Abstract
The hygiene hypothesis or “Old Friends” hypothesis proposes that inflammatory diseases are increasing in modern urban societies, due in part to reduced exposure to microorganisms that drive immunoregulatory circuits and a failure to terminate inappropriate inflammatory responses. Inappropriate inflammation is also emerging as a risk factor for anxiety disorders, affective disorders, and trauma-and stressor-related disorders, including posttraumatic stress disorder (PTSD), which is characterized as persistent re-experiencing of the trauma after a traumatic experience. Traumatic experiences can lead to long-lasting fear memories and fear potentiation of the acoustic startle reflex. The acoustic startle reflex is an ethologically relevant reflex and can be potentiated in both humans and rats through Pavlovian conditioning. Mycobacterium vaccae is a soil-derived bacterium with immunoregulatory and anti-inflammatory properties that has demonstrated to enhance fear extinction in the fear potentiated startle paradigm when given prior to fear conditioning. To determine if immunization with M. vaccae after fear conditioning also has protective effects, adult male Sprague Dawley rats underwent fear conditioning on days −37 and −36 followed by immunizations (3x), once per week beginning 24 h following fear conditioning, with a heat-killed preparation of M. vaccae NCTC 11659 (0.1 mg, s.c., in 100 μl borate-buffered saline) or vehicle, and, then, 3 weeks following the final immunization, were tested in the fear-potentiated startle paradigm (n = 12 per group). Rats underwent fear extinction training on days 1 through 6 followed by spontaneous recovery 14 days later (day 20). Rats were euthanized on day 21 and brain tissue was sectioned for analysis of Tph2, Htr1a, Slc6a4, Slc22a3, and Crhr2 mRNA expression throughout the brainstem dorsal and median raphe nuclei. Immunization with M. vaccae did not affect fear expression on day 1. However, M. vaccae-immunized rats showed enhanced between-session and within-session extinction beginning on day 2, relative to vehicle-immunized controls. Immunization with M. vaccae and fear-potentiated startle had minimal effects on serotonergic gene expression when assessed 42 days after the final immunization. Together with previous studies, these data are consistent with the hypothesis that immunoregulatory strategies, such as immunization with M. vaccae, have potential for both prevention and treatment of trauma- and stressor-related psychiatric disorders.
Keywords: dorsal raphe, extinction, fear-potentiated startle, posttraumatic stress disorder, serotonin
Graphical Abstract
1. Introduction
Lifetime prevalence of posttraumatic stress disorder (PTSD) is approximately 8.9% in the United States using the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria (Kilpatrick et al., 2013). A systematic review of randomized clinical trials for treatments for PTSD revealed that selective serotonin reuptake inhibitors had response rates that range from 17%-85% for treatment of PTSD (Ipser and Stein, 2012). This finding suggests that current treatments for PTSD are variable in response rates and that serotonin systems may be involved in PTSD symptomology.
Support for serotonin involvement in the etiology and pathophysiology of PTSD comes from studies demonstrating that single nucleotide polymorphisms of tryptophan hydroxylase (TPH) 1 (rs2108977) and 2 (rs11187997), rate-limiting enzymes for serotonin synthesis, were found to be associated with PTSD symptoms (Goenjian et al., 2012; Goenjian et al., 2015). Serotonin is also involved in animal models of anxiety (Ren et al., 2018) and fear conditioning (Johnson et al., 2015) (for review, see, (Bocchio et al., 2016; Hassell et al., 2018). Serotonin neurons in areas of the brain such as the dorsal raphe nucleus (DR), an area of the brain that gives rise to the majority of ascending serotonergic projections to forebrain corticolimbic structures, were found to be activated in a fear-potentiated startle (FPS) paradigm (Spannuth et al., 2011). Fear conditioning and fear extinction models such as FPS are useful tools in understanding stress-related disorders such as PTSD (VanElzakker et al., 2014).
Paradigms such as FPS have been used to investigate neural mechanisms of fear-related processes such as fear acquisition (Davis and Astrachan, 1978), between-session fear extinction (Falls et al., 1992), and within-session fear extinction (Davis et al., 1993). Extinction training is a form of inhibition of fear through a process of new learning (Myers et al., 2011; Rescorla, 2004). Individuals diagnosed with PTSD demonstrate a resistance to between-session extinction (Milad et al., 2009) as well as within-session extinction in a symptom severity dependent manner, with individuals with more severe symptoms of PTSD having more resistance to within-session extinction (Norrholm et al., 2011). Individuals with a diagnosis of PTSD and high severity of symptoms were unable to inhibit FPS in the presence of a safety signal (Jovanovic et al., 2009), suggesting that the neurocircuitry involved in inhibition of fear responses is dysregulated. In individuals diagnosed with PTSD with highest severity symptomology, a lack of inhibition of fear, as assessed in an FPS paradigm, has been proposed to differentiate between major depressive disorder and PTSD (Jovanovic et al., 2010).
Inflammatory markers such as interleukin (IL) 6 have been found to be elevated in individuals diagnosed with PTSD (Guo et al., 2012; Lindqvist et al., 2017a; Lindqvist et al., 2014). Additionally, white blood cell count is also higher in individuals diagnosed with PTSD suggesting an increase in immune cell activation (Lindqvist et al., 2017b). Specifically, T-cell populations such as CD45RA− CCR7− effector memory T-cells (Tern) were increased while CD4+CD25+Foxp3+ Treg cells were decreased in individuals with PTSD (Sommershof et al., 2009). In a separate study Morath et al. (2014), found that individuals diagnosed with PTSD that underwent Narrative Exposure Therapy had reduced Clinician Administered PTSD scores (CAPS) 4 months after therapy (Morath et al., 2014). Increases in Treg were found to correlate with reduced CAPS scores suggesting that immunoregulation to be involved in decreases in PTSD symptoms (Morath et al., 2014).
Immunizations with a heat-killed preparation of the saprophytic bacterium, Mycobacterium vaccae NCTC 11659, in mice exposed to chronic subordinate colony housing (CSC) stress, a murine model of PTSD (Reber et al., 2016a), induces a stress resilient phenotype (Reber et al., 2016b). M. vaccae prevented the stress-induced increase in secretion of IL-6 from mesenteric lymph node cells stimulated with anti-CD3 antibody ex vivo (Reber et al., 2016b). The protective effects of M. vaccae were prevented by pretreatment with an anti-CD25 antibody suggesting that M. vaccae’s protective effects were Treg-dependent (Reber et al., 2016b). In other previous studies, immunizations with M. vaccae prior to fear conditioning found enhanced between-session and within-session fear extinction (Fox et al., 2017; Loupy et al., 2018). Additionally, serotonergic genes and genes that control serotonergic signaling within the DR such as Tph2, Htr1a, and Slc22a3 and Crh, Crhr1, and Crhr2 genes in forebrain areas such as the central nucleus of the amygdala and bed nucleus of the stria terminalis were altered in animals that received M. vaccae immunizations that underwent FPS (Fox et al., 2017; Loupy et al., 2018). Therefore, treatments that can modulate serotonergic systems, lower inflammation or induce Treg are of interest for PTSD, anxiety- and fear-related disorders (Langgartner et al., 2019; Michopoulos et al., 2017).
In the current study, we hypothesized that M. vaccae immunizations after fear conditioning in a FPS paradigm would enhance between-session and within-session fear extinction. Additionally, we hypothesized that M. vaccae immunization after fear conditioning would modulate expression of serotonergic genes and genes that control serotonergic signaling within the DR.
2. Materials and Methods
2.1. Animals
Adult male Sprague Dawley® rats (Strain code 400, Crl:SD; Charles River, Wilmington, MA, USA) weighing 72-111 g (27-32 days old upon arrival) were pair-housed in standard polycarbonate rat cages (26 cm width × 47.6 cm length × 20.3 cm height; Alternative Design, Siloam Springs, AR, USA) containing an approximately 2.5 cm-deep layer of bedding (Cat. No. 7090; Teklad Sani-Chips; Harlan Laboratories, Indianapolis, IN, USA). This species, strain, and supplier were chosen for studies of FPS due to the extensive previous research conducted with these animals using this model (Davis, 1986; Fox et al., 2017). All rats were kept under standard laboratory conditions (12-h light/dark cycle, lights on at 0600 h, 22 °C) and had free access to tap water and standard rat diet (8640 Teklad 22/5 Rodent Diet, Harlan Laboratories). Cages were changed once per week. All studies were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (The National Academies Press, 2011) and the Institutional Animal Care and Use Committee at the University of Colorado Boulder approved all procedures. All possible efforts were made to minimize the number of animals used and their suffering.
2.1.1. Reagents
These studies used whole heat-killed M. vaccae National Collection of Type Cultures (NCTC) 11659 suspension [10 mg/ml solution, diluted to 1 mg/ml in 100 μl sterile borate-buffered saline (BBS), batch ENG#1, provided by BioElpida (Lyon, France)].
2.1.2. M. vaccae and vehicle immunization
Experimental rats received either subcutaneous (s.c.) immunization with 0.1 mg whole heat-killed M. vaccae suspension or received injections of 100 μl of the vehicle, sterile BBS, using 21-gauge needles and injection sites between the scapulae. The dose used in these experiments (0.1 mg) was 1/10 of the dose used in human studies (1 mg) (O'Brien et al., 2004) and identical to the dose used in previous studies in mice (Lowry et al., 2007; Reber et al., 2016b; Siebler et al., 2018) and rats (Fonken et al., 2018; Frank et al., 2018a; Loupy et al., 2018).
