Introduction
Drug addiction is a chronic and debilitating disorder with a high incidence of relapse. The lateral septum (LS) has been linked to the effects of abused drugs, but it is often overlooked in drug addiction research. Early intracranial self-stimulation experiments revealed that LS stimulation is highly reinforcing, as animals learn and maintain operant responding for electrical LS stimulation (Olds & Milner, 1954). LS projects to several limbic regions, including lateral hypothalamus and ventral tegmental area (VTA), and has been shown to regulate the mesolimbic dopamine (DA) system in both VTA and nucleus accumbens (Jonsson, Morud, Stomberg et al., 2017; Luo, Tahsili-Fahadan, Wise et al., 2011; Vega-Quiroga, Yarur & Gysling, 2017). Recently, it has been shown that inhibition of GABAAα1 receptor-containing VTA GABA neurons is necessary for LS to disinhibit VTA DA neurons (Vega-Quiroga, Yarur & Gysling, 2017).
Several studies indicate that LS neurons contribute to the rewarding (hedonic) and reinforcing effects of cocaine. LS neurons are Fos-activated following non-contingent injections of cocaine, cocaine self-administration, or exposure to cocaine-paired contexts (Franklin & Druhan, 2000; Zahm, Becker, Freiman et al., 2010). We have shown that inhibition of LS neurons reduces cocaine conditioned place preference (CPP) and context- or cue-induced reinstatement of cocaine seeking (McGlinchey & Aston-Jones, 2017; Sartor & Aston-Jones, 2012). We have also reported that LS input to VTA DA neurons is an important circuit for reinstatement of extinguished cocaine seeking (Luo, Tahsili-Fahadan, Wise et al., 2011). The role of LS in addiction appears to be complex, as genetic ablation of glucagon-like peptide-1 receptor (GLP-1R)-containing neurons in LS promotes cocaine CPP (Harasta, Power, von Jonquieres et al., 2015). These studies reveal that LS neurons are active during addiction-like behaviors and necessary for cocaine seeking.
However, LS neurons have also been shown to promote anxiety and interact with stress circuits (Anthony, Dee, Bernard et al., 2014; Sheehan, Chambers & Russell, 2004) Recently, several subpopulations of LS neurons have been identified that drive stress/anxiety. Optogenetic inhibition of type 2 CRF receptor (Crfr2)-containing LS neurons reduces anxiogenic behaviors and decreases plasma corticosterone levels (Anthony, Dee, Bernard et al., 2014). Similarly, knockdown of oxytocin receptor-containing LS neurons reduces a fear conditioned response following social defeat stress (Guzman, Tronson, Jovasevic et al., 2013). Finally, blocking GLP-1R signaling in LS attenuates stress-induced suppression of feeding (Terrill, Maske & Williams, 2018). Therefore, it is unclear if changes in drug seeking following LS manipulations are due to alterations in reward or anxiety.
To ascertain whether LS contributes to motivated drug taking, we utilized a within-session threshold procedure that uniquely assesses motivation for cocaine by measuring consumption at varying price points (Bentzley, Fender & Aston-Jones, 2013; Bentzley, Jhou & Aston-Jones, 2014). In this behavioral economics (BE) model of self-administration, the cost (number of presses) per mg of intravenous cocaine increases in successive 10-minute bins. Lever press data are fit to a demand curve using an exponential demand equation (Hursh & Silberberg, 2008). From this curve, we can extract two independent parameters of cocaine demand: demand elasticity (α) and free consumption (Q0). As α is the slope of the demand curve, it inversely scales with motivation, such that animals with high motivation for cocaine have lower α values.
Here we show that LS inhibition increased demand elasticity (decreased motivation) for cocaine in our BE paradigm without affecting consumption at low effort. The effects of LS inhibition on motivation were blocked by the benzodiazepine diazepam, which has been shown to disinhibit VTA DA neurons through inhibition of GABAAα1 receptor-containing VTA GABA neurons, and stimulate reward pathways (Heikkinen, Moykkynen & Korpi, 2009; Straub, Carlezon & Rudolph, 2010; Tan, Brown, Labouebe et al., 2010). Diazepam and LS inhibition similarly decreased anxiety but had opposing effects on drug taking, indicating that changes in motivation by LS inhibition were likely not due to changes in anxiety. Consequently, we conclude that LS regulates the motivational properties of cocaine during drug taking independently of effects on anxiety.
Materials and methods
Animals
Adult male Sprague-Dawley rats (n=88) weighing 325–350 grams were pair-housed on a 12:12 hour light:dark cycle in a temperature- and humidity-controlled animal facility with ad libitum access to standard rat chow and water. Animals were allowed to acclimate for 2 days, followed by handling for 3 days. All protocols and animal care procedures were approved by the Institutional Animal Care and Use Committee at Rutgers University.
Drugs
Diazepam (Sigma Aldrich, St. Louis, MO) was dissolved in 45% 2-hydroxypropyl-beta-cyclodextrin in sterile saline, and 1 or 2 mg/kg was given in a volume of 2 ml/kg (intraperitoneally). Each injection was given 30 minutes prior to the start of the behavioral economics (BE) paradigm. Cocaine HCl powder was provided by the National Institute of Drug Abuse (Research Triangle Park, NC) and was dissolved in 0.9% sterile saline.
