Abstract
Background/Aim: The problem of adequately marking any given lesion within a breast surgical site is commonly solved by introducing a titanium clip. However, clip dislocation and/or stereotactic hook-wire dislocation are common problems. An ideal solution would be a clip that can be easily found without the use of stereotactic intervention. This work reviews the available data on radiofrequency identification devices (RFID) in breast surgery, reports initial experience data in Europe and discusses surgical pitfalls, advantages and disadvantages. Patients and Methods: This study represents a single center, consecutively recruited, initiation trial with subsequent surgeon questionnaire for the first institution in Europe to report Faxitron LOCalizer™ chip data. Four patients with non-palpable tumors were marked with the system and were correlated via mammography, pre- and intra-operative ultrasound and pathology. Data were then compared to available literature and a literature review was added. Results: The four patients marked with this RFID system, displayed a 100% success location rate at a 0% complication rate. Surgeons evaluated the new system as being safe to use and only slightly more difficult to place compared to a standard clip. A significant improvement in ultrasound localization and intraoperative localization was also reported for the LOCalizer™ system when compared to a standard titanium clip. Conclusion: This trial added a small number of consecutively recruited patients to an existing number of available data, resulting in a total of 121 evaluated and reviewed Faxitron LOCalizer™ marked non-palpable in-breast lesions worldwide.
Keywords: Faxitron, LOCalizer™, Localizer, breast, non-palpable, nonpalpable, lesion, marking, radiofrequency identification device, RFID
The matter of adequately locating non-palpable breast lesions is a constant concern with breast surgeons around the world. The current state-of-the-art systems apply a plethora of different clips (1), which are generally placed following invasive breast biopsy in order to mark a specific location within the breast. While the system has been well established, several shortcomings have become apparent over the years. As any given clip may also migrate, break or dislodge completely, the main problem remains the dislocation of a stereotactic-guided wire used as a marker. Although modern ultrasound systems are sometimes capable of also locating clips intraoperatively, thus compensating this problem by intraoperative ultrasound, surgeons generally still turn to stereotactic guided hook wire marking in the preoperative setting (2). In addition to problems caused by wire dislodging, these wires can also break or, in some cases, not meet up with the previously placed clip (3). The latter can lead to discontinuation of the surgical procedure as the surgical site is improperly marked altogether. Most importantly, however, any patient with a clip-marked, non-palpable lesion is required to undergo an additional mammogram (for wire placement) as well the additional wire placement procedure itself. This causes an increase in patient morbidity, psychological stress and radiational burden. As this problem has become evident over the last decades, several attempts have been made to address this issue. The most promising technology seems to be the use of radiofrequency identification (RFID) systems, such as the Faxitron LOCalizer™. Very little data is available on this subject, although small prospective trials have shown its efficacy (4-7). No detailed description of the surgical process and/or experience has yet been reported (Table I) and none are from surgical sites in Europe. It was therefore the goal of this study group, in collaboration with the DZMGS (German Center for Material Science in Gynecology and Senology), to run a short feasibility trial at a European surgical setting. Additionally, a surgical guideline for the application of the Faxitron LOCalizer™ RFID system as well as a surgeon’s questionnaire and literature review were attempted, with the questions shown below:
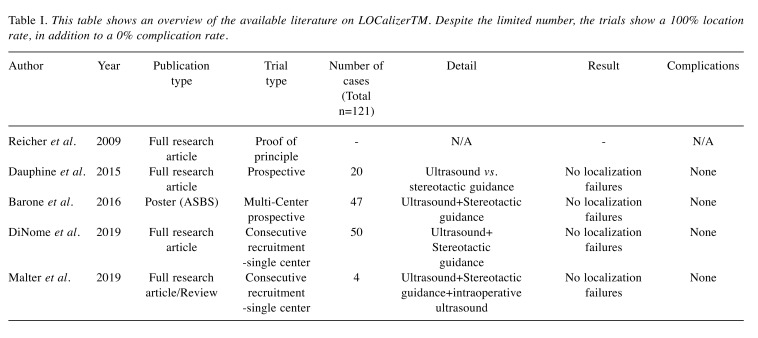
Table I. This table shows an overview of the available literature on LOCalizerTM. Despite the limited number, the trials show a 100% location rate, in addition to a 0% complication rate.
1) Is it possible to reproduce an internationally reported outcome in a European/German surgical setting?
2) How does this pilot trial compare to the available literature?
3) Does the Faxitron LOCalizer™ RFID system require specific surgical handling in complicated senological settings – i.e. augmentation, severe scarring, chronic infection?
