Abstract
Background/Purpose:
Synoptic, or standardized, reporting of surgery and pathology reports has been widely adopted in surgical oncology. Patients with Hirschsprung disease may experience morbidity related to surgical factors or underlying pathology and often undergo multiple operations. Our aim is to improve the postoperative outcome and care of patients with Hirschsprung disease by proposing a standardized set of data that should be included in every surgery and pathology report.
Methods:
Members of the American Pediatric Surgical Association Hirschsprung Disease Interest Group and experts in pediatric pathology of Hirschsprung disease participated in group discussions, performed literature review and arrived at expert consensus guidelines for surgery and pathology reporting.
Results:
The importance of accurate operative and pathologic reports and the implications of inadequate documentation in patients with Hirschsprung disease are discussed and guidelines for standardizing these reports are provided.
Conclusions:
Adherence to the principles of reporting for operations and surgical pathology may improve outcomes for Hirschsprung disease patients and will facilitate identification of correlations among morphology, function, genetics and outcomes, which are required to improve the overall management of these patients.
Keywords: Hirschsprung disease, aganglionosis, synoptic, surgery, pathology, enteric nervous system
Introduction
In an era of increasing focus on quality improvement in patient care, measures such as surgical safety checklists and resident duty hour restrictions have been developed and evaluated. However, one area that has been less well studied is surgical documentation and reporting. Historically, the format and details of operative notes have been dictated only by surgeons themselves. While certain components are shared across specialties and countries (e.g. procedure name and estimated blood loss), considerable variability remains. Many operative reports go unread, in large part due to the overall low complication rates associated with modern surgical practice. Although the details of an operative report can have important implications for patient care and medico-legal issues, there is a surprising paucity of research into the subject of surgical documentation. Guidelines for medical record documentation, such as those included in The Joint Commission Standards, only briefly mention requirements for operative reports, one being a “full description of the procedure”(1). Similarly, surgical pathology reports, which play a particularly crucial role in surgical management, lack standardization. In order to improve reporting and provide standardization, the concept of “synoptic”, or standardized, reporting has recently emerged for both surgical and pathologic reporting and has been widely adopted in oncology(2, 3). The importance of accurate operative and pathologic reports and the implications of inadequate documentation in patients with Hirschsprung disease (HSCR) are discussed here and recommendations for standardizing these reports are provided.
Effective surgical management of HSCR by removal of the distal, aganglionic segment of bowel with a pull-through and anastomosis of the bowel proximal to the affected segment was first described in 1949 by Orvar Swenson(4). Since then, many other methods have been described, with the Soave, Swenson or Duhamel techniques being commonly used in current clinical practice. Prior to one of these definitive operations, patients may undergo a leveling or diverting enterostomy (at the most distal site of ganglionated bowel). Operative reports for these procedures can vary with regard to the following details: exact anatomic levels where biopsies are taken, including the distances between biopsies; gross anatomical bowel caliber changes that suggest a transition zone and its location; the length of resected bowel; the exact level of ostomy creation; and the distance between the proximal margin and relevant landmarks (gross transition zone, ganglionic intraoperative biopsy site), the distance between the anastomosis and the dentate line, the length of the muscular cuff (for the Soave) and the length of the residual rectum (for the Duhamel).
Even the diagnostic methods for HSCR, which are generally well established, are not without limitations. Suction rectal biopsies, first introduced in the 1960’s, are the gold standard for diagnosing the disease in newborns and infants(5). A combination of advances in technology and special tissue staining techniques have increased the accuracy of this tool. However, it is imperfect and highly dependent on the experience of the pathologist reviewing the specimen. Limitations inherent in this procedure include inadequate or insufficient rectal suction biopsy specimens (reported rates vary between 8–26%)(6) and inconclusive biopsy specimens (less commonly reported and therefore difficult to quantify). Inadequate biopsies may occur when there is an insufficient amount of submucosa obtained or the specimen is taken too close to the dentate line. Inconclusive biopsies are those in which the pathologic findings are equivocal despite high clinical suspicion.