2.1.2.1. General experimental procedures
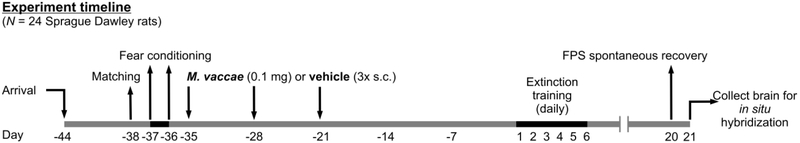
After arrival (Day −44) rats (N = 24) were housed in pairs assigned to receive subcutaneous injections of M. vaccae while the other half were housed in pairs assigned to receive subcutaneous injections of BBS vehicle on days −35, −28, and −21. Sample sizes were 1) BBS vehicle (n = 12), 2)M. vaccae (n = 12). We chose to initiate the immunization protocol on day – 35 days of the protocol in order to approximate the timing between the immunization protocol and behavioral testing in a previous study of the ability of M. vaccae, when given before fear conditioning, to enhance fear extinction (Fox et al., 2017). In this previous study, we also immunized rats beginning on day −35 of the protocol. The selection of timing for immunizations in the study by Fox and colleagues was in turn based on the timing of immunization in previous studies in mice (Reber et al., 2016b). In Reber et al., 2016, behavioral testing for anxiety-like defensive behavioral responses in the elevated plus-maze was done 39 days after the initial immunization with M. vaccae or vehicle. In Fox et al., 2017, extinction training likewise was initiated 39 days following the initial immunizations with M. vaccae or vehicle (i.e., after two days of baseline acoustic startle testing and two days of fear conditioning). In the present study, as we did not include baseline acoustic startle testing or fear conditioning immediately before extinction testing, extinction training was initiated 35 days following the initial immunizations with M. vaccae or vehicle. The stress resilience effects of M. vaccae are thought to be dependent in part on induction of Treg (Reber et al., 2016b), and the half-life of Treg in C57BL/6 mice is 27 days (Depis et al., 2016), which may explain the long-lasting stress resilience effects ofM. vaccae in different models.
2.1.2.2. Startle apparatus
The apparatus used consisted of a darkened sound-attenuated chamber (35.6 cm width × 27.6 cm depth × 49.5 cm height; SM100SP StartleMonitor Cabinet/Service Pack; Kinder Scientific, Poway, CA, USA). Inside the chamber was an aluminum base plate (Kinder Scientific) connected to a grounding cable to reduce electrical noise. An animal sensing plate for rats (SM2001; Kinder Scientific) was mounted onto the base plate, and contained a piezoelectric transducer. The transducer measured the startle response by converting the mechanical displacement of the animal sensing plate caused by the rat’s startle response into a voltage output, which was then amplified, presented to a 12-bit analog-to-digital (A/D) converter and recorded in newtons (N). Startle amplitude was defined as the maximum voltage output during the first 250 msec following each noise burst or during the first 500 msec after the onset of each noise burst during a conditioned stimulus present (CS+) trial. The transducer was calibrated prior to baseline and once again before the first FPS testing session using a Newton Impulse Calibrator (SMCAL; Kinder Scientific). The full-scale setting was determined experimentally so that the maximum startle response reached no more than 75-80% of the full-scale setting. An animal restrainer (9.5 cm width × 17.8 cm length × 17.8 cm height) was placed on top of the animal sensing plate. The animal restrainer had an adjustable ceiling that was positioned so the rat had adequate headroom but was unable to rear. This offered the added benefits of reducing the aversiveness of testing, while minimizing excessive movement. The ceiling level remained at the same height for the duration of FPS training and testing. The rat restrainer also contained 4 stainless steel bars (21.6 cm length; 0.6 cm diameter; 1.3 cm between each bar) used to deliver scrambled foot shocks (0.6 mA, 0.5 sec) that were generated by a Dual Programmable Shocker (scrambled output; DSCK-D; Kinder Scientific). The sound-attenuated chamber also contained a light bulb located 10 cm above the rat restrainer that was used to deliver the conditioned stimulus (CS) and two speakers (each 10 cm above the rat restrainer) to administer the startle stimulus and background white noise (60 dB, 3 – 32 kHz). Noise bursts and background white noise were delivered using a computer-generated sound file that was amplified through an auxiliary amplifier (AUXAMP-B; Kinder Scientific) and was administered to the rats through the two speakers in the sound-attenuated chamber. The Startle Monitor software (Build 06262-18; Kinder Scientific) was used to program the presentation, timing and sequencing of all auditory, tactile and visual stimuli. A control chassis (SM100CC Control Chassis; Kinder Scientific) with an independent microprocessor controller provided a stable interface between the data collection effort and the host personal computer. After each baseline startle testing, fear conditioning training and FPS testing session, rats were returned to their respective home cages in the vivarium. Prior to subsequent testing and/or training sessions, the apparatus was thoroughly cleaned with a soap and water-wetted paper towel, or paper towel with 95% alcohol for FPS startle so as to not interfere with electrical circuits, and the floor bars were cleaned free of dried urine/feces with medium-grit sandpaper to ensure that the current flow was not obstructed.
2.1.2.3. Baseline acoustic startle test for matching
Following six days of acclimation to the colony, on Day −38, (for experimental timeline, see Figure 1) each rat was placed into the animal restrainer inside the sound-attenuated chamber. After 5 min of acclimation to the restrainer, the rat was exposed to the first in a series of 30 acoustic startle (AS+) noise stimuli (10 trials each of 90, 95, and 105 dB noise stimuli) presented quasi-randomly (each intensity only occurred once in a block of 3 noise stimuli) with a 30-sec intertrial interval (ITI). The total session duration was 20 min. After the baseline startle test for matching, the subjects were matched into groups of 12 such that each group had equivalent baseline mean startle amplitudes.
Figure 1.
Diagrammatic illustration of experimental design and timeline. Days 1 through 6 are defined as days that rats underwent fear-potentiated startle (FPS) extinction training. Sample sizes (N = 24, vehicle, n = 12; M. vaccae, n = 12). All animals (N = 24) were matched for assignment of treatment groups on Day −38. Rats received fear conditioning on Days −37 and −36 and received subcutaneous injections of either M. vaccae or vehicle once a week for three weeks (on days −35, −28, and −21). Day −1 is defined as the day before fear extinction training; Day 1 is defined as the first day of fear extinction training. Abbreviations: FPS, fear-potentiated startle; s.c., subcutaneous.
2.1.2.4. Fear conditioning
Starting twenty-four hours after the baseline acoustic startle test for matching, i.e., on days −37 and −36, rats were each placed back into the same animal restrainer/chamber on each of 2 consecutive days (separated by 24 h) for fear conditioning (i.e., training for FPS). Fear conditioning consisted of 5 min of acclimation to the restrainer followed by the presentation of the first of 10 conditioned (CS, light; 115 lux) - unconditioned stimuli (US, footshock) pairings (variable ITI = 4 min; 3-5 min range). The light lasted for 3.7 sec and co-terminated with the footshock (0.6 mA, 0.5 sec). The total session duration was about 45 min.
2.1.2.5. Fear-potentiated startle (FPS) testing
On each of days 1-6, starting 36 d following the final day of two days of fear conditioning, rats were each returned to the same animal restrainer/chamber daily. After 5 min of acclimation to the restrainer, the rats were exposed to the first of 15 noise bursts (CS−/AS+ trials; 5 trials each at 90, 95 and 105 dB; presented quasi-randomly with each intensity only occurring once in a block of 3 trials; ITI = 30 sec), which were called “Leaders”. Thirty seconds after the last “Leader” trial, the rats were exposed to the first of 60 test trials (ITI = 30 sec). Half of the trials involved a 0.5 sec AS+ being administered 3.2 sec after the onset of the light (conditioned stimulus, CS+), which had been previously paired with shock, and co-terminated with the light (CS+/AS+ trials). For the other half of the trials, rats were presented with the AS+ (0.5 sec) in the dark, in the absence of the light (CS−/AS+ trials). There were 60 total trials for each of the CS+/AS+ (30 trials) and CS−/AS+ (30 trials) trials with 9–11 trials at each intensity of 90 (9 – 11 CS−/AS+ and 9 – 11 CS+/AS+), 95 (9 – 11 CS−/AS+ and 9 – 11 CS+/AS+) and 105 dB (9 – 11 CS−/AS+ and 9 – 11 CS+/AS+) noise stimuli and presented quasi-randomly with the restriction that each trial type only occurred once within each successive block of 6 trials. After the 60 trials there were an additional 15 acoustic noise bursts (CS−/AS+ trials; 5 trials each at 90, 95 and 105 dB; presented quasi-randomly with each intensity only occurring once in a block of 3 trials; ITI = 30 sec), which were called “Trailers”. Overall, each session lasted around 50 min. The daily assessment for FPS over the 6-day period served to determine the rate of extinction learning to the CS+. Within-session extinction was determined based on measurement of FPS response during the average of the first block of 90 dB, 95 dB, 105 dB FPS trials (3 CS+/AS+ trials and 3 CS−/AS+ trials per block) after Leaders and the average of the last block of 90 dB, 95 dB, and 105 dB FPS trials (3 CS+/AS+ trials and 3 CS−/AS+ trials per block) on each day.
2.2. Euthanasia and brain collection
Rats were euthanized 24 h following the last testing session using an overdose of sodium pentobarbital (Fatal Plus®, Vortech Pharmaceuticals Ltd., Dearborn, MI, USA; 200 mg/kg, i.p.) after which brains were dissected and immediately frozen on dry ice.
