Abstract
Aims
We examined whether late evening food consumption was prospectively associated with the risk of developing prediabetes or diabetes in a large observational study of individuals with normoglycaemia.
Methods
Participants were 2642 men and women with normoglycaemia (HbA1c < 39 mmol/mol; < 5.7%) from Whitehall II. Time of last eating episode (TLEE) before the examination day was assessed at baseline. We studied the associations of TLEE with 5-year changes in HbA1c and risk of developing prediabetes or diabetes (HbA1c ≥ 39 mmol/mol; ≥ 5.7%). Potential heterogeneity in the association between TLEE and prediabetes or diabetes was examined using recursive partitioning modelling for time-to-event outcomes.
Results
There was a tendency of an overall association of TLEE with change in HbA1c but with little effect size [β per 1-h increase in TLEE = 0.2 mmol/mol, 95% CI −0.0 to 0.3 (0.01%, −0.00 to 0.03); P = 0.055] and no association with the risk of developing prediabetes/diabetes (risk ratio per 1-h increase in TLEE = 1.03, 95% CI 0.94 to 1.13; P = 0.511). According to the recursive partitioning modelling, women with HbA1c ≤ 36 mmol/mol and TLEE after 21:00 had a 1.51 times (95% CI 1.16 to 1.93) higher 5-year risk of developing prediabetes or diabetes than those having their TLEE between 16:00 and 21:00 (35.4% vs. 23.5%; P = 0.003).
Conclusions
There was no overall association of TLEE with the development of prediabetes or diabetes in the Whitehall II population. However, explorative analyses suggested that eating late in the evening was associated with increased risk of developing prediabetes/diabetes among women with good glycaemic control. Whether restricting late evening food consumption is effective and feasible for the prevention of Type 2 diabetes needs testing in randomized controlled trials.
Introduction
Current prevention strategies and treatment of obesity and Type 2 diabetes focus on energy-restricted diets, increased physical activity or medication [1,2], but adherence to such strategies is challenging for most individuals. Timing of food intake and fasting periods affects the circadian rhythms of metabolic organs, and experimental data from animal studies suggest promising effects of timing of dietary intake on metabolic functions [3]. Also, in smaller human studies, restricting late evening food consumption seems to have beneficial effects on body weight and metabolism [4]. Whether timing of food intake is related to long-term changes in glucose metabolism is not known. In the current analysis, we examined whether late evening food consumption is prospectively associated with the risk of prediabetes and diabetes in a large observational study of individuals with normoglycaemia.
Methods
Data were from the Whitehall II study including 2642 men and women from the British civil service (no shift workers) who were free of diabetes or prediabetes in 2002–2004 and who participated in a 5-year follow-up examination in 2007–2009. At both visits, all participants attended a clinical examination after an overnight fast of at least 8 h and their ‘time of last eating episode’ (TLEE) prior to the examination was requested. The examinations were distributed equally across weekdays (21.0% Monday, 17.2% Tuesday, 21.3% Wednesday, 18.4% Thursday and 22.2% Friday).
HbA1c was measured in whole blood using high-performance liquid chromatography. Prediabetes (HbA1c 39–47 mmol/mol) and diabetes (HbA1c ≥ 48 mmol/mol) were classified according to the American Diabetes Association definition [5]. Diabetes could also be diagnosed by a doctor outside the study between the visits. Information on ethnicity, employment, smoking, sleep, physical activity, alcohol, family history of diabetes, medication and energy intake was obtained from questionnaires.
Using linear regression, we studied the association of TLEE (16:00–04:00) with 5-year changes in HbA1c. The analysis was adjusted for baseline HbA1c, age, sex, ethnicity, employment, family history of diabetes, anti-hypertensive treatment, lipid-lowering treatment, BMI, average sleep duration, smoking status, physical activity, alcohol intake, energy intake, waist circumference, triglycerides, total-, HDL-and LDL cholesterol, systolic and diastolic BP.
We further assessed the association between TLEE and the development of prediabetes or diabetes using Poisson regression with the same covariates and risk time as offset. Potential heterogeneity in the association between TLEE and prediabetes or diabetes was examined using recursive partitioning modelling for time-to-event outcomes (survival tree) [6] with the same explanatory variables as above.
