Abstract
Background
Wnt/β-catenin signaling has been reported to exert cytoprotective effects in a cellular model of Parkinson's disease (PD). Glutamate excitotoxicity has been suggested to contribute to the pathogenesis of PD, and excitatory amino acid transporters (EAATs) play a predominant role in clearing excessive glutamate. EAAT2 is mainly expressed in astrocytes, which are an important source of Wnt signaling in the brain.
Methods
Wnt1-overexpressing U251 astrocytes were indirectly cocultured with dopaminergic SH-SY5Y cells treated with 6-hydroxydopamine (6-OHDA). Cell toxicity was determined by cell viability and flow cytometric detection. Glutamate level in the culture medium was determined by enzyme-linked immunosorbent assay (ELISA). Western blot analysis was used to detect the expression of Wnt1, β-catenin, and EAAT2. Immunofluorescence was used to display the expression and translocation of NF-κB p65.
Results
6-OHDA treatment significantly decreased cell viability in both U251 cells and SH-SY5Y cells, inhibited the expression of Wnt1, β-catenin, and EAAT2 in U251 cells, and increased the glutamate level in the culture medium. Coculture with Wnt1-overexpressing U251 cells attenuated 6-OHDA-induced apoptosis in SH-SY5Y cells. Overexpression of Wnt1 decreased the glutamate level in the culture media, upregulated β-catenin, EAAT2, and NF-κB levels, and promoted the translocation of NF-κB from the cytoplasm to the nucleus in U251 cells.
Conclusion
Wnt1 promoted EAAT2 expression and mediated the cytoprotective effects of astrocytes on dopaminergic cells. NF-κB might be involved in the regulation of EAAT2 by Wnt1.
1. Introduction
Parkinson's disease (PD), characterized by the loss of dopaminergic neurons in the substantia nigra (SN), is the second most common neurodegenerative disease after Alzheimer's disease in elderly people. The neuropathological features of PD are progressive loss of dopaminergic neurons in the SN and aggregation of α-synuclein-positive Lewy bodies in surviving neurons [1]. Although PD has been heavily researched over the last two decades, the precise etiology and pathogenesis of the disease remain not very clear. Studies have shown that several mechanisms are involved in the pathogenesis of PD, including oxidative stress, mitochondrial dysfunction, and elevated brain iron levels [2–4]. In addition, a wide range of studies have suggested that glutamate excitotoxicity contributes to the pathogenesis of PD and that excitatory amino acid transporters (EAATs) play a predominant role in clearing the excessive glutamate in the synaptic cleft [5]. EAAT2 and EAAT1 (glutamate transporter 1 (GLT-1) and glutamate-aspartate transporter (GLAST) in rodents, respectively) are the main transporters that maintain optimal glutamate levels in the synaptic clefts [6]. Reduced expression and function of these transporters, especially EAAT2, have been reported in numerous neurodegenerative disorders, including amyotrophic lateral sclerosis, Alzheimer's disease, and PD [6–8].
Wnt signaling pathway is an autocrine-paracrine signal transduction pathway that has been demonstrated to participate in embryonic development, cell differentiation, and ontogenesis [9–11]. A main Wnt signaling pathway branch is the Wnt/β-catenin pathway, in which Wnt proteins bind to Frizzled receptors and to initiate the activation of Dishevelled [12]. The activation of Dishevelled results in the inhibition of glycogen synthase kinase-3β (GSK3β), which in turn stabilizes β-catenin [12]. Stabilized β-catenin accumulates and is taken into the nucleus where it regulates the expression of numerous genes [12]. Extensive research has confirmed the vital role of Wnt signaling in midbrain dopaminergic neuronal development. For example, Wnt1 and Wnt3a, which exert effects by the Wnt/β-catenin pathway, are key regulators in the development of dopaminergic neurons [13]. Cellular protective effects of the Wnt/β-catenin pathway have been demonstrated in animal and cellular models of Alzheimer's disease, retinal degeneration, cerebral ischemia, and PD [14–17]. Our previous studies demonstrated that the activation of the Wnt/β-catenin signaling pathway by exogenous Wnt1 or Wnt3a protects cells by restoring mitochondria function [18, 19].
Astrocytes are the most abundant glial cell type in the brain and exert protective effects on neurons. Impairment in astrocyte function can critically influence neuronal survival and lead to neurodegeneration [20]. Several studies have shown that astrocytes were able to express specific Wnt ligands, receptors, and regulators under physiological and pathological conditions, indicating the involvement of this family of proteins in astroglial functioning in the normal and damaged central nervous system [21–24]. Moreover, studies have shown that Wnt signaling could induce the expression of EAAT2 which is mainly expressed in astrocytes [25, 26]. Here, we cocultured dopaminergic SH-SY5Y cells and U251 astrocytes overexpressing Wnt1 and found that Wnt1 promoted the expression of EAAT2 in astrocytes and mediated the protective effects of astrocytes on dopaminergic cells.
2. Materials and Methods
2.1. Cell Culture
Human neuroblastoma SH-SY5Y cells and U251 astrocytes were obtained from the American Type Culture Collection (ATCC, Manassas, VA, U.S.A.), maintained in DMEM with high glucose (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), and cultured in a humidified incubator with 5% CO2 at 37°C. Cells with 20-30 passages were used. For experiments, cells were seeded at a density of 1 × 105/cm2 in plastic flasks or plates. Cells were treated with different concentrations of 6-hydroxydopamine (6-OHDA, Sigma-Aldrich, USA) according to the corresponding experiments and with vehicle as a control. To block the receptor of the Wnt signal, a Frizzled-1 antagonist, human recombinant Dickkopf- (DKK-) 1 protein (Sigma-Aldrich, USA), or vehicle was added to the cultures 10 min prior to 6-OHDA.
2.2. Transfection of Plasmid pCDNA3.1-Wnt1
The Wnt1 expression vector (pCDNA3.1-Wnt1) was constructed by Forevergen company (Guangzhou, China) using human nephridial tissue complimentary DNA (cDNA) as the template and confirmed by sequencing. pCDNA3.1-Wnt1 was transfected into U251 cells (U251-Wnt1), while the pCDNA3.1 empty vector was transfected as a control (U251-EV). All cell transfections were conducted using Lipofectamine 2000 reagent (Invitrogen, USA) following the manufacturer's protocol. Quantitative PCR and Western blot assays were used to identify the expression level of Wnt1.