For surgeons performing leveling colostomy/enterostomy or pull-through procedures for children with HSCR, no formal guidelines exist to delineate the key details that should be reported in an operative note. This lack of consistency in reporting and insufficient information available for review prior to re-operative procedures has major implications for both patient care and research aimed at improving long-term outcomes. For example, consistent reporting of the length of the proximal ganglionic bowel resected would allow surgeons to identify areas of variability and facilitate research aimed at improving outcomes for these patients. With respect to reoperation, which is required in at least 3% of patients(7), detailed reporting of the original surgery, including biopsy location, frozen section results, length of bowel resected, length of rectal cuff and whether or not the cuff was split would assist the operating surgeon doing a subsequent procedure, particularly if a different surgeon performed the index operation, or if the patient has had intervening procedures that might create an unusual or difficult change in the anatomy.
In recent literature, there have been reports on the quality, creation, and implementation of surgical and pathological synoptic reporting for breast, colon, ovarian and thyroid cancer, among other diseases(8–11). In contrast, there are virtually no existing recommendations or data to inform the creation of reporting guidelines in the treatment of HSCR. The Royal College of Surgeons published Good Surgical Practice in 2008, in which certain general standards are set for operative records(12). Institutional reviews have indicated variable compliance with these standards(8). Studies conducted on pathological specimens of patients who have had surgery for HSCR demonstrate several characteristics of transition zone anatomy which suggest that the length of proximal resection should be at least a specific distance proximal to a biopsy that contains ganglion cells(13–15). Routine, intra-operative, histological examination of frozen sections from the proximal resection margin has also been advocated to exclude transition zone pull-through(13, 15). Neither of these practices has been universally incorporated into standard surgical practice. Our aim is to improve the postoperative outcome and care of patients with HSCR by proposing a standardized set of data that should be included in every surgery and pathology report. This standardization will facilitate comparative studies examining functional outcomes in HSCR patients.
Recommendations: Surgical Reporting
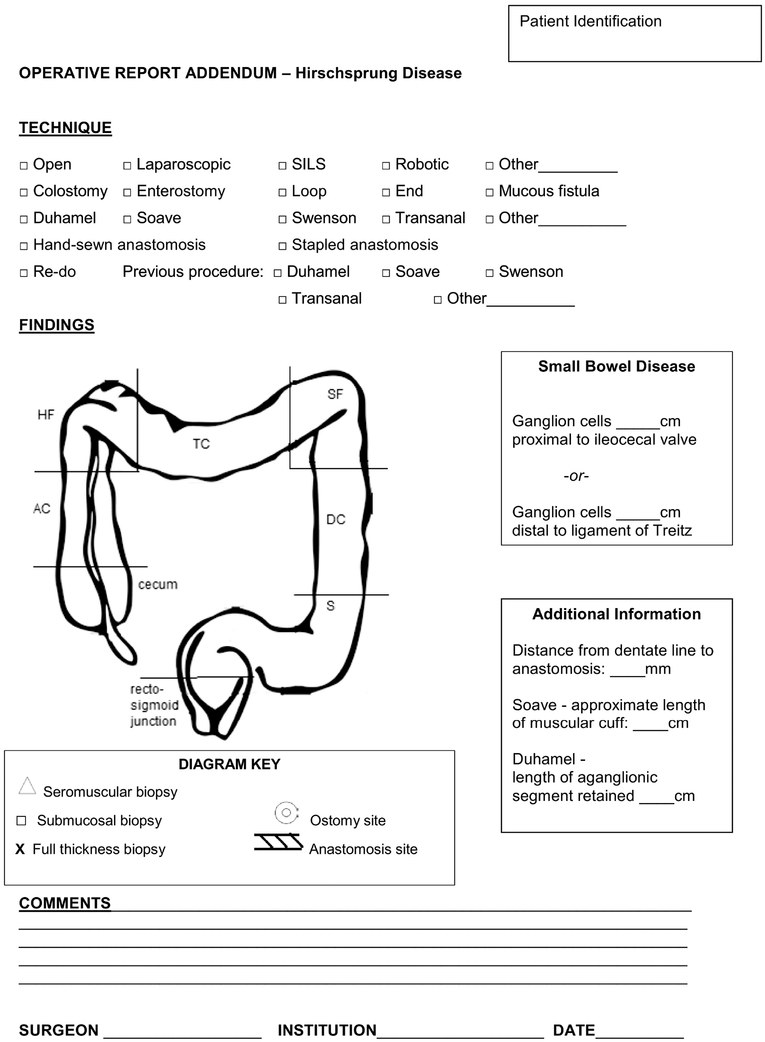
A model template for synoptic reporting of surgical procedures for HSCR is presented (Figure 1). This template addresses the key details for biopsies and operations in children with HSCR.