Results
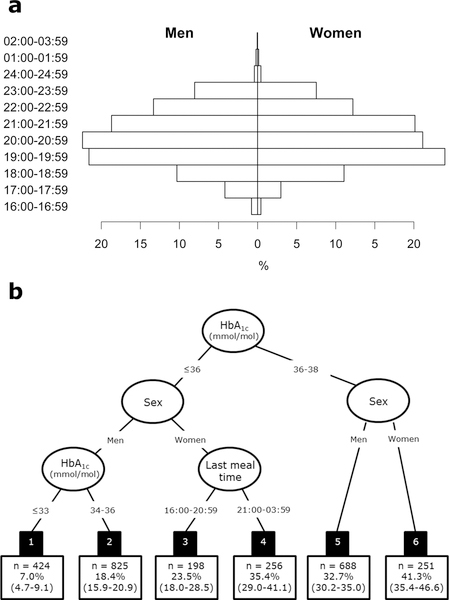
Mean (sd) age of participants at baseline was 60.2 (5.8) years, BMI was 26.3 (4.1) kg/m2 and 73.9% were men (Table 1). Median (range) TLEE at baseline was 21:00 (16:00–02:15) for men and 21:00 (16:00–02:20) for women (Fig. 1a). Median (IQR) population difference in TLEE between baseline and follow-up was 0.0 (−0.8; 1.5) h. At 5-year follow-up, 875 (33.1%) had developed prediabetes (n = 769, 29.1%) or diabetes (n = 106, 4.0%).
Table 1.
Baseline characteristics of the study population by sex
| Men | Women | |
|---|---|---|
| N | 1937 | 705 |
| White ethnicity (%) | 96.6 (95.7; 97.4) | 94.8 (92.8; 96.3) |
| Age (years) | 59.9 (5.7) | 59.7 (5.7) |
| BMI (kg/m2) | 26.0 (3.5) | 25.9 (4.8) |
| Waist circumference (cm) | 94.3 (10.0) | 85.0 (13.2) |
| HbA1c (mmol/mol) | 35 (3) | 35 (3) |
| HbA1c (%) | 5.4 (0.3) | 5.4 (0.3) |
| SBP (mmHg) | 126.9 (15.5) | 123.9 (18.0) |
| DBP (mmHg) | 74.3 (10.3) | 72.4 (10.8) |
| Total cholesterol (mmol/l) | 5.7 (1.0) | 5.9 (1.0) |
| LDL cholesterol (mmol/l) | 3.6 (0.9) | 3.5 (0.9) |
| HDL cholesterol (mmol/l) | 1.5 (0.4) | 1.9 (0.5) |
| Triglycerides (mmol/l) | 1.1 (0.8; 1.6) | 1.0 (0.7; 1.3) |
| Alcohol intake (units/week)* | 12.0 (5.0; 21.0) | 6.0 (2.0; 12.0) |
| Time of last meal intake | 21:00 (20:00; 22:00) | 21:00 (20:00; 22:00) |
| Nightly sleep < 7 h (%) | 38.4 (36.2; 40.6) | 43.7 (40.0; 47.4) |
| Daily energy intake (kcal∙103) | 2.25 (0.63) | 1.97 (0.6) |
| Current smoker (%) | 6.7 (5.6; 7.9) | 7.1 (5.3; 9.2) |
| Employment (%) | ||
| Administrative | 53.8 (50.1; 57.5) | 32.6 (27.1; 38.5) |
| Professional | 41.3 (37.7; 44.9) | 51.3 (45.2; 57.4) |
| Clerical | 5.0 (3.5; 6.8) | 16.1 (12.0; 21.0) |
| Physical activity ≥ 2.5 h/week | 31.5 (29.4; 33.6) | 25.6 (22.4; 29.1) |
| Family history of diabetes (%) | 8.4 (7.2; 9.7) | 8.7 (6.7; 11.0) |
| Antihypertensive treatment (%) | 17.6 (15.9; 19.3) | 18.7 (15.9; 21.8) |
| Lipid-lowering treatment (%) | 7.4 (6.3; 8.7) | 4.5 (3.1; 6.3) |
Data are shown as mean (sd), median (IQR) or proportion (95% confidence intervals).
A unit alcohol: 8 g of pure alcohol.
FIGURE 1.