4) How does the LOCalizer™ RFID system compare to a standard surgical clip when evaluated by surgeon questionnaire?
Patients and Methods
This pilot trial recruited 4 patients with non-palpable breast lesions in the first quarter of 2019 at the breast-cancer center of the University of Cologne, Germany and the DZMGS. All patients had prior invasively confirmed non-cancer lesion. Two patients had previously received titanium clip placement. The average age was 40.7±10.3 years. All patients were female. One patient could not be marked through guided hook wire due to prior augmentation. A second patient had experienced severe scarring following chronic infection.
Surgical procedures. All surgical procedures were performed by experienced oncology breast surgeons only. All lesions were preoperatively marked using the Faxitron (Hologic, Inc. Company, Marlborough, MA, USA) LOCalizer™ chip and ultrasound (GE Voluson E8 Expert, GE Healthcare Chicago, IL, USA). Chips were also correlated preoperatively via mammography (Hologic, Multicare Platinum, Hologic, Inc. Marlborough, MA, USA) when a prior clip was present. Intraoperative ultrasound (see above) was performed as well. All LOCalizer™ chips were placed on the day of the surgery. All chips (100%) were located and removed during surgery.
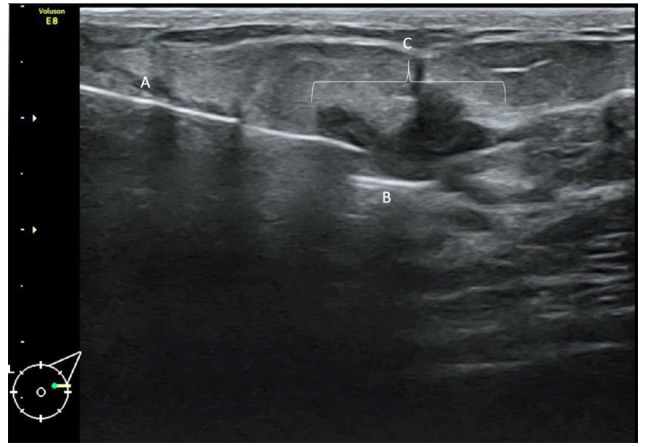
LOCalizer™ chip placement. For placing the chip a 2 mm incision was made prior to the insertion of the applicator, as the skin cannot be readily penetrated by the applicator itself. It should also be noted that placement of the chip is easily done in less dense breast tissue. When reaching a density level of ACR 3 (American College of Radiology) or higher, placing the chip is increasingly more difficult as the diameter of the applicator itself slightly impedes intra-tissue movement. Placement may be tracked by ultrasound as shown in Figure 1.
Figure 1. Ultrasound lesion marked using the LOCalizer™ system. The patient is a 55-year-old woman with an unclear lesion in the left outer quadrant. (A) Shows the application hollow needle system, (B) shows the LOCalizer™ chip and (C) is the non-palpable lesion.
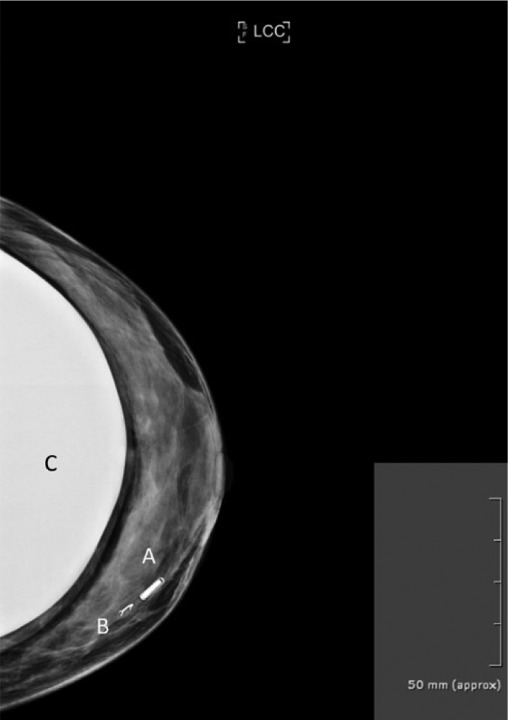
Preoperative mammography. Preoperative mammography was performed for cases where a prior clip had been placed after the initial invasive biopsy. Patients were given the option of receiving a preoperative wire marking versus a preoperative LOCalizer™ chip marking. In all cases patients decided against wire marking. One patient presented with a clip which could not be stereotactically hook-wire marked due to an earlier breast augmentation surgery. A preoperative mammogram had to be performed nonetheless in order to correlate the prior clip placement in relation to the current chip positioning. An example of a preoperative mammogram can be seen in Figure 2.
