Abstract
Background
Obtaining tumor specimens and re-evaluating targeted markers is recommended, if possible, in breast cancer patients who relapsed after curative treatment. The biomarker status changes in rebiopsied tumors have been demonstrated to have considerable clinical implications.
Objectives
To identify the changes of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status between the primary and recurrent lesions.
Materials and Methods
We conducted a study among 67 patients with recurrent breast cancer, recruited from January 2014 to September 2018 in the Vietnam National Cancer Hospital to compare ER, PR, and HER2 status between the primary and recurrent lesions. For each patient, a specimen of their primary tumor and another specimen of recurrent lesions underwent pathological assessment. Immunohistochemistry (IHC) was performed to determine ER, PR, and HER2 status in both specimens.
Results
Biomarker status conversion rates (in both directions) between primary and recurrent tumors were 26.9% for ER, 38.8% for PR, and 22.4% for HER2. Overall, IHC subtypes (hormone receptor positive, HER2 amplified, and triple-negative) changed in 25 out of 67 (37.3%) cases. Conversion rates were not statistically significantly different between patients with different recurrent sites and times of recurrence. Eight out of 13 initially triple-negative patients (61.5%) had a change to positive status of either ER, PR, or HER2.
Conclusion
A substantial discordance in ER, PR, and HER2 status were observed between primary breast cancer tissues and recurrent lesions. Rebiopsy could bring new therapeutic opportunities in the management of patients with recurrent breast cancer.
1. Introduction
Breast cancer has been recognized as the most common type of cancer and the leading cause of malignancy-related mortality in women worldwide [1]. In Vietnam, breast cancer incidence had an age-standardized rate of 29.9 per 100,000 women in 2010, which had doubled over the last two decades [2]. Despite the increasing trend in breast cancer incidence, there have been certain improvements in the prognosis and treatment thanks to the advances in the understanding of related biomarkers and the development of corresponding therapeutic approach [3, 4].
Among various biomarkers, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) play important roles in management and prognosis of patients with breast cancer [3]. Approximately 60%–70% of breast cancer patients are hormone-receptor positive and 20%–25% have amplified HER2 [5, 6]. According to the 2013 St Gallen Consensus Conference, based on ER, PR, HER2, and Ki67 status, breast cancer patients are divided into different subtypes, including Luminal A, Luminal B, HER2-amplified, and triple-negative [7]. Patients with different subtypes are treated differently in both early and advanced stages, and have different survival time [3, 8]. For example, in a study on 196,094 breast cancer patients, Luminal A group had a 4 year survival rate of 92.5%, followed by Luminal B (90.3%), HER2-amplified (82.7%), and finally worst survival for triple-negative subtype (77.0%) [9]. Patients with positive hormone receptor could be treated with endocrine therapy and those with HER2 overexpression have survival benefits from trastuzumab and/or pertuzumab treatment [10]. Meanwhile, in the triple-negative group, treatment options are usually limited to chemotherapy, and the polyadenosine diphosphate-ribose polymerase (PARP) inhibitor olaparib for selected cases with BRCA mutation [11]. Recently, androgen receptor (AR) expression has been evaluated in breast cancer, in which AR is associated with cell proliferation and metastasis in ER-negative breast cancer [12]. This evidence supports AR-targeted therapies, including bicalutamide and enzalutamide might be useful in patients with triple-negative breast cancer [12]. Therefore, biomarker status assessment has a significant clinical utility in guiding treatment decision-making in not only newly diagnosed breast cancer but also in recurrent settings. Recently, some studies have demonstrated a remarkable rate of conversion of these biomarker status in patients with recurrent breast cancer after curative treatment [13–17]. However, the conversion rate of each biomarker is inconsistent in the previous studies. The changes in receptor status, meanwhile, can result in changing the treatment approach, and also is a clinically significant prognostic factor of overall survival [15, 18, 19]. Therefore, international guidelines have encouraged to perform biopsy of recurrent lesions to re-evaluate biomarker status [10].
