Abstract
CONTEXT:
The most debilitating symptoms during oxaliplatin-based chemotherapy in patients with colorectal cancer (CRC) are neuropathy and fatigue. Inflammation has been suggested to contribute to these symptoms, and the anti-inflammatory agent minocycline is safe and readily available.
OBJECTIVES:
This proof-of-concept study investigated minocycline’s capacity to reduce treatment-related neuropathy and fatigue and its impact on inflammatory markers during chemotherapy in a phase II randomized, double-blind, placebo-controlled clinical trial.
METHODS:
Patients with locally advanced or metastatic CRC who were scheduled for oxaliplatin-based chemotherapy were randomly assigned to receive either minocycline (100 mg twice daily) or placebo over 4 months from started chemotherapy. Toxicity assessments and blood samples were prospectively collected monthly. The severity of fatigue and numbness/tingling were assessed weekly using the MD Anderson Symptom Inventory. The primary endpoint, area under the curve for numbness/tingling and fatigue over approximately 4 months, were compared between the 2 arms.
RESULTS:
Of 66 evaluable participants, 32 received minocycline and 34 placebo. There was no observed significant symptom reduction on both fatigue and numbness/tingling in either arm, nor was there a difference in levels of serum pro-inflammatory and anti-inflammatory markers between arms. No grade 3 adverse events, nor disparity mediating effect on intervention were observed.
CONCLUSION:
Minocycline treatment is feasible and has a low toxicity profile. However, with 200mg/day, it did not reduce numbness/tingling or fatigue nor moderate inflammatory biomarkers from this phase II randomized study. Our results do not support further exploration of minocycline for fatigue or neuropathy symptom intervention in patients treated for CRC.
Keywords: minocycline, colorectal cancer, chemotherapy-induced symptoms, neuropathy, fatigue, patient-reported outcomes
INTRODUCTION
Patients with advanced colorectal cancer (CRC) may experience substantial symptom burden that is often exacerbated by standard chemotherapy. The most bothersome symptom related to oxaliplatin-based chemotherapy for CRC is dose-dependent chemotherapy-induced peripheral neuropathy (CIPN), which often manifests as numbness and/or tingling in the hands and feet (1); fatigue is the most prevalent symptom related to both disease and therapy in these patients (2). Oxaliplatin-induced neuropathy and fatigue may interfere with daily functioning and can persist into survivorship, becoming a significant burden for both patients with early stage or metastatic CRC (3), and effective management of CIPN and fatigue is still very challenging. Thus, it is critical to develop an effective intervention that would allow patients to continue their cancer treatment without diminishing their quality of life during or after treatment.
Conceptually, modulation of inflammatory activity presents a potential mechanism for reducing treatment-induced symptoms (4–7). The anti-inflammatory effects of minocycline, a widely used second-generation tetracycline derivative, inexpensive antibiotic, manifest in both the peripheral and central nervous systems (8). Preclinical animal modeling has provided strong evidence that minocycline has a neuroprotective effect on oxaliplatin-induced (9) and taxane-induced hyperalgesia (10). Minocycline may also have long-lasting effects in preventing neuropathic pain (11, 12) and neurodegenerative disorders (13). Finally, a recent randomized placebo-controlled pilot study reported that minocycline reduced paclitaxel-related pain and fatigue over a 12 week period, although no effect on CIPN was noted (14) and a Phase II trial reported a positive effect of symptom reduction in head and neck cancer patient undergoing radiotherapy/chemoradiation (15). Based on this evidence, we proposed a proof-of-concept study to investigate minocycline’s capacity to reduce symptoms and their impact on biobehavioral mechanisms during oxaliplatin-based chemotherapy in a phase II randomized, double-blind, placebo-controlled clinical trial in patients with CRC. Our goal was to identify a signal of effective intervention and/or prevention of both CIPN and fatigue.
We previously showed that patients with advanced non-small cell lung cancer treated at a public hospital reported a significantly more severe treatment-related symptom burden than patients treated with the same standard chemotherapy at The University of Texas MD Anderson Cancer Center (16). Therefore, we were also interested in examining possible disparities in outcomes between medically underserved patients with CRC treated in a public hospital and patients treated in a tertiary cancer center.
