Abstract
Accumulating evidence has shown that N-methyl-D-aspartate (NMDA) glutamate receptors (NMDAR) are implicated in the pathophysiology of neurological and psychiatric disorders, and that patients with NMDAR antibody encephalitis develop psychopathological symptoms. Therefore, we hypothesized that NMDAR antibodies play a key role in the etiology of schizophrenia. In this study, we enrolled 110 first-episode patients with schizophrenia (FEP) and 50 healthy controls (HC). Cognitive function and psychopathology were assessed using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) and Positive and Negative Syndrome Scale (PANSS), respectively. NMDAR antibody levels were measured using enzyme-linked immunosorbent assay. Our results showed that FEP with schizophrenia exhibited cognitive deficits in all domains of the MCCB and had elevated levels of serum anti-NMDAR antibody compared with the healthy controls (9.2 ± 3.5 vs. 7.3 ± 2.9 ng / ml, t = 3.10, p = 0.002). Furthermore, serum antibody levels were positively correlated with PANSS positive, negative and total score, and inversely correlated with performances of verbal learning and memory, working memory, speed of processing and MCCB total score in the patient group. These results indicate that elevated levels of NMDAR antibody may play a role in the pathogenesis of schizophrenia, leading to NMDAR dysfunction, thereby inducing symptoms of psychosis and cognitive impairment. Therefore, NMDAR antibodies may serve as a biomarker and provide a new avenue for treatment of schizophrenia.
Keywords: anti-NMDA receptor antibody, first-episode patients with schizophrenia, autoimmunity, cognitive impairment, biomarker
1. Introduction
Schizophrenia is a complex and heterogeneous mental disorder. Although no unifying pathophysiologic abnormalities have been identified, neurodevelopmental, neuroimmunological, and neurotransmitter-based hypotheses have been proposed for the pathogenesis of the illness (Harrison and Weinberger, 2005). Accumulating evidence has demonstrated that schizophrenia is associated with immunological findings in the blood or cerebrospinal fluid (CSF) (Steiner et al., 2013), and that patients with schizophrenia exhibit increases in glial cell count and blood-brain/blood-CSF barrier dysfunction (Maxeiner et al., 2009; Bechter et al., 2010; Busse et al., 2012).
NMDA-type glutamate receptors are ligand-gated ion channels that mediate a major component of excitatory neurotransmission in the central nervous system (CNS). They are widely distributed in the CNS at all stages of development and are critically involved in neuronal development and synaptic plasticity. In the course of brain development, if the NMDA receptors receive proper stimulation, the neurons will survive. However, if the NMDA receptors are removed, apoptosis will be induced within a few hours (Ikonomidou et al., 1999). Under physiological conditions, the stimulation of NMDA receptors represents an important mechanism for synaptic pruning during brain development (Contestabile, 2000). The hypofunction of NMDA receptors has also been associated with multiple neurological and psychiatric disorders, such as ischemic stroke, traumatic brain injury, Alzheimer’s disease, epilepsy, mood disorders, and schizophrenia (Hansen et al., 2017; Warikoo et al., 2018). The NMDAR is an isotetramer that consists of three subunits: NR1, NR2, and NR3. NR1 contains eight different subunits (NR1-1a/b-4a/b); NR2 contains four different subunits (NR2A-2D); and NR3 contains two different subunits (NR3A-3B). While all eight subunits of NR1 are derived from the same gene yet generated from different parts of the shear; six different genes code NR2 and NR3. In mammalian nervous tissues, the functional NMDAR is composed of at least one NR1 and two NR2 subunits; but most NMDARs consist of two NR1 subunits and two NR2 subunits in the two-polymer combination. NR1 is the basic subunit of ion channels, while NR2 is a regulatory subunit. NMDARs consist of different types of NR2, which are distributed in different brain regions and give rise to different physiological characteristics (Gleichman et al., 2012). Convergent evidence indicates that NMDAR signaling is impaired in schizophrenia (Emamian et al., 2004; Ayalew et al., 2012; Weickert et al., 2013; Ohi et al., 2015), although the mechanism underlying the NMDAR hypofunction in patients with schizophrenia remains unclear. Growing evidence suggests that anti-NMDAR Abs is involved in schizophrenia.
However, studies on this have shown inconsistent results. Rhoads et al. and Masdeu et al. observed no anti-NMDAR autoantibodies in the serum of patients with schizophrenia (Rhoads et al., 2011; Masdeu et al., 2012), while Zandi et al. observed NMDAR serum antibodies in approximately 6% (3/46 patients) with first-episode schizophrenia (Zandi et al., 2011). In another study, immunoglobulin G (IgG)-class antibodies directed against NR1a were found in only two patients with an initial diagnosis of disorganized or catatonic schizophrenia, both of whom were subsequently rediagnosed with NMDAR encephalitis. In contrast, two other individuals with IgG antibodies received diagnoses of paranoid schizophrenia, and the antibodies did not bind to NR1a but were only reactive with NR1a/NR2b (Steiner et al., 2013). Lennox et al found 7 of 228 (3%) patients had NMDAR antibodies in first-episode psychosis (Lennox et al., 2017). Autoantibodies of the IgG isotype against NMDAR were also detected by Tsutsui et al. in 4 of 51 (8%) patients with schizophrenia (Tsutsui et al., 2012). In a review involving seven studies comprising 1,441 patients with schizophrenia and related psychoses, among whom 115 were positive for anti-NMDAR Abs (Pollak et al., 2014).