Figure 2. Left breast mammography of a 30-year-old woman. (A) Shows the LOCalizer™ chip which was placed in a non-palpable lesion. Note the direct comparisons in size when compared to a standard titanium clip (B). The patient received a bilateral sup-pectoral augmentation “C” 5 years earlier. In this case the non-palpable breast lesion had been clipped before, however the clip was dislocated. A stereotactic wire marking was also not possible due to the risk of breast implant damage. Thus, a LOCalizer™ chip was placed in the accurate position.
Surgical management. General surgical management does not differ from regular lumpectomy procedures. However, the absence of a guiding wire improves surgical movement and decreases the risk of injuring skin tissue through heat conduction while using electro-surgical tools. In addition, as the LOCalizer™ chip is covered in a glass casing, contact between the electro-cautery tool and the chip itself does not introduce a risk of local tissue damage. Locating the chip itself is best described as being similar to locating a sentinel lymph node with an appropriate detection probe. The LOCalizer™ detection probes do not require preoperative radioactive marking. They are also lighter compared to a common gamma probe. Noteworthy is the fact that the LOCalizer™ chip is inactive and could theoretically remain in situ for any given time and remain placed there. Figure 3 shows a surgical step-by-step for this type of procedure. Surgical tip: direct clamping of the chip itself should be avoided as the glass casing may break.
Figure 3. A series of the standard surgical proced ure in this trial. (A ) Shows a non-palpa ble lesion being loc ate d through the ski n following preo perati ve chi p pla cement. (B ) Show s an incis ion with t he LOC alizer ™ p ro be bein g place d in the wou nd cavi ty for an exa ct 3 - dimensi onal positi oning . (C) Show s a pre viously placed cl ip (white arrow) and chip (blue arrow) marked specimen for size comparison. (D) Shows an intraoperative radiogram of the excis ed speci men.
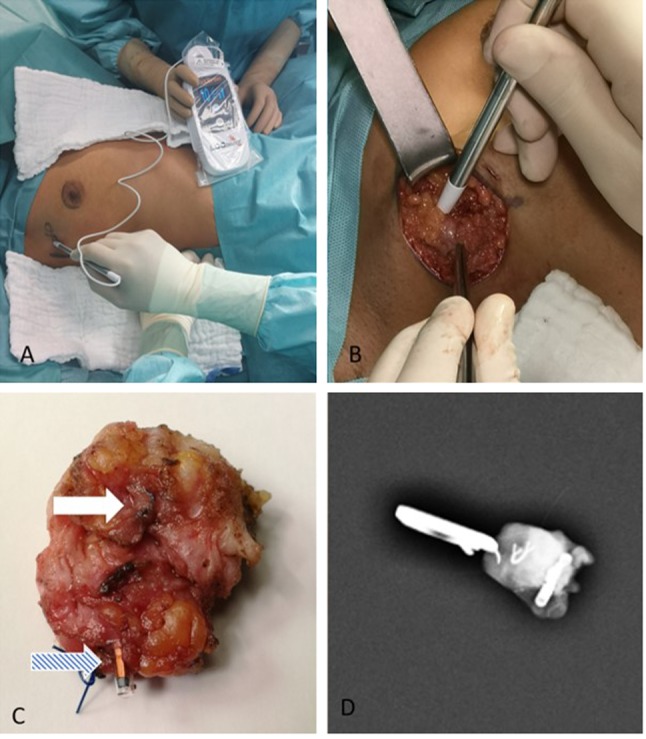
Intraoperative mammography. In order to validate this type of procedure it was necessary to confirm the complete chip and clip resection during surgery via radiography for patients who had received both. In patients where only a LOCalizer™ chip was used the intraoperative location of the chip was possible through the LOCalizer™ probe.
Postsurgical period. There were no complications during or following surgery. All excised tumors were benign. All margins were clean. No resubmission for surgery had to be performed.
Questionnaire. Four surgeons participated in this pilot trial. All surgeons had at least 5 years of surgical and ultrasound experience with standard titanium clips. A questionnaire was presented to all surgeons at the end of the trial day. Evaluated endpoints were subjective safety of the application, ease of application, ultrasound visibility of the applicator, ultrasound visibility of the Standard clip/chip, mammography visibility clip/chip, localization in surgery, macroscopic visibility (intraoperatively), palpation during surgery. In addition, they were asked whether they believed the product will be used in the future and whether they would recommend it to other surgeons.