METHODS
Patients
Eligible patients for this prospective, double-blind, placebo-controlled phase II randomized trial were adults who (a) had early stage or metastatic CRC and were scheduled for oxaliplatin-based chemotherapy (either FOLFOX or CAPOX) lasting at least 4 months and (b) were being treated either at MD Anderson, a tertiary academic hospital in Houston, Texas, or Lyndon B. Johnson Hospital (LBJ), a Harris County, Texas, public hospital that primarily treats underserved patients.
Patients were recruited between April 2013 and May 2018. All participants were required to have no baseline peripheral neuropathy (a clinician-rated sensory score of 0 assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events v4), adequate renal and hepatic function, and the ability to read and understand English. To select a more homogenous sample, we excluded patients who were using tetracycline (the same category of the trial agent, within 2 weeks; and patients potentially with high inflammatory status due to obesity (BMI>40). Pregnant patients or resectable metastatic disease for which metastasectomy was planned within 4 months of starting chemotherapy were excluded.
Baseline patient demographic and clinical variables were collected. Patient performance status before treatment was rated by the research staff according to the Eastern Cooperative Oncology Group scale. This study was approved by the MD Anderson and LBJ Institutional Review Boards, and all patients provided study-specific written informed consent to participate. The trial is registered at ClinicalTrials.gov: .
Randomization and Intervention
Participants were randomly assigned equally using a permuted block design to receive either minocycline capsules or matching placebo capsules in size and color during 4 months of chemotherapy. Patients were advised by the pharmacy to take the capsules with food in the morning and evening. The minocycline dose in 200 mg per day was based on the previous clinical trials with majority of the studies using this dose with an excellent safety profile (17). The 4-month intervention period for the primary endpoint was chosen because at 4 months patients would be receiving the minimum dosage of oxaliplatin chemotherapy that would be expected to reach a cumulative oxaliplatin dose (780 mg/m2) considered sufficient to produce CIPN.
Assessment of Patient-Reported Outcomes
Patients rated their symptoms and the subsequent interference with activities of daily living using the MD Anderson Symptom Inventory gastrointestinal cancer module (MDASI-GI), a psychometrically validated, 24-item questionnaire containing 13 core symptom items common to all cancer types and 6 interference items (18); both “numbness/tingling” and “fatigue” are among the core items. MDASI-GI symptom items are rated on a numeric scale ranging from 0 or “not present” to 10 or “as bad as you can imagine.”
Participants self-administered and completed the MDASI-GI before starting therapy (baseline, week 0) and then weekly during the trial by patient preferred time of the day. The MDASI-GI was completed on paper when participants were evaluated in the clinic areas; otherwise, assessments were done using a computerized, telephone-based interactive voice response system or via telephone interview when patients did not respond to the automated system.
The European Organization for Research and Treatment of Cancer CIPN20 questionnaire (19) was assessed each month during treatment to characterize 3 subscales of patient-reported neuropathy information.
Other Study Assessments
Toxic effects and any adverse events (AEs) were prospectively assessed every cycle during the study period and were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4. Attribution and severity of AEs were determined by the treating oncologist and further categorized as potentially related to study medication or chemotherapy. Safety and futility monitoring for both sites was overseen by MD Anderson’s Data Safety and Monitoring Board.
Inflammatory Marker Assay
Serum was prepared from blood samples to test cytokine and chemokine levels. Specifically, leukocytes isolated from peripheral blood were stimulated ex vivo with the T cell–stimulating antibodies anti-CD2/CD28, and their supernatants after centrifugation were analyzed to determine cytokine and chemokine production as we described before (20). Samples were collected at baseline, 2 months, and 4 months (the end of the trial). We assessed serum inflammatory markers that had been identified as potentially relevant to symptom development in animal and human studies: interleukin (IL)-1 receptor antagonist, IL-5, IL-6, IL-6 receptor, tumor necrosis factor-α receptors 1 and 2, and C-reactive protein (CRP). The ex vivo stimulated leukocyte supernatants were analyzed for interferon (IFN)-γ, IL-10, IL-17A, chemokine monocyte chemoattractant protein-1, and IFN-γ–induced protein 10 (21, 22).