As we know, immunofluorescence to detect NMDAR antibodies by their binding to the surface of HEK293A cells functionally expressing NMDAR was common and reliable detection method until recently (Ramberger et al., 2015). However, it is complex and difficult to implement and yields qualitative rather than quantitative results. NR1 is the basic subunit of ion channels and the functional subunit of NMDAR. Further, the amino terminal domain (ATD) of GluN1 is required for binding of anti-NMDAR Abs (Dalmau et al., 2008), and residues N368/G369 expressed in the N-terminal domain are crucial for antibody binding (Gleichman et al., 2012). The terminal sequence of NMDAR was known to be mature for the construction of polypeptide. The sequences were QKRLETLLEERESK (AA177-190) for the N-terminal of GluN1 (GluN1-NT), and SS FKRRRSSKDTST (AA889-902) for the C-terminal of GluN1 (GluN1-CT) (Fujita et al., 2012; Fukuyama et al., 2015; Ikura et al., 2016). And Sharma et al. (Sharma et al., 2018) found the ATD fusion protein also contains a Myc tag and a 6XHIS tag, which provide functionality for immunoassays and soluble antigen formats maintain the pathogenic anti-N-methyl-D-aspartate receptor encephalitis (ANRE) epitopes in ELISA binding assays, which makes quantitative research become reality. In recent years, ELISA has been continuously used to detect NMDAR antibody levels (Kowal and Diamond, 2012; Kalev-Zylinska et al., 2013; Ogawa et al., 2016; Ferensztajn-Rochowiak et al., 2019).
To our knowledge, there is still no published study that has examined the relationship between NMDAR antibody levels and clinical features in first-episode antipsychotics-naiïve patients. Studies have shown that the blood-brain barrier is damaged in patients with schizophrenia, thus increasing the number of Abs in the blood. Furthermore, one study (Castillo-Gomez et al., 2016) has demonstrated that Abs have crossed the blood-brain barrier to bind to the brain - with the brain acting as an immunoprecipitator - and are therefore unmeasurable in the CSF. Therefore, serum antibody levels may be a better option to reflect the levels of NMDAR Abs in brain. Therefore, in this study, we evaluated the presence of serum Abs directed against the NR1 subunit with ELISA.
2. Materials and Methods
2.1. Participants
One hundred and ten first-episode patients with schizophrenia were recruited for this study from Beijing Huilongguan Hospital. The inclusion criteria were: (a) a first psychotic episode meeting the Structured Clinical Interview of DSM-IV diagnostic criteria for schizophrenia; (b) age between 18 and 45 years; (c) Han Chinese ancestry; (d) total illness duration of less than 3 years; and (e) previous antipsychotic exposure time of less than 2 weeks. Exclusion criteria were: (a) DSM-IV Axis I diagnosis other than schizophrenia; (b) severe physical illness; (c) treatment with electroconvulsive therapy (ECT) within the preceding 3 months, repetitive transcranial magnetic stimulation (rTMS) within the preceding 2 weeks, or regular administration of neurotrophic agents; (d) treatment with immune modulators and antioxidants for more than 4 weeks; or (e) pregnancy or lactation. Healthy subjects were recruited from the local community by advertising, and screened by matching their age and gender to the patients. Axis I psychiatric disorders were ruled out in these controls by psychiatric review evaluation such as the Brief Psychiatric Rating Scale, Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD) conducted by a psychiatrist. Demographic data for patients and normal controls are summarized in Table 1. A complete medical history and family history were obtained from all participants. Also a physical examination, laboratory tests including urine and blood screening, and an electrocardiogram were performed on all participants. All subjects were physically healthy, without any neurological or other medical diseases. Neither the patients nor the control subjects had a diagnosis of alcohol or illicit drug abuse or dependence. All participants provided written informed consent, following a protocol approved by the Ethics Committee of the Beijing Huilongguan Hospital.
Table 1.
Participant demographics, clinical characteristics, and anti-NMDAR Ab levels
| First-Episode Schizophrenia (FEP) | Healthy Controls (HC) | Statistics t/χ2/F | P Value | |
|---|---|---|---|---|
| Gender, M/F | 55/55 | 27/23 | 0.22 | 0.64 |
| Age (yrs) | 27.6± 7.4 | 29.6±7.0 | −1.50 | 0.14 |
| Education (yrs) | 12.9±3.5 | 14.0±2.6 | −2.17 | 0.03* |
| Age of onset (yrs) | 26.2±7.3 | NA | NA | NA |
| Illness duration (mos) | 13.1±16.3 | NA | NA | NA |
| PANSS score | ||||
| PANSS Positive | 22.5±5.3 | NA | NA | NA |
| PANSS Negative | 17.4±6.2 | NA | NA | NA |
| PANSS General | 37.0±7.1 | NA | NA | NA |
| PANSS Total score | 77.0±13.0 | NA | NA | NA |
| MCCB score | ||||
| Speed of Processing | 45.3±8.8 | 57.6±7.7 | 46.07 | 6.66×10−16* |
| Attention/Vigilance | 42.7±9.1 | 58.0±6.6 | 60.28 | 2.50×10−19* |
| Working Memory | 47.2±10.4 | 57.9±5.5 | 32.64 | 3.03×10−12* |
| Verbal Learning | 48.0±11.9 | 56.7±7.6 | 14.07 | 2.90×10−6* |
| Visual Learning | 46.4±9.2 | 55.6±6.2 | 27.47 | 1.06×10−10* |
| Reason & Problem Solving | 49.1±10.2 | 57.2±5.9 | 16.09 | 5.59×10−7* |
| Social Cognition | 47.8±9.5 | 54.9±10.0 | 11.03 | 3.72×10−5* |
| MCCB total score | 45.7±9.2 | 58.9±4.9 | 61.79 | 1.20×10−19* |
| NMDAR Ab (ng/ml) | 9.2±3.5 | 7.3±2.9 | 3.10 | 2.00×10−3* |
All data are reported as mean±SD except gender. Abbreviations: NA, not applicable; PANSS, Positive and Negative Syndrome Scale; MCCB: MATRICS Consensus Cognitive Battery.