2.3. Real-Time PCR
Forty-eight hours after transfection, total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's protocol. Then, 1 μg of total RNA was reverse transcribed into first-strand cDNA using a reverse transcription (RT) kit (Takara, Japan) according to the manufacturer's protocol in a final volume of 20 μl. For analysis of Wnt1 expression with quantitative real-time RT-polymerase chain reaction (RT-PCR), human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control, and the sequences of the primers were as follows: Wnt1 forward: 5′-GGTTCCATCGAATCCTGCAC-3′ and Wnt1 reverse: 5′-GCCTCGTTGTTGTGAAGGTT-3′; GAPDH forward: 5′-GAAGGTGAAGGTCGGAGTC-3′ and GAPDH reverse: 5′-GAAGATGGTGATGGGATTTC-3′. One microliter of the synthesized cDNA was used in real-time PCR together with SYBR Green I Master Mix (SYBR® Premix Ex Taq™, Takara, Japan) on an MJ Research Opticon2 real-time thermocycler (Bio-Rad, Hercules, CA, USA). Fluorescent reading from the real-time PCR was quantitatively analyzed by determining the difference in Ct (delta Ct) between Ct of Wnt1 and its internal GAPDH, and Wnt1 gene expression was determined by the formula 2−deltaCt.
2.4. Indirect Astrocyte-Neuron Coculture System
SH-SY5Y cells and U251 cells were cocultured in 6-well Transwell Permeable Supports (polystyrene, pore size, 0.4 μm, Corning Company, USA) where cells shared medium coming into contact with one another. U251 cells were seeded on Transwell inserts, and SH-SY5Y cells were cultured in the lower compartment. Cells were divided into 6 groups: SH-SY5Y cells (control), SH-SY5Y cells treated with 50 μM 6-OHDA, SH-SY5Y cells cocultured with U251-Wnt1 cells and treated with 50 μM 6-OHDA, SH-SY5Y cells cocultured with U251-Wnt1 cells and treated with 50 μM 6-OHDA and 100 ng/ml DKK-1, and SH-SY5Y cells cocultured with U251-EV and treated with 50 μM 6-OHDA.
2.5. Cell Viability Assay
Cell viability was determined by colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide, Sigma) assay [27]. After treatment, SH-SY5Y cells or U251 cells were seeded in a 96-well plate at a density of 1 × 103 cells per well. After attachment, cells were incubated with 0.5 mg/ml MTT for 4 h at 37°C. Following aspiration of the MTT solution, the same volume of DMSO was added into each well to dissolve the purple formazan crystals. Absorbance was read in a microtiter plate reader at 490 nm. Cell viability was expressed as a percentage of the absorbance of control cells.
2.6. Flow Cytometric Detection of Apoptotic Cells
SH-SY5Y cell apoptosis was quantified by flow cytometry using fluorescein isothiocyanate- (FITC-) conjugated annexin V and propidium iodide (PI). The cells were seeded in 6-well Transwell Permeable Supports (5 × 105 cells/well). After the treatment described above, the cells were harvested, washed with cold PBS, and double-stained using an annexin V-FITC apoptosis detection kit (KeyGen, China). According to the manufacturer's instructions, cells resuspended in annexin V-FITC binding buffer were incubated with annexin V-FITC and PI for 10 min at room temperature in the dark. The number of cells under apoptosis and necrosis was evaluated by flow cytometry (BD, CA, USA). At least 10,000 cells were analyzed.
2.7. Glutamate Measurement
The concentrations of glutamate in the culture media were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Qincheng Biotechnology Company, China). The cells were seeded in 6-well Transwell Permeable Supports (5 × 105 cells/well). After the treatment described above, the conditioned media were collected and then centrifuged at 1,000 × RPM for 10 min to get rid of impurities. According to the manufacturer's instructions, 10 μl culture medium of each group was used for measurement. Absorbance was read in a microplate reader at 450 nm. The glutamate levels were expressed as the percentage versus the control group.
2.8. Western Blot Assay
Immunoblotting was performed in accordance with a standard procedure as described before [27]. Briefly, U251 cells in 6-well Transwell Permeable Supports were washed twice with prechilled PBS followed by lysis with the Mammalian Cell Extraction Kit (Biovision, CA, USA) mixed with PhosSTOP (Roche, Basel, Switzerland). After the protein concentrations were measured with the BCA protein assay kit (Thermo Fisher Scientific Inc., IL, USA), cell lysates were separated by 8%~12% SDS-polyacrylamide gels and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, MA, USA). After transfer, the membranes were blocked with 5% nonfat dry milk (Sigma, USA) in Tris-buffered saline (20 mM Tris-HCl pH 7.6, 137 mM NaCl) containing 0.01% Tween 20 (TBST) for 1 h at room temperature, and then, the membranes were probed with primary antibodies diluted according to the producers' datasheet at 4°C overnight. The following primary antibodies were used: rabbit anti-β-catenin (1 : 1,000 dilution, Abcam, Cambridge, UK), rabbit anti-EAAT2 (1 : 1,000 dilution, CST, USA), mouse anti-β-actin (1 : 1,000 dilution, Millipore, USA), and rabbit anti-Wnt1 (1 : 800 dilution, Abcam, Cambridge, UK). Subsequently, membranes were washed with TBST 3 times followed by incubation with HRP-labeled anti-mouse IgG or anti-rabbit IgG (KPL, MD, USA) secondary antibodies at room temperature for 1 h. After 3 washes with TBST, proteins were detected with the SuperSignal® West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., IL, USA), and membranes were exposed to X-ray films (Fujifilm Corporation, Japan), which were scanned and analyzed using Quantity One v4.62 for Windows software (Bio-Rad, CA, USA).
2.9. Immunofluorescence Staining
U251 cells, U251-Wnt1 cells, or U251-EV cells were treated with vehicle, 6-OHDA (50 μM), or/and DKK-1 (100 ng/ml) for 24 h, followed by immunofluorescent staining as described before [27]. Briefly, cells were washed three times with PBS and fixed in 4% paraformaldehyde-PBS for 15 min at room temperature. The fixed cells were permeabilized with 0.01 M PBS containing 0.1% Triton X-100 for 20 min and then blocked with 10% goat serum for 1 h at room temperature. After blocking, cells were incubated overnight at 4°C with primary antibodies diluted in 10% goat serum/rabbit anti-NF-κB p65 (1 : 800, CST, USA). Cells were washed three times with 0.01 M PBS for 5 min. Alexa Fluor 555-conjugated goat anti-rabbit antibody (CST, USA) was used as a secondary antibody at a dilution of 1 : 1,000 and incubated for 2 h at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, CST, USA) for 45 s at room temperature before cells were examined under a confocal laser scanning microscope LEICA TCS SP5 MP (Leica, Heidelberg, Germany).
2.10. Statistical Analysis
The results are presented as the mean ± standard deviation (SD). Differences among various time points or groups were examined by using analysis of variance (ANOVA) followed by Tukey-Kramer tests for post hoc multiple comparisons. We also used Student's t-test for pairwise comparisons at a given treatment. The level of significance was set at P < 0.05. All statistical analyses were performed with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Cytotoxic Effect of 6-OHDA on U251 Cells
Cell viability was measured after 0-200 μM 6-OHDA treatment for 24 h and showed a concentration-dependent reduction in cell viability. Compared with the cell viability of control cells, cell viability was 83.97 ± 3.07% with 10 μM 6-OHDA, 75.97 ± 2.26% with 20 μM, 62.65 ± 2.82% with 50 μM, 40.37 ± 1.62% with 100 μM, and 20.70 ± 1.31% with 200 μM (Figure 1), and 50 μM 6-OHDA was chosen for further experiments.