Figure 1. Recommended operative report addendum for surgical reporting of HSCR.
The form provides checkboxes for surgical technique, including open versus minimally-invasive approaches, variations on ostomies, specific types of pull-through operations, and details of prior operations if a re-do surgery has been performed. A diagram to annotate locations of biopsies, placement of ostomies, and site of anastomosis is provided. Space is provided for detailing distances that are important to note, such as length of small bowel disease (relative to either the ileocecal valve or ligament of Treitz), anastomosis distance from the dentate line, and the length of muscular cuff (Soave) or retained aganglionic segment (Duhamel).
Diagnostic Rectal Biopsies
Diagnostic rectal biopsies are approached trans-anally and performed as either suction biopsies (obtaining mucosa and submucosa) or open (obtaining deeper layers, often full-thickness). The site and type of these biopsies relative to the dentate (pectinate) line and circumferential location (e.g. anterior, right lateral, left lateral, posterior) should be indicated. The optimal distance from the dentate line at which biopsies should be obtained varies based on the age and size of the child.
For suction biopsies, which are typically performed in infants less than one year of age, ideally biopsies should be obtained at more than one level (e.g., estimated 1-, 2-, and 3-cm above the dentate line) and submitted separately. Biopsies performed too close to the dentate line may miss ganglion cells because of the normal paucity of ganglion cells in this region. Biopsies can be submitted in formalin, but if acetylcholinesterase histochemistry is anticipated, an additional unfixed biopsy (on moist saline pad) should be sent to the laboratory promptly for frozen section.
For older patients (e.g., >1 year), a single full-thickness biopsy is preferable to suction rectal biopsies. Since the former is obtained under anesthesia with direct visualization, a single biopsy beginning 1–1.5 cm above the dentate line and extending 0.5 cm in length cephalad is sufficient. If necessary, a full-thickness biopsy can be divided to provide sufficient tissue for enzyme histochemistry and routine formalin-fixed paraffin-embedded histology.
Leveling ostomy
Some patients undergo leveling ostomy as part of a staged approach to HSCR, or occasionally as a definitive operation. These may be performed open, laparoscopically, or using single-incision laparoscopic surgery (SILS). The report for a leveling ostomy should specify the location of each biopsy, including distance from the last biopsy, frozen section results of each biopsy, the location of the radiologic transition zone (if present), the gross transition zone (if visible) and the pathologic transition zone, the length of bowel resected (both total length and length of segment proximal to the most distal point where normal ganglion cells are first confirmed pathologically), and the anatomic level of ostomy creation. In the setting of small bowel disease, the distance of ganglion cells from either the ileocecal valve and/or ligament of Treitz should be included.
Pull-through Procedure
Multiple approaches to a definitive pull-through for HSCR exist. The report should indicate whether the operation was performed open, laparoscopically, single-incision laparoscopic surgery, robotically, trans-anally, or other (with a space to indicate how it was performed) and which surgical reconstruction was employed (Duhamel, Soave, Swenson, Trans-anal, other). The report should include the length of each segment of resected bowel, length of muscular cuff for the Soave procedure, length of retained aganglionic segment for a Duhamel, and frozen section results of any biopsies and proximal margin, if applicable. The distance of the anastomosis from the dentate line should be included. In the case of Duhamel operations, the report should include information about the type of anastomotic technique (hand-sewn vs. stapled) and the length of the pouch. Finally, the vascular supply to the bowel undergoing the pull-through should be described if it is dependent on an unexpected or unusual anatomy.
