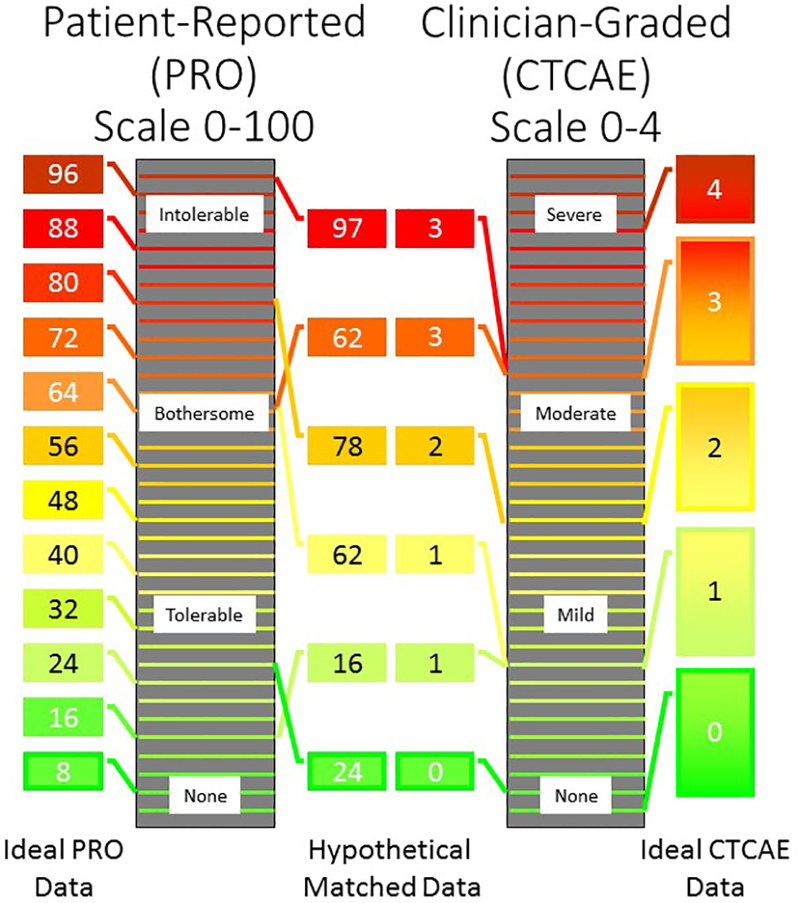
Schematic representation of the PRO (left) and CTCAE (right) grading scales for peripheral neuropathy (PN). The height of the box represents the extent of PN and the number inside the box is the tolerability assigned by the patient (left) or severity assigned by the clinician (right). Ideally, all patients (far left) and clinicians (far right) would have consistent scales, in which case PRO would likely be a superior endpoint for biomarker research due to its increased sensitivity. However, there is evidence that thresholds of severity are more consistent for clinicians (inner right) than patients (inner left)15. For example, two patients rated their PN a 62 on a scale of 0–100, but the actual extent of PN was very different between these patients. This increased inter-rater variability could be a critical drawback for using PRO as an endpoint in PN biomarker studies.
Empirical Comparison of CTCAE and PRO as Endpoints for Biomarker Studies
Biomarkers that are strongly predictive of treatment outcomes should be identifiable regardless of limitations of the endpoint selected. However, most clinical outcomes, including PN, seem to be multi-factorial and require highly reliable outcomes data to discover and replicate individual predictors that have relatively small effects on overall risk. In these cases it is critical that the outcomes data used in pharmacogenomics studies are as accurate as possible. We recently reported the results of a prospective observational clinical trial that characterized the relationship between systemic paclitaxel concentrations and PN. To our surprise, we did not detect an association with PRO PN, collected via EORTC CIPN-20, but detected a strong association with PN-induced treatment disruption (dose decreases, delays, or discontinuations due to PN)12. Treatment disruptions are assumed to reflect CTCAE grade, which we unfortunately did not collect within this cohort study due to our previously held confidence in the superiority of PRO data.
This finding, and the above described data suggesting greater overall concordance between clinicians than patients in assessing toxicity severity, should motivate the biomarker research community to more carefully consider which toxicity data source should be used as the primary endpoint for biomarker studies of PN. There are some best practices for collecting PN data that should be implemented regardless of the data source, such as collecting as many PN data types as feasible and assessing PN prior to and at the end of treatment, or at the time of a PN-induced treatment disruption. PN biomarker discovery analyses should a priori select a primary endpoint, account for cumulative dosing, and avoid unnecessarily collapsing continuous or ordinal scales into “cases” and “controls.” PN biomarker discovery studies could also consider including an objective measure of PN, such as thermal discrimination, vibration perception, or nerve conduction, which have been included in some comparisons of PRO and CTCAE, as indicated in Table 1. These quantitative measurements should further improve analytical power to discover PN predictors, however, it is unclear whether these highly controlled, experimental assessments reflect clinically relevant PN. An important next step in this field is to determine what magnitude of change in CTCAE, PRO, or an objective PN measure is “clinically-relevant,” since a biomarker must predict a clinically relevant endpoint to be useful in clinical practice. There is currently no established approach to translate changes in PRO to changes in CTCAE or to a clinically-relevant worsening of PN, necessitating investigators to select arbitrary thresholds in confirmatory studies. To that end, it could be valuable to the field to use the clinically-meaningful endpoint of PN-induced treatment disruption as a benchmark to estimate a clinically relevant magnitude of change in PRO PN.