Significant at P<0.05 or P < 0.05/7 in MCCB scores
2.2. Neurocognition and Psychopathology Assessment
Cognitive function was assessed using the validated Chinese version of the MATRICS Consensus Cognitive Battery (MCCB) (Kern et al., 2008; Nuechterlein et al., 2008; Shi et al., 2015). The MCCB includes seven cognitive domains: (1) Speed of Processing: Trail Making Test, Part A; Symbol Coding Subtest; and Category Fluency Test; (2) Attention and Vigilance: Continuous Performance Test–Identical Pairs; (3) Working Memory: spatial span and digit sequencing test of the Wechsler Memory Scale,; (4) Verbal Learning: Hopkins Verbal Learning Test - Revised; (5) Visual Learning: Brief Visuo-spatial Memory Test - Revised; (6) Reasoning and Problem Solving: Mazes Subtest of the Neuropsychological Assessment Battery, and; (7) Social Cognition: Managing Emotions Subtest of the Mayer-Salovey-Caruso Emotional Intelligence Test. The MCCB took approximately 60 to 90 minutes to complete. Raw scores were converted to Chinese-normalized T-scores, and seven domain T-scores as well as a composite T-score were computed. An attending psychiatrist using the Positive and Negative Syndrome Scale (PANSS) assessed clinical symptoms. All attending psychiatrists were trained for PANSS assessment with an intra-class correlation coefficient (ICC) above 0.80 prior to the study.
2.3. Measurement of Anti-NMDAR Abs
Serum samples from healthy controls and patients with schizophrenia were collected between 8 and 9 AM. The serum samples were centrifuged (4°C, 3,500 rpm, 10 min), aliquoted, and stored at −70°C. Anti-NMDAR Ab levels were measured via sandwich ELISA (Fujita et al., 2012; Fukuyama et al., 2015; Ikura et al., 2016) using a commercially available kit (Beijing Rongxin Zhihe Biotechnology Co. Ltd., Beijing, China). To minimize variance, the same technician, who was blinded to the clinical data, assayed all samples. The identity of the participants was coded and maintained by the investigator until all biochemical analyses were completed. Inter- and intra-assay variation coefficients were less than 7.2% and 5.5%, respectively.
2.4. Statistical analysis
Differences in continuous variables between patients and healthy controls were assessed using t-tests (normal distribution) or Mann-Whitney U-tests (non-normal distribution). Kolmogorov-Smimov tests were used to evaluate the distributions of continuous variables. Differences in the distributions of categorical variables were examined using χ2 tests. Multivariate analysis with general linear models was used to compare MCCB T scores and subunit T scores between the two groups using education level as a covariate. The corrected test level was P = 0.05 / 7. Relationships between the anti-NMDAR Ab level and the duration of illness/age at onset were assessed using Pearson’s correlation coefficients, while those between anti-NMDAR Ab levels and the PANSS score/MCCB T score were assessed via general linear regression with education, gender, and age as covariates. The test level was p = 0.05. All data are presented as the mean ± standard deviation (S.D). Three outliers of anti-NMDAR Ab level in patients (34.94 ng/ml, 40.81 ng/ml and 42.07 ng/ml) and one outlier in healthy controls (27.07 ng/ml) were excluded to avoid false-positive results.
3. Results
3.1. Demographics, Clinical Characteristics, and Serum Anti-NMDAR Ab Levels
The demographic and clinical characteristics of the participants are shown in Table 1. Age and gender were matched between patients and healthy controls (all P’s > 0.05). The patients had fewer years of education than controls (t = −2.165, P = 0.032), and years of education served as a covariate in data analyses. In the patient group, the mean age of onset of illness was 26.2 ± 7.3 years and the mean duration of illness was 13.1 ± 16.3 months. Serum anti-NMDAR Ab levels were significantly higher in the patient group than in the control group (9.2 ± 3.5 ng/ml vs. 7.3 ± 2.9 ng/ml, t = 3.10, P = 0.002) (Table 1). There was no significant correlation of serum anti-NMDAR Ab levels with either sex or age in both groups (all P’s > 0.05), and no significant correlation of serum anti-NMDAR Ab level with either age of onset or illness duration in patients (all P’s > 0.05).
3.2. Cognitive function
Compared with controls, patients with schizophrenia exhibited significantly lower scores on all seven domains of cognitive function, with education as a covariate (all P’s < 0.05/7 cognitive domains = 0.007, Table 1).
