Abstract
Background
Preterm birth is a global public health issue and rates in Puerto Rico are consistently among the highest in the USA. Exposures to environmental contaminants might be a contributing factor.
Methods
In a preliminary analysis from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort (n=1090), we investigated the association between urinary phthalate metabolite concentrations measured at three study visits (targeted at 20, 24, and 28 weeks of gestation) individually and averaged over pregnancy with gestational age at delivery and preterm birth. We additionally assessed differences in associations by study visit and among preterm births with a spontaneous delivery.
Results
Compared to women in the general USA population, urinary concentrations of metabolites of di-n-butyl phthalate (DBP) and di-isobutyl phthalate (DiBP) were higher among pregnant women in Puerto Rico. Interquartile range (IQR) increases in pregnancy-averages of urinary metabolites of DBP and DiBP were associated with shorter duration of gestation and increased odds of preterm birth. An IQR increase in mono-n-butyl phthalate (MBP), a metabolite of DBP, was associated with 1.55 days shorter gestation (95% confidence interval [CI]=−2.68, −0.42) and an odds ratio (OR) of 1.42 (95% confidence interval [CI]: 1.07, 1.88) for preterm birth. An IQR increase in mono-iso-butyl phthalate (MiBP), a metabolite of DiBP, was associated with 1.16 days shorter gestation (95% CI=−2.25, −0.08) and an OR of 1.32 (95% CI: 1.02, 1.71) for preterm birth. Associations were greatest in magnitude for urinary concentrations measured at the second study visit (median 23 weeks gestation). DiBP metabolite associations were greatest in magnitude in models of spontaneous preterm birth. No associations were detected with other phthalate metabolites, including those of di-2-ethylhexyl phthalate.
Conclusion
Among pregnant women in the PROTECT cohort, DBP and DiBP metabolites were associated with increased odds of preterm birth. These exposures may be contributing to elevated rates of preterm birth observed in Puerto Rico.
Keywords: phthalates, preterm birth, gestational age, Puerto Rico, Superfund
1. Introduction
Preterm birth, or delivery before 37 weeks completed gestation, is a global public health issue. Being born preterm increases the risk of neonatal mortality and various morbidities, adverse health consequences in childhood as well as later in life, and high economic costs (Butler and Behrman 2007). Preterm birth is a particular problem on the island of Puerto Rico, where rates are the highest in the USA, and among the highest worldwide (March of Dimes 2011, 2012). With a preterm birth rate of 11.5% in 2017, the March of Dimes report card issued an “F” to Puerto Rico (March of Dimes 2017).
The causes of the elevated preterm birth rates in Puerto Rico are unknown. We hypothesize that environmental exposures may be important contributing factors. Superfund waste sites are densely distributed on the island; and because of the unique karst aquifer system that supplies drinking water throughout Puerto Rico, contaminants leaching from these sites into groundwater could result in higher pollutant exposures to the local population. These pollutants could include legacy chemicals such as persistent organochlorine pesticides, heavy metals, and also modern exposures to non-persistent chemicals such as phthalates. The Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) birth cohort was established in 2011 to investigate the potential role of environmental contaminant exposures in the etiology of preterm birth within this uniquely vulnerable population.
Environmental phthalate exposure may occur through drinking water sources, diet, and use of various consumer products (Heudorf et al. 2007). In preliminary data from the PROTECT cohort, we observed that urinary concentrations of some phthalate metabolites, including mono-2-ethylhexyl phthalate (MEHP), a metabolite of di-2-ethylhexyl phthalate (DEHP), and mono-n-butyl phthalate (MnBP) were higher in this population compared to women of reproductive age from the National Health and Nutrition Examination Survey (NHANES) from the same time period (Cantonwine et al. 2014). In some, although not all, previous studies, maternal urinary concentrations of these phthalate metabolites during pregnancy have been associated with elevated odds of preterm birth (Adibi et al. 2009; Ferguson et al. 2014b; Meeker et al. 2009; Whyatt et al. 2009). The objective of the present study was to assess the association between maternal urinary phthalate metabolites measured at three study visits in pregnancy in relation to final gestational age as well as preterm birth. This analysis includes the first ~1000 participants, recruited between 2011 and 2017, representing approximately one-half of the targeted size for the full study.
2. Materials and Methods
2.1. Study population
Participants of the PROTECT cohort were recruited early in pregnancy (targeted 14 weeks of gestation) from two hospitals and 5 nearby clinics in the Northern Karst aquifer region of Puerto Rico (Ferguson et al. 2019). Exclusion criteria were: maternal age <18 or >40 years; residence outside of the Northern Karst aquifer region; use of oral contraceptives within the three months prior to pregnancy; use of in vitro fertilization to become pregnant; or any major preexisting medical conditions (e.g., diabetes) (Ferguson et al. 2019). At an initial screening, participants provided basic demographic information, reported the first day of their last menstrual period (LMP), and provided written informed consent to continue participation in the study.
At three subsequent study visits (targeted at 20, 24, and 28 weeks of gestation) participants provided spot urine specimens and questionnaire information. We collected questionnaire information on demographic and pregnancy characteristics. At the initial screening we collected maternal age, and at the first study visit participants self-reported the following information: educational attainment; family income; marital status; pre-pregnancy body mass index (BMI); employment status; current and former tobacco use; current and former alcohol use; and number of children (parity). At delivery, information on newborn sex was recorded. The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan School of Public Health, and Northeastern University. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subject research.