Distribution of time of last meal among 1937 men and 705 women with normoglycaemia at baseline (a). Recursive partitioning model (survival tree) for progression from normoglycaemia to prediabetes or diabetes (b). The black boxes are the six terminal subgroups of the tree, each with the number of participants (n) and their estimated 5-year probability of progressing to prediabetes or diabetes with 95% confidence intervals.
Results from the linear regression and Poisson regression analysis showed a tendency of an overall association of TLEE with change in HbA1c but with little effect size [β per 1-h increase in TLEE = 0.2 mmol/mol, 95% CI −0.0 to 0.3 (0.01%, −0.00 to 0.03); P = 0.055] and no association with the risk of developing prediabetes/diabetes (risk ratio per 1-h increase in TLEE = 1.03, 95% CI 0.94 to 1.13; P = 0.511).
Interestingly, the recursive partitioning model showed heterogeneity in the association between TLEE and development of prediabetes or diabetes (Fig. 1b). The strongest predictor of development of prediabetes or diabetes was HbA1c. Around half of the participants with HbA1c concentrations of 36–38 mmol/mol (5.4–5.6%) developed prediabetes or diabetes during follow-up (47% of men and 61% of women; nodes 5 and 6), and these progression rates were independent of TLEE. For those with HbA1c concentrations ≤ 36 mmol/mol (< 5.4%), sex was a significant predictor of progression to prediabetes or diabetes. Men with HbA1c ≤ 36 mmol/mol (< 5.4%) had an estimated 7–18% 5-year risk of progressing to prediabetes or diabetes (nodes 1 and 2). The probability of progressing to prediabetes or diabetes in individuals with a good glycaemic control was higher among women and was related to TLEE. Women with HbA1c ≤ 36 mmol/mol (< 5.4%) and TLEE after 21:00 (n = 256) had 1.51 times (95% CI 1.16 to 1.93) higher 5-year risk of developing prediabetes/diabetes than those having their TLEE between 16:00 and 21:00 (incidence 35.4% vs. 23.5%, P=0.003; nodes 3 and 4). Changes in body weight from baseline to follow-up did not explain the differences in incidence rates. Women in node 3 on average gained 0.52 kg (95% CI −0.12 to 1.16), whereas women in node 4 gained 0.38 kg (−0.18 to 0.94) (P for difference = 0.753). At baseline, body weight was also similar among women in nodes 3 and 4 (66.5 vs. 67.2 kg, P = 0.570).
In a sensitivity analysis, we used an HbA1c range of 42–47 mmol/mol (6.0–6.4%) for definition of prediabetes. In this analysis, TLEE after 21:00 was also associated with a higher risk of developing prediabetes/diabetes in women with a good glycaemic control (≤ 37 mmol/mol) (Fig. S1).
Conclusions
Our findings add to the evidence on late evening food consumption and cardiometabolic risk. Animal and smaller human studies have observed beneficial effects of timing of food intake on weight loss [3], but the effects on glucose regulation in the general population have not been known. One explanation for an increased diabetes risk associated with late evening food consumption could be circadian variation in hormones regulating glucose metabolism [7]. In healthy individuals, serum insulin concentration peaks between noon and 18:00 [8], whereas the appetite hormones leptin and ghrelin peak around midnight [9,10]. It has also been shown that the glucose response to identical meals is greater in the evening than in the morning [11]. Thus, it is possible that food intake late in the evening or during night-time is in misalignment with the circadian rhythm of the insulin response, which may result in a greater overall glycaemic exposure and thereby increased HbA1c levels over time.
We observed an association between TLEE and prediabetes/diabetes development among women with a good glycaemic control. Differences in food patterns between men and women are common [12], and we have previously observed sex-specific associations between lifestyle factors and glucose regulation in the Whitehall II cohort [13], supporting these findings. Whether late evening food consumption disturbs circadian rhythm differently in men and women needs further investigation.
This study has some limitations and needs confirmation in other studies with more detailed assessment of the time of dietary intake over several days. Information on time of dietary intake in Whitehall II was limited to 1 day at baseline and 1 day at follow-up. Although the TLEE measure was generally stable across the examinations conducted 5 years apart, there may still be misclassifications in participants’ eating pattern. The participants were instructed to fast for 8 h prior to the health examinations, and therefore our data do not capture night-time eating. We used the HbA1c criteria for prediabetes/diabetes, because fasting and 2-h plasma glucose are affected by the time of day and fasting duration prior to blood sampling [14]. Accordingly, these measures would not be independent of the TLEE prior to the examination.