2.3. In situ hybridization histochemistry
Previously published methods were used for in situ hybridization histochemistry (Day and Akil, 1996; Fox et al., 2017; Loupy et al., 2018). Brains were placed into a rat brain matrix and blocked to align with the coronal plane. After blocking brains were sectioned into 12-μm thick sections on a cryostat (Leica CM 1950, Leica Biosystems, Buffalo Grove, IL, USA), and slices were collected beginning approximately at −7.244 mm to −8.504 mm from bregma (Paxinos and Watson, 1998) and placed in a series of 7 sets of sections, thaw-mounted on Histobond® slides (Cat. No. 16,004-406; VWR, West Chester, PA, USA) and stored at −80 °C.
2.3.1. Riboprobe preparation
Riboprobes targeting were generated using standard transcription methods, as described previously (Day and Akil, 1996). Riboprobes used in this study were designed to detect: Tph2 mRNA, encoding the rate-limiting enzyme in the biosynthesis of serotonin; Htr1a mRNA, encoding the serotonin receptor subtype 1A; Slc6a4 mRNA, encoding the high-affinity, low-capacity, sodium-dependent serotonin transporter; Slc22a3 mRNA, encoding the corticosterone-sensitive, low-affinity, high-capacity, sodium-independent organic cation transporter 3 (OCT3); and Crhr2 mRNA, encoding the corticotropin-releasing hormone receptor 2.
A sulfur 35 uridine-5’-triphosphate ([[35S]-UTP)-labeled riboprobe was used for detection of Tph2 mRNA, i.e., a 462 base (1552-2013) antisense riboprobe complementary to the rat cDNA encoding Tph2 (Tph2, National Center for Biotechnology Information (NCBI) Reference Sequence: NC_005106.4, from C.A. Lowry (Gardner et al., 2009).
A [35S]-UTP-labeled riboprobe was used for detection of Htr1a (from Dr. Stanley J. Watson) mRNA, i.e., a 911 base (333-1243) antisense riboprobe complementary to the rat cDNA encoding Htr1a (i.e., Htr1a, NCBI Reference Sequence; NC 005101.4).
A [35S]-UTP-labeled riboprobe was used for detection of Slc6a4 (originally from Dr. Stanley J. Watson, re-subcloned by H.E.W. Day) mRNA, i.e., a 491 base (828-1318) antisense riboprobe complementary to the rat cDNA encoding Slc6a4 (i.e., Slc6a4, NCBI Reference Sequence; NC_005109.4).
A [35S]-UTP-labeled riboprobe was used for detection of Slc22a3 mRNA, i.e., a 1081 base (1313-2393) antisense riboprobe complementary to the rat cDNA encoding OCT3 (Slc22a3, NCBI Reference Sequence: NM_019230.1, designed by M.R. Arnold and H.E.W. Day). Sense and antisense riboprobes were created by adding the T7 phage and clamp sequences (5′-GCGTAATACGACTCACTATAGGGAGA-3′) to the 5′ end of the forward and reverse primer sequence (Eckalbar et al., 2012). The OCT3 forward primer sequence was AGTGCAATGGGAAACACCTCTCG and the reverse primer sequence was TGCCAGGCCTTGATATGTTCATCC.
A [35S]-UTP-labeled riboprobe was used for detection of Crhr2 mRNA, i.e., using a 464 base (330-793) antisense riboprobe (from Dr. Robert Thompson) complementary to the rat cDNA encoding Crhr2 (i.e., crhr2, NCBI Reference Sequence; NC_005103.4). Riboprobes were radiolabeled via in vitro transcription, incorporating [35S]-UTP. Briefly, a nucleotide mix of adenosine triphosphate (ATP), cytidine triphosphate (CTP), guanosine triphosphate (GTP) (1.1 μl of 10 mM each) and uridine-5’-triphosphate (UTP) (1.1 μl of 0.2 mM was added to 2.2 μl 10x transcription buffer (Cat. no. FP021; Promega, Madison, WI, USA), and 0.22 μl 1 M dithiothreitol (DTT). From this solution, 6.2 μl was withdrawn and added to 1 μl (2 μg) cut DNA (antisense or sense), 1.8 μl RNase inhibitor (RNaseOUT, Cat. no. 100000840; Invitrogen, Carlsbad, CA, USA), 4 μl [35S]-UTP (1250 Ci/mmol; Cat. no. NEG039H001MC; New England Nuclear-Perkin Elmer, Boston, MA, USA) and 2 μl of the appropriate RNA polymerase (T3 for antisense and T7 for sense [Cat. no. P208C and P207B, respectively; Promega]). The mixture was incubated at 37 °C for 1 h. The template DNA was then removed by digestion with 0.75 μl RNase-free DNase I (RQ1 DNase Stop Solution, Cat. no. M199A; Promega) for 15 min at 37 °C. The probe was precipitated by ethanol in the presence of 0.5 mg/ml glycogen carrier and redissolved in 100 μl water. Probe activity (1 μl) was counted in a 5-ml volume of scintillation fluid (Ready Safe, Cat. no. p/n484013-ae; Beckman Coulter, Fullerton, CA, USA) with a beta counter (Beckman LS 3801, ser. no. 7013835) and was typically 3–4 X 106 counts per minute (cpm).
2.3.2. In situ hybridization histochemistry
Tissue sections were fixed in 4% paraformaldehyde for 1 h, acetylated in 0.1 M triethanolamine hydrochloride with 0.25% acetic anhydride for 10 min, and dehydrated through graded alcohols. Sections were hybridized overnight at 55 °C with a [35S]-UTP-labeled riboprobe diluted in hybridization buffer containing 50% formamide, 10% dextran sulfate, 2× saline sodium citrate (SSC), 50 mM phosphate-buffered saline (PBS), pH 7.4, 1× Denhardt’s solution, and 0.1 mg/ml yeast tRNA. The following day, sections were treated with RNase A, 200 μg/ml at 37 °C for 1 h, and washed to a final stringency of 0.1× SSC at 65 °C (1 h). Dehydrated sections were exposed to x-ray film (BioMax MR; Eastman Kodak, Rochester, NY, USA) for region- and probe-appropriate times: Tph2, 3 days; Htr1a, 14 days; Slc6a4, 7 days; Slc22a3, 56 days; Crhr2, 6 days, prior to film development.
2.3.3. Imaging and densitometry of in situ hybridization histochemistry autoradiograms
Autoradiographic images of probes bound to Tph2, Htr1a, Slc6a4, Slc22a3, and Crhr2 mRNA, together with 14C-labeled standards, were measured using a computer-assisted image analysis system.
Analysis was performed on a personal computer using the publicly available National Institutes of Health (NIH)-developed image analysis software ImageJ (https://imagej.nih.gov/ij/). All digital images of the x-ray films were taken while blinded to the treatment groups. Virtual matrices in the shape of each subregion of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), pontomesencephalic reticular formation (PMRF), and B9 serotonergic cell group were created, overlaid with the image, and the “mean gray value (GV) x area” within each matrix, taking into account only the area of the above-threshold signal, was measured. During the entire analysis a constant threshold function was applied, which determined the area that was actually measured within each matrix. Thus, all pixels with a gray density below threshold were automatically excluded. The individual background of each image was measured using a control macro just lateral to the medial longitudinal fasciculus (mlf). Each subregion average mean gray value had this background value subtracted.
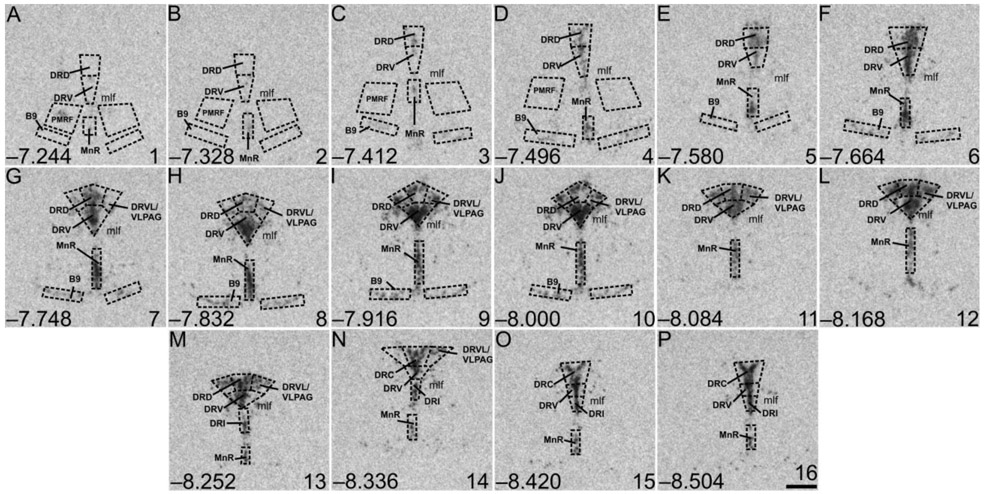
Rostrocaudal analysis atlases for Tph2, Htr1a, Slc6a4, Slc22a3, and Crhr2 expression in the DR, MnR, PMRF, and B9 serotonergic cell group were created by comparing the image of the tissue sections with a stereotaxic rat brain atlas (Paxinos and Watson, 1998). According to Paxinos and Watson (Paxinos and Watson, 1998), each rostrocaudal level of the DR was further divided into five subregions (Figure 3 (Tph2); Figures S5 (Htr1a), S8 (Slc6a4), S12 (Slc22a3), and S16 (Crhr2)). The MnR, PMRF, and serotonergic cell group B9 were not further divided. At each rostrocaudal level, the mean gray value x area values for the left and right sides of each dorsal raphe nucleus ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG), PMRF, and B9 serotonergic cell group were averaged and averaged values were used for statistical analyses. Overall mRNA expression within the five subregions of the DR, as well as in the MnR, PMRF, and B9 serotonergic cell group, were displayed by averaging background-corrected mean gray value x area values across all rostrocaudal levels per treatment group. A total of 16 rostrocaudal levels were studied for Tph2, Htr1a, and Slc6a4 mRNA expression, while 12 rostrocaudal levels were studied for Slc22a3 and Crhr2 mRNA (Figure 3 (Tph2); Figures S5 (Htr1a), S8 (Slc6a4), S12 (Slc22a3), and S16 (Crhr2)). The subdivisions studied were summarized into the following subregions and regions: B9, −7.244 mm to −8.000 mm from bregma; dorsal raphe nucleus, caudal part (DRC), −8.336 mm to −8.504 mm from bregma; dorsal raphe nucleus, dorsal part (DRD), −7.244 mm or −7.580 mm to −8.252 mm from bregma; dorsal raphe nucleus, interfascicular part (DRI), −8.252 mm to −8.504 mm from bregma; dorsal raphe nucleus, ventral part (DRV), −7.244 mm or −7.580 mm to −8.504 mm from bregma; DRVL/VLPAG, −7.748 mm to −8.336 mm from bregma; MnR, −7.244 mm or −7.580 mm to −8.504 mm from bregma; PMRF, −7.244 mm to −7.496 mm from bregma.