2.2. Gestational age at delivery
Following delivery, we recorded detailed information on complications of pregnancy and presentation at delivery. Following guidelines of ACOG (American College of Obstetricians and Gynecologists 2014), final gestational age was calculated based on LMP with verification by early pregnancy ultrasound when this information was available (76% of participants), as described in detail elsewhere (Ferguson et al. 2019). Briefly, LMP estimates were used as the gold standard, and were compared to ultrasound estimates of gestational age that were collected primarily before 14 weeks gestation (median 8.4 weeks gestation) (Ferguson et al. 2019). LMP estimates were changed to ultrasound estimates if the difference between the two was greater than a certain number of days (dependent on week during which the ultrasound was performed) (American College of Obstetricians and Gynecologists 2014; Ferguson et al. 2019). Overall, estimates were changed on less than 20 percent of the study population.
Preterm birth was defined as delivery before 37 weeks completed gestation. Additionally, because our previous work suggests that the association between urinary phthalate metabolites and preterm birth is driven by individuals who have a spontaneous preterm birth (Ferguson et al. 2014b), we examined associations with this subtype specifically. Spontaneous preterm birth was defined as preterm birth with presentation of premature rupture of the membranes, spontaneous preterm labor, or both (Ferguson et al. 2014b). We considered preterm births with preeclampsia or with both artificial membrane rupture and induced labor as non-spontaneous.
2.3. Urinary phthalate and phthalate alterative metabolites
Urine samples from 1–3 study visits were analyzed in batches on a rolling basis (i.e., as they were collected) for metabolites of phthalates and phthalate replacements at the CDC with online solid phase extraction high-performance liquid chromatography–isotope dilution tandem mass spectrometry, as described in detail elsewhere (Silva et al. 2007). Metabolites included in the present analysis are from nine batches analyzed between 2012 and 2018; metabolites as well as limits of detection (LOD) for some metabolites differed by batch.
Batches 1–3 included the following phthalate metabolites: monoethyl phthalate (MEP); mono-n-butyl phthalate (MBP); monobenzyl phthalate (MBzP); mono-isobutyl phthalate (MiBP); mono-2-ethyl-5-carboxypentyl phthalate (MECPP); mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP); mono-2-ethyl-5-oxohexyl phthalate (MEOHP); mono-2-ethylhexyl phthalate (MEHP); mono-3-carboxypropyl phthalate (MCPP); mono carboxy isooctyl phthalate (MCOP); and mono carboxy isononyl phthalate (MCNP). Beginning in batch 4, cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH), a metabolite of a phthalate replacement, was added to the analytic panel. Beginning in batch 7, mono-hydroxybutyl phthalate (MHBP), mono-hydroxyisobutyl phthalate (MHiBP), mono-isononyl phthalate (MNP), and cyclohexane-1 2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH) were added to the analytic panel. Finally, in batch 8, monooxononyl (MONP) and terephthalate metabolites mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP) and mono-2-ethyl-5-hydrohexyl terephthalate (MEHHTP) were added. Sample sizes thus differ across metabolites due to the continuous nature of laboratory analyses. LODs varied by batch as well; if concentrations below the LOD were reported they were used in analysis. For concentrations below LOD that were not reported as a numeric value, we imputed with the LOD corresponding to the batch of analysis divided by the square root of 2.
In addition to examining individual phthalate metabolites, we calculated the molar sum of DEHP metabolites (∑DEHP) using the following formula: ∑DEHP (nmol/L) = (MEHP/278.34) + (MEHHP/294.34) + (MEOHP/292.33) + (MECPP/308.33). Because of high correlation between DEHP metabolites, we only examined this summary DEHP measure and not individual phthalate metabolites in statistical models.
To account for urine dilution, we measured specific gravity using a digital handheld refractometer (ATAGO Co., Ltd., Tokyo, Japan) at the time of urine sample aliquoting. To examine distributions of metabolite concentrations, we corrected for specific gravity using the following formula: Pc=P[(1.019-1)/(SG-1)] where Pc is the SG-adjusted phthalate metabolite concentration (ng/mL), P is the observed phthalate or phthalate alternative metabolite concentration, 1.019 is the population median specific gravity, and SG is the specific gravity of the urine sample.
In order to create more stable estimates of individual exposure over the course of pregnancy, we created subject-specific geometric averages of all available measurements collected during gestation. For metabolites measured in all batches (e.g., ∑DEHP), the average reflects one measurement for 162 participants, two measurements for 406 participants, and three measurements for 522 participants. Geometric averages were calculated after correction for urinary specific gravity. Hereafter these will be referred to as pregnancy averages.
2.4. Statistical analysis
Distributions of demographic and pregnancy related characteristics were tabulated and examined in relation to pregnancy averages of urinary phthalate and phthalate alternative metabolites. We examined geometric mean metabolite concentrations by visit and for pregnancy averages and calculated Pearson correlation coefficients to examine pair-wise correlations between pregnancy averages. To assess variability in specific gravity-corrected metabolites over pregnancy we calculated intraclass correlation coefficients (ICC) among individuals with at least two urinary phthalate or phthalate alternative metabolites measured. ICC represent the ratio of within- to between- individual variability (Rosner 2000). ICC were only calculated for metabolites detected in >75% of samples at each of the three visits.
We created single pollutant statistical models for biomarkers of phthalates or phthalate alternatives measured at each study visit and for the pregnancy average, restricting our analysis to those biomarkers detected in >75% of samples at each of the three visits. All metabolite concentrations, including pregnancy averages, were natural log-transformed for consistency with previous studies. Crude models included gestational age at sample collection and specific gravity only for models from individual time points (i.e., where metabolite concentrations were uncorrected), and no covariates for models of metabolite averages (which were already corrected for specific gravity). We built full models using a forward stepwise procedure in which we added potential confounders one at a time and retained those that influenced effect estimates for multiple metabolites by >10%. Confounders that were considered for inclusion were identified by a directed acyclic graph (Figure S1) and included maternal age, education level, pre-pregnancy body mass index, and tobacco use in pregnancy. Results for both crude and adjusted models are presented as change in duration of pregnancy (days) in association with an interquartile range (IQR) difference in urinary metabolite concentration.