Intravenous catheter surgery
Rats were anesthetized with ketamine/xylazine (56.5/8.7 mg/kg, i.p., respectively) and given an analgesic (rimadyl 5 mg/kg, s.c.). Rats were implanted with chronic indwelling catheters into the jugular vein. Intracranial surgeries were performed following catheterization. Catheters were flushed with cefazolin (0.1 ml; 100 mg/ml) and heparin (0.1 ml; 100 U/ml) after surgery, and daily beginning 2 days after surgery and after each self-administration session. Rats were allowed to recover for 1 week following surgery before self-administration training.
Stereotaxic surgery
Immediately following catheter surgery, animals were placed in a stereotactic frame (Kopf, Tujunga, CA, USA) and implanted with bilateral stainless steel guide cannulae (26 gauge, 5 mm, Plastics One, Roanoke, VA, USA) 2 mm dorsal to rostral LS (coordinates relative to bregma from skull surface: +1.1 mm AP, ± 0.5 mm ML, −4.0 mm DV). Guide cannulae were secured to the skull using acrylic cement and jeweler screws. Rats were allowed to recover for 1 week after surgery before behavioral training began.
Intracranial microinjections
Control microinjections were performed first to ensure that behavioral changes following baclofen-muscimol were not due to dorsal diffusion up the cannula tract. Injectors projecting 0.2 mm below the tip of the guide cannula were used to deliver the GABAA/B agonists baclofen plus muscimol (0.3/0.03 nmol in artificial CSF, 0.5 ul) 1.8 mm dorsal to LS.
For LS microinjections, injectors were lowered 2 mm below the guide cannulae into LS to infuse artificial CSF (aCSF) or baclofen-muscimol (B-M). The injector cannulae were kept in place for 1 minute after infusion to allow for drug diffusion. A counterbalanced within-subjects design was used in bilateral LS microinjection experiments. Animals received no more than 6 microinjections during testing. Animals with misplaced cannula (n= 10 for LS B-M experiment, n=5 for LS B-M x diazepam experiment) were excluded from analyses. Animals with misplaced cannula in the lateral ventricle (n=6) failed to lever press following B-M microinjections. All other misses (n=4 for LS B-M experiment, n=5 for LS B-M x diazepam experiment) had cannulae dorsal to LS or off-center in LS.
Cocaine self-administration
Rats were trained on an FR1 cocaine self-administration paradigm (20-second timeout post-infusion) for 2 hours/session, 1 session/day. Sessions occurred in operant chambers in sound-attenuating boxes using Med-PC IV software (Med Associates). During training sessions, cocaine infusions (0.19 mg cocaine/infusion) were paired with discrete light and tone cues (white stimulus light above the active lever; 78-dB, 2900-Hz tone). After reaching criteria (≥ 10 infusions/session for 10 sessions), animals were trained on the within-session threshold BE procedure.
BE procedure
Following FR-1 training, rats were trained on a within-session threshold BE procedure, as described previously (Bentzley, Fender & Aston-Jones, 2013; Bentzley, Jhou & Aston-Jones, 2014). During the 110-minute session, the dose of cocaine per active lever press was reduced in successive 10-minute intervals by decreasing the duration of cocaine infusion on a quarter logarithmic scale (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2, 1.2 ug cocaine per infusion), such that animals had to work progressively harder (produce more lever presses) to receive the same amount of cocaine; this is equivalent to increasing the price per mg of cocaine across the session. Animals were trained for a minimum of six days (mean ± SEM of all tested animals: 7.3 ± 0.4 days, range: 6–15 days). When animals displayed stable behavior (Q0 and α values ≤ 30% variability across the last three sessions), they received a microinjection of baclofen-muscimol (B-M) above LS (control) or a microinjection of B-M or aCSF into LS (n=12), a systemic injection of diazepam or vehicle (n=11), or both (n=10). Animals were tested again when BE performance re-stabilized (after at least three BE daily sessions, mean ± SEM: 4.2 ± 0.2 days, range: 3–12 days). Animals completed testing on the BE procedure after receiving all treatments (mean ± SEM: 22.7 ± 0.9 days, range: 15–37 days).
Demand curve analysis
Lever pressing responses during each BE session were fit to a demand curve to calculate two demand parameters (Q0 and α), as described in a previous paper from our lab (Bentzley, Fender & Aston-Jones, 2013). Q0 represents baseline consumption at low effort, and α indicates demand elasticity or the rate of decline in consumption as price increases. Therefore, Q0 is a measure of low effort consumption of the drug, and α is a measure of motivation for drug. As α is the slope of the demand curve, it inversely scales with motivation, such that animals with high motivation for cocaine will have lower demand elasticity and α values. Following each testing session, Q0 and α values were compared to those the day previously to determine the percent change with LS B-M or diazepam treatment.
Sucrose self-administration
To determine if LS or diazepam manipulations impacted the motor ability of animals to lever press for cocaine, animals (n=15) were trained to self-administer sucrose. Separate cohorts of animals were used for LS B-M (n=7) and diazepam (n=8) experiments with sucrose self-administration; these also were separate animals from those studied with cocaine self-administration. Animals were trained on FR-1 sucrose self-administration for 2 hours/day for a minimum of 5 days, as previously described (Cason & Aston-Jones, 2014). Responses on the active lever resulted in a sucrose pellet (45 mg, Test Diet), with a 20 s timeout after each reward. Animals were tested when they displayed stable active lever responses across the last three days (≤ 25% variability).