3.3. Correlation of Clinical Symptoms with Anti-NMDAR Ab Concentration
In the patient group, total PANSS and subscale scores for positive symptoms, negative symptoms, and general psychopathology were 77.0 ± 13.0, 22.5 ± 5.3, 17.4 ± 6.2, and 37.0 ± 7.1, respectively. Serum anti-NMDAR Ab levels were positively correlated with PANSS positive sub-scores, negative sub-scores and total scores, with age, sex, and years of education as covariates (β = 0.38, p = 0.01; β = 0.53, p = 4.24 × 10−3; β = 1.01, p = 6.13 × 10−3, Table 2, Figure 1).
Table 2.
General Linear Regression Analysis Results of anti-NMDAR Ab level on cognitive performance
| FEP (anti-NMDAR Ab) |
HC (anti-NMDAR Ab) |
|||||
|---|---|---|---|---|---|---|
| Dependent | β | t | p | β | T | p |
| MCCB | ||||||
| Speed of Processing | −0.79 | −2.37 | 0.02* | −0.04 | −0.15 | 0.88 |
| Working Memory | −1.16 | −4.23 | 6.01 ×10−5* | −0.45 | −1.46 | 0.15 |
| Verbal Learning | −1.24 | −3.92 | 1.78×10−4* | 0.07 | 0.22 | 0.83 |
| Attention and Vigilance | −0.71 | −1.83 | 0.07 | −1.81 | −3.19 | 0.95 |
| Visual Learning | −0.66 | −2.01 | 0.05 | −0.09 | −0.36 | 0.72 |
| R/Problem Solving | −0.30 | −0.86 | 0.39 | −0.09 | −0.08 | 0.94 |
| Social Cognition | −0.35 | −0.94 | 0.35 | 0.02 | 0.05 | 0.96 |
| Total score | −0.96 | −3.65 | 4.63×10−4* | −0.22 | −0.79 | 0.44 |
| PANSS | ||||||
| Positive score | 0.38 | 2.55 | 0.01* | NA | NA | NA |
| Negative score | 0.53 | 2.94 | 4.24 ×10−3* | NA | NA | NA |
| General score | 0.13 | 0.63 | 0.53 | NA | NA | NA |
| Total score | 1.01 | 2.81 | 6.13 ×10−3* | NA | NA | NA |
Abbreviations: FEP, First Episode Patients with Schizophrenia; HC, Healthy Controls;MCCB, MATRICS Consensus Cognitive Battery; NA, not applicable; PANSS, Positive and Negative Syndrome Scale. General linear regression was used. The source was NMDAR antibody level (ng/ml), the dependents were subscores and total score of MCCB and PANSS, the covariate was age, sex and education, data on the final models were presented.
Significant at p<0.05
Figure 1.
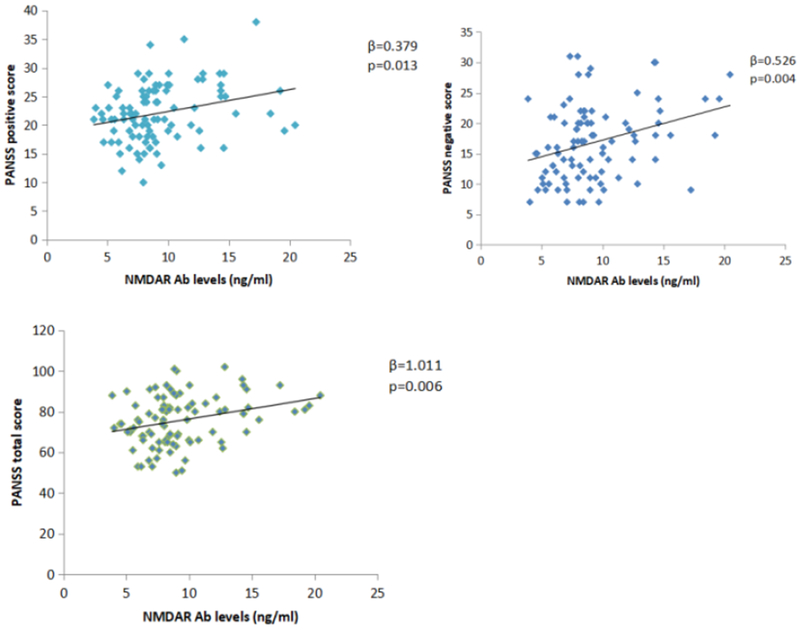
Correlation between Anti-NMDAR Ab concentration and PANSS scores
a: Correlations between NMDAR Ab level and the PANSS Positive score in patients. b: Correlations between NMDAR Ab level and the PANSS Negative score. c: Correlations between NMDAR Ab level and the PANSS Total score. The associations were significant at P<0.05 after adjusting for age, sex, education level.
3.4. Correlation of Cognition with Anti-NMDAR Ab Concentration
We conducted an exploratory analysis across all seven cognitive domains and serum anti-NMDAR Ab levels in each group. The results showed that, in the patient group, performances of speed of processing, working memory, verbal learning and memory, and MCCB total score were negatively correlated with serum anti-NMDAR Ab levels, with age, sex, and years of education as covariates (β = −0.79, p = 0.02; β = −1.16, p = 6.01 × 10−5; β = − 1.24, p = 1.78 × 10−4; β = −0.96, p = 4.63 × 10−4, respectively, Table 2 and Figure 2). In contrast, no significant correlations were observed between cognitive function and serum anti-NMDAR Ab levels in the control group.