We additionally assessed relationships between metabolites and preterm birth in crude and adjusted logistic regression models, utilizing the same covariates that were included in models where gestational age was the outcome. In addition to modeling overall preterm birth, we examined the associations restricting to spontaneous preterm births only (i.e., removing all other preterm births from the model). Results for these models are presented as the odds of preterm birth in association with an IQR difference in urinary metabolite concentration.
3. Results
The present analysis represents a preliminary investigation of this study question, since recruitment in PROTECT is ongoing. We included participants who were recruited through the end of July 2017 and had delivered live births, and who had at least one urinary phthalate or phthalate alternative measured prenatally as well as gestational age at delivery (N=1,090). Participants from the PROTECT cohort included in the present analysis were mostly below the age of 30 at the beginning of pregnancy (67%), had some college or technical school, a college degree, or higher education (82%), and were married or cohabitating (82%; Table 1) (Ferguson et al. 2019). Approximately half (49%) of the participants had a normal BMI (18.5–25 kg/m2) prior to pregnancy. In regard to previous and current pregnancy characteristics, approximately half (48%) of the participants were nulliparous, approximately half (47%) were carrying female fetuses, and 9% of the mothers had a preterm delivery (n=101). The only demographic characteristics associated with preterm delivery were related to socioeconomic status (income and education level) (Ferguson et al. 2019).
Table 1.
Population demographic and pregnancy characteristics among women from the PROTECT cohort (n=1090).
| n (%) | |
|---|---|
| Maternal age at enrollment (years) | |
| 18–24 | 388 (35.6) |
| 25–29 | 346 (31.8) |
| 30–34 | 223 (20.5) |
| >35 | 132 (12.1) |
| missing | 1 |
| Years of education | |
| <High school | 69 (6.44) |
| High school/equivalent | 126 (11.8) |
| Some college/technical school | 380 (35.5) |
| ≥College degree | 497 (46.4) |
| missing | 18 |
| Annual household income (US dollars) | |
| <20,000 | 418 (44.2) |
| 20,000–40,000 | 302 (31.9) |
| >40,000 | 226 (23.9) |
| missing | 144 |
| Marital status | |
| Single | 195 (18.1) |
| Married/cohabitating | 881 (81.9) |
| missing | 14 |
| Pre-pregnancy body mass index (kg/m2) | |
| <18.5 | 70 (6.81) |
| 18.5–24.9 | 500 (48.6) |
| 25.0–29.9 | 278 (27.0) |
| >30 | 180 (17.5) |
| missing | 62 |
| Employment | |
| Employed | 699 (65.2) |
| Unemployed | 373 (34.8) |
| missing | 18 |
| Smoking status | |
| Never | 917 (85.3) |
| Ever | 141 (13.1) |
| Current | 17 (1.58) |
| missing | 15 |
| Alcohol use during pregnancy | |
| None | 1004 (93.7) |
| Any | 68 (6.34) |
| missing | 18 |
| Newborn sex | |
| Female | 512 (47.2) |
| Male | 572 (52.8) |
| missing | 6 |
| Preterm delivery (<37 weeks gestation) | |
| Yes | 101 (9.27) |
| No | 989 (90.7) |
3.1. Urinary phthalate and phthalate alterative metabolite distributions
Sample sizes, percent detection, and geometric mean urinary metabolites by study visit and for the pregnancy average are presented in Table 2. Results are ordered by molecular weight of the parent compound (lowest to highest). Urinary metabolite concentrations for participants included in this analysis were similar, although slightly lower, than those observed among an earlier subset (Cantonwine et al. 2014). Of the metabolites that were added to the analytic panel since the time of that publication (MHBP, MHiBP, MNP, MONP, MCOCH, MECPTP, and MEHHTP), MHBP, MHiBP, metabolites of di-n-butyl phthalate (DBP) and di-iso-butyl phthalate (DiBP), respectively, were detected in over 80% of samples analyzed. Terephthalate metabolites MECPTP and MEHHTP were both detected in >95% of samples analyzed. Detection of MONP was also high (>90%), but MNP as well as di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH) metabolites MHiNCH and MCOCH were detected in <40% of samples. Missing phthalate measurements at visit 3 was highest (n=372) due to the fact that urine samples were analyzed in a rolling nature, so late pregnancy samples from the most recent deliveries may not have had visit 3 samples analyzed yet. However, the distribution of missingness by visit was similar in mothers who delivered preterm as well as term (data not shown).
Table 2.
Limits of detection (LOD), sample size, detection frequency, and specific gravity-corrected geometric mean concentrations (GM, ng/mL) of urinary metabolites from phthalate and phthalate alternatives by study visit and for pregnancy average in the PROTECT cohort.