Informed consent. Informed consent was provided by all patients. A written statement to this extent may be available to the editor at any time.
Results
This initiation trial showed for the first time that a Faxitron LOCalizer™ can be successfully used at the European/ German surgery theater. All of the chips used preoperatively were found during surgery via the LOCalizer™ system. All intraoperative mammograms showed both the previous clip (if one had been placed) as well as the current chip that was to be included within the specimen. No follow-up surgery and no wire marking had to be performed. When asked, all patients preferred a non-wire form of tumor localization. There were no significant complications, i.e. no hematoma, seroma or any wound infection. One patient had received breast augmentation several years prior to lumpectomy, however, also for this patient there was no complication i.e. no implant loss or revision surgery.
The surgeon questionnaire was answered at 100% completion rate, favoring the LOCalizer™ system. The safety of application did not differ between the titanium standard clip and the LOCalizer™ system with both receiving at least a 90% satisfaction rating. A slight difference in the “ease of application” could be shown with the gold standard titanium clip being easier to place (100% grade), while the LOCalizer™ chip only received a 75% grade. As numbers are low, these reported values are purely descriptive in nature. Other factors, such as ultrasound visibility of the chip or of the applicator as well as the ease of localization during surgery all favored the LOCalizer™ system. Especially noteworthy is the fact that the chip is easily visible using ultrasound and may thus also be located by intraoperative ultrasound should a probe fail. Table II shows the detailed results.
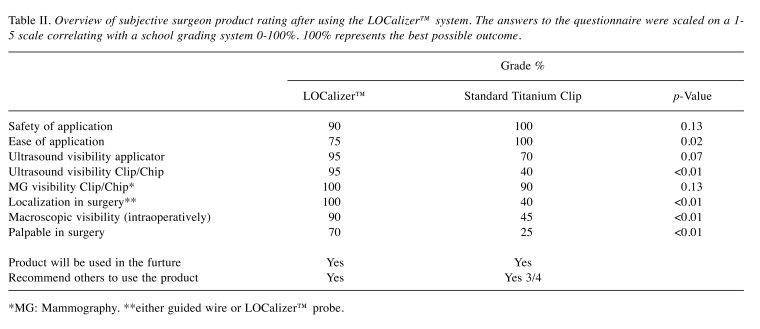
Table II. Overview of subjective surgeon product rating after using the LOCalizer™ system. The answers to the questionnaire were scaled on a 1- 5 scale correlating with a school grading system 0-100%. 100% represents the best possible outcome.
*MG: Mammography. **either guided wire or LOCalizer™ probe.
Discussion
The primary focus of this study was the evaluation of the overall applicability as well as effectiveness of the LOCalizer™ system. From the 121 cases described in the literature, this RFID system has been proved to be successful in 100% of all reported cases. In the initiation trial by our study group (DZMGS) we were able to add only 4 patients to this database, although the main goal of this work was to establish feasibility of the LOCalizer™ system, give a surgeon’s review on this type of procedure in a European surgery theater as well as review the available data.
Regarding the applicability of this system it should be noted that the LOCalizer™ chip is safe to place and easy to locate. Our surgeons were slightly less pleased with the “ease of application” for the LOCalizer™ system compared to what is published. This is due to the fact that a larger hollow needle is used for this RFID in comparison to a standard titanium clip. After consulting with the manufacturer, a thinner and sharper needle will become available soon, and this should alleviate this shortcoming. Furthermore, the ease with which this chip may be located during surgery, either by using the LOCalizer™ probe or even intraoperative ultrasound (8-11) led our surgeons to recommend its further use. It is also noteworthy, that the titanium standard clip seemed to be less than adequate compared to the chip, since an extreme level of dissatisfaction become apparent when we asked surgeons about the intraoperative handling of the titanium clip.
Currently, the LOCalizer™ chip may only remain inside the body for 30 days, which poses for an obstacle in a neoadjuvant setting. Nonetheless, for suspected DCIS (ductal carcinoma in situ) and or benign/uncertain lesions this type of chip could be recommended. When asked, patients at our institution generally dislike repeating a mammography in combination with stereotactic wire placement in a pre-surgical marking.