However, in Vietnam, rebiopsy of recurrent lesions has not been routinely performed. The objective of this study is to evaluate the changes of ER, PR, and HER2 status between the primary and recurrent lesions in Vietnamese patients with breast cancer.
2. Materials and Methods
2.1. Study Design
This is a retrospective and observational study, conducted at the Vietnam National Cancer Hospital. Convenience sampling method was used to enroll patients during a 4 year period from January 2014 to September 2018. This study was approved by the research committee of the Vietnam National Cancer Institute.
2.2. Study Population
We included patients who were diagnosed with recurrent breast cancer after curative treatment at our institution during the study period. The staging of primary tumor was based on the AJCC Cancer Staging Manual, 7th edition [20]. All patients were clinically or radiologically suspected of recurrence by their primary oncologists and underwent biopsy or surgical resection of the recurrent lesions for confirmation. Recurrent lesions (RL) were defined as any local, regional, or distant recurrence. For superficial lesions, core or excisional biopsy was performed. For internal lesions, the most amenable site of biopsy was determined in consultation with an interventional radiologist, and core biopsy was carried out under radiologic guidance (i.e., ultrasound for liver lesions and axillary or supraclavicular lymph nodes, CT scan for lung lesions, mediastinal lymph nodes, and bone lesions). One patient with seizure and suspected brain tumor in MRI scan underwent surgery for brain tumor resection which revealed a metastasis originating from the breast. Tissue samples were evaluated by hematoxylin and eosin staining and an immunohistochemical panel that includes ER, PR, HER2, and Ki67. Exclusion criteria included (1) patients with de novo metastatic breast cancer, (2) patients with contralateral tumor or pathological results suggesting a new primary tumor, and (3) patients with incomplete pathological and immunohistochemical information.
2.3. ER, PR, and HER2 Determination
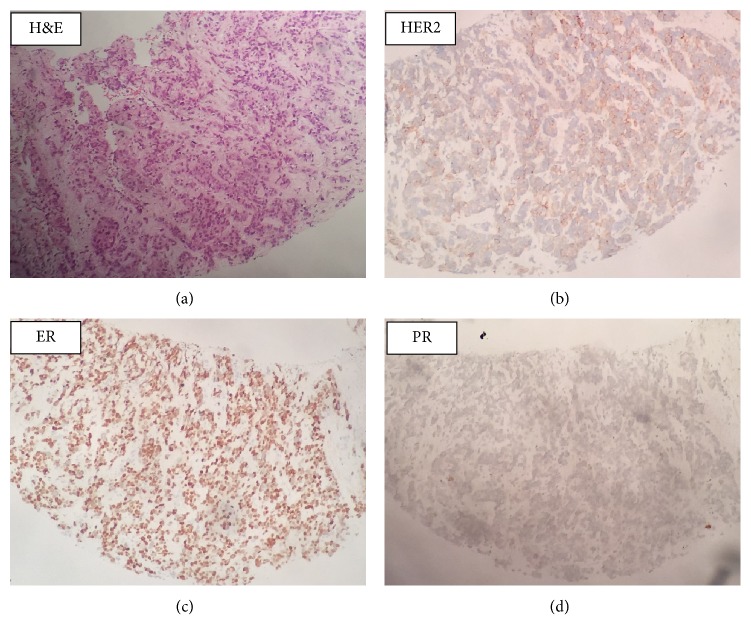
The ER, PR, and HER2 status for the primary tumors were obtained from medical chart/pathology report reviews. The rebiopsy tumor tissue samples from recurrent lesions were stained for ER, PR, and HER2 using formalin-fixed and paraffin-embedded (FFPE) sections following Avidin-Biotin Complex (ABC) method, in which tetravalent strept (avidin) and biotinylated antibodies were used. ER, PR, and HER2 classifications were made based on the American Society of Clinical Oncology/College of American Pathologists guideline recommendations for IHC testing of ER, PR, and HER2 in breast cancer [21, 22]. If ≥1% of tumor cells show positive ER/PR staining of any intensity, the ER/PR interpretation is positive. Negative hormone receptor is defined as <1% of tumor cells with ER/PR staining of any intensity. Meanwhile, a standard 0–3+ scoring system was used to evaluate HER2 status, in which 0 and 1+ scores were considered negative and 3+ was considered positive. If IHC HER2 scored 2+, HER2 FISH test was conducted. HER2/CEP17 ratio ≥2 was defined as HER2 amplified (Figure 1).