| Visit 1 | Visit 2 | Visit 3 | Average | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent | Metabolite | LODa | N | %>LOD | GM | N | %>LOD | GM | N | %>LOD | GM | N | GM |
| DEP | MEP | 0.6–1.2 | 908 | 99.8 | 44.1 | 919 | 99.7 | 44.7 | 713 | 99.6 | 45.8 | 1090 | 44.7 |
| DiBP | MiBP | 0.2–0.8 | 908 | 99.2 | 9.61 | 919 | 98.5 | 9.80 | 713 | 97.9 | 9.90 | 1090 | 9.94 |
| MHiBP | 0.4 | 668 | 98.4 | 4.26 | 665 | 97.6 | 4.10 | 513 | 96.9 | 4.04 | 827 | 4.31 | |
| DBP | MBP | 0.4 | 908 | 99.3 | 14.3 | 919 | 99.0 | 14.4 | 713 | 99.3 | 14.5 | 1090 | 14.9 |
| MHBP | 0.4 | 668 | 85.3 | 1.35 | 665 | 82.0 | 1.25 | 513 | 80.1 | 1.25 | 827 | 1.34 | |
| BzBP | MBzP | 0.3 | 908 | 95.3 | 2.73 | 919 | 95.2 | 2.68 | 713 | 93.3 | 2.52 | 1090 | 2.74 |
| MEHP | 0.5–0.8 | 908 | 84.1 | 2.22 | 919 | 83.2 | 2.39 | 713 | 79.9 | 2.21 | 1090 | 2.30 | |
| DEHP | MECPP | 0.2–0.4 | 908 | 100 | 13.3 | 919 | 99.9 | 12.9 | 713 | 100 | 13.2 | 1090 | 13.3 |
| MEHHP | 0.2–0.4 | 908 | 99.5 | 7.36 | 919 | 99.2 | 7.24 | 713 | 99.3 | 7.04 | 1090 | 7.31 | |
| MEOHP | 0.2 | 908 | 99.7 | 6.19 | 919 | 99.6 | 6.33 | 713 | 100 | 6.40 | 1090 | 6.38 | |
| DEHTP | MECPTP | 0.2 | 367 | 100 | 27.2 | 379 | 100 | 26.5 | 300 | 100 | 31.7 | 428 | 27.9 |
| MEHHTP | 0.4 | 367 | 98.1 | 4.94 | 379 | 96.6 | 4.29 | 300 | 97.0 | 5.03 | 428 | 4.64 | |
| DNOP | MCPP | 0.2–0.4 | 908 | 87.9 | 1.39 | 919 | 85.2 | 1.28 | 713 | 83.3 | 1.32 | 1090 | 1.38 |
| MNP | 0.9 | 668 | 30.7 | 0.71 | 665 | 31.0 | 0.72 | 513 | 25.7 | 0.63 | 827 | 0.72 | |
| DiNP | MONP | 0.4 | 367 | 93.2 | 1.91 | 379 | 92.1 | 2.04 | 300 | 91.7 | 2.08 | 428 | 2.00 |
| MCOP | 0.2–0.3 | 908 | 99.9 | 10.9 | 919 | 100 | 10.1 | 713 | 100 | 10.2 | 1090 | 10.7 | |
| DDP | MCNP | 0.2 | 908 | 99.2 | 1.83 | 919 | 98.2 | 1.73 | 713 | 98.6 | 1.73 | 1090 | 1.79 |
| DINCH | MHiNCH | 0.4 | 811 | 37.5 | 0.37 | 818 | 35.7 | 0.38 | 631 | 37.2 | 0.42 | 989 | 0.38 |
| MCOCH | 0.5 | 668 | 20.7 | 0.37 | 665 | 18.7 | 0.39 | 513 | 23.0 | 0.42 | 827 | 0.37 | |
Ranges reflect LOD values which differed across multiple batches.
Correlations between pregnancy averages of phthalate and phthalate alternative metabolites ranged from low to moderate (Table S1), with the lowest correlations observed between the terephthalate metabolites MECPTP and MEHHTP and other metabolites (R=−0.03 to 0.24) and the highest correlations observed between compounds from the same parent compound. Phthalate and phthalate alternative metabolites showed low to moderate reliability across pregnancy, with an obvious decrease in ICCs with increasing molecular weight of the parent compound (Table 3, results ordered by molecular weight of the parent compound). For example, MEP, MiBP, and MHiBP had the lowest molecular weight parent compounds and showed the most stable concentrations (ICC=0.48, 0.53, and 0.58, respectively) while the parent compound of MCNP had the highest molecular weight and had the least stable concentrations (ICC=0.18). ICC for terephthalate metabolites ranged from 0.25–0.27. ICC among individuals with measurements from all three visits were similar to those among individuals who only had measurements at two visits (data not shown).
Table 3.
Intraclass correlation coefficients (95% confidence intervals) for specific gravity-corrected urinary phthalate and phthalate alternative metabolites in the PROTECT cohort.
| Parent | Metabolite | n with >1 measure | ICC (95% CI) |
|---|---|---|---|
| DEP | MEP | 928 | 0.48 (0.44, 0.52) |
| DiBP | MiBP | 928 | 0.53 (0.49, 0.56) |
| MHiBP | 645 | 0.58 (0.53, 0.62) | |
| DBP | MBP | 928 | 0.40 (0.36, 0.45) |
| MHBP | 645 | 0.40 (0.35, 0.46) | |
| BzBP | MBzP | 928 | 0.49 (0.45, 0.53) |
| DEHP | ∑DEHP | 928 | 0.36 (0.31, 0.40) |
| DEHTP | MECPTP | 374 | 0.27 (0.20, 0.35) |
| MEHHTP | 374 | 0.25 (0.19, 0.34) | |
| DNOP | MCPP | 928 | 0.34 (0.29, 0.39) |
| DiNP | MONP | 374 | 0.09 (0.04, 0.19) |
| MCOP | 928 | 0.32 (0.27, 0.37) | |
| DDP | MCNP | 928 | 0.18 (0.14, 0.23) |
Most metabolite concentrations were higher among individuals in younger age groups, who had lower educational attainment, those in lower income categories, and who were unemployed as compared to employed (Table S2). The notable exception to this pattern were the terephthalate metabolites (MECPTP and MEHHTP) that were highest among women who were older, who had a college degree or higher, who fell into the highest income category, and who were employed.
3.2. Associations with gestational age at delivery
Final models of pregnancy averages included specific gravity-corrected averages and were adjusted for maternal age (continuous) and education level. In linear regression models adjusted for maternal age and education level, average concentrations of DBP and DiBP metabolites over pregnancy were associated with shorter duration of gestation (Table 4, results ordered first by the number of batches in which metabolites were analyzed and second by molecular weight of the parent compound). For DBP metabolites, an interquartile range (IQR) difference in average MBP was associated with 1.55 days shorter gestation (95% confidence interval [CI]: −2.68, −0.42) and MHBP was associated with 1.84 days shorter gestation (95% CI: −3.13, −0.55). For DiBP metabolites, an IQR difference in average MiBP was associated with 1.16 days shorter gestation (95% CI: −2.25, −0.08) and MHiBP was associated with 1.43 days shorter gestation (95% CI: − 2.82, −0.05). Terephthalate metabolites MECPTP and MEHHTP were associated with longer gestational duration; an IQR increase in MECPTP was associated with 1.78 days longer gestation (95% CI: 0.10, 3.46) and MEHHTP was associated with 1.75 days longer gestation (95% CI: 0.00, 3.51).