CTCAE and PRO PN data each have benefits and limitations when used as the endpoint in biomarker discovery, and, in my opinion, it is unclear which is superior for this purpose. It may be possible to empirically test whether CTCAE or PRO are the optimal endpoint for biomarker discovery studies of PN, and other subjective AEs. In our paclitaxel pharmacokinetics project we conducted parallel analyses of pharmacokinetics as a predictor of two endpoints; EORTC CIPN-20, a PRO, and PN-induced treatment disruption, a surrogate of CTCAE. Our results, in a relatively small pilot study, indicate that PK is more strongly predictive of CTCAE than PRO. Using a conceptually similar approach of conducting parallel analyses of multiple endpoints but employing a single validated predictor variable could be a reasonable approach to determining which endpoint should be used in future analyses to discover additional predictor variables. For example, for paclitaxel-induced PN, perhaps cumulative dose, which is an established predictor of PN severity during paclitaxel treatment, could be used as an indicator variable in a secondary analysis of a large prospective clinical trial to empirically determine whether PRO or CTCAE data should be used as the endpoint in future pharmacogenetic discovery efforts.
In conclusion, although PRO have demonstrable benefits in clinical research and practice, it has not been adequately established that these benefits extend to biomarker research. There is suggestive evidence that the increased frequency and severity of toxicity detected via PRO reflects differences in patient’s assessments of severity, or perhaps tolerability, making its use as an endpoint for pharmacogenomics research problematic. While researchers continue to develop improved approaches to collect toxicity data, I recommend the biomarker research community pause to consider whether PRO or CTCAE data should be used as the primary endpoint within pharmacogenomics studies of PN and more generally in biomarker studies of subjective treatment-related toxicities.
Acknowledgements:
I would like to acknowledge Lynn Henry, Karen Farris, Steve Erickson, Ellen Lavoie Smith, and Jane Perlmutter for their critical review and feedback.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
References:
- 1.Atkinson TM, Li Y, Coffey CW, Sit L, Shaw M, Lavene D, et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res 2012; 21(7): 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nature reviews Clinical oncology 2016; 13(5): 319–325. [DOI] [PubMed] [Google Scholar]
- 3.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017; 318(2): 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol 1998; 9(7): 739–744. [DOI] [PubMed] [Google Scholar]
- 5.Curcio KR. Instruments for Assessing Chemotherapy-Induced Peripheral Neuropathy: A Review of the Literature. Clin J Oncol Nurs 2016; 20(2): 144–151. [DOI] [PubMed] [Google Scholar]
- 6.Hertz DL, Owzar K, Lessans S, Wing C, Jiang C, Kelly WK, et al. Pharmacogenetic Discovery in CALGB (Alliance) 90401 and Mechanistic Validation of a VAC14 Polymorphism that Increases Risk of Docetaxel-Induced Neuropathy. Clin Cancer Res 2016; 22(19): 4890–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res 2012; 18(18): 5099–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, et al. Genome-Wide Association Studies for Taxane-Induced Peripheral Neuropathy in ECOG-5103 and ECOG-1199. Clinical cancer research : an official journal of the American Association for Cancer Research 2015; 21(22): 5082–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler AS, Kulkarni AA, Kanwar R, Klein CJ, Therneau TM, Qin R, et al. Sequencing of Charcot-Marie-Tooth disease genes in a toxic polyneuropathy. Annals of Neurology 2014; 76(5): 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, et al. Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol 2017; 28(11): 2733–2740. [DOI] [PubMed] [Google Scholar]
- 11.Chen EI, Crew KD, Trivedi M, Awad D, Maurer M, Kalinsky K, et al. Identifying Predictors of Taxane-Induced Peripheral Neuropathy Using Mass Spectrometry-Based Proteomics Technology. PloS one 2015; 10(12): e0145816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertz DL, Kidwell KM, Vangipuram K, Li F, Pai MP, Burness M, et al. Paclitaxel Plasma Concentration After the First Infusion Predicts Treatment-Limiting Peripheral Neuropathy. Clin Cancer Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan ME, El Charif O, Wheeler HE, Gamazon ER, Ardeshir-Rouhani-Fard S, Monahan P, et al. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res 2017; 23(19): 5757–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 2009; 101(23): 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson TM, Rogak LJ, Heon N, Ryan SJ, Shaw M, Stark LP, et al. Exploring differences in adverse symptom event grading thresholds between clinicians and patients in the clinical trial setting. J Cancer Res Clin Oncol 2017; 143(4): 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]