Figure 2.
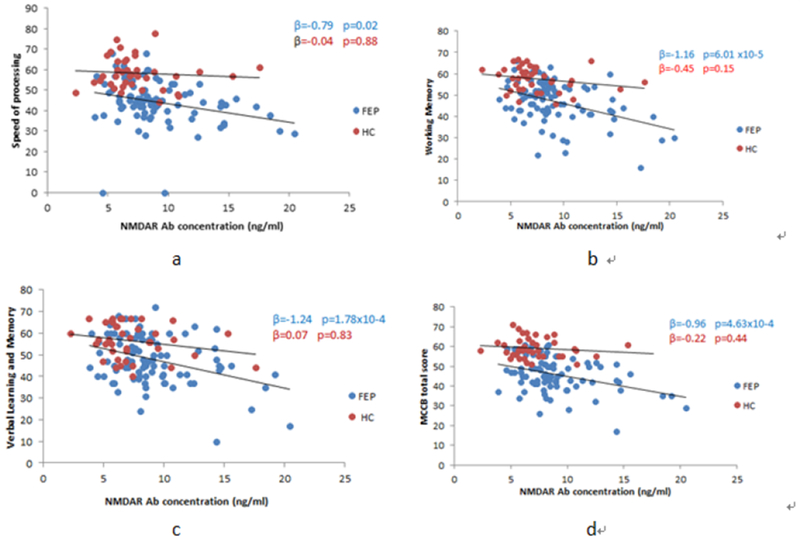
Correlation between Anti-NMDAR Ab concentration and cognitive performance
a: Correlations between NMDAR Ab level and speed of processing. b: Correlations between NMDAR Ab level and working memory. c: Correlations between NMDAR Ab level and verbal learning and memory. d: Correlations between NMDAR Ab level and MCCB total score. The FEP was represented by blue dots while the HC was represented by res dots. The associations were significant at P<0.05 after adjusting for age, sex, education level.
4. Discussion
Our results indicated that serum anti-NMDAR Ab levels were significantly elevated in patients with schizophrenia compared with age and gender-matched controls. This is consistent with a conclusion in a previous review of seven studies comprising 1,441 patients with schizophrenia and related psychoses that 115 (8%) of patients were positive for anti-NMDAR Abs (Poliak et al., 2014). These suggest that the NMDAR Abs may play a role in partially number of patients with schizophrenia(Teixeira, Rocha, & Zhang, 2017).
While our study provide some consistent findings, there were earlier studies using qualitative methods such as immunofluorescence or immunohistochemistry have reported lack of anti-NMDAR Abs in patients with schizophrenia (Rhoads et al., 2011; Masdeu et al., 2012). In a study of schizophrenia 80 cases and 40 healthy controls, anti-NR1 IgG antibodies were not detected in either group, but other unidentified sera reactive neuronal surface antigens were found in small number of both cases and controls (Masdeu et al., 2012). In addition, we found that patients with schizophrenia exhibited reduced cognitive function across all seven domains of the MCCB, consistent with previous reports (IIa et al., 2012; She et al., 2017). An earlier study indicated NMDAR antibodies in the early course of illness in schizophrenia patients but none in the large case series of chronic schizophrenia (Zandi et al., 2011), which could have been due to that NMDAR antibodies drop with time. However, our study did not observe significant correlations between serum Ab levels and the duration of illness or age of onset in the antipsychotic-naive first-episode schizophrenia. It remains to be seen whether disease progression or treatment may be associated with changes in NMDAR Ab levels.
So far, there have been only few studies on the mechanism of NMDAR antibody in schizophrenia. Previously study showed binding of NMDAR Abs leads to capping and cross-linking of NMDARs, resulting in receptor dimerization and internalization, leading to NMDAR hypofunction (Hughes et al., 2010; Mikasova et al., 2012). It has been reported that residual surface NMDARs exhibit no substantial change in functionality, that Abs induce no preferential loss of synaptic receptors or extrasynaptic receptors, and that there is no preferential loss of NMDARs associated with localization or with the presence of a particular GluN2 subunit (Warikoo et al., 2018). However, other studies have observed robust Ab signaling in the hippocampus, mild signaling in the forebrain, and a lack of signaling in the cerebellum (Dalmau et al., 2007).