Table 4.
Adjusteda associations (95% confidence intervals) between an interquartile range difference in urinary phthalate or phthalate alternative metabolite concentrations and gestational age at birth (days).
| Visit 1 | Visit 2 | Visit 3 | Pregnancy average | ||
|---|---|---|---|---|---|
| Parent | Metabolite | 863 | 883 | 669 | 1071 |
| DEP | MEP | 0.10 (−1.02, 1.22) | 0.29 (−0.80, 1.38) | 0.28 (−0.90, 1.47) | 0.60 (−0.61, 1.80) |
| DBP | MBP | −0.51 (−1.58, 0.56) | −1.31 (−2.32, −0.30) | −0.96 (−2.13, 0.21) | −1.55 (−2.68, −0.42) |
| BzBP | MBzP | −0.72 (−1.76, 0.32) | −0.92 (−1.95, 0.11) | −0.54 (−1.70, 0.62) | −0.93 (−2.03, 0.17) |
| DiBP | MiBP | −0.80 (−1.85, 0.25) | −0.82 (−1.86, 0.22) | −1.08 (−2.25, 0.08) | −1.16 (−2.25, −0.08) |
| DEHP | ∑DEHP | 0.49 (−0.52, 1.50) | −0.48 (−1.47, 0.52) | 0.17 (−0.91, 1.25) | −0.11 (−1.25, 1.03) |
| DNOP | MCPP | −0.56 (−1.48, 0.36) | −0.45 (−1.39, 0.49) | −0.14 (−1.08, 0.79) | −0.87 (−1.90, 0.16) |
| DiNP | MCOP | −0.23 (−1.14, 0.69) | −0.43 (−1.39, 0.52) | 0.08 (−0.92, 1.08) | −0.59 (−1.67, 0.49) |
| MCNP | −0.05 (−0.87, 0.77) | −0.42 (−1.24, 0.39) | −0.31 (−1.21, 0.58) | −0.57 (−1.62, 0.48) | |
| n | 640 | 640 | 483 | 813 | |
| DBP | MHBP | −0.87 (−2.14, 0.40) | −1.62 (−2.86, −0.38) | −0.86 (−2.14, 0.42) | −1.84 (−3.13, −0.55) |
| DiBP | MHiBP | −0.76 (−2.16, 0.63) | −1.52 (−2.94, −0.11) | −1.58 (−3.08, −0.08) | −1.43 (−2.82, −0.05) |
| n | 354 | 369 | 295 | 419 | |
| DEHTP | MECPTP | 1.71 (0.32, 3.09) | 0.20 (−1.18, 1.58) | 0.80 (−0.59, 2.18) | 1.78 (0.10, 3.46) |
| MEHHTP | 1.43 (−0.08, 2.93) | 0.75 (−0.74, 2.23) | 0.97 (−0.54, 2.47) | 1.76 (0.00, 3.51) | |
| DiNP | MONP | −0.05 (−1.37, 1.27) | 0.09 (−1.11, 1.29) | −0.08 (−1.41, 1.24) | −0.76 (−2.48, 0.96) |
Pregnancy averages are corrected for urinary specific gravity and models are additionally adjusted for maternal age (continuous) and education (4 level ordinal variable). Models for individual visits 1–3 include uncorrected phthalate or phthalate alternative metabolites and are additionally adjusted for gestational age at urine sample collection and specific gravity. Associations with p value <0.05 are bolded.
The median (range) gestational age in weeks at sample collection by visit was as follows: visit 1=17.6 (16.6, 19.3); visit 2=23.4 (22.0, 25.1); visit 3=27.6 (25.9, 30.3). Visit-specific models included the same covariates (age and education), but modeled phthalate or phthalate alternative metabolite concentrations that were not corrected for specific gravity, and additionally included specific gravity levels and gestational age at sample collection as covariates. The associations observed with urinary DBP and DiBP metabolites were greatest in magnitude with concentrations measured at visit 2 (Table 4). The effect estimates that were greatest in magnitude for the positive associations between terephthalate metabolites were observed at visit 1.
3.3. Associations with preterm birth
Associations between phthalate and phthalate alternative metabolites and preterm birth showed a similar pattern (Table 5). Averaged DBP metabolites over pregnancy were associated with an increased odds of preterm birth (OR for MBP=1.42, 95% CI: 1.07, 1.88; OR for MHBP=1.33, 95% CI: 0.98, 1.81). DiBP metabolite averages were also associated with increased odds of preterm birth (OR for MiBP=1.32, 95% CI: 1.02, 1.71; OR for MHiBP=1.44, 95% CI: 1.04, 2.01). OR for spontaneous preterm birth were greater in magnitude than OR for overall preterm birth for DiBP, but not DBP, metabolites. MiBP was associated with an increased OR of 1.46 (95% CI: 1.07, 1.99) and MHiBP was associated with an increased OR of 1.60 (95% CI: 1.08, 2.37). By visit, associations between DBP and DiBP metabolites and preterm birth were greater in magnitude for concentrations measured at visit 2 compared to visits 1 or 3 (Figure 1; Table S3). For example, at visit 2, an IQR increase in MBP was associated with an OR of 1.38 (95% CI: 1.05, 1.81) but at visit 1 (OR=1.11, 95% CI: 0.82, 1.49) and visit 3 (OR=1.15, 95% CI: 0.84, 1.59) the OR were slightly lower.