Blood sampling was scheduled for the morning of the patient’s routine clinic visit, concurrent with the patient’s MDASI-GI assessment. After serum isolation by centrifugation, samples were stored at −80°C for batch analysis. All serum proteins were measured in 25 μL of serum using MILLIPLEX map assays (EMD Millipore Corporation, Billerica, MA, USA), and data were acquired using a Luminex 100 Analyzer (Luminex Corporation, Austin, TX, USA). The Millipore nonmagnetic Human Soluble Cytokine Receptor Panel was used to measure soluble IL-1 receptors 1 and 2, soluble IL-2 receptor antagonist, soluble IL-6 receptor, and soluble tumor necrosis factor-α receptors 1 and 2. CRP was measured in 1:27,000 diluted serum sample prior to analysis using the Millipore Human Cardiovascular Disease Panel 2. IL-1 receptor antagonist was measured in 100 μL of serum by enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN, USA).
Statistical Methods
The overall goal of this study was to generate estimates of the clinical efficacy of minocycline in reducing symptom burden during oxaliplatin-based chemotherapy in patients with CRC to inform future clinical trials. The primary outcome variable, the area under the curve (AUC) for numbness/tingling and fatigue over approximately 4 months, were compared between the 2 arms. The AUC was calculated using trapezoidal approximation. (23) All patients who completed the intervention were included in the analysis. The missing of outcome variables were imputed via Last-Observation-Carried-Forward for AUC calculation. Our preliminary data in CRC patients undergoing chemotherapy yielded an average 4-month numbness AUC of 205.76 (equivalent to a mean daily AUC of 1.7), with a standard deviation of 168.3. Therefore, with 42 evaluable patients per arm, we expected to be able to detect a difference of 103.5 (standardized difference of 0.61) on the symptom AUC between the 2 arms with 80% power and a 2-tailed 5% significance test.
Descriptive analysis were used to demonstrate patients’ demographic and clinical characteristics and symptom burden. Clinical characteristics were compared between treatment arms using independent t-tests for continuous variables and chi-square tests for categorical variables. CIPN-20, levels of inflammatory markers were tabulated between arms, with means, SDs, effect sizes and p-values. For the longitudinal component, linear mixed-effect models provided the effect of symptom reduction for fatigue and numbness/tingling by treatment arm and the changes over time. Group-based trajectory models were used to identify high vs. low symptom development patterns over time, with each group of patients presented a consistent trend on symptom severity during the trial (24, 25). Logistic regression analysis provided the predictive value of severity of each baseline symptom for persistent high severity. We mapped MDASI-numbness/tingling with three EORTC-CIPN 20 subscales via general mixed effects models, with adjustment of time and treatment. SAS 9.4 (Gary, NC) was used for all analysis.
RESULTS
Patient Characteristics
Demographic and clinical characteristics by study arm are shown in Table 1. Of the 66 patients, 62% had stage III and 33% had stage IV disease.
Table 1.
Baseline patient, tumor, and treatment characteristics of evaluable patients, by treatment arm (n=66)
| Minocycline (n=32) | Placebo (n=34) | P-value | |
|---|---|---|---|
| Mean age, years (SD) | 53.4 (10.1) | 51.4 (10.1) | 0.43 |
| Median age, years (range) | 52 (33–76) | 54 (31–69) | |
| Educational level, n (%) | 0.14 | ||
| 12th grade and below | 17 (53.1) | 24 (70.6) | |
| Beyond 12th grade | 15 (46.9) | 10 (29.4) | |
| Race, n (%) | 0.66 | ||
| White non-Hispanic | 13 (40.6) | 12 (35.3) | |
| Hispanic | 19 (59.4) | 22 (64.7) | |
| Sex, n (%) | 0.57 | ||
| Female | 11 (34.4) | 14 (41.2) | |
| Male | 21 (65.6) | 20 (58.8) | |
| ECOG performance status, n (%) | 0.77 | ||
| Grade 0 | 25 (78.1) | 26 (76.5) | |
| Grade 1 | 6 (18.8) | 8 (23.5) | |
| Grade 2 | 1 (3.1) | 0 (0.0) | |
| Cancer stage, n (%) | 0.42 | ||
| II | 2 (6.3) | 1 (2.9) | |
| III | 22 (68.8) | 19 (55.9) | |
| IV | 8 (25.0) | 14 (41.2) | |
| Charlson comorbidity index, n (%) | 0.87 | ||
| 0 | 25 (78.1) | 26 (76.5) | |
| 1+ | 7 (21.9) | 8 (23.5) | |
| Treatment site, n (%) | 0.63 | ||
| MDA | 15 (46.9) | 18 (52.9) | |
| LBJ | 17 (53.1) | 16 (47.1) |
Abbreviations: SD, standard deviation; ECOG, Eastern Cooperative Oncology Group performance status; MDA, MD Anderson Cancer Center; LBJ, Lyndon B. Johnson Hospital.