Figure 1.
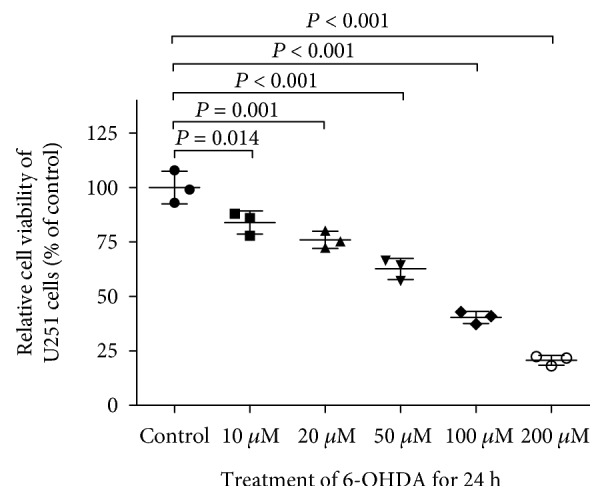
Cytotoxic effect of 6-OHDA on U251 cells. U251 cells were treated with 0-200 μM 6-OHDA for 24 h, and cell viability was measured by MTT assay and expressed as a percentage of that in the vehicle group. All of the data are presented as the mean ± SD from 3 independent experiments. Differences among groups were examined by using ANOVA followed by Tukey-Kramer tests for post hoc multiple comparisons. F value = 112.6, degrees of freedom between groups (dfbetween groups) = 5, degree of freedom within groups (dfwithin groups) = 12, and the total degree of freedom (dftotal) = 17.
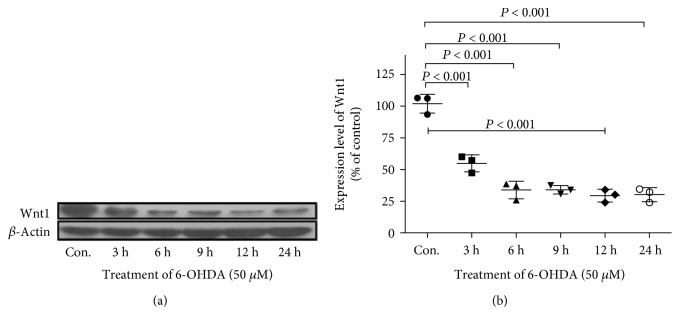
3.2. 6-OHDA Treatment Caused Downregulation of Wnt1 in U251 Cells
6-OHDA is an endogenous oxidative metabolite of dopamine commonly used to generate experimental models of PD. A previous study demonstrated that the Wnt signal in the central nervous system mainly originates from astrocytes [21]. U251 astrocyte cells were used to study the change in Wnt1 expression after 6-OHDA treatment. U251 cells were treated with 50 μM 6-OHDA for 3, 6, 9, 12, and 24 h. Then, total protein was extracted, and the level of Wnt1 was determined by Western blot (Figure 2). 6-OHDA treatment caused a time-dependent decrease in Wnt1. Compared to Wnt1 expression in the control, the Wnt1 level decreased to 54.82 ± 6.69% at 3 h, 33.84 ± 4.00% at 6 h, and remained relatively stable from 9 h to 24 h.
Figure 2.
6-OHDA treatment downregulated the protein level of Wnt1 in U251 cells. (a) U251 cells were treated with vehicle or 6-OHDA (50 μM) for 3, 6, 9, 12, and 24 h, and the protein level of Wnt1 was detected by Western blot with β-actin as an internal control. (b) The relative band intensities of Wnt1 were measured by Quantity One software and normalized to the expression of β-actin in U251 cells. All of the data are presented as the mean ± SD from three independent experiments. Differences among various time points were examined by using ANOVA followed by Tukey-Kramer tests for post hoc multiple comparisons. F value = 67.10, dfbetween groups = 5, dfwithin groups = 12, and dftotal = 17.
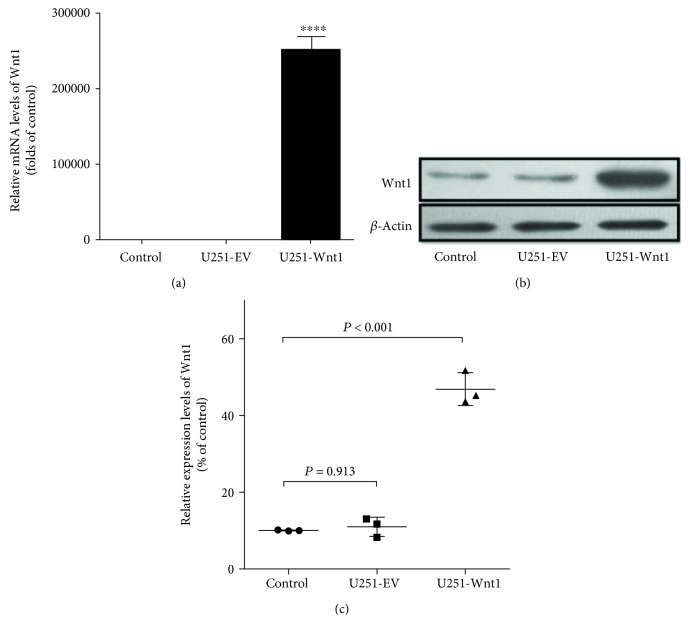
3.3. Overexpression of Wnt1 in U251 Cells
To investigate the effects of Wnt1 in astrocytes, we constructed U251 cell lines overexpressing Wnt1. As shown in Figure 1, the mRNA (Figure 3(a)) and protein (Figure 3(b)) levels of Wnt1 were significantly higher in U251 cells transfected with the Wnt1 expression vector than in control cells, while cells transfected with the empty vector did not show a change in Wnt1 mRNA and protein expression.
Figure 3.
Evaluation of the Wnt1 overexpression vector in U251 cells by Western blot and PCR. Forty-eight hours after transfection with the Wnt1 expression vector or empty vector, U251 cells were harvested, and the mRNA and protein levels of Wnt1 were determined by real-time PCR and Western blot. The mRNA (a) and protein (b, c) levels of Wnt1 were significantly higher in U251 cells overexpressing Wnt1 than in control cells, whereas the empty vector did not influence Wnt1 expression. ∗∗∗∗P < 0.0001. For Western blot assay, differences among groups were examined by using ANOVA followed by Tukey-Kramer tests for post hoc multiple comparisons (c). F value = 161.42, dfbetween groups = 2, dfwithin groups = 6, and dftotal = 8.