Figure 1.
Representative examples of IHC analysis of the studied biomarkers on a biopsied specimen. ER was markedly expressed (ER score 2+) and PR staining status was negative. The staining score of HER2 was 2+ (FISH test showed that the tumor was HER2-amplified). H&E, hematoxylin and eosin stain; IHC, immunohistochemistry; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; FISH, Fluorescence in situ hybridization.
2.4. Subtype Definition
The patients were divided into three IHC subtypes based on primary tumor, including HR-positive (ER positive and/or PR positive and HER2 negative), HER2-amplified (HER2 positive/any ER, PR status), and triple-negative (ER negative, PR negative, and HER2 negative).
2.5. Statistical Analysis
All data were presented descriptively as a median (interquartile range) or number (percentage). Comparisons between different groups were done using Fisher's exact test or Chi square test where appropriate. p values < 0.05 were considered statistically significant. Data were analyzed using STATA SE 12.0 for Windows (STATA Corp., College Station, TX 77845).
3. Results
3.1. Patients' Baseline Characteristics
During the study period, a total of 67 patients were diagnosed with recurrent breast cancer at our institution. The IHC profile of primary tumors was ER/PR-positive in 30 (44.8%) patients, HER2-amplified in 24 (35.8%) patients, and triple-negative in 13 (19.4%) patients.
A summary of patient characteristics is presented in Table 1. Ductal carcinoma accounted for a majority of cases (80.6%, 54/67), followed by lobular carcinoma (9.0%, 6/67). After initial diagnosis, all patients underwent surgery, and 61/67 (91.0%) patients then received either adjuvant chemotherapy, endocrine therapy, or target therapy prior to recurrences. The median time from diagnosis of the primary tumor to identification of recurrences was 45 months (interquartile range (IQR) 24–59 months). The most common site of recurrence was regional lymph nodes (36/67, 53.7%), followed by chest wall (17/67, 25.4%).
Table 1.
Patient demographics and clinical characteristics.
| Characteristics | Number (N = 67) | Percentage |
|---|---|---|
| At time of initial diagnosis | ||
| Age (median (IQR)) | 44 | (38–53) |
| Menopause status | ||
| Pre-menopause | 38 | 56.7% |
| Post-menopause | 29 | 43.3% |
| Stage at diagnosis | ||
| 0 | 2 | 3.0% |
| I | 11 | 16.4% |
| II | 27 | 40.3% |
| III | 27 | 30.3% |
| Pathological types | ||
| Ductal | 54 | 80.6% |
| Lobular | 6 | 9.0% |
| Other | 7 | 10.4% |
| Therapy | ||
| Chemotherapy | 53 | 79.1% |
| Endocrine therapy | 37 | 55.2% |
| Trastuzumab | 5 | 7.5% |
| At time of recurrence | ||
| Duration from primary to recurrent disease | ||
| <24 months | 17 | 25.4% |
| 24–36 months | 8 | 11.9% |
| >36–60 months | 28 | 41.8% |
| >60 months | 14 | 20.9% |
| Locoregional recurrence | 48 | 71.6% |
| Chest wall | 17 | 25.4% |
| Regional lymph nodes | 36 | 53.7% |
| Distant metastasis | ||
| Lung | 15 | 22.4% |
| Liver | 5 | 7.5% |
| Bone | 10 | 14.9% |