Figure 3.
Atlas of rat tryptophan hydroxylase 2 (Tph2) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Tph2 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.244 mm bregma through (P) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (1-16) correspond to rostrocaudal levels on the x-axis for Figure 4A and Supplemental Figure 1A-H. Dotted lines delineate different subdivisions of the DR, the MnR, B9 serotonergic cell group, and PMRF analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; PMRF pontomesencephalic reticular formation. Scale bar, 500 μm
2.4. Statistical analysis
Statistical analyses were conducted using the software package IBM statistical package for the social sciences (version 24.0, SPSS., Inc Chicago, IL, USA). All tests were two-tailed with a level of significance of 0.05. To account for missing data and individual differences, we used a linear mixed model (LMM) approach, which has been recommended for analysis of repeated measures (Judd et al., 2012). Extreme statistical outliers were identified using Grubbs’ test for single outliers using two-sided α = 0.05 (Grubbs, 1969) and were removed from the analysis. A survey of LMMs with different covariance structures was performed and the model with the best −2 log-likelihood value, an information criterion function used for goodness of fit, was selected (i.e., the covariance structure with the lowest −2 log-likelihood value was used). For analysis of FPS data, M. vaccae and day were used as fixed effects. Post hoc between-subjects comparisons were made using Fisher’s least significant difference (LSD) test and post hoc within-subjects comparisons were made using paired t-tests. For analysis of in situ hybridization histochemistry data, we used a random intercept and slope model, modeling mRNA expression (mean background-corrected gray values x area) for each rostrocaudal level within subregions of the DR, MnR, PMRF, as well as B9 serotonergic cell group. Sixteen covariate structures were assessed, including: diagonal; compound symmetry; correlation compound symmetry; heterogeneous compound symmetry; Huynh-Feldt; first-order ante-dependence; first-order autoregressive; heterogeneous first-order autoregressive; first-order analytic (constant diagonal offset); first-order analytic (heterogeneous diagonal offset); identity; toeplitz; heterogeneous toeplitz; ARMA (1,1); unstructured; and unstructured correlations. The level-one model is as follows:
Random intercepts and slopes for the covariates were modeled on each individual participant. The individual β values in the above equation explain the partial effects of M. vaccae treatment (β1 either M. vaccae or BBS vehicle), rostrocaudal level (β2, 84 μm-thick coronal slices) raphe subregion (β3, DRD, DRV, DRVL/VLPAG, DRC, DRI, PMRF, B9 serotonergic cell group, MnR) and the interaction of treatment, rostrocaudal level and subregion of the DR.
Results
Effects of M. vaccae on between-session fear extinction
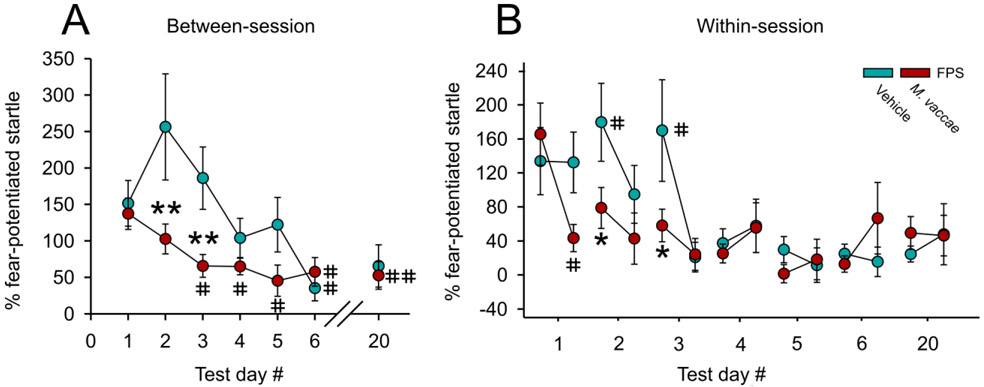
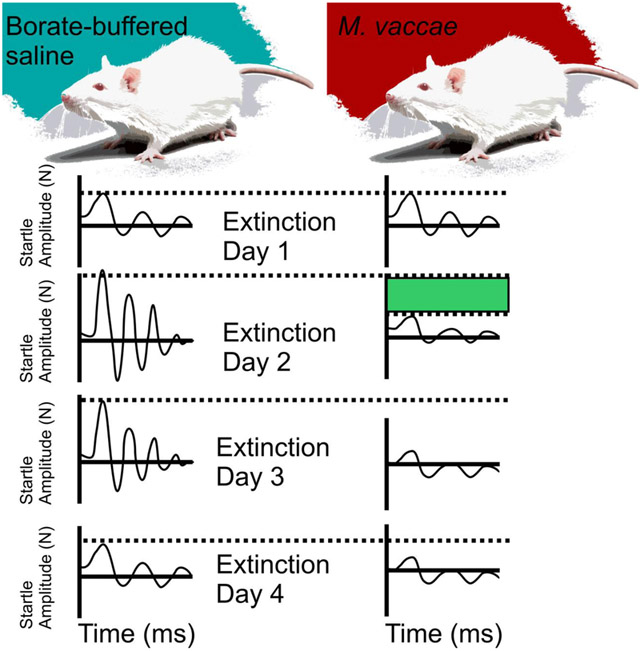
Linear mixed model analysis of between-session fear extinction revealed a main effect of M. vaccae (F(1,19.6) = 9.1; p < 0.01) and a main effect of extinction training day (F(6,20.3) = 5.0; p < 0.01) (Figure 2A). Post hoc analysis of between-session fear extinction revealed that there were no differences in FPS between M. vaccae- and vehicle-immunized controls on day 1 of fear extinction training (Figure 2A). In contrast, M. vaccae-immunized rats showed decreased FPS, relative to vehicle-treated controls, on days 2 (p < 0.001) and 3 (p < 0.007) of fear extinction. Among rats treated with M. vaccae, paired t-tests revealed a difference between extinction day 1 and the following extinction days: extinction day 3 (t(10) = 2,70, p < 0.022); extinction day 4 (t(10) = 2.41, p < 0.037); extinction day 5 (t(11) = 2.90, p < 0.014); extinction day 6 (t(11) = 2.85, p < 0.016); and spontaneous recovery on day 20 (t(11) = 3.79, p < 0.003; Figure 2A). In comparison, among vehicle-treated rats, paired t-tests revealed a difference in FPS between extinction day 1 and extinction day 6 (t(11) = 2.98, p < 0.012; Figure 2A).
Figure 2.
M. vaccae prevents fear extinction resistance and enhances both between-session and within-session extinction of conditioned fear in male rats. (A) Between-session fear-potentiated startle (FPS) extinction, measured on Days 1-6 and spontaneous recovery of fear measured on Day 20, starting 36 days after fear conditioning (FPS extinction training). (B) Within-session extinction of conditioned fear measured on Days 1-6 and spontaneous recovery of fear, measured on Day 20. Data are presented as means ± standard error of means (SEMs). Data were analyzed using (A and B), linear mixed model (LMM) analysis with fixed effects of M. vaccae and day, with block nested within day; post hoc pairwise comparisons were made using Fisher’s least significant difference (LSD) tests for between-subjects comparisons and paired t-tests for within-subjects comparisons. *p < 0.05, **p < 0.01, between-subjects effects; #p < 0.05, ##p < 0.01, within-subjects effects. A) between-session fear extinction on Days 1-6, and spontaneous recovery of fear on Day 20; B) within-session fear extinction between the first 6 and last 6 (CS−/AS+) trials within the session. Sample sizes (N = 24, vehicle, n = 12; M. vaccae, n = 12).
Effects of M. vaccae on within-session fear extinction
Linear mixed model analysis of within-session extinction of FPS across days 1– 6 and spontaneous recovery on day 20 revealed an M. vaccae x trial block within day interaction (F(54,219) = 1.68, p < 0.005). On day 1, the first day of fear extinction training, paired t-tests revealed within-session extinction in M. vaccae-treated rats (t(10) = 3.04, p < 0.012; Figure 2B) but not vehicle-treated rats. On days 2 (p < 0.05) and 3 (p < 0.05) M. vaccae-immunized animals had a lower FPS compared to vehicle-treated rats at the beginning of the extinction testing session. Vehicle-treated rats showed within-session extinction on day 2 (t(10) = 2.30, p < 0.05) and day 3 (t(10) = 2.74, p < 0.05; Figure 2B).