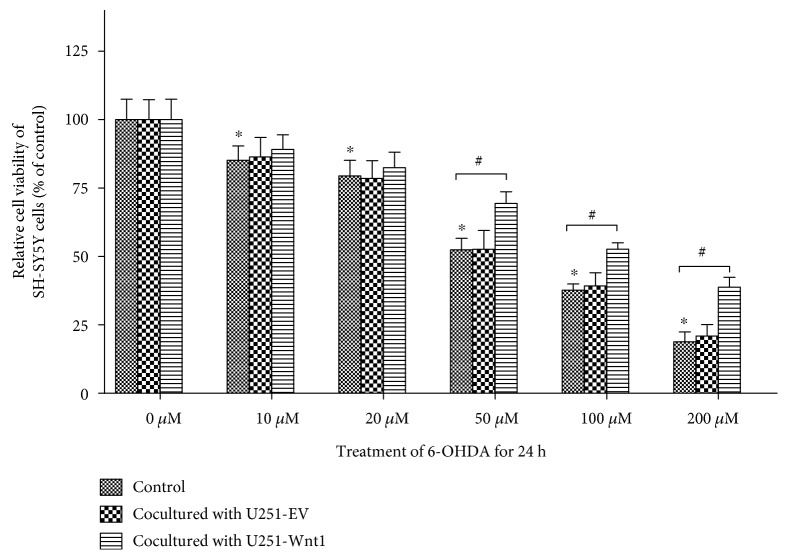
3.4. Coculturing with U251-Wnt1 Cells Attenuated 6-OHDA-Induced SH-SY5Y Cell Injury
Treatment with 6-OHDA for 24 h caused a concentration-dependent reduction in cell viability in SH-SY5Y cells (Figure 4). Compared with the cell viability of controls, cell viability was 85.12 ± 5.31% with 10 μM 6-OHDA, 79.43 ± 5.69% with 20 μM, 52.41 ± 4.23% with 50 μM, 37.67 ± 2.34% with 100 μM, and 18.77 ± 3.61% with 200 μM.
Figure 4.
Coculturing with U251-Wnt1 cells attenuated 6-OHDA-induced injury in SH-SY5Y cells. SH-SY5Y cells were indirectly cocultured with U251-Wnt1 cells or U251-EV cells, with SH-SY5Y cells cultured alone as the control, and then treated with 50 μM 6-OHDA for 24 h; cell viability was assessed using the MTT assay. The data are expressed as percentages relative to the control group with 0 μM 6-OHDA and are presented as the mean ± SD from four independent experiments. ∗P < 0.05 compared to the control with 0 μM 6-OHDA; #P < 0.05 compared to the control group at the corresponding 6-OHDA concentration.
When cocultured with U251-EV cells or U251-Wnt1 cells, treatment with 6-OHDA for 24 h also caused a concentration-dependent reduction in cell viability in SH-SY5Y cells (Figure 4). Coculturing with U251-EV cells did not change SH-SY5Y cell viability. When cocultured with U251-Wnt1, the cell viability of SH-SY5Y cells was significantly higher than that of the isolated SH-SY5Y cells after treatment with 50 μM, 100 μM, and 200 μM 6-OHDA.
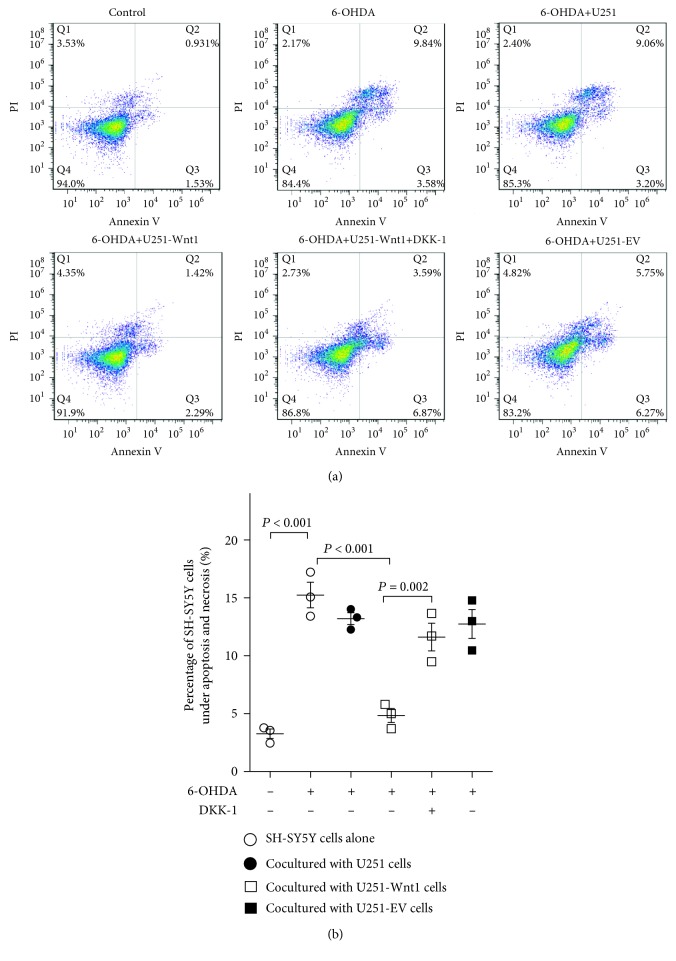
Then, we further examined the presence of cells under apoptosis and necrosis using the FITC-annexin-V/PI staining assay (Figure 5). Annexin V has a strong affinity for phosphatidylserine that translocates from the inner surface of the plasma membrane to the cell surface upon initiation of apoptosis and is widely used as a marker of apoptosis. When used in combination with PI, apoptotic cells can easily be differentiated from necrotic and living cells. The percentage of cells under apoptosis and necrosis in the control group was 3.27 ± 0.71%, while that in 6-OHDA-treated cells was 15.24 ± 1.90%. However, coculturing with U251-Wnt1 cells reduced the 6-OHDA-induced injury to 4.84 ± 0.61%, and this reduction was partly blocked by 100 ng/ml DKK-1, an antagonist of the Wnt/β-catenin pathway. Coculturing with U251 cells or U251-EV cells did not significantly reduce 6-OHDA-induced SH-SY5Y cell injury.
Figure 5.