The 33 patients from MD Anderson were primarily white non-Hispanic (64%), whereas the 33 patients from LBJ were primarily Hispanic/Latino (85%). There were no differences by age, sex, or CRC stage in patients between the 2 institutions, but years of education, employment rate, and income were all significantly lower in patients from LBJ (all P<0.001).
Compliance and Toxicity
Compliance with Symptom Assessment
The overall MDASI-GI compliance rate, calculated as the ratio of the actual number of MDASI-GI questionnaires completed by patients divided by the number of possible assessments, was 84.7% from baseline to 4 months for patients in both treatment arms. All patients contributed patient-reported outcomes (PRO) data at baseline and at 2 or more additional points.
Compliance with Intervention
From April 2013 to May 2018, 120 of 971 screened patients were identified as eligible and provided study-specific informed consent. Of the 120 enrolled patients, 28 from the minocycline arm and 26 from the placebo arm dropped out of the study after randomization. Reasons that patients did not complete the study included cancelled chemotherapy at start or later (transition from chemotherapy to hospice), switching to definitive therapy with surgery or radiation after 2–3 cycles treatment, AE-related discontinuation of chemotherapy (infection, nausea, fatigue, severe CIPN), not reachable/lost to follow-up, and noncompliance with study medication protocols. Only 1 patient refused to complete the PRO questionnaires. One patient assigned to the minocycline arm was removed from the study because our investigational pharmacy erroneously distributed placebo to this patient instead of minocycline. The 66 evaluable patients achieved a good compliance rate. Overall study medication compliance based on pill count was 72.4% (SD=28.1%). No significant differences between 2 arms were found for any of these reasons. There was no significant difference in compliance by arm (P=0.83).
Toxicity of the Trial
No grade 3 or higher AEs that were potentially related to study medication were reported. All chemotherapy-related issues (7 grade 3 AEs) were addressed within a few days. Two grade 1 AEs reported in the minocycline arm and 2 in the placebo arm (P=0.95) were judged to be potentially related to the study medication. Five grade 2 AEs in the minocycline arm and 7 in the placebo arm were related to chemotherapy, not the trial agent.
Primary Endpoint for Minocycline Intervention
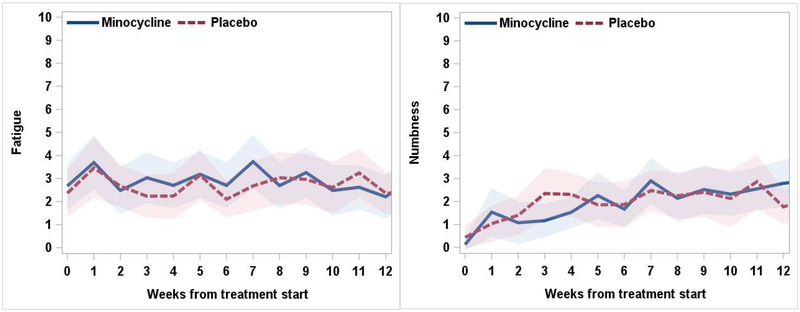
We observed no significant differences between minocycline and placebo in their impact on PROs, nor were there significant differences in toxic effects by treatment site. Figure 1 presents the severity of the 2 worst symptoms reported by our cohort (neuropathy [MDASI-GI-reported numbness/tingling] and fatigue) by treatment arm. Between arms, the AUCs for numbness/tinging (35.4±27.4 vs. 31.5±27.8, Cohen’s d = 0.14, P=0.57) and fatigue (47.9±36.4 vs. 40.3±39.3, Cohen’s d = 0.23, P=0.36) did not differ, see Table 2.
Figure 1.
Longitudinal symptom severity on fatigue and the chemotherapy-induced peripheral neuropathy symptom numbness/tingling over the course of the trial, by treatment arm (Mean, 95% CI)
Table 2.