Re-do Pull-through Procedure
Unfortunately, a number of children require re-operative surgery for HSCR. Careful analysis of prior operative reports and pathology specimens is critically important in planning for these operations. In the case of re-do procedures, the report should include the type of prior operation performed, the amount of colon that had previously been resected, the indications for the re-do procedure, and integrity of the dentate line (intact, partially intact, absent), and location of original anastomosis relative to the distal resection margin, in addition to the standard information about the new pull-through procedure described above.
Additional Information
All operative reports should indicate variations of normal anatomy, any deviation from standard procedure, any drains or tubes left in, and any complications encountered.
Recommendations: Pathology Reporting
Standards for evaluation and reporting of surgical pathology results are an important complement to clinical documentation when a patient undergoes surgery for HSCR. The following types of surgical specimens need to be considered: intraoperative leveling biopsies, distal bowel resections, ostomy takedown/closure specimens in multi-stage procedures, and redo resections. Each of these specimens poses unique issues and specific data, which should be reported (Table 1).
Table 1:
Pathology Data to be Reported from HSCR Surgical Specimens
| Specimen Type | Required (Impact Management) | Considerations (May Impact Management) |
|---|---|---|
| Diagnostic rectal biopsy | Adequacy Ganglion cells present or absent Submucosal nerve hypertrophy present or absent Active enterocolitis present or absent |
Results of calretinin immunohistochemistry, other immunohistochemistry, or acetylcholinesterase histochemistry |
| Leveling seromuscular or full-thickness biopsy | Adequacy Ganglion cells present or absent |
Features suggestive of transition zone (e.g. large myenteric or submucosal nerves, sparse tiny myenteric ganglia) Active enterocolitis |
| Distal Bowel Resection | Type of pull-through procedure Portion of colon resected Total length Length of mucosal sleeve (Soave only) Positions of intraoperative biopsy sites Length of aganglionic segment Neuromuscular anatomy at the proximal surgical margin Active enterocolitis or skip areas, if present |
Histological features and length of transition zone Non-specific histopathological findings (e.g. eosinophilic gangliomuscular inflammation, adventitial fibromuscular dysplasia, gangliosclerosis) |
| Ostomy T akedown/Closure | Total length Presence/absence of stenosis, aganglionic segment or transition zone histology Neuromuscular anatomy at the proximal surgical margin |
|
| Re-do Pull-through Resection | Total length Location of original surgical anastomosis Presence/absence of stricture Presence/absence of aganglionic bowel, transition zone histology, or active enterocolitis proximal to the anastomosis Neuromuscular anatomy at the proximal surgical margin |
Non-specific histopathological findings (e.g. eosinophilic gangliomuscular inflammation, adventitial fibromuscular dysplasia, gangliosclerosis) Comment comparing findings with pre-operative biopsies and/or proximal margin of original pull- through resection |
Diagnostic rectal biopsies (suction and incisional)
Key features that should be specified in the surgical pathology report for suction rectal biopsies are adequacy of the biopsy tissue and explicit statements regarding the presence or absence of identifiable submucosal ganglion cells, abnormally large and crowded submucosal nerves (“submucosal nerve hypertrophy”), ancillary histochemical (e.g. acetylcholinesterase) or immunohistochemical (calretinin, choline transporter, etc.) results that support or refute HSCR, or other significant diagnostic alterations (e.g., active colitis). For biopsies deemed “inadequate” or “inconclusive”, an explanation should be clearly provided in the diagnostic portion of the report. Common reasons for inadequacy are insufficient submucosa, squamous or transitional mucosa indicative of a low biopsy, or problems with procurement or processing (e.g., “crush artifact”) that compromise histological evaluation. When adequacy is in question, it may be helpful to specify the number of individual histological sections evaluated and whether the available tissue was exhaustively sectioned. Inconclusive biopsies typically have conflicting diagnostic features (e.g., intact calretinin immune-reactive mucosal innervation in a biopsy with nerve hypertrophy and no identifiable ganglion cells). The specified location and results for each biopsy should be documented separately.