In addition, our findings showed that the positive and negative subscores as well as total score of the PANSS were positively associated with Ab levels. The dopamine hypothesis of schizophrenia posits that striatal hyperdopaminergia contributes to positive symptoms, while frontocortical hypodopaminergia contributes to negative symptoms and cognitive dysfunction (Howes and Kapur, 2009). Kazuhito et al. demonstrated that genetic NMDAR subunit type 1 gene (Grin1) deletion in corticolimbic parvalbumin (PV+) GABAergic neurons during early postnatal development causes striatal hyper- and medial prefrontal cortex (mPFC) hypo-sensitivity to amphetamine-induced dopamine release (Nakao et al., 2018). The authors further suggested that NMDAR hypofunction in cortical and hippocampal PV neurons (but not neurons in other subcortical areas) is responsible for the emergence of dopamine phenotypes. These findings are consistent with the distribution of anti-NMDAR Abs, which exhibited robust signaling in the hippocampus, modest signaling in the forebrain, and a lack of signaling in the cerebellum (Dalmau et al., 2007). Taken together, these findings suggest that anti-NMDAR Abs may selectively affect the NMDARs of PV+ GABAergic central neurons in the hippocampus and forebrain, resulting in NMDAR hypofunction and abnormal release of dopamine, causing symptoms of schizophrenia. In addition, NMDAR hypofunctioning may lead to the emergence of auditory verbal hallucinations (AVH) by affecting inter-hemispheric connectivity between the bilateral auditory cortices in the gamma-band frequency range (Thiebes et al., 2018). Furthermore, deficiencies in NMDAR-mediated second messengers may play a significant role in the emergence of psychotic and cognitive symptoms. Interestingly, Nakashima et al. demonstrated that phosphodiesterase antagonists can improve cognitive function and negative symptoms of schizophrenia, particularly social withdrawal, which is induced by NMDAR antagonists (Nakashima et al., 2018).
Our findings indicated that performance of verbal learning and memory, working memory, speed of processing and MCCB total score was negatively correlated with serum anti-NMDAR Ab levels in the patient group, but no significant in the control group. Indeed, deficits in processing speed and working memory represent core cognitive dysfunction in schizophrenia (Keefe et al., 2005; Dickinson et al., 2007; Knowles et al., 2010; Kochunov et al., 2017). Decades of research has revealed that cognitive impairment may reflect NMDAR dysfunction (Kantrowitz and Javitt, 2010). In healthy individuals, NMDAR antagonists induce not only psychotic symptoms, but also cognitive symptoms similar to those observed in patients with schizophrenia (Krystal et al., 1994; Morgan and Curran, 2006). NMDAR bind to scaffold and signaling molecules in the postsynaptic density (PSD), yielding a vast protein complex that physically links the NMDAR to kinases, the cytoskeleton, and downstream signaling pathways (Rao and Finkbeiner, 2007). NMDAR opening leads to an influx of sodium ions, which contributes to postsynaptic depolarization, and calcium ions, which promotes synaptic plasticity by activating signaling cascades including long term potentiation (LTP) in the post-synaptic densities (Hardingham and Bading, 2003). Critical to learning and memory, LTP in the hippocampus is an objective indicator of neuronal activity during memory formation and consolidation. During early hippocampal development, NMDAR in glutamate neurons participate in the establishment of LTP. LTP-dependent NMDA receptors are mainly induced in Schaffer collateral commissural synapses (SCS) in the hippocampal CA1 region, following the influx of free calcium into postsynaptic neurons mediated by NMDA receptors. The NR1 subunit is a functional unit of the NMDAR and an essential part of the NMDAR channel, and thus may contribute critically to learning and memory. Nakazawa et al. found that mice with knockout of the NMDAR NR1 subunit in hippocampal CA3 pyramidal cells exhibited significant impairments in forming associative memory (Nakazawa et al., 2002). Another study reported that NMDAR activity is necessary for recurrent excitation - a cellular mechanism for consolidation - in working memory circuits (Wang et al., 2013), in support of a link between autoimmune-induced NMDAR dysfunction and working memory impairments.
While the NMDARs antibodies was elevated in schizophrenia, the source of the antibody is not determined. The detection of NMDARantibodies could be caused by various parasite and virus infections. NMDAR antibodies have been regarded as a mechanistic links between Toxoplasma gondii infection and schizophrenia through involving Anti-T gondii immune responses (Lucchese, 2017). Kannan et al. also reported that T. gondii causes sustained increases in IgG-class antibodies to the NMDAR, in addition to compromising the blood-gut and blood-brain barriers (Kannan et al., 2017). In addition, NMDAR antibodies synthesis can be found after herpes simplex virus–1 infection (Leypoldt et al., 2013). In addition, other virus infections, autoimmunity, metabolomic and environmental toxic factors may cause the detection of NMDAR antibodies. These highly heterogeneous sources could explain the inconsistent findings of NMDAR antibodies and schizophrenia. Zandi et al. performed plasmapheresis to reduce levels of NMDAR Abs in a patient with schizophrenia, who exhibited significant improvements in clinical symptoms 3 weeks later. Further improvements were noted following treatment with prednisolone. Clinical and functional improvements were maintained at the 7-month follow-up without antipsychotic medications (Zandi et al., 2011). These findings suggest that the study of antibodies will be of great significance in the early identification and treatment of schizophrenia. In addition, other type of Glutamate receptor antibodies have been implciated in neurological diseases including that anti-NMDA-NR2A/B antibodies are present in patients with Systemic Lupus Erythematosus (SLE) (Levite, 2014).
One major limitation of the present study is that the control group was not ideal in our study because of their higher years of education. However, getting perfect controls is very hard, and in the follow-up data analysis, education was used as a covariant to exclude the intervention of educational differences on the results. Meanwhile, as shown in the patients group, levels of anti-NMDAR antibody were significantly associated with lower level of cognition. However, the mechanism of cognitive impairment in schizophrenia patients is not clear, and it could be due to multiple causes, for example, a disruption in frontocortical GABAergic interneuron(Lewis, et al., 2011), dysfunction in 5-HT6 receptors(Meffre, et al., 2012), furthermore, combination of genetic and developmental or environmental factors. Therefore, we speculate that NMDAR antibody is an important link for the cognitive impairment in schizophrenia patients through induced dysfunction in NMDAR. The cognitive function of the healthy control group appeared not affected and there was no association between cognition and antibody levels in controls. Persons with higher antibody levels may have a higher risk of cognitive impairment during follow-up.