Table 5.
Adjusteda odds ratios (95% confidence intervals) between an interquartile range difference in urinary phthalate or phthalate alternative metabolite concentrations averaged over pregnancy and preterm or spontaneous preterm birth.b
| Overall preterm birth | Spontaneous preterm birth | ||
|---|---|---|---|
| Parent | Metabolite | n=100 preterm, 971 term | n=65 spontaneous preterm, 971 term |
| DEP | MEP | 0.98 (0.73, 1.32) | 1.29 (0.92, 1.82) |
| DBP | MBP | 1.42 (1.07, 1.88) | 1.36 (0.97, 1.91) |
| BzBP | MBzP | 1.09 (0.84, 1.42) | 1.06 (0.77, 1.47) |
| DiBP | MiBP | 1.32 (1.02, 1.71) | 1.46 (1.07, 1.99) |
| DEHP | ∑DEHP | 0.92 (0.69, 1.22) | 0.92 (0.65, 1.29) |
| DNOP | MCPP | 1.18 (0.92, 1.51) | 1.17 (0.87, 1.59) |
| DiNP | MCOP | 1.08 (0.83, 1.41) | 1.18 (0.86, 1.62) |
| MCNP | 1.14 (0.88, 1.47) | 1.23 (0.91, 1.68) | |
| n (preterm, term) | 75, 738 | 49, 738 | |
| DBP | MHBP | 1.33 (0.98, 1.81) | 1.17 (0.80, 1.70) |
| DiBP | MHiBP | 1.44 (1.04, 2.01) | 1.60 (1.08, 2.37) |
| n (preterm, term) | 38, 381 | 24, 381 | |
| DEHTP | MECPTP | 0.65 (0.41, 1.04) | 0.67 (0.38, 1.19) |
| MEHHTP | 0.70 (0.44, 1.11) | 0.62 (0.35, 1.10) | |
| DiNP | MONP | 0.89 (0.58, 1.36) | 0.77 (0.45, 1.30) |
Adjusted for maternal age (continuous) and education (4 level ordinal variable).
Spontaneous preterm birth is defined as preterm birth preceded by premature rupture of membranes and/or spontaneous labor, and other cases of preterm birth are excluded from the model. Associations with p value <0.05 are bolded.
Figure 1.
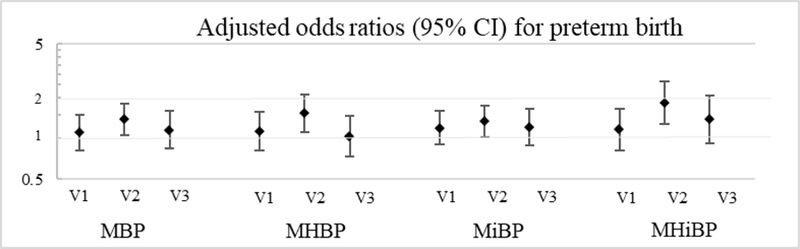
Adjusteda odds ratios (95% confidence intervals) between an interquartile range difference in urinary phthalate or phthalate alternative metabolite concentrations by study visit and preterm birth.
aAdjusted for maternal age (continuous), education (4 level ordinal variable), urinary specific gravity, and gestational age at sample collection.
3.4. Sensitivity analyses
We examined the robustness of our results to adjustment for different sets of covariates in models of phthalate or phthalate alternative metabolites and gestational age at delivery. Effect estimates were comparable, although generally greater in magnitude, in crude models (Table S4). Maternal pre-pregnancy BMI and tobacco use were identified as confounders based on our DAG but were not included in final models due to a minimal influence on effect estimates as well as missingness (Table S5). Finally, when we adjusted for year of delivery, which was associated with urinary phthalate metabolites but not with gestational age at delivery (Ferguson et al. 2019; Rodríguez-Carmona et al. [accepted]), point estimates and confidence intervals varied slightly but interpretation of our results remained largely the same for both gestational age at delivery (Table S6).
4. Discussion
In PROTECT, a prospective birth cohort of pregnant women in Puerto Rico, urinary metabolites of the phthalates DBP and DiBP, particularly in samples collected in mid to late pregnancy, were associated with reduced gestational age and increased odds of preterm birth. There was also evidence that the associations observed with DiBP metabolites were greater in magnitude among women delivering a spontaneous preterm birth compared to overall preterm birth.
These findings are consistent with our previous work demonstrating an association between DBP and DiBP metabolites and spontaneous preterm birth in pregnant women from the Boston area (Ferguson et al. 2014b), as well as findings from a small case-control study of pregnant women in Mexico City (Meeker et al. 2009). However, no other studies measuring phthalate metabolites prenatally detected a statistically significant association between these metabolites and preterm birth or gestational age at delivery (Bloom et al. 2019; Casas et al. 2015; Polanska et al. 2016; Shoaff et al. 2016; Suzuki et al. 2010; Watkins et al. 2016; Weinberger et al. 2014; Wolff et al. 2008). It should be noted that the sample sizes for those other studies ranged from 68–391 participants, with rather small numbers of preterm births in each study, and three out of those seven studies observed inverse, although non-significant, associations between DBP or DiBP metabolites and gestational age at delivery, consistent with what we observed here (Suzuki et al. 2010; Watkins et al. 2016; Weinberger et al. 2014). The present analysis is the largest prospective cohort study to examine the association between urinary phthalate or phthalate alternative metabolites and gestational age at delivery, and it is possible that smaller studies, and studies with few adverse pregnancy outcomes, cannot accurately capture this association.