| Mediastinal lymph node | 9 | 13.4% |
| Data: distant metastasis/other | 5 | 7.5% |
| Site of biopsy | ||
| Locoregional | 45 | 67.2% |
| Lung | 8 | 11.9% |
| Liver | 4 | 6.0% |
| Bone | 2 | 3.0% |
| Brain | 1 | 1.5% |
| Mediastinal lymph node | 1 | 1.5% |
| Others | 6 | 9.0% |
3.2. Change in Receptor Profile between the Primary and Recurrent Lesions
The concordance and discordance of tumor biomarkers between primary and recurrent lesions are shown in Tables 2 and 3. Among the three receptors, PR status had the highest conversion rate (38.8%, 95% CI 26.8%–50.8%) between primary and recurrent lesions; the majority of which was from PR-positive to PR-negative 17/67 (25.4%), compared to 9/67 (13.4%) in the opposite direction. ER status conversion was seen in 26.9% patients (95% CI: 16.0%–37.8%); however, it comprised comparable numbers of gain vs. loss (8 vs. 10 patients, respectively). The overall conversion rate for HR was 25.4% (17/67 patients, 95% CI: 15.5%–37.5%), similarly distributed between two directions (8 gains vs. 9 losses). Among the discordant subset, ER and PR conversions were most commonly observed in the HR-positive group (38.9%, 7/18 for ER and 69.2%, 18/26 for PR) (Table 3). There were 5/13 (38.5%) triple-negative patients that switched hormone receptor profile (either ER or PR).
Table 2.
Distribution of ER, PR, and HER2 status between the primary tumor and recurrent lesions (N = 67).
| Biomarkers | ConcordanceN (%) | DiscordanceN (%) | Total discordanceN (%) | ||
|---|---|---|---|---|---|
| PL (+) | PL (−) | PL (+) | PL (−) | ||
| RL (+) | RL (−) | RL (−) | RL (+) | ||
| ER | 27 (40.3%) | 22 (32.8%) | 8 (11.9%) | 10 (14.9%) | 18 (26.9%) |
| PR | 11 (16.4%) | 30 (44.8%) | 17 (25.4%) | 9 (13.4%) | 26 (38.8%) |
| HER2 | 19 (29.7%) | 33 (49.3%) | 5 (7.5%) | 10 (14.9%) | 15 (22.4%) |
PL: Primary lesions; RL: Recurrent lesions.
Table 3.
Conversion rates of receptor status between primary tumor and metastasis.
| Markers | Total | HR-positive N (%) | HER2 amplified N (%) | Triple-negative N (%) |
|---|---|---|---|---|
| ER | ||||
| No conversion | 49 | 23 (76.7%) | 18 (75.0%) | 8 (61.5%) |
| From (+) to (−) | 8 | 6 (20.0%) | 2 (8.3%) | 0 |
| From (−) to (+) | 10 | 1 (3.3%) | 4 (16.7%) | 5 (38.5%) |
| Total | 67 | 30 (100%) | 24 (100%) | 13 (100%) |
| PR | ||||
| No conversion | 41 | 12 (40.0%) | 20 (83.3%) | 9 (69.2%) |
| From (+) to (−) | 17 | 14 (46.7%) | 3 (12.5%) | 0 |
| From (−) to (+) | 9 | 4 (13.3%) | 1 (4.2%) | 4 (30.8%) |
| Total | 67 | 30 (100%) | 24 (100%) | 13 (100%) |
| HER2 | ||||
| No conversion | 52 | 24 (80.0%) | 19 (79.2%) | 9 (69.2%) |
| From (+) to (−) | 5 | 0 | 5 (20.8%) | 0 |
| From (−) to (+) | 10 | 6 (20.0%) | 0 | 4 (30.8%) |
| Total | 67 | 30 (100%) | 24 (100%) | 13 (100%) |
In terms of HER2, the conversion rate was 22.4% (95% CI: 12.1%–32.6%), including 5 (7.5%) patients from positive to negative and 10 (14.9%) patients in the reverse. HER2 gain proportions were 20% (6/30) and 30.8% (4/13) in HR-positive and triple-negative groups, respectively.