For incisional biopsies, the gross description should include dimensions of the specimen(s), how it was divided and oriented, and whether any was frozen for histochemistry or other testing. In addition to documentation of the features described above, descriptions of incisional biopsies should clearly indicate the extent to which the muscularis propria and myenteric plexus was sampled and the presence/absence of myenteric ganglia. Use of diagnostic phrases like “no diagnostic alteration” implies that not only are ganglion cells are present, but other neuropathic and myopathic findings that might explain a patient’s dysmotility have been excluded.
Rectal biopsies obtained after a pull-through procedure are usually done to exclude transition zone pull-through because of persistent post-operative obstructive symptoms. Pathology reports for these biopsies are similar to pre-operative incisional biopsies, but the diagnostic interpretation must account for age-related changes in nerve caliber and potential histopathological changes (e.g. fibromuscular reaction in the submucosa) due to the trauma of prior surgery(16). The latter changes occur at site of anastomosis between ganglionic and aganglionic bowel, where focal absence or paucity of ganglion cells and nerve hypertrophy can falsely suggest transition zone. Such biopsies do not sufficiently sample the pull-through bowel proximal to the anastomosis and therefore are inadequate to exclude transition zone pull-through. Accordingly, biopsies following pull-through procedure should be performed above the prior anastomotic site and the surgical and pathology approaches recommended above for full-thickness diagnostic rectal biopsies apply.
Leveling biopsy
Leveling biopsies may be full-thickness or seromuscular. In either case, adequacy requires a generous length (typically >3 mm) of bowel wall that includes both layers of the muscularis propria. Orientation of the specimen so that sections are obtained perpendicular to the serosal surface is highly advantageous, as it eliminates the need to step section through the block to find the plane between the muscle layers where the myenteric plexus normally resides. Frozen sections may be stained with H&E, Diff-Quik, or other methods; most important is the pathologist’s experience with the stain used and the quality of the section and staining. The minimal goal is to confidently identify or exclude ganglion cells, which can generally be accomplished with 10 or fewer well-oriented frozen sections. The presence of large nerves with extrinsic innervation morphology (higher density/more compact, wavy peripheral nerve-like appearance and conspicuous perineurium) in aganglionic biopsies or tiny ganglia composed of one or two ganglion cells with minimal surrounding neuropil may suggest transition zone. In permanent section, extrinsic innervation can be highlighted by Glut-1 staining and size of the nerve trunks measured. Therefore, the only obligate reporting options for a leveling biopsy are “inadequate or insufficient”, “ganglion cells present”, or “ganglion cells absent”.
Distal bowel resection
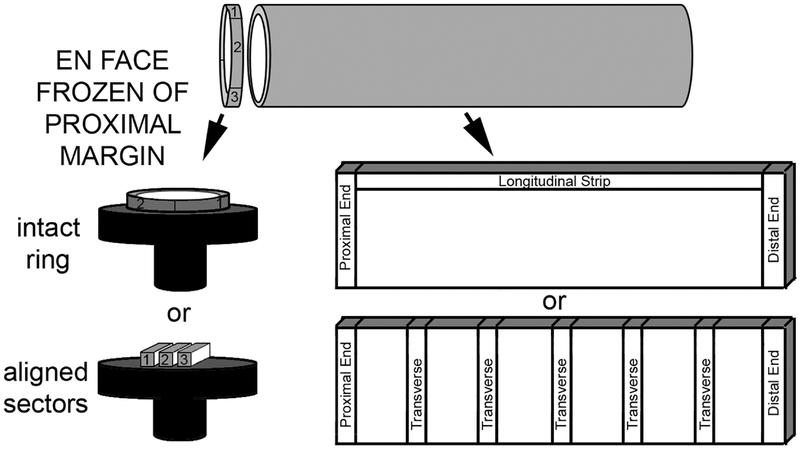
Definitive surgery for HSCR requires resection of the aganglionic segment and transition zone. The resultant specimen is usually a length of bowel with distal aganglionosis, neuroanatomically normal proximal bowel, and intervening ganglionic bowel with one or more of the following neuropathological features of transition zone: partial circumferential aganglionosis (absent myenteric and/or submucosal ganglion cells in a contiguous eighth of the circumference), myenteric hypoganglionosis (as defined above of ≥1/8th circumference), or submucosal nerve hypertrophy (e.g., >2 submucosal nerves >40 μm thick in one high power field). Nerve hypertrophy diminishes at greater distances from the distal rectum and generally is not present proximal to the splenic flexure(17). Resection should be performed at least 5 cm proximal to a ganglionic leveling biopsy and the entire proximal resection margin should be evaluated to reduce the likelihood of transition