The AUCs comparison for numbness/tinging and fatigue between arms
| Treatment | P-value | Cohen’s d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minocycline (n=32) | Placebo (n=34) | |||||||||||
| Mean | Std | Median | Min | Max | Mean | Std | Median | Min | Max | |||
| AUC_MDASI-Fatigue | 47.92 | 36.42 | 42.50 | 0.00 | 120.50 | 40.31 | 30.92 | 39.25 | 0.00 | 101.00 | 0.3624 | 0.23 |
| AUC_MDASI-Numbness/tingling | 35.41 | 27.35 | 30.00 | 1.00 | 116.50 | 31.50 | 27.80 | 26.25 | 0.00 | 113.00 | 0.5673 | 0.14 |
Consistent with the MDASI numbness/tinging item, the 3 subscales of European Organization for Research and Treatment of Cancer CIPN20 questionnaire (sensory, motor, and autonomic) did not differ between arms over time, see Table 3. By a longitudinal analysis, patient-reported numbness/tingling on MDASI was highly relevant to CIPN-Sensory subscale score overtime, regardless treatments (est=.0557, P<.0001), although numbness/tingling also significantly related to scores of CIPN-Motor and CIPN-Autonomic subscales (P<.0001 and P=.0103).
Table 3.
Comparison of subscales of CIPN-20 by timepoints between arms
| CIPN-20 | P-value | Cohen’s d | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||||||
| Minocycline | Placebo | |||||||||||||
| N | Mean | Std | Median | P25 | P75 | N | Mean | Std | Median | P25 | P75 | |||
| Sensory | ||||||||||||||
| Month 0 | 25 | 1.03 | 0.08 | 1.00 | 1.00 | 1.00 | 22 | 1.00 | 0.02 | 1.00 | 1.00 | 1.00 | 0.114 | 0.51 |
| Month 1 | 31 | 1.26 | 0.33 | 1.17 | 1.00 | 1.33 | 27 | 1.24 | 0.39 | 1.17 | 1.00 | 1.33 | 0.628 | 0.06 |
| Month 2 | 27 | 1.32 | 0.35 | 1.17 | 1.00 | 1.50 | 25 | 1.33 | 0.35 | 1.25 | 1.08 | 1.50 | 0.846 | 0.03 |
| Month 3 | 26 | 1.32 | 0.30 | 1.29 | 1.08 | 1.42 | 26 | 1.29 | 0.35 | 1.17 | 1.00 | 1.42 | 0.497 | 0.03 |
| Month 4 | 21 | 1.34 | 0.35 | 1.17 | 1.17 | 1.42 | 24 | 1.34 | 0.40 | 1.17 | 1.08 | 1.50 | 0.809 | 0.00 |
| Motor | Minocycline | Placebo | ||||||||||||
| N | Mean | Std | Median | P25 | P75 | N | Mean | Std | Median | P25 | P75 | |||
| Month 0 | 25 | 1.04 | 0.07 | 1.00 | 1.00 | 1.06 | 22 | 1.01 | 0.04 | 1.00 | 1.00 | 1.00 | 0.050 | 0.53 |
| Month 1 | 31 | 1.09 | 0.15 | 1.00 | 1.00 | 1.13 | 27 | 1.15 | 0.26 | 1.00 | 1.00 | 1.21 | 0.580 | 0.28 |
| Month 2 | 27 | 1.25 | 0.34 | 1.08 | 1.00 | 1.33 | 25 | 1.22 | 0.60 | 1.00 | 1.00 | 1.19 | 0.232 | 0.06 |
| Month 3 | 26 | 1.15 | 0.25 | 1.00 | 1.00 | 1.19 | 26 | 1.13 | 0.22 | 1.00 | 1.00 | 1.25 | 0.754 | 0.08 |
| Month 4 | 21 | 1.19 | 0.31 | 1.00 | 1.00 | 1.25 | 24 | 1.18 | 0.33 | 1.03 | 1.00 | 1.13 | 0.951 | 0.03 |
| Autonomic | Minocycline | Placebo | ||||||||||||
| N | Mean | Std | Median | P25 | P75 | N | Mean | Std | Median | P25 | P75 | |||
| Month 0 | 25 | 1.31 | 0.43 | 1.00 | 1.00 | 1.50 | 22 | 1.27 | 0.46 | 1.00 | 1.00 | 1.50 | 0.534 | 0.09 |
| Month 1 | 31 | 1.37 | 0.40 | 1.33 | 1.00 | 1.67 | 27 | 1.40 | 0.45 | 1.33 | 1.00 | 2.00 | 0.921 | 0.07 |
| Month 2 | 27 | 1.43 | 0.50 | 1.33 | 1.00 | 2.00 | 25 | 1.45 | 0.74 | 1.00 | 1.00 | 1.50 | 0.569 | 0.03 |
| Month 3 | 26 | 1.49 | 0.54 | 1.50 | 1.00 | 1.67 | 26 | 1.42 | 0.55 | 1.00 | 1.00 | 2.00 | 0.508 | 0.13 |
| Month 4 | 21 | 1.40 | 0.49 | 1.00 | 1.00 | 2.00 | 24 | 1.47 | 0.59 | 1.33 | 1.00 | 1.83 | 0.715 | 0.13 |