Effect of coculturing with U251-Wnt1 cells on 6-OHDA-induced damage in SH-SY5Y cells measured by flow cytometry. SH-SY5Y cells were indirectly cocultured with U251 cells, U251-Wnt1 cells, or U251-EV cells and then treated with or without 50 μM 6-OHDA or 100 ng/ml DKK-1 for 24 h, and a combination of annexin V-FITC and PI staining was performed followed by flow cytometry. (a) Representative set of flow cytometric two-parameter dot plots in the corresponding groups. The lower right quadrant, which shows annexin V-FITC-positive and PI-negative cells, represents early apoptotic cells. The upper right quadrant, which shows both annexin V-FITC- and PI-positive cells, represents cells in the end stages of apoptosis and necrosis. (b) The percentage of cells under apoptosis and necrosis. Data are presented as the mean ± SD from 3 independent experiments. Differences among groups were examined by using ANOVA followed by Tukey-Kramer tests for post hoc multiple comparisons. F value = 28.59, dfbetween groups = 5, dfwithin groups = 12, and dftotal = 17.
3.5. Wnt1 Overexpression Decreased the Glutamate Level in Culture Medium
To confirm the effect of Wnt1 overexpression on the toxicity of excitatory amino acids, the glutamate level in culture medium was detected. Treatment with 50 μM 6-OHDA for 24 h significantly increased the glutamate level in the culture medium of SH-SY5Y cells to 115.28 ± 7.62% of that in the control group (P = 0.011). Coculturing with U251-Wnt1 cells could decrease the glutamate level to 79.97 ± 6.16%, which could be blocked by the antagonist of Wnt signaling, DKK-1 (Figure 6).
Figure 6.
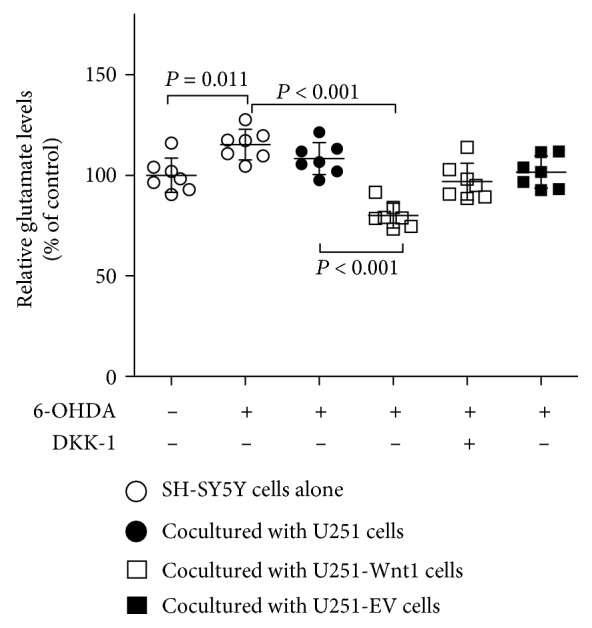
Wnt1 overexpression decreased the glutamate level in culture medium. SH-SY5Y cells were indirectly cocultured with U251 cells, U251-Wnt1 cells, or U251-EV cells and then treated with or without 50 μM 6-OHDA or 100 ng/ml DKK-1 for 24 h, and the glutamate levels in culture media were detected by ELISA. The data are expressed as percentages relative to the control group. All of the data are presented as the mean ± SD from 7 independent experiments. Differences among groups were examined by using ANOVA followed by Tukey-Kramer tests for post hoc multiple comparisons. F value = 15.87, dfbetween groups = 5, dfwithin groups = 36, and dftotal = 41.
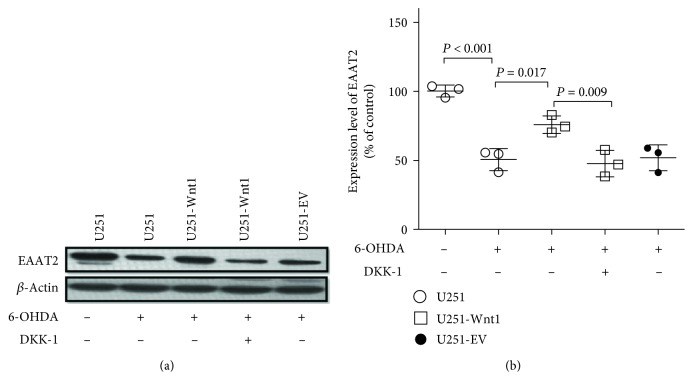
3.6. Wnt1 Overexpression Upregulated EAAT2 Expression
Reduced expression of EAAT2 has been reported in PD [28]. Here, Western blotting was used to test the effects of 6-OHDA and/or Wnt1 overexpression on EAAT2 levels in U251 cells (Figure 7). Treatment with 50 μM 6-OHDA for 24 h decreased the protein level of EAAT2 in U251 cells to 50.57 ± 7.98% of the protein level of EAAT2 in the control. However, the protein level of EAAT2 in U251-Wnt1 cells was increased to 75.84 ± 6.39% of the control level after 6-OHDA treatment, which indicated that the overexpression of Wnt1 attenuated the inhibitory effect of 6-OHDA on EAAT2 expression. The effect of Wnt1 upregulation on EAAT2 expression could be partly blocked by DKK-1.
Figure 7.
Wnt1 overexpression attenuated the inhibitory effect of 6-OHDA on EAAT2 expression in U251 cells. U251 cells, U251-Wnt1 cells, or U251-EV cells were treated with vehicle, 6-OHDA (50 μM), or/and DKK-1 (100 ng/ml) for 24 h, and the protein levels of EAAT2 (a) were detected by Western blot with β-actin as an internal control. The relative band intensities of EAAT2 (b) were measured by Quantity One software and normalized to the expression of β-actin. Differences among groups were examined using ANOVA followed by using Tukey-Kramer tests for post hoc multiple comparisons. Data are presented as the mean ± SD of 3 experiments. F value = 25.29, dfbetween groups = 4, dfwithin groups = 10, and dftotal = 14.
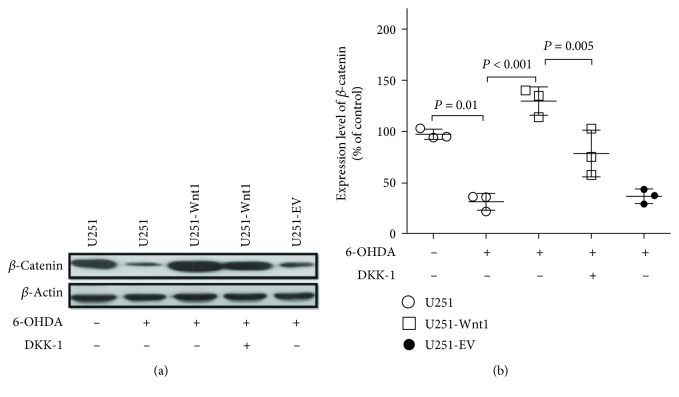
3.7. Wnt1 Overexpression Activated the Wnt/β-Catenin Pathway
To confirm the activation effect of endogenous Wnt1 overexpression on the Wnt/β-catenin pathway, Western blotting was used to detect the expression of β-catenin in U251 cells. Treatment with 50 μM 6-OHDA for 24 h decreased the β-catenin level to 32.36 ± 5.93% of that in control cells, while overexpression of Wnt1 reversed the inhibitory effect of 6-OHDA treatment on β-catenin expression to 129.6 ± 7.99% of the control level. This reversal effect of Wnt1 overexpression on β-catenin expression was blocked by DKK-1, indicating that endogenous Wnt1 might exert effects via the autocrine pathway (Figure 8).