In situ hybridization histochemistry
In situ hybridization histochemistry was used to evaluate the effects of M. vaccae immunization on expression of a panel of genes thought to be important for control of serotonergic neurotransmission, including Tph2, Htr1a, Slc6a4, Slc22a3, and Crhr2.
Tph2 mRNA expression
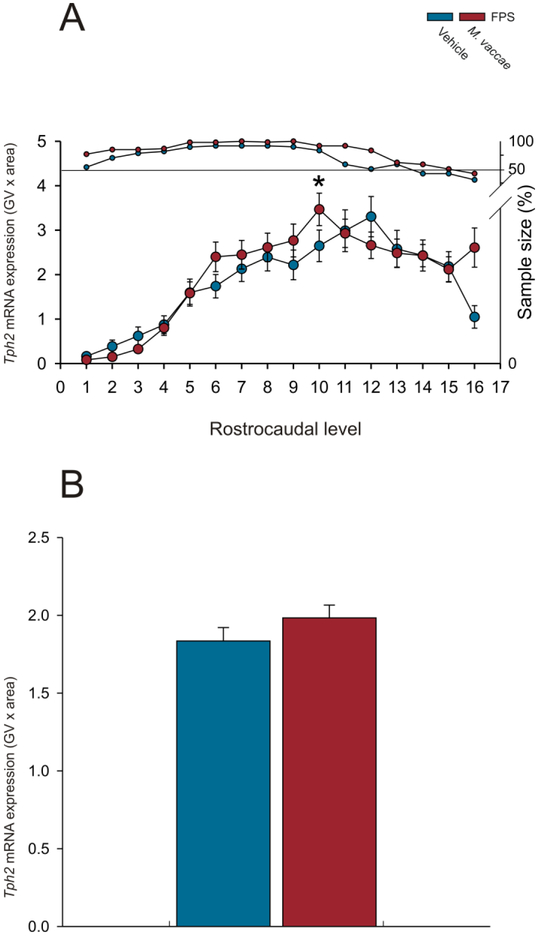
Analysis of Tph2 mRNA expression using the overall LMM revealed an M. vaccae by rostrocaudal level interaction (F(15,127.8) = 2.6; p < 0.01; Figure 3A-P; Figure 4A-B; Supplemental Figure 1A-H; Supplemental Figure 1A-H; Supplemental Table 1). When Tph2 mRNA expression was averaged at each rostrocaudal level throughout the extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF, M. vaccae increased Tph2 mRNA at rostrocaudal level −8.000 mm from bregma (Figure 4A; p < 0.05). There were no effects of M. vaccae on Tph2 mRNA expression within individual subregions of the DR, MnR, B9, or PMRF when Tph2 mRNA expression was averaged across all rostrocaudal levels of each subregion (Supplemental Figure 2A-H) or on overall Tph2 mRNA expression across all rostrocaudal levels of all subregions studied (Figure 4B).
Figure 4.
Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Tph2 mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean (A) ± SEM or (B) + SEM of Tph2 mRNA expression, i.e., (A) mean ± SEM of Tph2 mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF. (B) Overall mean + SEM Tph2 mRNA expression within the DR, MnR, B9 serotonergic cell group, and PMRF. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. *p < 0.05, M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Figure 3.
Htr1a mRNA expression
Analysis of Htr1a mRNA expression using the overall LMM revealed an M. vaccae by rostrocaudal level interaction (F(15,134.3) = 3.9; p < 0.001; Supplemental Figure 3A-P Supplemental Figure 5A-H; Supplemental Table 2). Secondary LMMs were used to determine effects of treatment within each subregion of the DR, MnR, PMRF, and B9 serotonergic cell group. When Htr1a mRNA expression was averaged at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF, M. vaccae decreased Htr1a mRNA expression at rostrocaudal level −8.000 mm from bregma (***p < 0.001; Supplemental Figure 4A). Linear mixed model analysis of specific subregions revealed an M. vaccae x rostrocaudal level interaction in the DRD (F(12,30.8) = 2.2, p < 0.05) (Supplemental Figure 5A). Specifically, M. vaccae decreased Htr1a mRNA expression at −8.000 mm from bregma in the DRD (p < 0.05; Supplemental Figure 5A). M. vaccae also increased the mean Htr1a mRNA expression within the MnR (Supplemental Figure 6F).
Slc6a4 mRNA expression
Analysis of Slc6a4 mRNA expression using the overall LMM revealed an M. vaccae by rostrocaudal level interaction (F(15,149.0) = 2.1; p < 0.015; Supplemental Figure 7A-P; ; Supplemental Figure 9A-H; Supplemental Table 3). Secondary LMM were used to determine effects of treatment within each subregion of the DR, MnR, PMRF, and B9 serotonergic cell group. Linear mixed model analysis of specific subregions revealed an M. vaccae x rostrocaudal level interaction in the B9 serotonergic cell group (F(11,42.3) = 2.3, p < 0.05; Supplemental Figure 9G, Supplemental Table 3). M. vaccae decreased Slc6a4 mRNA at −8.168 mm (p < 0.05) and – 8.252 mm (p < 0.01) from bregma (Supplemental Figure 9G). Additionally, M. vaccae increased overall mean Slc6a4 mRNA expression across all regions studied (t(1385) = 2.26, p < 0.05; Supplemental Figure 8B).
Slc22a3 mRNA expression
Analysis of Slc22a3 mRNA expression using the overall LMM revealed no effects of M. vaccae or interactions among M. vaccae, raphe subregion, or rostrocaudal level within the DR or MnR (Supplemental Figure 11A-L; Supplemental Figure 12A-B; Supplemental Figure 13A-F; Supplemental Figure 14A-F; Supplemental Table 4).
Crhr2 mRNA expression
Analysis of Crhr2 mRNA expression using the overall LMM revealed no effects of M. vaccae or interactions among M. vaccae, raphe subregion, or rostrocaudal level within the DR or MnR (Supplemental Figure 15A-L; Supplemental Figure 16A-B;Supplemental Figure 17A-G; Supplemental Figure 18A-G; ; Supplemental Table 5).
Discussion
Treatment with a heat-killed preparation of M. vaccae NCTC 11659, with three weekly immunizations initiated 24 h after fear conditioning, enhanced both between-session and within-session fear extinction in a rat FPS paradigm. Immunization with M. vaccae had no effect on the acquisition of FPS; i.e., the M. vaccae-immunized and vehicle-immunized rats showed the same level of FPS on the first day of fear extinction training, assessed 36 days after fear conditioning. However, M. vaccae-immunized rats had enhanced between-session fear extinction, with lower FPS compared to vehicle-immunized controls on days 2 and 3 of fear extinction training. Furthermore, within-subject comparisons revealed reduced FPS in M. vaccae-immunized rats by day 3 of fear extinction training; in contrast, vehicle-immunized rats did not show reductions in FPS until day 6 of fear extinction training. M. vaccae-immunized rats also showed enhanced within-session fear extinction relative to vehicle-immunized controls on day 1 of fear extinction training. When assessed 41 days after the final immunization (i.e., 15 days after the final day of extinction training), M. vaccae had no consistent effects on expression of serotonergic genes (Tph2, Htr1a, Slc6a4, Slc22a3), or genes that control serotonergic signaling (Crhr2), within the brainstem dorsal and median raphe nuclei.
Immunization with M. vaccae had no effect on the acquisition of FPS, i.e., the M. vaccae-immunized and vehicle-immunized rats showed the same level of FPS on the first day of fear extinction training. The findings in the current study are consistent with previous studies in which immunizations with M. vaccae prior to fear conditioning had no effect on either acoustic startle or fear acquisition in the FPS paradigm, but instead enhanced between-session and within-session fear extinction (Fox et al., 2017; Loupy et al., 2018).
Treatment with a heat-killed preparation of M. vaccae NCTC 11659 with immunizations initiated 24 h after fear conditioning enhanced both between-session and within-session fear extinction in the rat FPS paradigm, effects that may be dependent on enhanced immunoregulation. The effects of M. vaccae to enhance fear extinction were long-lasting (i.e., 21 [current study] to 25 days between the final M. vaccae immunization and fear-extinction training (Fox et al., 2017; Loupy et al., 2018)). Similar long-lasting effects of M. vaccae were observed on anxiety-like defensive behavioral responses in a chronic subordinate colony housing (CSC) model in mice (Reber et al., 2016b). The protective effects of M. vaccae on anxiety-like behaviors in the CSC model were absent following depletion of Treg, suggesting that the stress-protective effects are dependent on Treg (Reber et al., 2016b). These findings parallel previous findings that immunization with the same strain of M. vaccae prevents allergic airway inflammation in a murine model of asthma in a Treg-dependent manner (Zuany-Amorim et al., 2002). The protective effects of M. vaccae in allergic airway inflammation have also been demonstrated to be dependent on the production of anti-inflammatory cytokines, including interleukin (IL) 10 and transforming growth factor beta (Zuany-Amorim et al., 2002). The half-life of newly differentiated Treg is estimated to be 27 days in C57BL/6 mice (Depis et al., 2016). Therefore, induction of Treg is a reasonable hypothesis to explain the long-lasting stress-protective and anti-inflammatory effects observed in diverse model systems. An alternative, but not mutually exclusive, hypothesis is that M. vaccae induces long-lasting anti-inflammatory responses in the central nervous system (Fonken et al., 2018; Frank et al., 2018a). As a final alternative, we recently characterized a novel triacylglycerol that seems to be unique to mycobacteria, 1,2,3-tri [Z-10-hexadecenoyl] glycerol, the free fatty acid form of which, 10(Z)-hexadecenoic acid, acts as a ligand at host peroxisome proliferator-activated receptor alpha (PPARα)(Smith et al., 2019). Recent studies have demonstrated that the endocannabinoid, palmitoylethanolamide (PEA), also acts as an agonist at PPARα (Guida et al., 2017; Lo Verme et al., 2005). PEA increases the biosynthesis of allopregnanolone, an endocannabinoid, in the hippocampus and amygdala, effects that are associated with faster fear extinction learning in mice (Locci and Pinna, 2017; Pinna 2018; Sasso et al., 2012)). Future studies should determine if lipid constituents of M. vaccae, i.e., 1,2,3-tri [Z-10-hexadecenoyl] glycerol or 10(Z)-hexadecenoic acid, are sufficient to induce the enhanced fear extinction learning demonstrated using whole, heat-killed M. vaccae, and to what extent these effects are mediated by PPARα.