Exploratory Analyses
Inflammatory Marker Testing
For patients who contributed both PRO data and blood samples, from which levels of inflammatory markers were determined, we did not observe a significant difference for any marker between arms at baseline (minocycline group N=14; placebo=15), at the end of month 2 (minocycline=18; placebo=18), or at the end of the study (end of month 4, minocycline=20, placebo=17), see Figure 2. All serum samples were detectable, including IL-1R, IL-5, Il-6, IL-6R, TNF-alpha, sTNF-R1, sTNF-R2.
Figure 2.
Serum of inflammatory markers by treatment groups (Mean, 95% CI)
Prevalence of the High-Severity Symptom Burden
Patients had no existing neuropathy at enrollment. Fatigue, pain, and disturbed sleep were the most severe MDASI-GI-reported symptoms for all patients during the first cycle of therapy. Regardless of trial groups, no significant differences in severity of these 4 symptoms was detected between the 2 institutions (public versus tertiary) over time.
Figure 3 presents the high- and low-severity group trajectories for all patients with numbness/tingling, fatigue, pain, and disturbed sleep over time during therapy, identified by trajectory analysis. During the 4-month study period, 56.4% of all patients were in the high-severity fatigue group, with mean severity greater than or equal to 4; 44.7% of patients were in the high-severity numbness group, and severity of numbness increased significantly over time (est=0.94, P<0.001); 44.0% were in the high-severity disturbed-sleep group; and 46.2% were in the high-severity pain group. The average scores for MDASI-GI pain by group were in the mild range, 3.12 (SD=2.64) in the high-severity pain group and 0.32 (SD=1.29) for the low-severity pain group; the difference was significant (P<0.001). There were 41% of all patients presents both high-severity CIPN and fatigue, and 33% of all patients presents both high-severity CIPN and pain, who likely suffered from neuropathic pain.
Figure 3.
Trajectories of major symptom burden over treatment period (n=66)
Higher baseline fatigue predicted high-severity fatigue during treatment (odds ratio 1.22, 95% confidence interval 1.00–1.49, P=0.04); and higher baseline pain was predictive of high-severity pain (odds ratio 1.35, 95% confidence interval 1.09–1.67, P=0.002).
DISCUSSION
Although minocycline was well tolerated, in this phase II randomized, placebo-controlled trial, we did not observe effectiveness to reduce patient-reported numbness/tingling or fatigue by minocycline, compare to placebo. In addition, paralleling the negative clinical results, none of our findings suggested that minocycline modulated the level of inflammatory markers. Therefore, further clinical study of minocycline for treatment-related symptom management in patients with CRC undergoing standard oxaliplatin-based chemotherapy is not encouraged. While the expectation for an effective reduction of major symptom burden, CIPN and fatigue, by one medication might be not the reality, given these outcomes and the current fact that oxaliplatin is a cornerstone in the treatment of CRC, clinical studies to investigate effective ways to better manage these bothersome symptoms are still urgently needed.