Figure 8.
Wnt1 overexpression activated the Wnt/β-catenin pathway in U251 cells. U251 cells, U251-Wnt1 cells, or U251-EV cells were treated with vehicle, 6-OHDA (50 μM), or/and DKK-1 (100 ng/ml) for 24 h, and the protein levels of β-catenin (a) were detected by Western blot with β-actin as an internal control. The relative band intensities of β-catenin (b) were measured by Quantity One software and normalized to the expression of β-actin. Differences among groups were examined using ANOVA followed by using Tukey-Kramer tests for post hoc multiple comparisons. Data are presented as the mean ± SD of 3 experiments. F value = 30.23, dfbetween groups = 4, dfwithin groups = 10, and dftotal = 14.
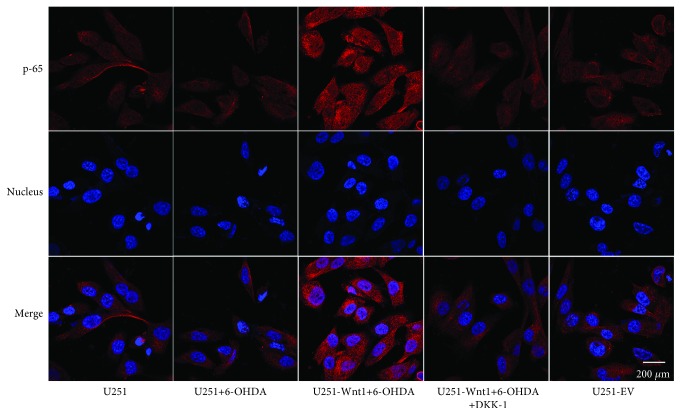
3.8. Wnt1 Overexpression Activated the NF-κB Signaling Pathway
Previous studies have demonstrated that NF-κB contributes to the neuron-dependent induction of GLT-1 (the rodent homologue for EAAT2) expression [29]. To determine whether NF-κB participates in the regulation of Wnt1 on EAAT2 expression, p65 and DAPI double staining was assessed. We found a small amount of expression of NF-κB p65 in the cytoplasm and nucleus of U251 cells with or without 6-OHDA treatment, whereas Wnt1 overexpression caused an upregulation in expression both in the cytoplasm and the nucleus, indicative of NF-κB signaling pathway activation (Figure 9).
Figure 9.
Immunofluorescence staining of p65 protein in U251 cells. U251 cells, U251-Wnt1 cells, or U251-EV cells were treated with vehicle, 6-OHDA (50 μM), or/and DKK-1 (100 ng/ml) for 24 h, and the expression and distribution of p65 were examined using an antibody against p65 (red). Nuclei were counterstained with DAPI (blue). All images were captured using a confocal laser scanning microscope.
4. Discussion
The study investigated the protective effects of astrocytes on dopaminergic cells mediated by Wnt signaling. Our present study demonstrated that Wnt1 levels in U251 cells were decreased after 6-OHDA treatment and that coculture with Wnt1-overexpressing U251 astrocytes attenuated the toxic effect of 6-OHDA on dopaminergic SH-SY5Y cells. Coculture with Wnt1-overexpressing U251 astrocytes also could decrease the glutamate level in the culture medium. Moreover, the Wnt1-mediated protective effects of astrocytes on dopaminergic cells might activate the NF-κB signaling pathway and upregulate the expression of EAAT2.
We cocultured SH-SY5Y cells with U251 astrocytes to simulate an in vivo environment. Both SH-SY5Y and U251 are human cell lines. The SH-SY5Y cell line was chosen in this study for its expression of tyrosine hydroxylase, which leads to its consideration as a dopaminergic cell line used to simulate dopaminergic neurons [30]. As an endogenous oxidative metabolite of dopamine, 6-OHDA has been found to be taken up by the plasma membrane dopamine transporter. Once in the cytoplasm, the cytotoxicity of 6-OHDA has been thought to be based primarily on dopaminergic neuron damage by mechanisms similar to those that have been proposed for patients with PD. For example, 6-OHDA inhibits mitochondrial complex I, produces large amounts of free radicals, induces cell death, and has been widely used to study the neurodegenerative process in PD [31, 32]. It has also been shown that 6-OHDA induces apoptosis in various cell types that do not express dopaminergic transporters, such as PC12 cells and astrocytes [33–35]. For example, Gupta et al. reported a significantly decreased mitochondrial dehydrogenase activity, mitochondrial membrane potential, augmented reactive oxygen species (ROS) level, caspase-3 mRNA level, and chromatin condensation, and DNA damage was observed in 6-OHDA-treated astrocytes [33]. Kaddour et al. also reported that 6-OHDA treatment caused cell death in cultured rat astrocytes through enhancing the level of ROS, associated with a reduction of both superoxide dismutase and catalase activity, and exposure of cultured astrocytes to 6-OHDA concentrations above 30 μM reduced astrocyte viability in a concentration- and time-dependent manner [34]. A higher concentration of 6-OHDA may increase the permeability of the cell membrane, enter the cytoplasm, and play a toxic role.
Wnt1 is a cysteine-rich glycosylated protein that promotes neuronal cell and astrocyte crosstalk as a mechanism of neuroprotection in PD models [21]. The involved neuroprotective effect may consist of two aspects. As demonstrated by previous studies, Wnt1 is secreted by astrocytes, binds to Frizzled receptors, and exerts a direct protective effect on dopaminergic neurons. For example, a recent study by L'Episcopo et al. showed that exogenous Wnt1 exerts robust neuroprotective effects against caspase-3 activation, loss of tyrosine hydroxylase-positive neurons, and [3H]dopamine uptake induced by DA-specific insults, including serum and growth factor deprivation, 6-OHDA and 1-methyl-4-phenyl-1,2,4,5-tetrahydropyridine (MPTP)/the 1-methyl-4-phenylpyridinium ion (MPP+) [22]. Our previous study also found that exogenous Wnt1 could increase the β-catenin protein level, potentially contributing to the protection of SH-SY5Y cells against 6-OHDA toxicity by inhibiting mitochondrial stress and endoplasmic reticulum stress [18]. On the other hand, Wnt1 might also bind to Frizzled receptors on astrocytes by autocrine effects and regulate the protective effects of astrocytes on dopaminergic neurons (indirect protective effect). In the present study, we found that Wnt1 regulated the EAAT2 level in astrocytes and protected dopaminergic cells against 6-OHDA toxicity.