zone pull-through. In longer-segment disease, the hypoganglionic transition zone may be longer than 5 cm and evaluation of the entire proximal resection margin will ensure completeness of resection. The surgeon should resist removing a proximal ring of tissue at the time of definitive resection and send the entirety of the specimen to the lab asking for frozen section evaluation of the entire circumference of the proximal margin. If frozen section analysis is not available, then biopsy for permanent sectioning should be obtained and definitive pull-through deferred until pathology results are available. In order to obtain well-oriented sections of the entire circumference, a ~5 mm transverse full-thickness strip is removed from the proximal end of the resection, and a full en face cross-section is prepared, if the diameter allows. Proximal margins with large diameters may be divided into two or three linear segments, aligned to support each other (like books on a shelf), and frozen and sectioned as a group (Figure 2). Frozen sections are stained with H&E and/or Diff-Quik and assessed for any of the three features of transition zone listed above. If transition zone is identified at the proximal margin, additional bowel should be resected, and the process repeated.
Figure 2. Recommended surgical pathology work-up for pull-through resection specimens.
Intraoperative frozen section evaluation of the entire circumference of the proximal surgical margin should be performed to exclude histological features of transition zone. An en face section of the margin can be frozen entirely either as a concentric ring (“donut”) or divided into linear sectors (easier to orient with large diameter specimens). The remaining bowel should be opened longitudinally and sampled by some variation of either of the two illustrated methods to include full-circumference samples of the proximal and distal ends, along with either a longitudinal strip or closely spaced (1–2 cm) transverse sections.
After frozen section, the gross anatomy of the specimen should be assessed. Key features that require documentation include the length of the resected bowel, caliber or circumference (noting areas of significant transition which may correlate with preoperative imaging or histological transition zone), length of the mucosal sleeve at the distal end of the resection (applicable only to Soave resections), and the integrity/appearance of the serosal surface. Locations of intraoperative biopsy sites relative to the proximal or distal margin should be specified and inked to facilitate histologic correlation. The specimen should be opened longitudinally and the mucosal surface inspected to identify any ulcers, polyps (e.g., ganglioneuromatous polyps in patients with MEN2A and HSCR), or preoperative biopsy sites. A photograph of the opened inked specimen is recommended.
When sampling for histology, the surgical pathologist’s goals at a minimum are to evaluate innervation at the proximal and distal ends of the specimen and to establish the approximate lengths of the ganglionic and aganglionic segments. In most cases, the distal margin and some contiguous length of bowel will be aganglionic, but the full circumference of the proximal margin will show no significant neuropathology. In addition to full-circumference sections from the proximal and distal ends of the specimen, either of two approaches to define the length of the aganglionic segments is acceptable. One option is one or more full-thickness longitudinal strip, between the proximal and distal sections. The strip may be subdivided into 7–8 cm-long segments and each segment rolled in the longitudinal plane (“jelly-roll”) after inking the proximal and distal margins. If preferred, the entire strip can be submitted as shorter linear segments marked (e.g., inked for orientation) to reduce the number of cassettes required. Alternatively, serial consecutive transverse sections taken at closely spaced intervals (e.g., 1–2 cm) in sequential blocks along the entire length of the specimen also suffices and allows for a better “map” of the transition zone.