Between our 2 study sites, we found that the level of patient care was roughly equivalent (oncology management at LBJ is provided by MD Anderson oncologists), disease conditions of the study sample were similar, and similar therapy was provided. Although the patients treated at LBJ were more likely to be members of underserved groups, the reasons that patients dropped out of the trial, the rates of early withdrawal from standard care due to toxicity, and the major symptom burden were similar for the 2 sites. Thus, this study revealed no mediating disparity effect of socioeconomic status on the severity of CIPN and fatigue in patients with CRC during standard oxaliplatin-based chemotherapy.
To identify patients who would likely to report significant symptom burden during standard chemotherapy over 4 months among patients with CRC, by trajectory analysis with the weekly MDASI symptom assessment, we observed that 44.7% of patients reported significantly more severe CIPN (numbness/tingling determined by MDASI), and 56.4% of patients reported persistently more severe fatigue, compared with other patients under this trial.
The outcome results from the trial’s primary PRO outcome measure, the single MDASI-GI item “numbness/tingling” (a core symptom of CIPN) on a 0 to 10 scale were consistent with results from 3 subscales of the European Organization for Research and Treatment of Cancer CIPN20 questionnaire items (sensory, motor, and autonomic) in this cohort of patients who had no preexisting neuropathy. We observed that single-item numbness/tingling on the MDASI could well represent the longitudinal change on sensory scale of CIPN 20, meanwhile this single item also significantly relevant to the severity of motor and autonomic subscale of CIPN. For the sake of simplicity and less burden for patients, it supports the value for further study of the utility of the single item in a clinical trial that uses multiple assessment points.
In addition, for the purpose of better patient care with attention to the most bothersome symptom burden in patients with CRC, severe pretherapy fatigue and pain, perhaps driven by a combination of the disease and previous therapy, may be indicative of risk for the development of high symptom burden during chemotherapy. These might even be considered as simple screening items for use in routine care before chemotherapy is employed.
The study was limited by non dose dependent trial design for intervention or prevention of CIPN and fatigue. The lack of an impact on inflammatory markers indicates that the dose may have been suboptimal. The dosage (200 mg/day) was chosen on the basis of minocycline’s known safety in other clinical applications, which have used this daily dose. However, the lack of any positive signal does not encourage dosing studies at higher levels. The second limitation is lack of information of drug bioavailability and effects of an ingested compound in the study. This might provide more useful interpretation of the reason of this negative trial.
CONCLUSION
Minocycline for the management of treatment-related symptoms is feasible and has a low toxicity profile at 100 mg twice a day. However, the use of minocycline at this dose yielded no positive evidence of its capacity to reduce either fatigue or neuropathy on MDASI symptom ratings associated with oxaliplatin-based therapy. Furthermore, we did not observe the impact of treatment site on symptom development nor the mediating effect on efficacy of minocycline intervention. The absence of evidence of reduced inflammation by minocycline on any tested marker suggests that the trial provides little new information on the role that inflammation might play in the development of neuropathy or fatigue in patients with CRC undergoing standard oxaliplatin-based chemotherapy, and the results do not support further trials of minocycline for treatment-related symptom reduction in these patients. However, this study does demonstrate the feasibility of a pathway from basic animal behavioral studies to early-phase clinical trials as we learn more about the mechanisms responsible for treatment-related symptoms.
Acknowledgments:
The authors extend special thanks to both Jeanie F. Woodruff, BS, ELS, in the Department of Symptom Research and Kathryn L. Hale in the Department of Scientific Publications for editorial comments. We also appreciate the kind support for testing of inflammatory markers by Dr. Cobi Heijnen’s laboratory.
Funding: This work was supported by the American Cancer Society (grant number 125079-RSG), National Cancer Institute (grant numbers R01 CA026582 and P01 CA124787), and MD Anderson Cancer Center Support Grant (P30 CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Cancer Society, the National Cancer Institute, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The MD Anderson Symptom Inventory (MDASI) and its modules are copyrighted and licensed by The University of Texas MD Anderson Cancer Center and Charles S. Cleeland. Charles Cleeland and Xin Shelley Wang have a financial interest in the MDASI and its modules. The authors have no other financial conflicts of interest to disclose.