Glutamate excitotoxicity has been shown to participate in the pathogenesis of PD. For example, Li and colleagues found higher levels of glutamate both in the cerebrospinal fluid and the striatum in a 6-OHDA-induced PD model [36]. Excessive glutamate in the synaptic cleft overstimulates the ionotropic and metabotropic glutamate receptors in the postsynaptic membrane and mediates excitotoxicity [37]. Previous studies have revealed that glutamate excitotoxicity induces dopamine neuron death, movement disorder, and cognitive impairment; thus, glutamate excitotoxicity plays an important role in the pathogenesis of PD [37–39]. Glutamate transporters prevent the overstimulation of postsynaptic glutamate receptors that leads to excitotoxic neuronal injury. To date, five subtypes of glutamate transporters have been identified. EAAT1 and EAAT2 are predominantly expressed in astrocytes and are responsible for the uptake of excess glutamate from the extracellular space [6, 37]. In the adult brain, EAAT2 accounts for >90% of extracellular glutamate clearance [6]. Impaired glutamate uptake and reduced EAAT2 expression are found in PD animal models constructed by 6-OHDA or MPTP [28, 38, 39]. Our present study also showed that treatment with 6-OHDA caused a decrease in EAAT2 expression and an increase of glutamate level in the culture medium.
A previous study showed that Wnt1 could induce expression of GLT-1 (the rodent homologue for EAAT2) in rat C6 glioma cells [26]. Here, we also obtained a similar result, showing that Wnt1 overexpression increased EAAT2 levels in U251 astrocytes. Then, increased EAAT2 level attenuated the increase of glutamate level in the culture medium and consequently reduced the toxicity of excitatory amino acids. Previous studies have shown that the NF-κB pathway is critical for positive transcription of EAAT2. NF-κB binds to the EAAT2 promoter, activates the NF-κB signaling pathway, and elevates EAAT2 transcription [6, 40]. Our present results also showed that overexpression of Wnt1 increased the level of NF-κB p-65 both in the cytoplasm and nucleus, indicative of the NF-κB pathway activation. A series of studies have demonstrated that NF-κB is involved in the protective effects of Wnt1. For example, Bournat et al. reported that Wnt1 promotes cell survival by activating NF-κB in PC12 cells [41]; Shang and colleagues reported that microglial cell survival determined by Wnt1 during oxidative stress requires NF-κB p65 [42]. The above results indicate that the crosstalk of the Wnt signal with the NF-κB signal participates in the regulation of EAAT2 expression.
In conclusion, we found that 6-OHDA treatment decreased Wnt1 levels in U251 cells. Wnt1 promoted EAAT2 expression and mediated the cytoprotective effects of astrocytes on dopaminergic cells. Furthermore, NF-κB might be involved in the regulation of EAAT2 by Wnt1.
5. Highlights
We conclude the following:
6-OHDA inhibits the expression of Wnt1, β-catenin, and EAAT2 in U251 cells
Coculture with Wnt1-overexpressing U251 astrocytes attenuated the toxic effect of 6-OHDA on dopaminergic SH-SY5Y cells
Wnt1 activated NF-κB, promoted EAAT2 expression, and mediated the cytoprotective effects of astrocytes on dopaminergic cells
Acknowledgments
This work was supported by grants from the Nature Natural Science Foundation of China (81401058), the Natural Science Foundation of Guangdong Province (2014A030310105), and the Medical Scientific Research Foundation of Guangdong Province (B2014139).
Abbreviations
- 6-OHDA:
6-Hydroxydopamine
- DKK-1:
Dickkopf-1
- EAAT:
Excitatory amino acid transporter
- GAPDH:
Glyceraldehyde-3-phosphate dehydrogenase
- GLAST:
Glutamate aspartate transporter
- GLT-1:
Glutamate transporter 1
- GSK3β:
Glycogen synthase kinase-3β
- MPP+:
1-Methyl-4-phenylpyridinium ion
- MPTP:
1-Methyl-4-phenyl-1,2,4,5-tetrahydropyridine
- NF-κB:
Nuclear factor-κB
- PD:
Parkinson's disease
- SN:
Substantia nigra.
Contributor Information
Pingyi Xu, Email: pingyixu@sina.com.
Zhengqi Lu, Email: luzhengqi2018@163.com.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Lei Wei, Chuan Chen, Li Ding, Pingyi Xu, and Zhengqi Lu conceived and designed the experiments. Lei Wei, Chuan Chen, Li Ding, Mingshu Mo, Jing Zou, and Zhenze Lu performed the experiments. Haiyan Li, Haotian Wu, Yongqiang Dai, and Pingyi Xu analyzed the data. Lei Wei, Chuan Chen, Li Ding, and Zhengqi Lu wrote the paper. Lei Wei, Chuan Chen, Li Ding, and Mingshu Mo contributed equally to this work.
References
- 1.Lill C. M. Genetics of Parkinson’s disease. Molecular and Cellular Probes. 2016;30(6):386–396. doi: 10.1016/j.mcp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. Mitochondrial complex I deficiency in Parkinson’s disease. The Lancet. 1989;1(8649):p. 1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 3.Andersen J. K. Oxidative stress in neurodegeneration: cause or consequence? Nature Medicine. 2004;10(S7):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 4.Oakley A. E., Collingwood J. F., Dobson J., et al. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology. 2007;68(21):1820–1825. doi: 10.1212/01.wnl.0000262033.01945.9a. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., He X., Meng X., et al. Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model. Cell Death & Disease. 2017;8(2, article e2574) doi: 10.1038/cddis.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karki P., Smith K., Johnson J., Jr., Aschner M., Lee E. Y. Genetic dys-regulation of astrocytic glutamate transporter EAAT2 and its implications in neurological disorders and manganese toxicity. Neurochemical Research. 2015;40(2):380–388. doi: 10.1007/s11064-014-1391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldon A. L., Robinson M. B. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochemistry International. 2007;51(6-7):333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C.-L. G., Kong Q., Cuny G. D., Glicksman M. A. Glutamate transporter EAAT2: a new target for the treatment of neurodegenerative diseases. Future Medicinal Chemistry. 2012;4(13):1689–1700. doi: 10.4155/fmc.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F., Chong Z. Z., Maiese K. Winding through the WNT pathway during cellular development and demise. Histology and Histopathology. 2006;21(1):103–124. doi: 10.14670/HH-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazemondet O., Hubner R., Frahm J., et al. Quantitative and kinetic profile of Wnt/β-catenin signaling components during human neural progenitor cell differentiation. Cellular and Molecular Biology Letters. 2011;16(4):515–538. doi: 10.2478/s11658-011-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schinzari V., Timperi E., Pecora G., et al. Wnt3a/β-catenin signaling conditions differentiation of partially exhausted T-effector cells in human cancers. Cancer Immunology Research. 2018;6(8):941–952. doi: 10.1158/2326-6066.CIR-17-0712. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbluh J., Wang X., Hahn W. C. Genomic insights into WNT/β-catenin signaling. Trends in Pharmacological Sciences. 2014;35(2):103–109. doi: 10.1016/j.tips.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castelo-Branco G., Wagner J., Rodriguez F. J., et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapia-Rojas C., Inestrosa N. C. Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. Journal of Neurochemistry. 2018;144(4):443–465. doi: 10.1111/jnc.14278. [DOI] [PubMed] [Google Scholar]