When assessed 41 days after the final immunization, M. vaccae had no consistent effects on expression of serotonergic genes, or genes that control serotonergic signaling, within the brainstem dorsal and median raphe nuclei. In a previous study, M. vaccae immunization prior to fear conditioning, as opposed to after fear conditioning, as done here, altered serotonergic gene expression. For example, immunization with M. vaccae prior to fear conditioning decreased Tph2 mRNA expression, encoding tryptophan hydroxylase 2, the rate-limiting enzyme for serotonin synthesis, within the MnR and decreased Slc22a3 mRNA expression (which encodes organic cation transporter 3, a corticosterone-sensitive, low-affinity, high-capacity monoamine transporter), within the DRD and DRV. However, the timelines between the final M. vaccae immunization and tissue collection were different in the two studies, i.e., 31 days in Fox et al. (2017) versus 41 days in this study. Also of potential relevance, mRNA expression was assessed 24 h following the final day of fear extinction testing in Fox et al. (2017), and, due to testing for spontaneous recovery 14 days following the final day of fear extinction training, mRNA expression was assessed 15 days following the final day of fear extinction testing in the current study. Nevertheless, studies suggest that immunoregulation is an important determinant of brain serotonergic signaling as depletion of Treg decreases hippocampal concentrations of serotonin and the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA) (Kim et al., 2012). Serotonergic projections from the MnR to the hippocampus have been suggested to comprise a stress resilience circuit, as proposed by Deakin and Graeff (Deakin and Graeff, 1991; Paul and Lowry, 2013). Indeed, lesions or pharmacological inhibition of the MnR interfere with contextual fear conditioning, but leave cued fear conditioning intact, supporting a functional connection between the MnR and hippocampal-dependent learning processes (Silva et al., 2004). Reber et al. (2016) found that immunization with M. vaccae increased Tph2 mRNA expression within the rostral portion of the DRD when measured 48 hrs after the last day of CSC stress (Reber et al., 2016b). Future studies are required to determine the time course of the effects of immunizations of M. vaccae, or other immunoregulatory and anti-inflammatory interventions, on serotonergic signaling.
Conclusions and future directions
The studies described here show that immunization with M. vaccae following fear conditioning, as opposed to before fear conditioning, as shown in previous studies (Fox et al., 2017; Loupy et al., 2018), is able to enhance both between-session and within-session fear extinction. These studies are consistent with previous studies showing that stress-induced enhancement of the acoustic startle reflex was attenuated in RAG2−/− mice, suggesting a role for peripheral mature T cells in modulating stress-induced enhancement of anxiety- and fear-like defensive behavioral responses (Clark et al., 2014; Clark et al., 2016). These findings suggest that immunization with M. vaccae, or other immunoregulatory approaches, might be considered as novel therapeutic approaches to treatment of anxiety disorders, or trauma- and stressor-related disorders, such as PTSD. Persons with a diagnosis of PTSD have been shown to have enhanced FPS (Morgan et al., 1995) as well as fear extinction resistance in a number of paradigms (Norrholm et al., 2011), suggesting a need for translational studies. Individuals with a diagnosis of PTSD have also been found to have increased secretion of proinflammatory cytokines, including IL-1β, IL-6, and TNF, from peripheral blood mononuclear cells prior to and after LPS stimulation (Gola et al., 2013). Furthermore, neuroinflammatory genes have been associated with individuals diagnosed with PTSD and comorbid conditions (Zass et al., 2017). The current study utilized a cue, light, in the FPS paradigm and future studies would benefit from exploring the effects of immunization with M. vaccae in traditional contextual and cued-fear conditioning paradigms. Future studies of the effects of immunization with M. vaccae on stress-induced enhancement of fear acquisition, or stress-induced enhancement of resistance to fear extinction are needed to further validate potential use of M. vaccae or other immunoregulatory approaches in the prevention and treatment of trauma- and stressor-related disorders including PTSD.
Supplementary Material
Supplemental Figure 1. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Tph2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Tph2 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36 followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Tph2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF). Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Figure 3.
Supplemental Figure 2. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Tph2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Tph2 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36 followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Tph2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF).
Supplemental Figure 3. Atlas of rat serotonin receptor subtype 1A (Htr1a) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Htr1a mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.244 mm bregma through (P) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (1-16) correspond to rostrocaudal levels on the x-axis for Figure 4A and Supplemental Figure 2. Dotted lines delineate different subdivisions of the DR, the median raphe nucleus, (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF) analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; PMRF pontomesencephalic reticular formation. Scale bar, 500 μm.
Supplemental Figure 4. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Htr1a mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the (A) mean ± SEM or (B) + SEM Htr1a mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). (B) Overall mean + SEM Htr1a mRNA expression within the DR, MnR, B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. ***p < 0.001. M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Supplemental Figure 3.
Supplemental Figure 5. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Htr1a mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Htr1a mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Htr1a mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF); *p < 0.05 M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 3.
Supplemental Figure 6. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Htr1a mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Htr1a mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Htr1a mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF).
Supplemental Figure 7. Atlas of rat serotonin transporter (Slc6a4) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Slc6a4 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.244 mm bregma through (P) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (1-16) correspond to rostrocaudal levels on the x-axis for Figure 4A and Supplemental Figure 4. Dotted lines delineate different subdivisions of the DR, the median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and PMRF analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; PMRF, pontomesencephalic reticular formation. Scale bar, 500 μm.
Supplemental Figure 8. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Slc6a4 mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean (A) ± SEM or (B) + SEM of Slc6a4 mRNA expression, i.e., (A) mean ± SEM of Slc6a4 mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF. (B) Overall mean + SEM Slc6a4 mRNA expression with the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Supplemental Figure 7. *p < 0.05, M. vaccae versus vehicle.
Supplemental Figure 9. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc6a4 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Slc6a4 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc6a4 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF); *p < 0.05, **p < 0.01 M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 7.
Supplemental Figure 10. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc6a4 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Slc6a4 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc6a4 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF).
Supplemental Figure 11. Atlas of rat organic cation transporter 3 (Slc22a3) mRNA expression in the midbrain raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Slc22a3 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.580 mm bregma through (L) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (5-16) correspond to rostrocaudal levels on the x-axis for Supplemental Figure 6 and Supplemental Figure 7. Dotted lines delineate different subdivisions of the DR and MnR analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus. Scale bar, 500 μm.
Supplemental Figure 12. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Slc22a3 mRNA expression in the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Each graph represents the (A) mean ± SEM or (B) + SEM of Slc22a3 mRNA expression, i.e., (A) the mean ± SEM of Slc22a3 mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR and MnR. (B) Overall mean + SEM of Slc22a3 mRNA expression within the DR and MnR, Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Supplemental Figure 11.
Supplemental Figure 13. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc22a3 mRNA expression in subdivisions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Each graph represents the mean ± SEM of Slc22a3 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc22a3 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR). Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 11.
Supplemental Figure 14. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc22a3 mRNA expression in subdivisions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Each graph represents the mean ± SEM of Slc22a3 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc22a3 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), and (F) median raphe nucleus (MnR).
Supplemental Figure 15. Atlas of rat corticotropin-releasing hormone type 2 receptor (Crhr2) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR), median raphe nucleus, (MnR), and B9 supralemniscal serotonergic cell group with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Crhr2 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.580 mm bregma through (L) −8.504 mm bregma; bregma levels are listed in the lower left or each panel. Numbers in the lower right of each panel (5-16) correspond to rostrocaudal levels on the x-axis for Figure 10 and Supplemental Figure 9. Dotted lines delineate different subdivisions of the DR, the median raphe nucleus, (MnR), and B9 serotonergic cell group analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, B9 supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus. Scale bar, 500 μm.
Supplemental Figure 16. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Crhr2 mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), and B9 serotonergic cell group. Each graph represents the mean (A) ± SEM or (B) + SEM of Crhr2 mRNA expression, i.e., (A) mean ± SEM of Crhr2 mRNA at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, and B9 serotonergic cell group. (B) Overall mean + SEM Crhr2 mRNA expression with the DR, MnR, and B9 serotonergic cell group. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-L of Supplemental Figure 15.
Supplemental Figure 17. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Crhr2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), and B9 serotonergic cell group. Each graph represents the mean ± SEM of Crhr2 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Crhr2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), and (G) B9 supralemniscal serotonergic cell group (B9); Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 15.
Supplemental Figure 18. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Crhr2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), and B9 supralemniscal serotonergic cell group. Each graph represents the mean ± SEM of Crhr2 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Crhr2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus, (MnR), and (G) B9 supralemniscal serotonergic cell group (B9). *p < 0.05, M. vaccae versus vehicle.
Highlights.
Treatment with Mycobacterium vaccae NCTC 11659 does not influence fear acquisition.
Treatment M. vaccae enhances between-session fear extinction.
Treatment with M. vaccae enhances within-session fear extinction.