With the above conversion of receptor status, the IHC subtype was changed in 25/67 (37.3%) patients. Changing from HR-positive to HER2 amplified and to Triple-negative had the highest frequency (6/67 patients in each change, 9.0%). Meanwhile, changes from HER2 amplified to HR-positive, from triple-negative to HR-positive and to HER2 amplified occurred in 4 patients (6.0%) each. Only 1 patient (1.5%) switched from HER2 amplified to triple-negative. In contrast, a change to positive of either ER, PR, or HER2 was observed in 8/13 triple-negative patients (61.5%).
Regarding the biopsy of recurrent lesions, changes in ER, PR, and HER2 status of locoregional recurrences were recorded in 26.7%, 40.0%, and 20.0%, respectively. Among distant metastasis biopsies, discordance rate was also highest in PR status (36.4%), followed by ER and HER2 (27.3% each) (Table 4).
Table 4.
Distribution of ER, PR, and HER2 status according to biopsied site.
| Sites of biopsy | Total | Change in ER | Change in PR | Change in HER2 status | |||
|---|---|---|---|---|---|---|---|
| DiscordantN (%) | ConcordantN (%) | DiscordantN (%) | ConcordantN (%) | DiscordantN (%) | ConcordantN (%) | ||
| Locoregional lesions | 45 | 12 (26.7%) | 33 (73.3%) | 18 (40.0%) | 27 (60.0%) | 9 (20.0%) | 36 (80.0%) |
| Metastasis lesions | 22 | 6 (27.3%) | 16 (72.7%) | 8 (36.4%) | 14 (63.6%) | 6 (27.3%) | 16 (72.7%) |
| Lung | 8 | 3 (37.5%) | 5 (62.5%) | 2 (25.0%) | 6 (75.0%) | 2 (25.0%) | 6 (75.0%) |
| Liver | 4 | 0 | 4 (100%) | 0 | 4 (100%) | 1 (25.0%) | 3 (75.0%) |
| Bone | 2 | 1 (50%) | 1 (50%) | 1 (50%) | 1 (50%) | 0 | 2 (100%) |
| MLN | 1 | 0 | 1 (100%) | 1 (100%) | 0 | 1 (100%) | 0 |
| Others | 7 | 2 (28.6%) | 5 (71.4%) | 4 (57.1%) | 3 (42.9%) | 2 (28.6%) | 5 (71.4%) |
MLN: Mediastinal lymph nodes.
3.3. Effects of the Duration between Primary and Recurrent Disease and the Recurrent Site on Conversion Rates
The proportions of receptor discordance were similar between locoregional and distant metastatic sites, which was presented in all three IHC subtype groups (p-values > 0.05, see details in Table 4). Significant differences were also not observed between patients with recurrences that occurred >36 months and ≤36 months after primary disease (ER: 32% vs. 27%; PR: 32 vs. 43%; HER2: 20 vs. 24%, p-values > 0.05).
4. Discussion
Our study included 67 cases of recurrent breast cancer after curative treatment. RL biopsies with IHC showed considerable rates of receptor status conversions, including 26.9% in ER, 38.8% in PR, and 22.4% in HER2. There were no statistical differences in conversion rates in regard to different sites and times of recurrence. Eight out of 13 triple-negative patients (61.5%) had a change to positive of either ER, PR, or HER2 status compared to the primary tumors. To our knowledge, this is one of the few reports in Vietnam as well as in other developing countries [18, 23–25], focusing on the conversion of breast cancer biomarkers between primary tumors and recurrence lesions.