In conclusion, there was no overall association between TLEE and development of prediabetes/diabetes in the Whitehall II population. However, explorative analyses suggested that eating late in the evening was associated with increased risk of developing prediabetes/diabetes among women with good glycaemic control. Whether restricting late evening food consumption is effective and feasible for the prevention of Type 2 diabetes [3] needs testing in randomized controlled trials.
Supplementary Material
Figure S1. Recursive partitioning model (survival tree) for progression from normoglycaemia to prediabetes or diabetes.
What’s new?
Eating within a specific time window each day, i.e. time-restricted eating, reduces cardiometabolic risk in rodents.
In humans, timing of meal intake has acute effects on glucose regulation, but the long-term effects of meal timing on diabetes risk are less clear.
In more than 3500 men and women from the Whitehall II study, we found no associations between timing of last eating episode and 5-year development of prediabetes or diabetes.
However, explorative analyses suggested that late evening food consumption was associated with increased 5-year risk of developing prediabetes or diabetes among women with good glycaemic control.
Whether restricting late evening food consumption is effective and feasible for the prevention of Type 2 diabetes needs testing in randomized controlled trials.
Acknowledgements
The authors thank all participating women and men in the Whitehall II Study, as well as all Whitehall II research scientists, study and data managers and clinical and administrative staff who make the study possible.
Funding sources
The UK Medical Research Council (K013351), British Heart Foundation (RG/13/2/30098), and the US National Institutes of Health (R01HL36310, R01AG013196) have supported collection of data in the Whitehall II Study. K.F. is supported by a grant from the Novo Nordisk Foundation. A.H. and D.R.W. are supported by the Danish Diabetes Academy, which is funded by an unrestricted grant from the Novo Nordisk Foundation. M.K. is supported by the Medical Research Council (K013351, R024227), NordForsk and the Academy of Finland (311492). The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the report.
K.F and M.E.J. have received research grants from AstraZeneca (investigator-initiated research). S.P has published a book The Circadian Code.
Footnotes
Competing interests
The other authors declare no competing interests.
Supporting information
Additional Supporting Information may be found in the online version of this article:
References
- 1.World Health Organization. Diet, Nutrition and the Prevention of Chronic Disease. Report of a Joint FAO/WHO Expert Consultation Geneva: WHO, 2003. [Google Scholar]
- 2.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001; 74: 579–584. [DOI] [PubMed] [Google Scholar]
- 3.Melkani GC, Panda S. Time restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol 2018; 595: 3691–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 2015; 22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017; 40(Suppl. 1): S11–S24. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HS. Recursive Partitioning and Applications New York: Springer, 2010. [Google Scholar]
- 7.Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol 2012; 349: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol 1996; 271: E246–E252. [DOI] [PubMed] [Google Scholar]
- 9.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 1996; 97: 1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birketvedt GS, Geliebter A, Kristiansen I, Firgenschau Y, Goll R, Florholmen JR. Diurnal secretion of ghrelin, growth hormone, insulin binding proteins, and prolactin in normal weight and overweight subjects with and without the night eating syndrome. Appetite 2012; 59: 688–692. [DOI] [PubMed] [Google Scholar]
- 11.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol 1992; 262: E467–E475. [DOI] [PubMed] [Google Scholar]
- 12.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med 2004; 27: 107–116. [DOI] [PubMed] [Google Scholar]
- 13.Færch K, Witte DR, Brunner EJ, Kivimaki M, Tabak A, Jørgensen ME et al. Physical activity and improvement of glycemia in prediabetes by different diagnostic criteria. J Clin Endocrinol Metab 2017; 102: 3712–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulman A, Faerch K, Vistisen D, Karsai J, Nyari TA, Tabak AG et al. Effect of time of day and fasting duration on measures of glycaemia: analysis from the Whitehall II Study. Diabetologia 2013; 56: 294–297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recursive partitioning model (survival tree) for progression from normoglycaemia to prediabetes or diabetes.