Reviewing the literature, we found 121 cases (117 excluding our 4 new cases), of both randomized and consecutively recruited patients, yielding a zero percent failure to localize the chip. This itself is an important enough reason to readily apply this type of in-breast marking, as wire dislocation or wrongful wire placement leads to failure to dissect out up to 10% (12) of all stereotactically marked lesions. It should also be noted that the actual number of failures to dissect might be higher since institutions generally do not report their failed stereotactic marking. We must therefore face the question, whether it is still ethical to expose patients to a stereotactic mammography-based, i.e. radiation-dependent marking scenario when such an alternative is available.
Knowing that stereotactic marking may at times be difficult or inaccurate for in-breast lesions, we must immediately note that this is also and perhaps even more so the case for targeted lymph node dissection. In general, a titanium clipped lymph node is very difficult to mark through a guided hook-wire. We therefore propose that it might be useful to also apply the LOCalizer™ system to targeted lymph node dissection (13). Our workgroup has therefore initiated a LOCalizer™ lymph node trial i.e. the TargEx trial (chip localization of lymph nodes in breast cancer patients).
Following this initiation trial, two shortcomings may be mentioned from a surgeon’s point of view. Firstly, chip placement is slightly more difficult (compared to a titanium clip) in dense tissue or stiff tumors. After conferring with the manufacturer, a thinner and sharper needle will be available for chip placement soon, thus this problem will be solved. Secondly, one might disagree with the chip size at 2 mm × 10.6 mm. It is, as seen in Figure 2, roughly twice as large as a common titanium clip. Therefore, small lesions (below 5 mm) are not ideally marked with this chip. Larger micro-calcifications/DCIS areas are easily marked using the LOCalizer™ system.
Noteworthy is the fact that the LOCalizer™ chip will produce an MRI artifact (1). Thus, we cannot recommend the use of the LOCalizer™ chip for tumors which have to be MRI marked and/or MRI evaluated under neoadjuvant chemotherapy (5).
Current price listings show this product at €150 for the applicator. This is slightly more expensive when compared to a regular clip. The same is true for removing the product as the localizing equipment is currently priced also at around €150 per surgery (probe only). However, since in the standard scenario the patient must undergo a separate mammogram while receiving a pre-surgical stereotactic marking the cost effectiveness of this procedure becomes apparent. The mammography step may completely be omitted as the surgeon is able to locate the Faxitron LOCalizer™ chip without the aid of a radiologist. In addition, intra-surgical radiographs of the specimens are no longer required.
We were thus able to answer the following questions:
1) Is it possible to reproduce internationally reported outcomes in a European/German surgical setting?
- The product was safely and successfully used in this European setting.
2) How does this pilot trial compare to available literature?
- Literature shows a 100% successful location rate. This was reproduced here as well.
3) Does the Faxitron LOCalizer™ RFID system require specific surgical handling in complicated senological settings – i.e. augmentation, severe scarring, chronic infection.
- Severe scarring, as well as dense tissue may present a challenge as the LOCalizer™ application is slightly more difficult compared to a stranded titanium clip. Patients with augmentation will profit from this system as the LOCalizer™ chip is much more visible compared to a standard clip.
4) How does the LOCalizer™ RFID system compare to a standard surgical clip when evaluated by the surgeon questionnaire?
- The LOCalizer™ chip is safe to use and easily detected for lesions above 5mm. Due to a detection rate of 100% without required presurgical x-ray exposure our group of surgeon were led to favor its use compared to standard titanium clips.
In conclusion, this trial was able to show that use of the LOCalizer™ RFID represents a sensible alternative for larger, non-palpable in-breast lesions and complement the currently available data. The authors especially recommend its use for large microcalcifications/suspected DCIS areas, as patients may omit an unnecessary stereotactic guidewire marking. Future work must also evaluate this product in targeted lymph node dissection as is already taking place in our workgroup.
Conflicts of Interest
The Authors declare no conflicts of interest.
Authors’ Contributions
Wolfram Malter: writing, editing, data collection, statistical analysis, trial development; Johannes Holtschmidt: writing, editing, trial development; Fabinshy Thangarajah: writing, editing; Peter Mallmann: writing, editing. Barbara Krug: writing, editing, trial development; Mathias Warm: writing, editing; Christian Eichler: writing, editing, data collection, statistical analysis, trial development.
Acknowledgements
The Authors would like to thank Faxitron for providing the LOCalizer™ RFID system.