The diagnostic portion of the surgical pathology report should specify the nature of the specimen (e.g., rectosigmoid colon), surgical procedure (e.g., Soave pull-through), and length of the resection, as well as explicit statements as to the presence/absence of aganglionosis, length of the aganglionic segment, and presence or absence of significant neuromuscular pathology (features of transition zone) at the proximal margin (or the distance between the proximal end of the transition zone to the final surgical proximal margin). Other clinically significant diagnoses (e.g., active enterocolitis, skip areas, ganglioneuromas) should also appear in this portion of the report. Additional useful, but not obligate information, may be included elsewhere in the report (e.g., Microscopic Description), such as length or sections with histological features of transition zone, location and severity of eosinophilic neuromuscular inflammation, muscular hypertrophy, gangliosclerosis (fibrosis in and around myenteric ganglia), arterial fibromuscular dysplasia, ectopic ganglia in the muscularis propria or other findings with uncertain clinical significance. Special histochemical or immunohistochemical stains are generally not required, but if applied, the results and interpretation should be recorded.
Ostomy takedown/closure
In multistage surgical approaches to HSCR, resection of a diversion or protective enterostomy and bowel anastomosis is often a final procedure. In some instances, bowel immediately proximal to the ostomy is pulled through and the proximal margin of the ostomy takedown/closure represents the proximal-most bowel removed from the patient. In this situation, intraoperative frozen section analysis of the entire proximal margin, as described above, may be warranted. The lumen of the ostomy should be probed to identify any site of obvious stenosis. The length of these specimens is usually relatively short and it is feasible to generously sample the mid and distal portions of the ostomy with either transverse or longitudinal sections.
Re-do pull-through resection
A significant subset of patients who undergo pull-through surgery for HSCR have post-operative persistent obstructive symptoms or other complications. In some instances, repeat pull-through surgery is deemed necessary to address their problems. The principles for surgical pathology evaluation are similar to those described in preceding sections, but it is very important that the pathologist understands what type of surgery was done initially, the clinical indication for the redo (e.g., stricture vs. transition zone pull-through), and the type of re-do procedure. Whenever possible, every effort should be made to review the original pathology reports, well in advance, as part of the decision to perform a re-do procedure. Particular attention should be given to the length of the ganglionic bowel that was resected previously and the margin that was adjacent to bowel used for the original pull-through anastomosis, as these data may provide the best indices of prior transition zone pull-through. Similarly, pathology from any biopsies of the pulled through neo-rectal tissue should be examined prior to re-do.
Although pathological handling and examination of a re-do specimen is similar to an initial pull-through resection (described above), the following important difference should be considered. Most re-do resections include the original anastomosis and a short length (sometimes only a few mm) of native aganglionic distal rectum inferior to the anastomosis. Depending in part upon the interval between original surgery and re-do, the anastomosis may be difficult to identify grossly, but is readily apparent histologically. It is advisable to thoroughly sample the distal end of the resection with longitudinal tissue samples, which will extend from the aganglionic distal rectum through the anastomotic scar and into the pulled through neorectum. In the case of a prior Soave procedure, portions of the native rectal cuff may encircle the distal neorectum such that the muscularis propria appears duplicated, typically with internal ganglionic bowel and external aganglionic myenteric plexus.
Histological evaluation of the re-do resection should consider the presence and length of the native aganglionic rectum inferior to the anastomosis, the thickness and cellular/matrix composition of the anastomotic scar, and the properties of the supra-anastomotic bowel. With regard to the latter, features of transition zone (aganglionosis, myenteric hypoganglionosis, nerve hypertrophy) should be explicitly addressed, and the presence/absence of such features at the proximal margin (new anastomotic site) must be documented.
Conclusions
Adherence to the principles of reporting for operations and surgical pathology outlined briefly in this manuscript will likely improve the outcome for HSCR patients in several respects. For individual patients, relevant details concerning the native anatomy of their resected bowel will be well documented in a manner that should make clear to current or future caregivers how much aganglionic and ganglionic bowel were resected and the likelihood that transition zone was removed completely. For the field of HSCR research, more standardized surgical and pathology reporting will facilitate identification of correlations among morphology, function, outcomes and genetics, all of which are required as we aim to improve the overall management of these patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Level of Evidence: V
References
- 1.Commission TJ. The Joint Commission 2018. [Available from: https://www.jointcommission.org/.