Biopsy of metastatic and recurrent lesions in breast cancer plays an important role, not only to achieve a final diagnosis, but also to re-evaluate ER, PR, and HER2 status. Evidence has shown that the conversion of these markers between primary and RL could be useful in the clinical management of patients with breast cancer [16, 17]. Previous studies reported that 39%–46% of treatment plans were modified according to the conversion of rebiopsy IHC [16, 17]. In the Breast Recurrence In Tissues Study (BRITS) which prospectively investigated the receptor status of 137 paired tissue samples of primary and recurrent tumors, ER, PR, and HER2 status were changed in 10%, 25%, and 3% patients, respectively [16]. Meanwhile, a prospective cohort study (DESTINY) of 121 women with recurrent or metastatic breast cancer reported a conversion rate of 16% in ER, 40% in PR, and 10% in HER2 [17]. A pooled analysis of these two studies yielded ER, PR, and HER2 discordant rates of 13%, 31%, and 6%, respectively [15]. These proportions are lower than results from our study (26.9% for ER, 38.8% for PR, and 22.4% for HER2). The variation of discordance rates between different studies might be due to laboratory artifacts, tissue handling and processing, time of specimen preparation as well as result interpretation [13, 15]. In a prospective study on 184 patients with recurrent or metastatic breast cancer in Spain, receptor discordance rates were different when tested at central vs local laboratories (13% vs. 21% for ER, 28% vs. 35% for PR, and 3% vs. 16% for HER2) [13]. In a prospective observational study in 178 patients, the discordance rates between primary and metastasis lesions were 13%, 28%, and 3% for ER, PR, and HER2, respectively. In our study, rates of ER and HER2 status conversion from negative to positive were both approximately 15%, which are slightly higher than in other studies [15, 26]. In these patients, re-evaluation of tumor biomarkers had provided new treatment options, especially for triple-negative group. Moreover, according to Vietnam Law in Health Insurance, 80% of the treatment cost can be covered by insurance in most treatment modalities, but for certain drugs, patients need to pay at least one-half of the cost, such as trastuzumab (US$10,000–40,000 per individual) [2]. If there is conversion to HER2-negative in recurrent disease, continuation of trastuzumab might not be as effective as expected and cause major economic burden on patients, especially those living on poverty. Therefore, rebiopsy of recurrent lesions might be necessary to assess tumor receptor status.
There are several possible mechanisms for the conversion in ER, PR, and HER-2 expression. Firstly, technical artifacts and the variability in the accuracy of IHC tests may contribute to the difference of biomarker status between primary and recurrent tumors [27]. However, if laboratory issues were the main cause of status changes, the discordance rates would be expected to be approximately equal among ER, PR, and HER2 as well as between two directions. Meanwhile, in our study, the conversion rate was significantly different among ER, PR, and HER2 (26.9%, 38.8%, and 22.4%, respectively), which is consistent with previous studies [15–17]. Another possible etiology of receptor status changes is the clonal genome evolution and biological heterogeneity of the tumor, in which the more aggressive cell clones might be more likely to be involved in the micro-metastatic and recurrent process [28–30]. Besides, the discordance might also be a result of a biological drift due to clonal selection under the pressure of therapy. Some studies demonstrated the association between the use of hormonal therapy and the disappearance of ER/PR-positive cells [31, 32] as well as the effect of previous trastuzumab treatment on HER2 conversion [33, 34]. Finally, although newly acquired genomic mutations appear to be rare, it is still possible that the genuine changes in tumor biology can contribute to receptor status discordance [35].
Discordances of receptor status between primary tumor and recurrent lesions may lead to difficulty in determining tumor status and planning treatment accordingly [15]. In this case, reanalyzing of the primary specimen would be helpful to confirm the tumor profile. In the DESTINY study, three primary tumors initially categorized as ER-negative were found to be ER-positive after reanalysis [17]. Two triple-negative tumors in the initial pathology reports were eventually ER-positive after reassessment [17]. This suggests the clinical importance of storing primary tumor specimens to recheck the receptor profile of the tumor when needed, which has not been well-recognized in resource-limited settings, like in Vietnam.