Previous presentation: Presented in part at the American Society for Clinical Oncology (ASCO) Annual Meeting; Chicago, Illinois, 06/2018
Contributor Information
Xin Shelley Wang, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Qiuling Shi, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Nishin A. Bhadkamkar, Department of General Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Charles S. Cleeland, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Araceli Garcia-Gonzalez, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jonathan R. Aguilar, Office of Protocol Support and Management, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Cobi Heijnen, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Cathy Eng, Department of GI Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
REFERENCES
- 1.Grothey A Reintroduction of oxaliplatin: a viable approach to the long-term management of metastatic colorectal cancer. Oncology 2010;79:389–399. [DOI] [PubMed] [Google Scholar]
- 2.Wang XS, Shi Q, Williams LA, Komaki R, et al. Prospective study of patient-reported symptom burden in patients with non-small cell lung cancer undergoing proton or photon chemoradiation therapy. J Pain Symptom Manage 2016;51:832–838.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thong MS, Mols F, Wang XS, et al. Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Eur J Cancer 2013;49:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav Immun 2011;25:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AH. Cytokines and sickness behavior: Implications for cancer care and control. Brain Behav Immun 2003;17:S132–S134. [DOI] [PubMed] [Google Scholar]
- 6.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun 2010;24:968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: Evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum 2006;33:535–542. [DOI] [PubMed] [Google Scholar]
- 8.Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem 2008;105:1582–1595. [DOI] [PubMed] [Google Scholar]
- 9.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain 2011;152:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res 2008;1229:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol 2008;601:79–87. [DOI] [PubMed] [Google Scholar]
- 12.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003;306:624–630. [DOI] [PubMed] [Google Scholar]
- 13.Noble W, Garwood CJ, Hanger DP. Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion 2009;3:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pachman DR, Dockter T, Zekan PJ, Fruth B, Ruddy KJ, Ta LE, Lafky JM, Dentchev T, Le-Lindqwister NA, Sikov WM, Staff N, Beutler AS, Loprinzi CL. A pilot study of minocycline for the prevention of paclitaxel-associated neuropathy: ACCRU study RU221408I. Support Care Cancer. 2017. November; 25 (11):3407–3416. [DOI] [PubMed] [Google Scholar]
- 15.Gunn GB, Mendoza TR, Garden AS, Wang XS, Shi Q, Morrison WH, Frank SJ, Phan J, Fuller CD, Chambers MS, Hanna EY, Lu C, Rosenthal DI, Cleeland CS. Minocycline for symptom reduction during radiation therapy for head and neck cancer: a randomized clinical trial. Support Care Cancer. 2019. April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleeland CS, Mendoza TR, Wang XS, et al. Levels of symptom burden during chemotherapy for advanced lung cancer: differences between public hospitals and a tertiary cancer center. J Clin Oncol 2011;29:2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilley BC, Alarcón GS, Heyse SP, Trentham DE, Neuner R, Kaplan DA, Clegg DO, Leisen JC, Buckley L, Cooper SM, Duncan H, Pillemer SR, Tuttleman M, Fowler SE. Minocycline in rheumatoid arthritis. A 48- week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann Intern Med. 1995. January 15;122(2):81–9. [DOI] [PubMed] [Google Scholar]
- 18.Wang XS, Williams LA, Eng C, et al. Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer 2010;116:2053–2063. [DOI] [PubMed] [Google Scholar]
- 19.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 2005;41:1135–1139. [DOI] [PubMed] [Google Scholar]
- 20.van Zuiden M, Heijnen CJ, van de Schoot R, Amarouchi K, Maas M, Vermetten E, Geuze E, Kavelaars A. Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One. 2011;6(12):e29142. doi: 10.1371/journal.pone.0029142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol 2012;9:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonelli RM, Hodl AK, Hofmann P, Kapfhammer HP. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol 2004;19:337–342. [DOI] [PubMed] [Google Scholar]
- 23.Cappelleri JC, Bushmakin AG, Zlateva G, Sadosky A. Pain responder analysis: use of area under the curve to enhance interpretation of clinical trial results. Pain Pract. 2009. Sep-Oct;9(5):348–53. [DOI] [PubMed] [Google Scholar]
- 24.Haviland A, Jones B, & Nagin D (2011) Group-based Trajectory Modeling Extended to Account for Nonrandom Participant Attrition. Sociological Methods & Research, 40 (2), 367–390. DOI: 10.1177/0049124111400041 [DOI] [Google Scholar]
- 25.Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res 22(9):2331–9, 11/2013. e-Pub 3/2013. [DOI] [PMC free article] [PubMed] [Google Scholar]