Locomotor testing
Animals (n=25) trained on the threshold BE procedure underwent general locomotor activity testing with B-M or aCSF microinjected into LS (n=8), diazepam or vehicle given ip (n=8), or both (n=9) to determine if effects on motivation were due to sedation. All animals had previously undergone BE testing for cocaine, except animals used for the combined LS B-M/diazepam experiment, in which a separate cohort of animals was used. At least 24 hours following the final BE testing session, rats were habituated to locomotor boxes (clear acrylic, 40 × 40 × 30 cm) equipped with Digiscan monitors (AccuScan Instruments) for 2 hours/day for 3 days. After three days of habituation, rats received a microinjection of aCSF or B-M into LS and/or a systemic injection of diazepam/vehicle prior to testing. Rats received each drug once in a randomized and counterbalanced fashion. In between each testing day, rats were given a 1-day washout session, in which they were placed in the locomotor box but did not receive any treatment. Total locomotor activity and center time were recorded using beam breaks, and the amount of time spent within an 8×8 in square matrix in the center of the chamber was determined with Fusion SuperFlex software.
Elevated plus maze
Animals were tested on an elevated plus maze (EPM) to measure anxiety-like behavior following LS inhibition (n=5/group) or diazepam treatment (n=7/group). LS inhibition animals had previously undergone BE testing. To obtain enough animals for the three diazepam treatments (vehicle, 1 or 2 mg/kg diazepam), we tested animals that had undergone BE testing with diazepam treatment (n=7) and an additional cohort of cocaine-experienced animals that underwent cocaine FR-1 training (n=14). The EPM (Med Associates, Inc.) was made of black, opaque Plexiglas elevated 60 cm above the floor of the testing room. The apparatus consisted of four arms (10 × 50 cm) perpendicular to one another: two opposing closed arms (with 40-cm walls) and two opposing open arms (with no walls). Two infrared photo-beams were positioned at the entrance of each of the four runways of the maze to track subjects’ movements and record entry time, based on when subjects broke and released both infrared photo-beams. Animals were placed at the junction of the open and closed runways 5 minutes after intracranial injections or 30 minutes after systemic diazepam treatment. Animals explored the apparatus for 15 minutes, and the percent time that the animals spent in the open, closed, and junction areas was recorded by Med-PC IV software.
Localization of microinjections
Following the last behavioral test, animals were deeply anesthetized with isoflurane and decapitated. Brains were dissected, flash-frozen in 2-methylbutane, and sectioned into 40 um sections on a cryostat. Sections were slide-mounted, Nissl-stained with neutral red, and coverslipped with DPX mounting medium to localize injection sites. We excluded data from animals that had extensive damage near the injection site as seen with Nissl staining (n=2).
Data analysis
Statistical analyses were performed with GraphPad Prism 7. BE data were analyzed using repeated measures one-way analyses of variance (ANOVA) with Bonferroni’s multiple comparisons test as appropriate. Locomotor, elevated plus maze, and sucrose self-administration tests were analyzed using paired samples t-tests to compare performance following aCSF or B-M microinjections. Non-parametric Friedman or Kruskal-Wallis test with post-hoc Dunn’s test, or Wilcoxon matched-pairs signed rank tests, was used when data were not normally distributed, as determined by a Shapiro-Wilk normality test. All statistics were two-tailed.
Results
Animals implanted with guide cannulae above LS (Figure 1A) were trained on cocaine self-administration and the within-session BE procedure. Prior to testing the effects of LS inhibition on motivation for cocaine, there were no significant differences in the animals’ baseline α (Shapiro Wilk normality test, W=0.76, p<0.01; Friedman test, Q=0.500, p=0.78) or Q0 values (one-way repeated measures ANOVA, F2,22=1.81, p=0.19) preceding each of the three treatments. Inhibition of LS with B-M increased demand elasticity (α; decreased motivation) compared to baseline (one-way repeated measures ANOVA, F2,22=9.89, p<0.01; Figure 1B). No changes were observed following microinjections of aCSF into LS, or of B-M 1.8 mm dorsal to LS. A one-way repeated measures ANOVA determined that none of the intracranial manipulations altered cocaine consumption at low effort (Q0; one-way repeated measures ANOVA, F2,22=0.23, p= 0.76; Figure 1C). Representative demand curves for LS aCSF and B-M manipulations are shown in Figure 1D. These effects were not due to sedation, as there was a trend towards increased locomotor activity during open field testing (paired t test; t7= 2.05, p=0.08; Figure 1E). For locomotor data analyzed in 5min bins, there was a significant effect of time (two-way repeated measures ANOVA, F23,161=6.99, p<0.0001; Figure 1F) and a time x treatment interaction (F23,161=1.94, p<0.01), but no effect of treatment (F1,7=4.19, p=0.08). Animals had greater locomotor activity following LS B-M microinjections at 40–50 (Bonferroni’s multiple comparisons test, p<0.05) and at 60 min of testing (p<0.01).
Figure 1.
Intra-lateral septum (LS) baclofen-muscimol (B-M) microinjections reduced motivation for cocaine. (A) Schematic of bilateral microinjector placements in LS in animals tested on behavioral economics (BE; n=12). (B) B-M microinjection into LS increased α compared to dorsal control B-M or intra-LS aCSF microinjections. (C) None of these treatments altered low effort consumption of cocaine (Q0; p>0.05). (D) Representative demand curves constructed from an animal’s BE performance following microinfusion of aCSF (solid line) or B-M (dotted line) into LS. (E) B-M microinjections did not significantly affect locomotor activity (p>0.05). (F) Binned data from locomotor activity testing. Locomotor activity following B-M microinjections was higher than after aCSF microinfusions between 40–50 minutes and at 60 minutes of testing. (G) B-M microinjections increased active lever presses for sucrose pellets during FR1 sucrose self-administration. *p<0.05, **p<0.01. Bar graphs and error bars indicate mean ± SEM.