Acknowledgements
We gratefully acknowledge Zachary D. Barger for proofreading the article. Funding: This work was supported by the National Institute of Mental Health (grant number 1R21MH116263). Dr. Christopher A. Lowry is supported by the National Institute of Mental Health (grant number 1R21MH116263), Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (grant number N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development (VA-ORD) RR&D Small Projects in Rehabilitation Research (SPiRE) (I21) (grant number 1 I21 RX002232-01), Colorado Clinical & Translational Sciences Institute (CCTSI) Center for Neuroscience (grant number CNSTT-15-145), the Colorado Department of Public Health and Environment (CDPHE; grant number DCEED-3510), and the Alfred P. Sloan Foundation (grant number, G-2016-7077). Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd.
List of Abbreviations
- A/N
analog to digital
- AS−
acoustic startle absent
- AS+
acoustic startle present
- ATP
adenosine triphosphate
- B9
B9 serotonergic cell group
- BBS
borate-buffered saline
- CAPS
Clinician administered PTSD score
- cpm
counts per minute
- Crhr2
corticotropin-releasing hormone receptor 2
- CS
conditioned stimulus
- CS−
conditioned stimulus absent
- CS+
conditioned stimulus present
- CTP
cytidine triphosphate
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfasicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL
dorsal raphe nucleus, ventrolateral part
- DTT
dithiothreitol
- FPS
fear-potentiated startle
- GV
gray value
- GTP
guanosine triphosphate
- Htr1a
serotonin receptor subtype 1A
- i.p.,
intraperitoneal
- ITI
intertrial interval
- LSD
least significant difference
- LMM
linear mixed model
- MnR
median raphe nucleus
- Mv
Mycobacterium vaccae NCTC 11659
- N
Newtons
- NCBI
National Center for Biotechnology Information
- NCTC
National Collection of Type Cultures
- NIH
National Institutes of Health
- PBS
phosphate-buffered saline
- PMRF
pontomesencephalic reticular formation
- SSC
saline sodium citrate
- s.c.
subcutaneous
- Slc6a4
solute carrier family 6 member 4
- Slc22a3
solute carrier family 22 member 3
- Tph2
tryptophan hydroxylase 2
- UTP
uridine-5’-triphosphate
- US
unconditioned stimulus
- Veh
vehicle
- VLPAG
ventrolateral periaqueductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M, 2016. Serotonin, amygdala and fear: assembling the puzzle. Front Neural Circuits 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Sand J, Francis TC, Nagaraju A, Michael KC, Keegan AD, Kusnecov A, Gould TD, Tonelli LH, 2014. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain Behav Immun 38, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Soroka JA, Song C, Li X, Tonelli LH, 2016. CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(−/−) mice. Stress 19, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 1986. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci 100, 814–824. [DOI] [PubMed] [Google Scholar]
- Davis M, Astrachan DI, 1978. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process 4, 95–103. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M, 1993. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58, 175–198. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H, 1996. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology 63, 207–218. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Graeff FG, 1991. 5-HT and mechanisms of defence. J Psychopharmacol 5, 305–315. [DOI] [PubMed] [Google Scholar]
- Depis F, Kwon HK, Mathis D, Benoist C, 2016. Unstable FoxP3+ T regulatory cells in NZW mice. Proc Natl Acad Sci U S A 113, 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckalbar WL, Lasku E, Infante CR, Elsey RM, Markov GJ, Allen AN, Corneveaux JJ, Losos JB, DeNardo DF, Huentelman MJ, Wilson-Rawls J, Rawls A, Kusumi K, 2012. Somitogenesis in the anole lizard and alligator reveals evolutionary convergence and divergence in the amniote segmentation clock. Dev Biol 363, 308–319. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M, 1992. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12, 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, D'Angelo HM, Heinze JD, Watkins LR, Lowry CA, Maier SF, 2018. Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol Aging 71, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Hassell JE Jr., Siebler PH, Arnold MR, Lamb AK, Smith DG, Day HEW, Smith TM, Simmerman EM, Outzen AA, Holmes KS, Brazell CJ, Lowry CA, 2017. Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun 66, 70–84. [DOI] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Schmidt D, Watkins LR, Maier SF, Lowry CA, 2018a. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav Immun 73, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Watkins LR, Maier SF, Lowry CA, 2018b. Could probiotics be used to mitigate neuroinflammation? ACS Chem Neurosci 10, 13–15. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA, 2009. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience 163, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenjian AK, Bailey JN, Walling DP, Steinberg AM, Schmidt D, Dandekar U, Noble EP, 2012. Association of TPH1, TPH2, and 5HTTLPR with PTSD and depressive symptoms. J Affect Disord 140, 244–252. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Noble EP, Steinberg AM, Walling DP, Stepanyan ST, Dandekar S, Bailey JN, 2015. Association of COMT and TPH-2 genes with DSM-5 based PTSD symptoms. J Affect Disord 172, 472–478. [DOI] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT, 2013. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 13, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE, 1969. Procedures for detecting outlying observations in samples. Technometrics 11, 1–21. [Google Scholar]
- Guida F, Luongo L, Boccella S, Giordano ME, Romano R, Bellini G, Manzo I, Furiano A, Rizzo A, Imperatore R, Iannotti FA, D’Aniello E, Piscitelli F, Sca Rossi F, Cristino L, Di Marzo V, de Novellis V, Maione S, 2017. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: involvement of the CB2 receptor. Sci Rep 7, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS, 2012. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med 5, 323–325. [DOI] [PubMed] [Google Scholar]
- Hassell JE Jr., Nguyen KT, Gates CA, Lowry CA, 2018. The impact of stressor exposure and glucocorticoids on anxiety and fear. Curr Top Behav Neurosci. 2018 October 25. doi: 10.1007/7854_2018_63. [Epub ahead of print], 1–51. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ, 2014. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A 111, 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Stein DJ, 2012. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int J Neuropsychopharmacol 15, 825–840. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Arendt D, Deehan GA, Federici LM, Bernabe C, Engleman EA, Rodd ZA, Lowry CA, Shekhar A, 2015. Pharmacological depletion of serotonin in the basolateral amygdala complex reduces anxiety and disrupts fear conditioning. Pharmacol Biochem Behav 138, 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ, 2010. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 27, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ, 2009. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res 167, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Westfall J, Kenny DA, 2012. Treating stimuli as a random factor in social psychology: A new and comprehensive solution to a pervasive but largely ignored problem. J Pers Soc Psychol 103, 54–69. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ, 2013. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim T, Bae H, 2012. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One 7, e42054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langgartner D, Lowry CA, Reber SO, 2019. Old Friends, immunoregulation, and stress resilience. Pflugers Arch 471, 237–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Dhabhar FS, Mellon SH, Yehuda R, Grenon SM, Flory JD, Bierer LM, Abu-Amara D, Coy M, Makotkine T, Reus VI, Bersani FS, Marmar CR, Wolkowitz OM, 2017a. Increased pro-inflammatory milieu in combat related PTSD - A new cohort replication study. Brain Behav Immun 59, 260–264. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Mellon SH, Dhabhar FS, Yehuda R, Grenon SM, Flory JD, Bierer LM, Abu-Amara D, Coy M, Makotkine T, Reus VI, Aschbacher K, Bersani FS, Marmar CR, Wolkowitz OM, 2017b. Increased circulating blood cell counts in combat-related PTSD: Associations with inflammation and PTSD severity. Psychiatry Res 258, 330–336. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer M, Abu-Amara D, Coy M, Neylan TC, Makotkine T, Reus VI, Yan X, Taylor NM, Marmar CR, Dhabhar FS, 2014. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 42, 81–88. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D, 2005. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67, 15–19 [DOI] [PubMed] [Google Scholar]
- Locci A, Pinna G, 2017. Stimulation of the endocannabinoid system by PEA engages neurosteroid biosynthesis to improve anxiety and fear in a PTSD mouse model. Soc. Neurosci. Abstr. [Google Scholar]
- Loupy KM, Arnold MR, Hassell JE Jr., Lieb MW, Milton LN, Cler KE, Fox JH, Siebler PH, Schmidt D, Noronha S, Day HEW, Lowry CA, 2018. Evidence that preimmunization with a heat-killed preparation of Mycobacterium vaccae reduces corticotropin-releasing hormone mRNA expression in the extended amygdala in a fear-potentiated startle paradigm. Brain Behav Immun. 2018 December 28. pii: S0889–1591(18)31248-0. doi: 10.1016/j.bbi.2018.12.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hollis JH, de Vries A, Pan B, Brunet LR, Hunt JR, Paton JF, van Kampen E, Knight DM, Evans AK, Rook GA, Lightman SL, 2007. Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience 146, 756–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T, 2017. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42, 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morath J, Gola H, Sommershof A, Hamuni G, Kolassa S, Catani C, Adenauer H, Ruf-Leuschner M, Schauer M, Elbert T, Groettrup M, Kolassa IT, 2014. The effect of trauma-focused therapy on the altered T cell distribution in individuals with PTSD: evidence from a randomized controlled trial. J Psychiatr Res 54, 1–10. [DOI] [PubMed] [Google Scholar]
- Morgan CA 3rd, Grillon C, Southwick SM, Davis M, Charney DS, 1995. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry 38, 378–385. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA Jr., Davis M, 2011. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ, 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 69, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien ME, Anderson H, Kaukel E, O'Byrne K, Pawlicki M, Von Pawel J, Reck M, Group S-O-S, 2004. SRL 172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced nonsmall-cell lung cancer: phase III results. Ann Oncol 15, 906–914. [DOI] [PubMed] [Google Scholar]
- Paul ED, Lowry CA, 2013. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol 27, 1090–1106. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego. [Google Scholar]
- Pinna G, 2018. Biomarkers for PTSD at the Interface of the Endocannabinoid and Neurosteroid Axis. Front Neurosci 12, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO, Langgartner D, Foertsch S, Postolache TT, Brenner LA, Guendel H, Lowry CA, 2016a. Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment-2016 Curt Richter Award Paper. Psychoneuroendocrinology 74, 221–230. [DOI] [PubMed] [Google Scholar]
- Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, Lowe KR, Wheeler KJ, Fox JH, Hassell JE Jr., Greenwood BN, Jansch C, Lechner A, Schmidt D, Uschold-Schmidt N, Füchsl AM, Langgartner D, Walker FR, Hale MW, Lopez Perez G, Van Treuren W, González A, Halweg-Edwards AL, Fleshner M, Raison CL, Rook GA, Peddada SD, Knight R, Lowry CA, 2016b. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A 113, E3130–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, Weissbourd B, Neve RL, Huguenard J, Horowitz MA, Luo L, 2018. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175, 472–487 e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, 2004. Spontaneous recovery. Learn Mem 11, 501–509. [DOI] [PubMed] [Google Scholar]