References
- 1.Santiago L, Candelaria RP, Huang ML. Mr imaging-guided breast interventions: Indications, key principles, and imaging-pathology correlation. Magn Reson Imaging Clin N Am. 2018;26(2):235–246. doi: 10.1016/j.mric.2017.12.002. PMID: 29622128. DOI: 10.1016/j.mric.2017. 12.002. [DOI] [PubMed] [Google Scholar]
- 2.Patel J, Jenkins S. A technique for marking oncolinogical breast tissue specimens. Ann Med Surg (Lond) 2016;7:7–8. doi: 10.1016/j.amsu.2016.02.012. PMID: 27158488. DOI: 10.1016/j.amsu.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods RW, Camp MS, Durr NJ, Harvey SC. A review of options for localization of axillary lymph nodes in the treatment of invasive breast cancer. Acad Radiol. 2019;26(6):805–819. doi: 10.1016/j.acra.2018.07.002. PMID: 30143401. DOI: 10.1016/j.acra.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol. 2015;204(6):W720–W723. doi: 10.2214/AJR.14.13201. PMID: 30143401. DOI: 10.1016/j.acra.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Reicher JJ, Reicher MA, Thomas M, Petcavich R. Radiofrequency identification tags for preoperative tumor localization: Proof of concept. AJR Am J Roentgenol. 2008;191(5):1359–1365. doi: 10.2214/AJR.08.1023. PMID: 17406988. DOI: 10.1007/s10877-007-9069-9. [DOI] [PubMed] [Google Scholar]
- 6.DiNome ML, Kusske AM, Attai DJ, Fischer CP, Hoyt AC. Microchipping the breast: An effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat. 2019 doi: 10.1007/s10549-019-05143-w. PMID: 30689105. DOI: 10.1007/s10549-019-05143-w. [DOI] [PubMed] [Google Scholar]
- 7.Rupp CC, Kagarise MJ, Nelson SM, Deal AM, Phillips S, Chadwick J, Petty T, Meyer AA, Kim HJ. Effectiveness of a radiofrequency detection system as an adjunct to manual counting protocols for tracking surgical sponges: A prospective trial of 2,285 patients. J Am Coll Surg. 2012;215(4):524–533. doi: 10.1016/j.jamcollsurg.2012.06.014. PMID: 22770865. DOI: 10.1016/j.jamcollsurg.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Ramos M, Diez JC, Ramos T, Ruano R, Sancho M, Gonzalez-Orus JM. Intraoperative ultrasound in conservative surgery for non-palpable breast cancer after neoadjuvant chemotherapy. Int J Surg. 2014;12(6):572–577. doi: 10.1016/j.ijsu.2014.04.003. PMID: 24735893. DOI: 10.1016/j.ijsu.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Sikosek NC, Dovnik A, Arko D, Takac I. The role of intraoperative ultrasound in breast-conserving surgery of nonpalpable breast cancer. Wien Klin Wochenschr. 2014;126(3-4):90–94. doi: 10.1007/s00508-013-0470-8. PMID: 24442857. DOI: 10.1007/s00508-013-0470-8. [DOI] [PubMed] [Google Scholar]
- 10.Colakovic N, Zdravkovic D, Skuric Z, Mrda D, Gacic J, Ivanovic N. Intraoperative ultrasound in breast cancer surgery-from localization of non-palpable tumors to objectively measurable excision. World J Surg Oncol. 2018;16(1):184–184. doi: 10.1186/s12957-018-1488-1. PMID: 30205823. DOI: 10.1186/s12957-018-1488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esgueva A, Rodriguez-Revuelto R, Espinosa-Bravo M, Salazar JP, Rubio IT. Learning curves in intraoperative ultrasound guided surgery in breast cancer based on complete breast cancer excision and no need for second surgeries. Eur J Surg Oncol. 2019;45(4):578–583. doi: 10.1016/j.ejso.2019.01.017. PMID: 30737056. DOI: 10.1016/j.ejso. 2019.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Tardioli S, Ballesio L, Gigli S, DIP F, D'Orazi V, Giraldi G, Monti M, Amabile MI, Pasta V. Wire-guided localization in non-palpable breast cancer: Results from monocentric experience. Anticancer Res. 2016;36(5):2423–2427. PMID: 27127152. [PubMed] [Google Scholar]
- 13.Ersoy YE, Kadioglu H. Review of novel sentinel lymph node biopsy techniques in breast cancer patients treated with neoadjuvant chemotherapy. Clin Breast Cancer. 2018;18(4):e555–e559. doi: 10.1016/j.clbc.2018.01.004. PMID: 29429940. DOI: 10.1016/j.clbc.2018.01.004. [DOI] [PubMed] [Google Scholar]