In our sub-analyses examining windows of vulnerability to exposure and associations with spontaneous preterm birth, we noted consistency with our previous findings. In PROTECT, we found that the associations between DBP and DiBP metabolites and preterm birth differed slightly by study visit, with effect estimates that were greatest in magnitude observed at visit 2, (median 23 weeks gestation). This is consistent with our previous work in the case-control study from Boston, where we also observed that urinary MBP and MiBP metabolites measured later in pregnancy (median 26 weeks gestation), rather than early (10 weeks) or middle (18 weeks) pregnancy, had the greatest effect estimates for the association with preterm (Ferguson et al. 2014a). In that study we also reported that the association between MBP and MiBP and preterm birth was driven by the associations with spontaneous preterm birth. For example, the OR for the association between average MiBP over pregnancy and overall preterm birth was 0.98 (95% CI: 0.72, 1.34), whereas the OR for spontaneous preterm birth was 1.52 (95% CI: 0.97, 2.38). In PROTECT, we similarly saw that associations were strongest for DiBP metabolites among spontaneous cases of preterm birth, although we did not observe the same association for DBP metabolites. While these differences need to be confirmed, these findings support the hypothesis that select phthalates and/or their metabolites may play a role in the etiology of preterm birth by activating maternal inflammatory and oxidative stress networks that are in the pathway to preterm parturition (Challis 2000; Ferguson et al. 2016; Latini et al. 2005). This hypothesis is also substantiated by animal and in vitro data which indicates the ability of some phthalates to cause increased inflammation and oxidative stress (Robinson and Miller 2015).
We did not observe associations between DEHP metabolites and preterm birth in PROTECT, which has been reported in previous studies (Ferguson et al. 2014b; Meeker et al. 2009; Weinberger et al. 2014; Whyatt et al. 2009), although again there is inconsistency across the literature (Adibi et al. 2009; Casas et al. 2015; Polanska et al. 2016; Shoaff et al. 2016; Suzuki et al. 2010; Watkins et al. 2016; Wolff et al. 2008). This might be attributable to lower exposure in pregnant women from Puerto Rico. Urinary concentrations of MECPP were much lower in PROTECT (GM=13.3 ng/mL) compared to other studies where associations between DEHP metabolites and shortened gestation were observed. This includes a case-control study from Boston (GM=41.3 ng/mL) (Ferguson et al. 2014b), a case-control study from Mexico City (GM=29.7 ng/mL) (Meeker et al. 2009), and an inner-city cohort of pregnant women from New York (GM=38.9 ng/mL) (Whyatt et al. 2009). One likely reason for the lower metabolite concentrations in the ongoing PROTECT pregnancy cohort is that DEHP exposure has decreased considerably in the USA over time (Zota et al. 2014), as DEHP has been replaced with other compounds, such as DINCH (Silva et al. 2013). This may be interpreted as a public health success, although the relationship between exposure to DINCH in pregnancy with gestational age at delivery is unknown. The frequency of detection of DINCH metabolites in the PROTECT population was insufficient to adequately investigate such an association here. An alternative explanation for our inability to detect associations between DEHP metabolites and preterm birth could be the higher variability (i.e., lower ICC) observed for these compounds compared to others; although, other studies that have detected an effect have observed similar instability in DEHP measurements across pregnancy (Ferguson et al. 2014a).
This is the first study to investigate the association between terephthalate metabolites and preterm birth, and we observed protective associations. Di-2-ethylhexyl terephthalate (DEHTP) is a structural isomer of DEHP that has similar applications, e.g., in food packaging materials, but use likely increasing as the use of DEHP declines, and urinary concentrations have increased in the general US population from 2000 to 2016 (Silva et al. 2017). We detected both metabolites in most PROTECT women, and urinary concentrations were similar to those observed in women from NHANES (Centers for Disease Control and Prevention 2019). Interestingly, we observed higher urinary concentrations of terephthalate metabolites among women with higher socioeconomic status (e.g., higher education, income, and among women who were employed). This could potentially be explained by women in these groups having more access to new products that contain these phthalate replacements. Because terephthalate metabolites were added to the CDC analytical panel later in the study, only a subset of women (n=419) from PROTECT had terephthalate metabolites measured during gestation. Thus, the sample size for these estimates was relatively small, especially for the number of preterm births (n=38), and these findings should be interpreted with caution.
There are some limitations to our study. It is important to note that the PROTECT study is ongoing, with continued recruitment, and this is an analysis performed among what is anticipated to be half of the full cohort. Thus, some of the results from our analysis should be interpreted with caution, including: 1) the associations observed with terephthalate metabolites and other phthalate and phthalate alternative metabolites added later in the study; 2) the associations observed with spontaneous preterm birth specifically where power was more limited; and 3) the associations with visit 3 metabolite measurements, since, due to the rolling nature of analysis, fewer samples were available from that time point for the current analysis (n for visit 1=908, n for visit 2=919, n for visit 3=713). Another limitation that we must consider is co-pollutant confounding. Exposure to phthalates and phthalate alternatives may be correlated with other environmental exposures that may be pertinent to preterm birth. Future work from our study will explore the isolated effect of phthalates as well as the effects of cumulative exposure to a mixture of environmental chemical exposures.
There were a number of strengths to our study. First, this is the largest prospective birth cohort to date to examine the association between prenatal urinary concentrations of metabolites of phthalates and phthalate alternatives and preterm birth. Second, we were able to more stable estimates of average exposure over pregnancy and examine windows of vulnerability by measuring urinary metabolites at up to three time points in pregnancy per participant. Third, we studied a population that was restricted to pregnant women who did not have comorbidities such as diabetes that have been associated with poor pregnancy outcomes. While this may have limited the generalizability of our study somewhat, it enabled us to better examine the association between urinary metabolites and preterm birth without the additional heterogeneity created by differing susceptibilities to toxicants for individuals with these preexisting conditions. Finally, using information from medical records we identified cases of spontaneous preterm birth in order examine associations within this more homogeneous subtype.