- Sasso O, Russo R, Vitiello S, Raso GM, D'Agostino G, Iacono A, Rana GL, Vallee M, Cuzzocrea S, Piazza PV, Meli R, Calignano A, 2012. Implication of allopregnanolone in the antinociceptive effect of N-palmitoylethanolamide in acute or persistent pain. Pain 153, 33–41. [DOI] [PubMed] [Google Scholar]
- Siebler PH, Heinze JD, Kienzle DM, Hale MW, Lukkes JL, Donner NC, Kopelman JM, Rodriguez OA, Lowry CA, 2018. Acute administration of the nonpathogenic, saprophytic bacterium, Mycobacterium vaccae, induces activation of serotonergic neurons in the dorsal raphe nucleus and antidepressant-like behavior in association with mild hypothermia. Cell Mol Neurobiol 38, 289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RC, Gargaro AC, Brandao ML, 2004. Differential regulation of the expression of contextual freezing and fear-potentiated startle by 5-HT mechanisms of the median raphe nucleus. Behav Brain Res 151, 93–101. [DOI] [PubMed] [Google Scholar]
- Smith DG, Martinelli R, Besra GS, Illarionov PA, Szatmari I, Brazda P, Allen MA, Xu W, Wang X, Nagy L, Dowell RD, Rook GAW, Rosa Brunet L, Lowry CA, 2019. Identification and characterization of a novel anti-inflammatory lipid isolated from Mycobacterium vaccae, a soil-derived bacterium with immunoregulatory and stress resilience properties. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT, 2009. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun 23, 1117–1124. [DOI] [PubMed] [Google Scholar]
- Spannuth BM, Hale MW, Evans AK, Lukkes JL, Campeau S, Lowry CA, 2011. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience 179, 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM, 2014. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 113, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zass LJ, Hart SA, Seedat S, Hemmings SM, Malan-Muller S, 2017. Neuroinflammatory genes associated with post-traumatic stress disorder: implications for comorbidity. Psychiatr Genet 27, 1–16. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine AL, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C, 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med 8, 625–629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Tph2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Tph2 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36 followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Tph2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF). Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Figure 3.
Supplemental Figure 2. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Tph2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Tph2 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36 followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Tph2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF).
Supplemental Figure 3. Atlas of rat serotonin receptor subtype 1A (Htr1a) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Htr1a mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.244 mm bregma through (P) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (1-16) correspond to rostrocaudal levels on the x-axis for Figure 4A and Supplemental Figure 2. Dotted lines delineate different subdivisions of the DR, the median raphe nucleus, (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF) analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; PMRF pontomesencephalic reticular formation. Scale bar, 500 μm.
Supplemental Figure 4. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Htr1a mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the (A) mean ± SEM or (B) + SEM Htr1a mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). (B) Overall mean + SEM Htr1a mRNA expression within the DR, MnR, B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. ***p < 0.001. M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Supplemental Figure 3.
Supplemental Figure 5. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Htr1a mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Htr1a mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Htr1a mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF); *p < 0.05 M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 3.
Supplemental Figure 6. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Htr1a mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Htr1a mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Htr1a mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF).
Supplemental Figure 7. Atlas of rat serotonin transporter (Slc6a4) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Slc6a4 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.244 mm bregma through (P) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (1-16) correspond to rostrocaudal levels on the x-axis for Figure 4A and Supplemental Figure 4. Dotted lines delineate different subdivisions of the DR, the median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and PMRF analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; PMRF, pontomesencephalic reticular formation. Scale bar, 500 μm.
Supplemental Figure 8. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Slc6a4 mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean (A) ± SEM or (B) + SEM of Slc6a4 mRNA expression, i.e., (A) mean ± SEM of Slc6a4 mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF. (B) Overall mean + SEM Slc6a4 mRNA expression with the DR, MnR, B9 supralemniscal serotonergic cell group, and PMRF. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Supplemental Figure 7. *p < 0.05, M. vaccae versus vehicle.
Supplemental Figure 9. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc6a4 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Slc6a4 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc6a4 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF); *p < 0.05, **p < 0.01 M. vaccae versus vehicle. Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 7.
Supplemental Figure 10. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc6a4 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), B9 supralemniscal serotonergic cell group, and pontomesencephalic reticular formation (PMRF). Each graph represents the mean ± SEM of Slc6a4 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc6a4 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), (G) B9 supralemniscal serotonergic cell group (B9), and (H) pontomesencephalic reticular formation (PMRF).
Supplemental Figure 11. Atlas of rat organic cation transporter 3 (Slc22a3) mRNA expression in the midbrain raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Slc22a3 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.580 mm bregma through (L) −8.504 mm bregma; bregma levels are listed in the lower left of each panel. Numbers in the lower right of each panel (5-16) correspond to rostrocaudal levels on the x-axis for Supplemental Figure 6 and Supplemental Figure 7. Dotted lines delineate different subdivisions of the DR and MnR analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus. Scale bar, 500 μm.
Supplemental Figure 12. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Slc22a3 mRNA expression in the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Each graph represents the (A) mean ± SEM or (B) + SEM of Slc22a3 mRNA expression, i.e., (A) the mean ± SEM of Slc22a3 mRNA expression at each rostrocaudal level throughout the rostrocaudal extent of the DR and MnR. (B) Overall mean + SEM of Slc22a3 mRNA expression within the DR and MnR, Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-P of Supplemental Figure 11.
Supplemental Figure 13. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc22a3 mRNA expression in subdivisions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Each graph represents the mean ± SEM of Slc22a3 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc22a3 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR). Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 11.
Supplemental Figure 14. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Slc22a3 mRNA expression in subdivisions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Each graph represents the mean ± SEM of Slc22a3 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Slc22a3 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), and (F) median raphe nucleus (MnR).
Supplemental Figure 15. Atlas of rat corticotropin-releasing hormone type 2 receptor (Crhr2) mRNA expression in the brainstem raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR), median raphe nucleus, (MnR), and B9 supralemniscal serotonergic cell group with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Crhr2 mRNA expression from a single rat in this study. The levels chosen for analysis ranged from (A) −7.580 mm bregma through (L) −8.504 mm bregma; bregma levels are listed in the lower left or each panel. Numbers in the lower right of each panel (5-16) correspond to rostrocaudal levels on the x-axis for Figure 10 and Supplemental Figure 9. Dotted lines delineate different subdivisions of the DR, the median raphe nucleus, (MnR), and B9 serotonergic cell group analyzed in this study, based on a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Abbreviations: B9, B9 supralemniscal serotonergic cell group; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus. Scale bar, 500 μm.
Supplemental Figure 16. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on overall mean Crhr2 mRNA expression in the dorsal raphe nucleus (DR), median raphe nucleus (MnR), and B9 serotonergic cell group. Each graph represents the mean (A) ± SEM or (B) + SEM of Crhr2 mRNA expression, i.e., (A) mean ± SEM of Crhr2 mRNA at each rostrocaudal level throughout the rostrocaudal extent of the DR, MnR, and B9 serotonergic cell group. (B) Overall mean + SEM Crhr2 mRNA expression with the DR, MnR, and B9 serotonergic cell group. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and spontaneous recovery 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Rostrocaudal levels 1 through 16 correspond to those illustrated in panels A-L of Supplemental Figure 15.
Supplemental Figure 17. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Crhr2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), and B9 serotonergic cell group. Each graph represents the mean ± SEM of Crhr2 mRNA expression throughout the rostrocaudal extent of the subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Crhr2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus (MnR), and (G) B9 supralemniscal serotonergic cell group (B9); Rostrocaudal levels 1 through 16 correspond to those illustrated in Supplemental Figure 15.
Supplemental Figure 18. Effects of repeated immunizations with a heat-killed preparation of M. vaccae or vehicle in rats exposed to fear conditioning, fear extinction, and spontaneous recovery on Crhr2 mRNA expression in subdivisions of the dorsal raphe nucleus (DR), median raphe nucleus (MnR), and B9 supralemniscal serotonergic cell group. Each graph represents the mean ± SEM of Crhr2 mRNA expression of each subregion indicated. Rats received fear conditioning on Days −37 and −36, followed by immunizations with a heat-killed preparation of M. vaccae or borate-buffered saline vehicle (Vehicle) on Days −35, −28, and −21, followed by fear extinction training on Days 1-6, and testing for spontaneous recovery of fear 14 days later (Day 20). Rats were euthanized 24 h later on Day 21. Graphs illustrate Crhr2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) median raphe nucleus, (MnR), and (G) B9 supralemniscal serotonergic cell group (B9). *p < 0.05, M. vaccae versus vehicle.