- 15.Patel A. K., Surapaneni K., Yi H., et al. Activation of Wnt/β-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology. 2015;91:1–12. doi: 10.1016/j.neuropharm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Chen J., Chen C., et al. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Scientific Reports. 2015;5(1, article 16151) doi: 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L'Episcopo F., Tirolo C., Testa N., et al. Wnt/β-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson’s disease. Stem Cells. 2014;32(8):2147–2163. doi: 10.1002/stem.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei L., Sun C., Lei M., et al. Activation of Wnt/β-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. Journal of Molecular Neuroscience. 2013;49(1):105–115. doi: 10.1007/s12031-012-9900-8. [DOI] [PubMed] [Google Scholar]
- 19.Wei L., Ding L., Mo M. S., et al. Wnt3a protects SH-SY5Y cells against 6-hydroxydopamine toxicity by restoration of mitochondria function. Translational Neurodegeneration. 2015;4(1, article 11) doi: 10.1186/s40035-015-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lev N., Barhum Y., Ben-Zur T., Melamed E., Steiner I., Offen D. Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. Journal of Molecular Neuroscience. 2013;50(3):542–550. doi: 10.1007/s12031-013-9984-9. [DOI] [PubMed] [Google Scholar]
- 21.L'Episcopo F., Tirolo C., Testa N., et al. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiology of Disease. 2011;41(2):508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L'Episcopo F., Serapide M. F., Tirolo C., et al. A Wnt1 regulated Frizzled-1/β-catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Molecular Neurodegeneration. 2011;6(1, article 49) doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., Guan Y., Chen Y., et al. Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnology Letters. 2013;35(8):1199–1207. doi: 10.1007/s10529-013-1199-1. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez P., Rodriguez F. J. Analysis of the expression of the Wnt family of proteins and its modulatory role on cytokine expression in non activated and activated astroglial cells. Neuroscience Research. 2017;114:16–29. doi: 10.1016/j.neures.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Parkin G. M., Udawela M., Gibbons A., Dean B. Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World journal of psychiatry. 2018;8(2):51–63. doi: 10.5498/wjp.v8.i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palos T. P., Zheng S., Howard B. D. Wnt signaling induces GLT-1 expression in rat C6 glioma cells. Journal of Neurochemistry. 1999;73(3):1012–1023. doi: 10.1046/j.1471-4159.1999.0731012.x. [DOI] [PubMed] [Google Scholar]
- 27.Luo F., Wei L., Sun C., et al. HtrA2/Omi is involved in 6-OHDA-induced endoplasmic reticulum stress in SH-SY5Y cells. Journal of Molecular Neuroscience. 2012;47(1):120–127. doi: 10.1007/s12031-011-9694-0. [DOI] [PubMed] [Google Scholar]
- 28.Chung E. K. Y., Chen L. W., Chan Y. S., Yung K. K. L. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. The Journal of Comparative Neurology. 2008;511(4):421–437. doi: 10.1002/cne.21852. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh M., Yang Y., Rothstein J. D., Robinson M. B. Nuclear factor-κB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. The Journal of Neuroscience. 2011;31(25):9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi T., Deng Y., Maruyama W., Dostert P., Kawai M., Naoi M. Uptake of a neurotoxin-candidate, (R)-1,2-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline into human dopaminergic neuroblastoma SH-SY5Y cells by dopamine transport system. Journal of Neural Transmission General Section. 1994;98(2):107–118. doi: 10.1007/BF01277014. [DOI] [PubMed] [Google Scholar]
- 31.Blum D., Torch S., Nissou M. F., Benabid A. L., Verna J. M. Extracellular toxicity of 6-hydroxydopamine on PC12 cells. Neuroscience Letters. 2000;283(3):193–196. doi: 10.1016/S0304-3940(00)00948-4. [DOI] [PubMed] [Google Scholar]
- 32.Soto-Otero R., Mendez-Alvarez E., Hermida-Ameijeiras A., Munoz-Patino A. M., Labandeira-Garcia J. L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson’s disease. Journal of Neurochemistry. 2000;74(4):1605–1612. doi: 10.1046/j.1471-4159.2000.0741605.x. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S., Goswami P., Biswas J., et al. 6-Hydroxydopamine and lipopolysaccharides induced DNA damage in astrocytes: involvement of nitric oxide and mitochondria. Mutation Research Genetic Toxicology and Environmental Mutagenesis. 2015;778:22–36. doi: 10.1016/j.mrgentox.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Kaddour H., Hamdi Y., Amri F., et al. Antioxidant and anti-apoptotic activity of octadecaneuropeptide against 6-OHDA toxicity in cultured rat astrocytes. Journal of Molecular Neuroscience. 2019;69(1):1–16. doi: 10.1007/s12031-018-1181-4. [DOI] [PubMed] [Google Scholar]
- 35.Blum D., Torch S., Nissou M. F., Verna J. M. 6-Hydroxydopamine-induced nuclear factor-kappa B activation in PC12 cells. Biochemical Pharmacology. 2001;62(4):473–481. doi: 10.1016/S0006-2952(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 36.Li C., Sun Y., Yang W., et al. Pilose antler extracts (PAEs) protect against neurodegeneration in 6-OHDA-induced Parkinson’s disease rat models. Evidence-Based Complementary and Alternative Medicine. 2019;2019:11. doi: 10.1155/2019/7276407.7276407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Tan F., Xu P., Qu S. Recent advance in the relationship between excitatory amino acid transporters and Parkinson’s disease. Neural Plasticity. 2016;2016:8. doi: 10.1155/2016/8941327.8941327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonsalla P. K., Albers D. S., Zeevalk G. D. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids. 1998;14(1-3):69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- 39.Meredith G. E., Totterdell S., Beales M., Meshul C. K. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Experimental Neurology. 2009;219(1):334–340. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karki P., Webb A., Smith K., et al. cAMP response element-binding protein (CREB) and nuclear factor κB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. Journal of Biological Chemistry. 2013;288(40):28975–28986. doi: 10.1074/jbc.M113.483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bournat J. C., Brown A. M., Soler A. P. Wnt-1 dependent activation of the survival factor NF-κB in PC12 cells. Journal of Neuroscience Research. 2000;61(1):21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Shang Y. C., Chong Z. Z., Hou J., Maiese K. Wnt1, FoxO3a, and NF-κB oversee microglial integrity and activation during oxidant stress. Cellular Signalling. 2010;22(9):1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.