As an additional motor control, we trained a separate cohort of animals on FR1 responding for sucrose pellets. We found that B-M microinfusions into LS increased active lever pressing for sucrose, confirming that the effect of LS B-M microinfusions to increase demand elasticity for cocaine was not due to decreased lever pressing abilities (Shapiro-Wilk test for normality: W=0.73, p<0.01;Wilcoxon matched-pairs signed rank test; W= 24.00, p<0.05; Figure 1G).
LS has an established role in anxiety, and therefore we sought to determine whether LS inhibition impacted anxiety in our animals. BE-experienced animals underwent locomotor testing in an open field, and the time spent in the center was determined following either microinjections of aCSF or B-M into LS. Intra-LS B-M microinfusions increased total center time compared to aCSF microinjections (Shapiro-Wilk test: W=0.81, p<0.05; Wilcoxon matched-pairs signed rank test; W= 36.00, p<0.01; Figure 2A). Microinfusions of B-M into LS also increased the percent time spent in the open arms of an elevated plus maze (unpaired t-test; t8=3.56, p<0.01; Figure 2B).
Figure 2.
Inhibition of LS reduced anxiety-like behaviors. (A) B-M microinjections into lateral septum (LS B-M) increased total center time during locomotor testing, compared to aCSF. (B) LS B-M increased the percentage of time animals spent in the open arms of an elevated plus maze. **p<0.01. Bar graphs and error bars indicate mean ± SEM.
Diazepam can increase cocaine self-administration (David, Gold, Koob et al., 2001; Maier, Ledesma, Seiwell et al., 2008). Although this effect was primarily attributed to reduced anxiety, previous research found that benzodiazepines also stimulated reward pathways via disinhibition of VTA DA neurons (Heikkinen, Moykkynen & Korpi, 2009; Tan, Brown, Labouebe et al., 2010). We tested animals in the BE procedure to determine if diazepam altered cocaine demand (α and Q0 values). When animals displayed stable baseline α and Q0 values, they were given systemic injections of diazepam (1 or 2 mg/kg) or vehicle IP thirty minutes prior to testing. There were no differences in animals’ baseline α (Shapiro-Wilk normality test, W=0.77, p<0.01; Friedman test, Q=0.00, p=0.99) or Q0 values (one-way repeated measures ANOVA, F2,20=0.022, p=0.97) preceding the three treatments. Diazepam significantly reduced α (increased motivation for cocaine) compared to vehicle treatment (one-way repeated measures ANOVA; F2,18= 4.20; p<0.05; Figure 3A), and this effect was specifically at the 2 mg/kg dose (Bonferroni’s multiple comparisons test, p<0.01). We also observed a trend towards a decrease in Q0, but this failed to reach statistical significance (one-way repeated measures ANOVA; F2,18=3.55, p=0.051; Figure 3B). A representative demand curve illustrating changes in α and Q0 is given in Figure 3C.
Figure 3.
Systemic diazepam increased motivation for cocaine. The day after animals displayed stable BE behavior, they were given vehicle, 1 or 2 mg/kg diazepam ip 30min prior to additional BE testing. (A) The 2 mg/kg dose of diazepam decreased cocaine demand elasticity (α; increased motivation) compared to vehicle. No significant change in demand elasticity was observed following the 1 mg/kg dose (p>0.05). (B) There was a trend towards a decrease in Q0 following 2 mg/kg diazepam, compared to vehicle (p=0.05). No significant effects were observed at the 1 mg/kg dose (p>0.05) (C) Representative demand curves from an animal showing a reduction in α (increase in motivation) and in Q0 following 2 mg/kg diazepam (dotted line) compared to vehicle (solid line). (D) Both doses of diazepam decreased locomotor activity compared to vehicle. (E) Both 1 and 2 mg/kg diazepam reduced locomotor activity in the first 10 minutes of testing (F) Both doses of diazepam increased the number of active lever presses during FR1 responding for sucrose pellets compared to vehicle. *p<0.05, **p<0.01, ****p<0.0001. Bar graphs and error bars indicate mean ± SEM.
Benzodiazepines also have been shown to have sedative properties (Rudolph, Crestani, Benke et al., 1999). Both doses of diazepam caused a significant reduction in locomotor activity (Shapiro Wilk, W=0.82, p<0.05; Friedman test, Q=12.00, p<0.01; Figure 3D). When locomotor activity was examined in 5-minute bins, there was a significant effect of time (two-way repeated measures ANOVA; F23,161=23.46, p<0.0001), treatment (F2,14=12.78, p<0.01), and a treatment x time interaction (F46,322=3.32, p<0.0001; Figure 3E). Animals had lower total locomotor activity following 1 or 2 mg/kg diazepam compared to vehicle during the first 10 min (Bonferroni multiple comparisons test, p<0.0001). Animals also significantly increased FR1 responding for sucrose following diazepam treatment (Shapiro-Wilk normality test, W=0.822, p<0.05; Friedman test: Q=9.25, p<0.01; Figure 3F). Therefore, although diazepam reduced habituated locomotor activity, it did not impair lever-pressing behavior.