In conclusion, we observed associations between maternal urinary DBP and DiBP metabolites measured during pregnancy and increased odds ratios of preterm birth, with evidence for highest risk with concentrations measured toward the end of the second trimester and especially among women delivering a spontaneous preterm birth. Exposure to select phthalates in pregnancy may contribute to risk of preterm birth in Puerto Rico, and should continue to be a focus in the ongoing PROTECT cohort study.
Supplementary Material
Highlights.
Pregnant women in Puerto Rico have an elevated risk of delivering preterm
PROTECT is a large prospective cohort of pregnant women in Puerto Rico
We measured phthalate and phthalate alternative metabolites in urine from three visits
DBP and DiBP, but not DEHP, metabolites were associated with preterm birth
Exposure to phthalates may be contributing to preterm birth risk in Puerto Rico
Acknowledgements
We thank the PROTECT participants for their contribution to this study. This work is supported by the National Institute of Environmental Health Sciences (NIEHS) grant P42ES017198. Funding for this work was also provided in part by the Intramural Research Program of the NIEHS, National Institutes of Health.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Footnotes
Conflict of interest statement: The authors declare that they have no actual or potential conflicts of interest.
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. 2009. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a us multicenter pregnancy cohort study. American journal of epidemiology 169:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. 2014. Committee opinion no 611: Method for estimating due date. Obstetrics and gynecology 124:863–866. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Wenzel AG, Brock JW, Kucklick JR, Wineland RJ, Cruze L, et al. 2019. Racial disparity in maternal phthalates exposure; association with racial disparity in fetal growth and birth outcomes. Environment international 127:473–486. [DOI] [PubMed] [Google Scholar]
- Butler AS, Behrman RE. 2007. Preterm birth: Causes, consequences, and prevention:National Academies Press. [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-González LO, Del Toro LVA, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in northern puerto rico: Distribution, temporal variability, and predictors. Environment international 62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernández MF, Garcia-Esteban R, et al. 2015. Exposure to bisphenol a and phthalates during pregnancy and ultrasound measures of fetal growth in the inma-sabadell cohort. Environmental health perspectives 124:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. National report on human exposure to environmental chemicals, updated tables january 2019 Available: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf [accessed 02/13/2019.
- Challis JR. 2000. Mechanism of parturition and preterm labor. Obstetrical & gynecological survey 55:650–660. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Rosario Z, McElrath T, Vélez Vega C, Cordero J, Alshawabkeh A, et al. 2019. Demographic risk factors for adverse birth outcomes in puerto rico in the protect cohort. PLoS ONE 14:e0217770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko Y-A, Mukherjee B, Meeker JD. 2014a. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environment international 70:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. 2014b. Environmental phthalate exposure and preterm birth. JAMA pediatrics 168:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Chen Y-H, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. 2016. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environmental health perspectives 125:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. 2007. Phthalates: Toxicology and exposure. International Journal of Hygiene and Environmental Health 210:623–634. [DOI] [PubMed] [Google Scholar]
- Latini G, Massaro M, De Felice C. 2005. Prenatal exposure to phthalates and intrauterine inflammation: A unifying hypothesis. Toxicological Sciences 85:743–743. [DOI] [PubMed] [Google Scholar]
- March of Dimes. 2011. March of dimes 2011 premature birth report card Available: http://media.graytvinc.com/documents/usmap.pdf [accessed 11/15/2017.
- March of Dimes. 2012. The global action report on preterm birth Available: https://www.marchofdimes.org/mission/global-preterm.aspx#tabs-1 [accessed 11/15/2017.
- March of Dimes. 2017. March of dimes 2017 premature birth report card Available: https://www.marchofdimes.org/materials/PrematureBirthReportCard-United-States-2017.pdf [accessed 11/15/2017.
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. 2009. Urinary phthalate metabolites in relation to preterm birth in mexico city. Environmental health perspectives 117:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Ligocka D, Sobala W, Hanke W. 2016. Effect of environmental phthalate exposure on pregnancy duration and birth outcomes. International Journal of Occupational Medicine and Environmental Health [DOI] [PubMed]
- Robinson L, Miller R. 2015. The impact of bisphenol a and phthalates on allergy, asthma, and immune function: A review of latest findings. Current Environmental Health Reports 2:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, P A, Calafat AM, X Y, Rosario Z, LD B, et al. [accepted]. Determinants and characterization of exposure to phthalates, dehtp and dinch among pregnant women in the protect birth cohort in puerto rico. Journal of Exposure Science and Environmental Epidemiology [DOI] [PMC free article] [PubMed]
- Rosner B 2000. The intraclass correlation coefficient. Fundamentals of biostatistics:562–566.
- Shoaff JR, Romano ME, Yolton K, Lanphear BP, Calafat AM, Braun JM. 2016. Prenatal phthalate exposure and infant size at birth and gestational duration. Environmental Research 150:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B 860:106–112. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Jia T, Samandar E, Preau JL Jr, Calafat AM. 2013. Environmental exposure to the plasticizer 1, 2-cyclohexane dicarboxylic acid, diisononyl ester (dinch) in us adults (2000—2012). Environmental Research 126:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Wong L-Y, Samandar E, Preau JL, Calafat AM, Ye X. 2017. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of us adults from 2000 to 2016. Archives of Toxicology 91:3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. 2010. Prenatal exposure to phthalate esters and pahs and birth outcomes. Environment international 36:699–704. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. 2016. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: A preliminary analysis. Reproductive Toxicology 65:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger B, Vetrano AM, Archer FE, Marcella SW, Buckley B, Wartenberg D, et al. 2014. Effects of maternal exposure to phthalates and bisphenol a during pregnancy on gestational age. The Journal of Maternal-Fetal Neonatal Medicine 27:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, et al. 2009. Prenatal di (2-ethylhexyl) phthalate exposure and length of gestation among an inner-city cohort. Pediatrics 124:e1213–e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. 2008. Prenatal phenol and phthalate exposures and birth outcomes. Environmental health perspectives 116:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environmental health perspectives 122:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