Highlights.
Serum anti-NMDAR Ab was higher in FEP with schizophrenia than in healthy controls.
FEP with schizophrenia showed cognitive deficits in all domains of the MCCB.
Serum Ab levels were positively correlated with PANSS subscores and total score.
Serum Ab levels were inversely correlated with performances of verbal learning and memory, working memory, speed of processing and total score of MCCB in the patient’s group.
Autoimmunity against NMDAR may be a major factor in schizophrenia.
Acknowledgments
Supports were received for this study from the National Key R & D Program of China (2016YFC1307000), Beijing Municipal Administration of Hospitals Clinical medicine Dengfeng plan funding support (DFL20151901), National Natural Science Foundation of China (81771452, 81761128021), Beijing Natural Science Foundation (7151005), and the National Institute of Health (R01MH112180 and R01MH116948).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ayalew M, Le-Niculescu H, Levey DF, et al. 2012. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry. 17(9): 887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. 2010. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res 44(5): 321–30. [DOI] [PubMed] [Google Scholar]
- Busse S, Busse M, Schiltz K, et al. 2012. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations. Brain Behav Immun 26(8): 1273–9. [DOI] [PubMed] [Google Scholar]
- Castillo-Gomez E, Kästner A, Steiner J, et al. 2016. The brain as immunoprecipitator of serum autoantibodies against N-Methyl-D-aspartate receptor subunit NR1. Ann Neurol 79(1): 144–51. [DOI] [PubMed] [Google Scholar]
- Contestabile A 2000. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev 32(2-3): 476–509. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Tüzün E, Wu HY, et al. 2007. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 61(1): 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. 2007. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 64(5): 532–42. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Karayiorgou M, Gogos JA. 2004. Decreased phosphorylation of NMDA receptor type 1 at serine 897 in brains of patients with Schizophrenia. J Neurosci 24(7): 1561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferensztajn-Rochowiak E, Kaczmarek M, Wójcicka M, et al. 2019. Glutamate-Related Antibodies and Peripheral Insulin-Like Growth Factor in Bipolar Disorder and Lithium Prophylaxis. Neuropsychobiology. 77(1): 49–56. [DOI] [PubMed] [Google Scholar]
- Fujita K, Yuasa T, Takahashi Y, et al. 2012. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt-Jakob disease patients. J Neuroimmunol 251(1-2): 90–3. [DOI] [PubMed] [Google Scholar]
- Fukuyama T, Takahashi Y, Kubota Y, et al. 2015. Semi-quantitative analyses of antibodies to N-methyl-d-aspartate type glutamate receptor subunits (GluN2B & GluN1) in the clinical course of Rasmussen syndrome. Epilepsy Res 113: 34–43. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. 2012. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci 32(32): 11082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Menniti FS, Traynelis SF. 2017. NMDA Receptors in the Central Nervous System. Methods Mol Biol 1677: 1–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. 2003. The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26(2): 81–9. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. 2005. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 10(1): 40–68; image 5. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. 2009. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull 35(3): 549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, et al. 2010. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 30(17): 5866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIa G, Shmukler AB, IuS Z. 2012. Dynamics of neurocognitive functioning in patients in early stages of schizophrenia and schizophrenia spectrum disorders. Zh Nevrol Psikhiatr Im S S Korsakova. 112(8): 7–14. [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, et al. 1999. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 283(5398): 70–4. [DOI] [PubMed] [Google Scholar]
- Ikura T, Katsuse O, Chiba Y, et al. 2016. Evaluation of titers of antibodies against peptides of subunits NR1 and NR2B of glutamate receptor by enzyme-linked immunosorbent assay in psychiatric patients with anti-thyroid antibodies. Neurosci Lett 628: 201–6. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, Symes W, Little KC, et al. 2013. Stroke patients develop antibodies that react with components of N-methyl-D-aspartate receptor subunit 1 in proportion to lesion size. Stroke. 44(8): 2212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Gressitt KL, Yang S, et al. 2017. Pathogen-mediated NMDA receptor autoimmunity and cellular barrier dysfunction in schizophrenia. Transl Psychiatry. 7(8): e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. 2010. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia. Brain Res Bull 83(3-4): 108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Eesley CE, Poe MP. 2005. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 57(6): 688–91. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, et al. 2008. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 165(2): 214–20. [DOI] [PubMed] [Google Scholar]