We then tested animals on general anxiety measures to confirm that diazepam was anxiolytic. Diazepam increased center time in an open field (Shapiro-Wilk test, W=0.80, p<0.05; Friedman test, Q=6.25, p<0.05, Figure 4A); this was specifically at the 2 mg/kg dose (Dunn’s multiple comparisons test, p<0.05). Diazepam also increased percent time in the open arms of an EPM (Shapiro-Wilk test, W=0.79, p<0.05; Kruskal-Wallis test; H3.21=8.28, p<0.05; Figure 4B), and this effect was only for the 2 mg/kg dose (Dunn’s multiple comparisons test, p<0.01). Therefore, diazepam treatment was anxiolytic during general anxiety measures, like we found for LS inhibition.
Figure 4.
Diazepam was anxiolytic. (A) 2 mg/kg dose of diazepam increased center time during open field testing and (B) increased the percentage of time spent in the open arms of the elevated plus maze relative to vehicle. No significant changes were observed at the 1 mg/kg dose in either open field or elevated plus maze testing (p>0.05). *p<0.05, **p<0.01. Bar graphs and error bars indicate mean ± SEM.
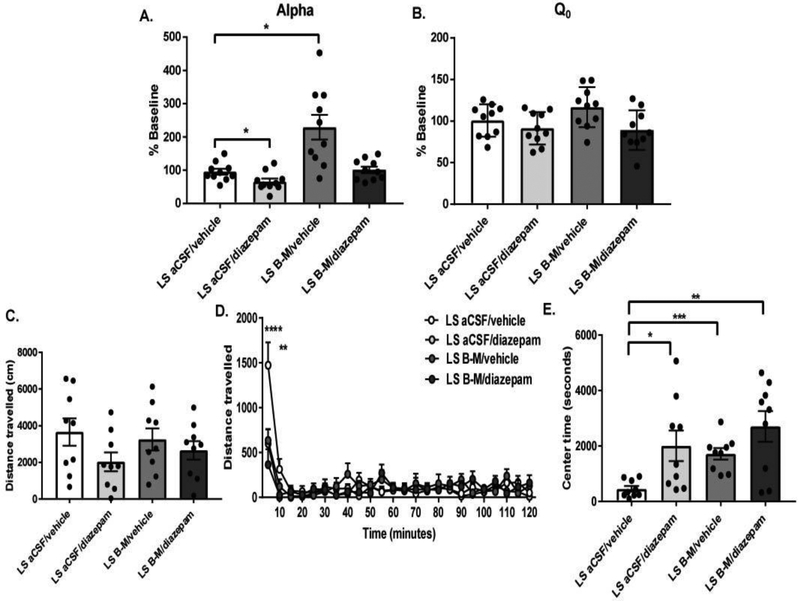
The above tests show that LS inhibition and diazepam are both anxiolytic but have opposite effects on motivation for cocaine. Both LS stimulation and diazepam have opposing effects on VTA DA neurons (Tan, Brown, Labouebe et al., 2010; Vega-Quiroga, Yarur & Gysling, 2017). To test whether diazepam could reverse the effects of LS inhibition on cocaine demand elasticity, we gave animals with stable BE behavior either 2 mg/kg diazepam or vehicle IP, followed by an intracranial microinjection of B-M or aCSF into LS 30 minutes later; animals were re-tested on a BE paradigm immediately after the intracranial injection. Animals’ baseline α and Q0 values did not differ across the four treatments (α: Shapiro-Wilk test, W=0.78, p<0.01; Friedman test, Q=2.28, p=0.52; Q0: one-way repeated measures ANOVA, F3,27= 1.68, p=0.22). Microinjections of aCSF into LS and systemic diazepam (LS aCSF/diazepam) increased motivation, whereas LS B-M and systemic vehicle treatment (LS B-M/vehicle) decreased motivation relative to vehicle, consistent with our observations above (one-way repeated measures ANOVA; F3,27=13.4, p<0.01; Figure 5A). However, we found that motivation following LS B-M microinjections plus systemic diazepam treatment (LS B-M/diazepam) did not differ from that following vehicle treatment (LS aCSF/vehicle; Bonferroni’s multiple comparisons test, p=0.99). None of the manipulations altered Q0 (one-way repeated measures ANOVA; F3,27=2.70, p=0.085; Figure 5B).
Figure 5.
Diazepam reversed the effects of LS inhibition on motivation for cocaine, but not on anxiety. (A) Pretreatment with 2 mg/kg diazepam decreased cocaine demand elasticity (increased motivation), whereas intra-LS B-M increased cocaine demand elasticity (decreased motivation) compared to vehicle. In contrast, combined LS B-M/diazepam treatment did not significantly differ from vehicle treatment. (p>0.05) (B) No effects on free consumption (Q0) were observed following any of these treatments. (C) None of the manipulations altered the total distance animals traveled during locomotor testing. (D) LS aCSF/diazepam, LS B-M/vehicle, and LS B-M/diazepam reduced locomotor activity in the first 5 minutes of testing. LS aCSF/diazepam and LS B-M/diazepam also reduced locomotor activity in the first 10 minutes of testing. (E) Both diazepam and intra-LS B-M microinjections significantly increased total center time, as did the combined treatment. There was no difference between the combined treatment and LS aCSF/diazepam or LS B-M/vehicle (p>0.05). *p<0.05, **p<0.01, ****p<0.0001. Bar graphs and error bars indicate mean ± SEM.