- 2.Cundy TP, Kirby CP, Kirby ML. Synoptic operative reports for quality improvement in pediatric cancer care. Pediatr Blood Cancer. 2018;65(10):e27238. [DOI] [PubMed] [Google Scholar]
- 3.Edhemovic I, Temple WJ, de Gara CJ, Stuart GC. The computer synoptic operative report--a leap forward in the science of surgery. Ann Surg Oncol. 2004;11(10):941–7. [DOI] [PubMed] [Google Scholar]
- 4.Swenson O My early experience with Hirschsprung's disease.1989. August 839–44- discussion 44–5 p. [DOI] [PubMed]
- 5.Friedmacher F, Puri P. Rectal suction biopsy for the diagnosis of Hirschsprung’s disease: a systematic review of diagnostic accuracy and complications. Pediatr Surg Int. 2015;31(9):821–30. [DOI] [PubMed] [Google Scholar]
- 6.Muise ED, Cowles RA. Rectal biopsy for Hirschsprung’s disease: a review of techniques, pathology, and complications. World J Pediatr. 2016;12(2):135–41. [DOI] [PubMed] [Google Scholar]
- 7.Ralls MW, Coran AG, Teitelbaum DH. Redo pullthrough for Hirschsprung disease. Pediatr Surg Int. 2017;33(4):455–60. [DOI] [PubMed] [Google Scholar]
- 8.Maniar RL, Hochman DJ, Wirtzfeld DA, McKay AM, Yaffe CS, Yip B, et al. Documentation of quality of care data for colon cancer surgery: comparison of synoptic and dictated operative reports. Ann Surg Oncol. 2014;21(11):3592–7. [DOI] [PubMed] [Google Scholar]
- 9.Slodkowska J, Cierniak S, Patera J, Kopik J, Baranowski W, Markiewicz T, et al. Functional Assessment of Synoptic Pathology Reporting for Ovarian Cancer. Pathobiology. 2016;83(2–3):70–8. [DOI] [PubMed] [Google Scholar]
- 10.Lam E, Vy N, Bajdik C, Strugnell SS, Walker B, Wiseman SM. Synoptic pathology reporting for thyroid cancer: a review and institutional experience. Expert Rev Anticancer Ther. 2013;13(9):1073–9. [DOI] [PubMed] [Google Scholar]
- 11.Donahoe L, Bennett S, Temple W, Hilchie-Pye A, Dabbs K, Macintosh E, et al. Completeness of dictated operative reports in breast cancer--the case for synoptic reporting. J Surg Oncol. 2012;106(1):79–83. [DOI] [PubMed] [Google Scholar]
- 12.England RCoSo. Good Surgical Practice. London, UK: Royal College of Surgeons of England; 2008. [Google Scholar]
- 13.Kapur RP. Histology of the Transition Zone in Hirschsprung Disease. The American journal of surgical pathology. 2016;40(12):1637–46. [DOI] [PubMed] [Google Scholar]
- 14.White FV, Langer JC. Circumferential distribution of ganglion cells in the transition zone of children with Hirschsprung disease. Pediatr Dev Pathol. 2000;3(3):216–22. [DOI] [PubMed] [Google Scholar]
- 15.Kapur RP, Kennedy AJ. Histopathologic delineation of the transition zone in short-segment Hirschsprung disease. Pediatr Dev Pathol. 2013;16(4):252–66. [DOI] [PubMed] [Google Scholar]
- 16.Kapur RP. Submucosal nerve diameter of greater than 40 μm is not a valid diagnostic index of transition zone pull-through. Journal of pediatric surgery. 2016;51(10):1585–91. [DOI] [PubMed] [Google Scholar]
- 17.Meier-Ruge W, Hunziker O, Tobler HJ, Walliser C. The pathophysiology of aganglionosis of the entire colon (Zuelzer-Wilson syndrome). Morphometric investigations of the extent of sacral parasympathetic innervation of the circular muscles of the aganglionic colon. Beitr Pathol. 1972;147(3):228–36. [DOI] [PubMed] [Google Scholar]