- Knowles EE, David AS, Reichenberg A. 2010. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 167(7): 828–35. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Coyle TR, Rowland LM, et al. 2017. Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry. 74(9): 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal C, Diamond B. 2012. Aspects of CNS lupus: mouse models of anti-NMDA receptor antibody mediated reactivity. Methods Mol Biol 900: 181–206. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 51(3): 199–214. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Palmer-Cooper EC, Pollak T, et al. 2017. Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. Lancet Psychiatry. 4(1): 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M 2014. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 121(8): 1029–75. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Fish KN, Arion D, Gonzalez-Burgos G. 2011. Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Curr Opin Neurobiol 21(6): 866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F, Titulaer MJ, Aguilar E, et al. 2013. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology. 81(18): 1637–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Hung YY, Tsai MC, Huang TL. 2017. Increased serum anti-N-methyl-D-aspartate receptor antibody immunofluorescence in psychiatric patients with past catatonia. PLoS One. 12(10):e0187156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G 2017. From Toxoplasmosis to Schizophrenia via NMDA Dysfunction: Peptide Overlap between Toxoplasma gondii and N-Methyl-d-Aspartate Receptors As a Potential Mechanistic Link. Front Psychiatry. 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masdeu JC, González-Pinto A, Matute C, et al. 2012. Serum IgG antibodies against the NR1 subunit of the NMDA receptor not detected in schizophrenia. Am J Psychiatry. 169(10): 1120–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner HG, Rojewski MT, Schmitt A, Tumani H, Bechter K, Schmitt M. 2009. Flow cytometric analysis of T cell subsets in paired samples of cerebrospinal fluid and peripheral blood from patients with neurological and psychiatric disorders. Brain Behav Immun 23(1): 134–42. [DOI] [PubMed] [Google Scholar]
- Meffre J, Chaumont-Dubel S, Mannoury lCC, et al. 2012. 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol Med 4(10): 1043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. 2006. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl). 188(4): 408–24. [DOI] [PubMed] [Google Scholar]
- Nakao K, Jeevakumar V, Jiang SZ, et al. 2018. .Schizophrenia-Like Dopamine Release Abnormalities in a Mouse Model of NMDA Receptor Hypofunction. Schizophr Bull [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Imada H, Shiraishi E, et al. 2018. Phosphodiesterase 2A Inhibitor TAK-915 Ameliorates Cognitive Impairments and Social Withdrawal in N-Methyl-d-Aspartate Receptor Antagonist-Induced Rat Models of Schizophrenia. J Pharmacol Exp Ther 365(1): 179–188. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, et al. 2002. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 297(5579): 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, et al. 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 165(2): 203–13. [DOI] [PubMed] [Google Scholar]
- Ogawa E, Nagai T, Sakuma Y, Arinuma Y, Hirohata S. 2016. Association of antibodies to the NR1 subunit of N-methyl-D-aspartate receptors with neuropsychiatric systemic lupus erythematosus. Mod Rheumatol 26(3): 377–83. [DOI] [PubMed] [Google Scholar]
- Ohi K, Hashimoto R, Ikeda M, et al. 2015. Glutamate Networks Implicate Cognitive Impairments in Schizophrenia: Genome-Wide Association Studies of 52 Cognitive Phenotypes. Schizophr Bull 41(4): 909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak TA, McCormack R, Peakman M, Nicholson TR, David AS. 2014. Prevalence of anti-N-methyl-D-aspartate (NMDA) receptor [corrected] antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol Med 44(12): 2475–87. [DOI] [PubMed] [Google Scholar]
- Ramberger M, Peschl P, Schanda K, et al. 2015. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-D-aspartate receptor antibodies in serum using a live cell-based assay. PLoS One. 10(3): e0122037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. 2007. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci 30(6): 284–91. [DOI] [PubMed] [Google Scholar]
- Rhoads J, Guirgis H, McKnight C, Duchemin AM. 2011. Lack of anti-NMDA receptor autoantibodies in the serum of subjects with schizophrenia. Schizophr Res 129(2-3): 213–4. [DOI] [PubMed] [Google Scholar]
- Sharma R, Al-Saleem FH, Puligedda RD, Rattelle A, Lynch DR, Dessain SK. 2018. Membrane-bound and soluble forms of an NMDA receptor extracellular domain retain epitopes targeted in auto-immune encephalitis. BMC Biotechnol 18(1): 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She S, Zhang B, Li X, et al. 2017. Face-related visual search deficits in first-episode schizophrenia. Psychiatry Res 256: 144–149. [DOI] [PubMed] [Google Scholar]
- Shi C, Kang L, Yao S, et al. 2015. The MATRICS Consensus Cognitive Battery (MCCB): Co-norming and standardization in China. Schizophr Res 169(1-3): 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Walter M, Glanz W, et al. 2013. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 70(3): 271–8. [DOI] [PubMed] [Google Scholar]
- Thiebes S, Steinmann S, Curic S, et al. 2018. Alterations in interhemispheric gamma-band connectivity are related to the emergence of auditory verbal hallucinations in healthy subjects during NMDA-receptor blockade. Neuropsychopharmacology. 43(7): 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AL, Rocha NP, & Zhang X 2017. Anti-NMDAR antibodies as a new piece in schizophrenia’s puzzle. Future Sci OA, 3(2), FSO178. doi: 10.4155/fsoa-2017-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Kanbayashi T, Tanaka K, et al. 2012. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, et al. 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 77(4): 736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warikoo N, Brunwasser SJ, Benz A, et al. 2018. Positive Allosteric Modulation as a Potential Therapeutic Strategy in Anti-NMDA Receptor Encephalitis. J Neurosci 38(13): 3218–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Fung SJ, Catts VS, et al. 2013. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 18(11): 1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi MS, Irani SR, Lang B, et al. 2011. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 258(4): 686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