These manipulations did not alter locomotor activity in a separate cohort of animals (one-way repeated measures ANOVA; F3,24=2.36, p=0.10; Figure 5C). When locomotor data was binned in 5min intervals, there was a significant effect of time (F23,184= 18.5, p<0.0001; Figure 5D) and an interaction between time x treatment (F69, 184=3.37, p<0.0001). In the first 5 min, animals had higher locomotor activity following LS aCSF/vehicle than after LS aCSF/diazepam (Bonferroni’s multiple comparison test, p<0.0001), LS B-M/vehicle (p<0.0001), or LS B-M/diazepam (p<0.0001), and after 10 minutes with LS aCSF/diazepam (p<0.01) or LS B-M diazepam (p<0.01). All three manipulations significantly increased open field center time compared to vehicle (one-way repeated measures ANOVA; F3,24=5.48; p<0.05; Figure 5E), although there was no additive effect of the combined LS B-M/diazepam treatment on center time when compared to either LS aCSF/diazepam (Bonferroni’s multiple comparisons test, p=0.99) or LS B-M/vehicle (Bonferroni’s multiple comparisons test, p=0.70).
Discussion
Here, we extended recent work on the role of LS in addiction to show that LS is a critical region for effortful responding for cocaine. We found that pharmacological inhibition of LS reduced motivation for cocaine, as measured by demand elasticity in the BE paradigm. These effects were not present after intra-LS microinjections of aCSF or B-M dorsal to LS, indicating that changes in motivation were due specifically to inhibition of LS neurons. LS B-M microinjections did not alter free consumption of cocaine (Q0). Effects of LS inhibition were not likely due to anxiolytic actions, as the anxiolytic diazepam and LS inhibition had similar effects on anxiety but opposing effects on motivation for cocaine. The reduction in cocaine motivation following LS inhibition was blocked with pretreatment of diazepam. These results demonstrate that LS inhibition reduces cocaine demand elasticity by decreasing motivation for the drug.
LS inhibition increases cocaine demand elasticity
Previous studies reported that LS is an important region for cocaine seeking, yet it was unclear how the region contributes to this behavior. Our lab and others have implicated LS in stimulus-associated drug seeking including cue- or context-induced reinstatement of extinguished cocaine seeking, or CPP (Franklin & Druhan, 2000; Harasta, Power, von Jonquieres et al., 2015; Luo, Tahsili-Fahadan, Wise et al., 2011; McGlinchey & Aston-Jones, 2017; Sartor & Aston-Jones, 2012). Most recently, our lab found that inhibiting LS with B-M (as in the present studies) attenuated both context- and cue-induced reinstatement of cocaine seeking. We also showed that hippocampal input to LS drove context-induced reinstatement behavior (McGlinchey and Aston-Jones, 2017), implicating LS in cue- and context-associated cocaine seeking and relapse. To our knowledge, ours is the first study to demonstrate that LS also specifically mediates motivation for cocaine. In light of these studies, we hypothesize that inhibition of the region reduces the motivational properties of cocaine.
We saw no effect of LS B-M on general locomotor activity, indicating that the effects of LS inhibition on motivation were not due to sedation. These results are also consistent with a previous study from our lab showing no change in general locomotor activity following intra-LS B-M microinjections (McGlinchey & Aston-Jones, 2017). We tested animals on FR-1 responding for sucrose to determine if the effects of inhibition on cocaine demand elasticity were due to impaired lever-pressing. We observed no decrease (and in fact an increase) in active lever responding for sucrose, indicating that LS inhibition did not compromise animals’ ability to lever-press. These results agree with recent studies showing that LS inhibition promotes feeding behavior (Sweeney & Yang, 2015, 2016). Thus, our data support a role for LS in motivation for cocaine; future studies are needed to examine whether LS is also involved in effortful responding for natural rewards.
Pretreatment with diazepam increases motivation for cocaine
Human cocaine addicts will take benzodiazepines to alleviate the anxiogenic effects of cocaine withdrawal (Motta-Ochoa, Bertrand, Arruda et al., 2017). However, benzodiazepines have their own addictive properties, which make them poorly suited to treat addiction. In contrast to the effects on drug taking following LS inhibition, we observed that diazepam at the 2 mg/kg dose decreased α (increased motivation) and Q0 in the BE paradigm. These results may be due either to diazepam’s ability to ameliorate cocaine’s anxiogenic properties or to its ability to facilitate reward (Ettenberg & Geist, 1991; Geist & Ettenberg, 1997; Paine, Jackman & Olmstead, 2002; Reynolds, Engin, Tantillo et al., 2012; Straub, Carlezon & Rudolph, 2010). Our results support the latter hypothesis for two reasons. If diazepam reduced anxiety during BE performance, we would expect to see enhanced consumption in early BE bins, reflected by increased Q0. However, the trend towards a reduction in Q0 after diazepam indicates that animals need less drug to reach satiety, pointing to elevated rewarding effects of cocaine (Caine & Koob, 1994; Koob, Le & Creese, 1987; Maldonado, Robledo, Chover et al., 1993). Also, if LS inhibition and diazepam treatment both acted on cocaine demand elasticity by reducing anxiety, we would expect to see similar effects on drug taking, as both were found to be anxiolytic. However, the two had opposing effects on demand elasticity and similar effects on general anxiety. Our results do not discount that diazepam reduces drug-induced anxiety but provide support for the idea that the increased motivation it causes is due instead to enhanced activation of dopaminergic reward circuits following treatment.