Our results, consistent with previous studies, did not observe any associations between receptor conversion and the duration from primary tumor diagnosis to recurrence, sites of recurrence, or tumor molecular subtypes [13, 15]. This, again, emphasizes the importance of rebiopsy of recurrent lesions in patients with breast cancer, regardless of the above-mentioned characteristics. Our study has some limitations. Firstly, due to the retrospective design and inadequate storage capacity on initial specimens, we were unable to reanalyze to confirm primary tumor receptor status. Secondly, effects of receptor conversion on overall survival and treatment alteration were not assessed in this study due to limited follow-up duration. Finally, the sample size was not large enough to evaluate the effect of previous therapeutic approach on tumor biomarker conversion. Therefore, there is a need for further prospective and more comprehensive studies to thoroughly investigate the receptor conversion rate and its impacts.
5. Conclusion
There is a substantial conversion rate of receptor status between primary and recurrent tumors in breast cancer. Rebiopsy could help confirm the recurrence and may play an important role in clinical management. The conversion from receptor-negative to positive may provide new therapeutic opportunities for patients with recurrent breast cancer, including endocrine and targeted therapies for those who were previously not indicated.
Acknowledgments
We gratefully acknowledge the Department of Pathology and Molecular Biology, Vietnam National Cancer Hospital and other staffs at Vietnam National Cancer Hospital for their valuable assistance during our study.
Data Availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethical Approval
This study was approved by the ethics committee of the Vietnam National Cancer Institute. Informed consent was waived due to the retrospective nature of this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Nguyen Thi Hoa and Phung Thi Huyen are equal contributors and co-primary authors.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins C., Minh L. N., Anh T. T., et al. Breast cancer services in Vietnam: a scoping review. Global Health Action. 2018;11(1) doi: 10.1080/16549716.2018.1435344.1435344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy M. J., Harbeck N., Nap M., et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European group on tumor markers (EGTM) European Journal of Cancer (Oxford, England: 1990) 2017;75:284–298. doi: 10.1016/j.ejca.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Mirabelli P., Incoronato M. Usefulness of traditional serum biomarkers for management of breast cancer patients. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/685641.685641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens M. A., Horten B. C., Da Silva M. M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clinical Breast Cancer. 2004;5(1):63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 6.Onitilo A. A., Engel J. M., Greenlee R. T., Mukesh B. N. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clinical Medicine & Research. 2009;7(1–2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbeck N., Thomssen C., Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care. 2013;8(2):102–109. doi: 10.1159/000351193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotiriou C., Neo S.-Y., McShane L. M., et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howlader N., Cronin K. A., Kurian A. W., Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by American Society of Preventive Oncology. 2018;27(6):619–626. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 10.Goetz M. P., Gradishar W. J., Anderson B. O., et al. NCCN guidelines insights: breast cancer, version 3.2018. Journal of the Natural Comprehensive Cancer Network. 2019;17(2):118–126. doi: 10.6004/jnccn.2019.0009. [DOI] [PubMed] [Google Scholar]
- 11.Robson M. E., Tung N., Conte P., et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Annals of Oncology: Offical Journal of the European Society for Medical Oncology. 2019;30(4):558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannelli P., Di Donato M., Galasso G., Di Zazzo E., Bilancio A., Migliaccio A. The androgen receptor in breast cancer. Frontiers in Endocrinology. 2018;9 doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Dueñas E. M., Hernández A. L., Zotano A. G., et al. Prospective evaluation of the conversion rate in the receptor status between primary breast cancer and metastasis: results from the GEICAM 2009–03 ConvertHER study. Breast Cancer Research and Treatment. 2014;143(3):507–515. doi: 10.1007/s10549-013-2825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arapantoni-Dadioti P., Valavanis C., Gavressea T., Tzaida O., Trihia H., Lekka I. Discordant expression of hormone receptors and HER2 in breast cancer. A retrospective comparison of primary tumors with paired metachronous recurrences or metastases. Journal of B.U.ON.: Official Journal of the Balkan Union of Oncology. 2012;17(2):277–283. [PubMed] [Google Scholar]