Animal studies have predominantly attributed diazepam’s actions on cocaine self-administration to its role as an anxiolytic. It has been reported that diazepam increases cocaine self-administration across sessions or within a single session (David, Gold, Koob et al., 2001; Maier, Ledesma, Seiwell et al., 2008). In addition, diazepam has been shown to decrease the latency to start the self-administration session or to enter a goal box to receive cocaine infusions (Ettenberg & Geist, 1991; Geist & Ettenberg, 1997; Maier, Ledesma, Seiwell et al., 2008). Still, other studies have shown that benzodiazepines decrease cocaine intake (Augier, Vouillac & Ahmed, 2012; Goeders, McNulty & Guerin, 1993; Weerts, Froestl & Griffiths, 2005). These differences may be dependent on dose or the type of benzodiazepine administered. Lower doses of diazepam similar to those utilized in our study have consistently been shown to increase cocaine self-administration (David, Gold, Koob et al., 2001; Maier, Ledesma, Seiwell et al., 2008). We administered diazepam acutely, which is sufficient to increase activity of VTA DA neurons (Heikkinen, Moykkynen & Korpi, 2009). It remains unclear how chronic treatment would impact performance in the BE paradigm. Previous studies highlighting diazepam’s role as an anxiolytic have shown the largest increases in cocaine intake occur late in the self-administration session (David, Gold, Koob et al., 2001) and decreases in latency to start the self-administration session after two days of diazepam treatment (Maier, Ledesma, Seiwell et al., 2008). Therefore, investigation of the effects of chronic treatment on cocaine intake, particularly in the early bins (and in Q0), or the latency to start the session, might point to an anxiolytic role for diazepam in effects on motivation for cocaine.
Increased lever pressing during BE following diazepam treatment is not due to sedation
Diazepam also has sedative properties and can attenuate locomotor activity (Soderpalm, Svensson, Hulthe et al., 1991). Although both doses of diazepam used in this study reduced general locomotor activity, FR-1 responding for sucrose increased. Moreover, following diazepam treatment the number of presses for sucrose was greater than during BE, indicating that diazepam did not prevent lever-pressing behavior. The increase in lever-pressing for sucrose is consistent with previous studies demonstrating that diazepam increases sucrose palatability and consumption (Pecina & Berridge, 1996; Treit & Berridge, 1990), which is opioid-dependent and can be blocked with the opioid receptor antagonist naltrexone (Richardson, Reynolds, Cooper et al., 2005). Taken together, these results indicate that although sedation by diazepam reduces general locomotor activity, it does not interfere with lever pressing ability.
Diazepam reverses the effects of LS inhibition on cocaine demand elasticity
LS inhibition and diazepam treatment produced opposing effects on drug taking, and diazepam pretreatment blocked the effects of LS inhibition on motivation for cocaine. A possible mechanism for these differences is through different effects on the activity of VTA DA neurons. Diazepam’s rewarding/reinforcing effects are thought to occur through its binding to the GABAAα1 receptor on VTA GABAergic neurons, thereby disinhibiting VTA DA neurons (Heikkinen, Moykkynen & Korpi, 2009; Tan, Brown, Labouebe et al., 2010). Other receptor subunits of the GABAA receptor, including the α2 and α3 subunits, have also been implicated in diazepam’s rewarding effects (Reynolds, Engin, Tantillo et al., 2012). Recently, LS stimulation was found to promote disinhibition of DA neurons via the GABAAα1 receptor on VTA GABAergic neurons (Vega-Quiroga, Yarur & Gysling, 2017). Our laboratory found that inhibiting LS input to VTA reduces context-induced reinstatement, indicating that LS disinhibition of VTA DA neurons is an important connection that drives drug seeking (Luo, Tahsili-Fahadan, Wise et al., 2011). Therefore, we hypothesize that the inhibitory LS to VTA GABAergic interneuron pathway may be downregulated following LS inhibition, but potentiated by diazepam treatment.
LS inhibition and diazepam similarly reduce anxiety
Both LS inhibition and diazepam decreased anxiety-like behaviors, as indicated by increased center time in the open field and open arm time in the elevated plus maze, and combined treatment did not have an additive effect on anxiolytic behaviors. Experiments investigating the role of LS on anxiety have yielded differing results. Optogenetic inhibition of LS neurons containing Crfr2 was anxiolytic and reduced corticosterone levels (Anthony, Dee, Bernard et al., 2014). However, other studies found that inhibiting or lesioning LS was anxiogenic, giving rise to the conclusion that LS neuronal activity is anxiolytic (Sheehan, Chambers & Russell, 2004). These differences across studies may result from the subpopulations of LS neurons impacted by each study’s manipulation. Notably, early experiments linked the anxiolytic properties of LS to those seen after benzodiazepine treatment (Clarke & File, 1982; Drugan, Skolnick, Paul et al., 1986; Yadin, Thomas, Grishkat et al., 1993). We observed a similar but non-additive effect of LS inhibition and diazepam on anxiety-like behaviors, indicating that diazepam may act on LS neurons to reduce anxiety. Although it is possible that LS neurons mediate some of the anxiogenic properties of cocaine, our results provide strong evidence for a role for LS in motivation for cocaine.
Taken together, our study implicates LS in promoting high effort cocaine seeking, and points to an important role for LS in motivation for cocaine. Inhibiting LS prior to BE testing reduced motivation for cocaine (increased α) without impacting low effort consumption (Q0) or general locomotor activity. These findings add to a growing literature that LS neurons contribute to cue-dependent, motivated drug seeking. The benzodiazepine diazepam blocked the effects of LS inhibition on motivation for cocaine, pointing to opposing roles for the two manipulations on VTA DA neurons. Collectively, these studies indicate that LS regulates motivated drug taking, making it an attractive region to manipulate for the treatment of addiction.
Acknowledgments
We would like to thank Dr. Morgan James for valuable suggestions on this manuscript and Griffin Poole for assistance with behavioral procedures and histology. These experiments were supported by U.S. Public Health Service awards from the National Institute of Drug Abuse to GAJ (R01 DA006214) and CBP (F31DA042588). CBP and GAJ declare that they have no conflict of interests.
References
- Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, Anderson DJ (2014) Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 156:522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier E, Vouillac C, Ahmed SH (2012) Diazepam promotes choice of abstinence in cocaine self-administering rats. Addict Biol 17:378–391. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G (2013) The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111:11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF (1994) Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270:209–218. [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G (2014) Role of orexin/hypocretin in conditioned sucrose-seeking in female rats. Neuropharmacology 86:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, File SE (1982) Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviours. Pharmacol Biochem Behav 17:623–628. [DOI] [PubMed] [Google Scholar]
- David V, Gold LH, Koob GF, Cazala P (2001) Anxiogenic-like effects limit rewarding effects of cocaine in balb/cbyj mice. Neuropsychopharmacology 24:300–318. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Skolnick P, Paul SM, Crawley JN (1986) Low doses of muscimol produce anticonflict actions in the lateral septum of the rat. Neuropharmacology 25:203–205. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD (1991) Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 103:455–461. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP (2000) Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 12:2097–2106. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A (1997) Concurrent positive and negative goalbox events produce runway behaviors comparable to those of cocaine-reinforced rats. Pharmacol Biochem Behav 57:145–150. [DOI] [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Guerin GF (1993) Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol Biochem Behav 44:471–474. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J (2013) Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci 16:1185–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M (2015) Septal Glucagon-Like Peptide 1 Receptor Expression Determines Suppression of Cocaine-Induced Behavior. Neuropsychopharmacology 40:1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen AE, Moykkynen TP, Korpi ER (2009) Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology 34:290–298. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115:186–198. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Morud J, Stomberg R, Ericson M, Soderpalm B (2017) Involvement of lateral septum in alcohol’s dopamine-elevating effect in the rat. Addict Biol 22:93–102. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le HT, Creese I (1987) The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci Lett 79:315–320. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G (2011) Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ledesma RT, Seiwell AP, Duvauchelle CL (2008) Diazepam alters cocaine self-administration, but not cocaine-stimulated locomotion or nucleus accumbens dopamine. Pharmacol Biochem Behav 91:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF (1993) D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav 45:239–242. [DOI] [PubMed] [Google Scholar]
- McGlinchey EM, Aston-Jones G (2017) Dorsal Hippocampus Drives Context-Induced Cocaine Seeking via Inputs to Lateral Septum. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta-Ochoa R, Bertrand K, Arruda N, Jutras-Aswad D, Roy E (2017) “I love having benzos after my coke shot”: The use of psychotropic medication among cocaine users in downtown Montreal. Int J Drug Policy 49:15–23. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47:419–427. [DOI] [PubMed] [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC (2002) Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol 13:511–523. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC (1996) Brainstem mediates diazepam enhancement of palatability and feeding: microinjections into fourth ventricle versus lateral ventricle. Brain Res 727:22–30. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Engin E, Tantillo G, Lau HM, Muschamp JW, Carlezon WA Jr., Rudolph U (2012) Differential roles of GABA(A) receptor subtypes in benzodiazepine-induced enhancement of brain-stimulation reward. Neuropsychopharmacology 37:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DK, Reynolds SM, Cooper SJ, Berridge KC (2005) Endogenous opioids are necessary for benzodiazepine palatability enhancement: naltrexone blocks diazepam-induced increase of sucrose-’liking’. Pharmacol Biochem Behav 81:657–663. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H (1999) Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature 401:796–800. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS (2012) A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci 32:4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS (2004) Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev 46:71–117. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Svensson L, Hulthe P, Johannessen K, Engel JA (1991) Evidence for a role for dopamine in the diazepam locomotor stimulating effect. Psychopharmacology (Berl) 104:97–102. [DOI] [PubMed] [Google Scholar]
- Straub CJ, Carlezon WA Jr., Rudolph U (2010) Diazepam and cocaine potentiate brain stimulation reward in C57BL/6J mice. Behav Brain Res 206:17–20. [DOI] [PubMed] [Google Scholar]
- Sweeney P, Yang Y (2015) An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun 6:10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney P, Yang Y (2016) An Inhibitory Septum to Lateral Hypothalamus Circuit That Suppresses Feeding. J Neurosci 36:11185–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C (2010) Neural bases for addictive properties of benzodiazepines. Nature 463:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill SJ, Maske CB, Williams DL (2018) Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiol Behav 192:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Berridge KC (1990) A comparison of benzodiazepine, serotonin, and dopamine agents in the taste-reactivity paradigm. Pharmacol Biochem Behav 37:451–456. [DOI] [PubMed] [Google Scholar]
- Vega-Quiroga I, Yarur HE, Gysling K (2017) Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: Role of GABA-A alpha 1 receptors. Neuropharmacology 128:76–85. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Griffiths RR (2005) Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend 80:369–376. [DOI] [PubMed] [Google Scholar]
- Yadin E, Thomas E, Grishkat HL, Strickland CE (1993) The role of the lateral septum in anxiolysis. Physiol Behav 53:1077–1083. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, Meredith GE, Marinelli M (2010) Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology 35:445–463. [DOI] [PMC free article] [PubMed] [Google Scholar]