- 15.Amir E., Clemons M., Purdie C. A., et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treatment Reviews. 2012;38(6):708–714. doi: 10.1016/j.ctrv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Thompson A. M., Jordan L. B., Quinlan P., et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the breast recurrence in tissues study (BRITS) Breast Cancer Research: BCR. 2010;12(6) doi: 10.1186/bcr2771.R92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir E., Miller N., Geddie W., et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. Journal of Clinical Oncology. 2012;30(6):587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y.-F., Liao Y.-Y., Yang M., Peng N.-F., Xie S.-R., Xie Y.-F. Discordances in ER, PR and HER2 receptors between primary and recurrent/metastatic lesions and their impact on survival in breast cancer patients. Medical Oncology (Northwood, London, England) 2014;31(10):214. doi: 10.1007/s12032-014-0214-2. [DOI] [PubMed] [Google Scholar]
- 19.Simmons C., Miller N., Geddie W., et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2009;20(9):1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge S. B., Compton C. C. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Hammond M. E. H., Hayes D. F., Dowsett M., et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Archives of Pathology & Laboratory Medicine. 2010;134(7):e48–e72. doi: 10.1043/1543-2165-134.7.e48. [DOI] [PubMed] [Google Scholar]
- 22.Wolff A. C., Hammond M. E. H., Schwartz J. N., et al. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Archives of Pathology & Laboratory Medicine. 2007;131(1):18–43. doi: 10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Meng X., Song S., Jiang Z.-F., et al. Receptor conversion in metastatic breast cancer: a prognosticator of survival. Oncotarget. 2016;7(44):71887–71903. doi: 10.18632/oncotarget.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y.-Y., Si W., Ji T.-F., Guo X.-Q., Hu Y., Yang J.-L. The variation and clinical significance of hormone receptors and Her-2 status from primary to metastatic lesions in breast cancer patients. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(6):7675–7684. doi: 10.1007/s13277-015-4649-7. [DOI] [PubMed] [Google Scholar]
- 25.Santosh T., Patro M. K., Nayak J., et al. Receptor conversion in carcinoma breast metastatic to the bone marrow. Indian Journal of Hematology & Blood Transfusion: An Official Journal of Indian Society of Hematology and Blood Transfusion. 2014;30(suppl 1):338–340. doi: 10.1007/s12288-014-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAnena P. F., McGuire A., Ramli A., et al. Breast cancer subtype discordance: impact on post-recurrence survival and potential treatment options. BMC Cancer. 2018;18(1):203. doi: 10.1186/s12885-018-4101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allred D. C. Commentary: hormone receptor testing in breast cancer: a distress signal from Canada. The Oncologist. 2008;13(11):1134–1136. doi: 10.1634/theoncologist.2008-0184. [DOI] [PubMed] [Google Scholar]
- 28.Aurilio G., Disalvatore D., Pruneri G., et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. European Journal of Cancer (Oxford, England: 1990) 2014;50(2):277–289. doi: 10.1016/j.ejca.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Shipitsin M., Campbell L. L., Argani P., et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Navin N., Kendall J., Troge J., et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472(7341):90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurbel S. Selective reduction of estrogen receptor (ER) positive breast cancer occurrence by estrogen receptor modulators supports etiological distinction between ER positive and ER negative breast cancers. Medical Hypotheses. 2005;64(6):1182–1187. doi: 10.1016/j.mehy.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Duchnowska R., Dziadziuszko R., Trojanowski T., et al. Polish Brain Metastasis Consortium., Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Research: BCR. 2012;14(4) doi: 10.1186/bcr3244.R119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtit E., Nerich V., Mansi L., et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. The Oncologist. 2013;18(6):667–674. doi: 10.1634/theoncologist.2012-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura R., Yamamoto N., Onai Y., Watanabe Y., Kawana H., Miyazaki M. Importance of confirming HER2 overexpression of recurrence lesion in breast cancer patients. Breast Cancer. 2013;20(4):336–341. doi: 10.1007/s12282-012-0341-6. [DOI] [PubMed] [Google Scholar]
- 35.Shah S. P., Morin R. D., Khattra J., et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461(7265):809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.