Abstract
Background/Aim: C-Reactive protein (CRP) is a common marker of inflammation. Elevated CRP levels have been associated with increased risk of development of type 2 diabetes mellitus (T2DM). This study aimed to evaluate the association of CRP gene polymorphisms with early-onset T2DM and the effect of genetic variants on CRP level. Materials and Methods: In total, 948 individuals with early-onset (n=271) or late-onset (n=677) T2DM were enrolled in the study. Five single-nucleotide polymorphisms (SNPs) in the CRP gene, namely rs3093077, rs2808630, rs1800947, rs11265263, and rs11265265, were selected for genotyping, and CRP levels were measured. Results: Genotypic, allelic, and haplotype frequencies of these five SNPs were not significantly different between patients with early- and those with late-onset. T2DM Higher serum CRP levels were independently associated with the C-allele of rs3093077 and T-allele of rs11265265 (p<0.001). Furthermore, the C-allele of rs3093077 was associated with higher CRP level in both early- (p=0.016) and late-onset (p<0.001) T2DM. Conclusion: CRP gene variants may contribute to the risk of early-onset T2DM by affecting the serum CRP level.
Keywords: C-Reactive protein, polymorphism, early-onset, type 2 diabetes, Taiwan
Type 2 diabetes mellitus (T2DM) is currently a major public health concern worldwide (1). During the past two decades, the prevalence of T2DM in young adults has steadily increased (2-4). A major risk factor strongly associated with early-onset T2DM is obesity in children, adolescents and young adults (5-7). From extensive experimental, clinical, and epidemiological studies, obesity has been linked to activation of innate immunity-related inflammatory signaling pathways. Inflammatory cytokines, such as interleukin-6 (IL6) and tumor necrosis factor-alpha (TNFα), and acute-phase reactants such as C-reactive protein (CRP) can block major anabolic cascades downstream of insulin signaling, thereby disrupting insulin homeostasis and increasing the risk of T2DM (8,9). Studies have shown that reduction of inflammation and the ensuing acute-phase reactant responses through exercise, medication, or nutrition improves insulin sensitivity and delays disease onset (8).
Current evidence indicates that the risk of developing T2DM is regulated by lifestyle and genetic factors. Heterogeneity in the genetic determinants of T2DM development has been identified by candidate gene and genome-wide association studies (GWAS) across multiple populations (10-13). The influence of innate immunity-related inflammation genes combined with various lifestyle factors may affect serum levels of cytokines and inflammatory markers, that may play a pivotal role in susceptibility to T2DM. Therefore, investigation of the association between variants of inflammation-related genes and the risk of T2DM may help develop better approaches for early detection and prevention.
Recent evidence from linkage and GWAS studies has implicated a region of chromosome 1q21-23 encompassing the CRP gene with risk of T2DM development in various ethnic populations (14-16). CRP, a common marker of inflammation, is an acute-phase reactant regulated by cytokines, predominantly IL6 and TNFα. CRP plays a critical role in T2DM by its action on pancreatic β-cells and is thought to be an early risk factor for T2DM (17). In vitro studies have shown an association between the serum level of high-sensitivity CRP and β-cell dysfunction and insulin resistance (18,19). Moreover, elevation of high-sensitivity CRP in diabetic patients has been associated with an increased risk of diabetic vasculopathy (18,20-22). Furthermore, several single-nucleotide polymorphisms (SNPs) in the CRP gene have been reported to be associated with serum CRP level, insulin sensitivity, and T2DM incidence (23). Based on these observations, we investigated the association between variants of the CRP gene and early-onset T2DM in the Han Chinese population of Taiwan.
Materials and Methods
Patient and data collection. In total, 948 patients with T2DM (age >20 years) from the China Medical University Hospital in Taiwan were enrolled in the study, and informed consent was acquired from all patients. Diabetes was diagnosed based on the patient medical records and fasting plasma glucose level using the American Diabetes Association Criteria (24). Patients with type 1 diabetes, gestational diabetes, and maturity-onset diabetes of the young were excluded from this study. According to the age recommended by the American Diabetes Association for T2DM screening in adults, patients with type 2 diabetes were segregated into two subgroups: (i) early-onset diabetes (n=271; age at diagnosis, at least 20 years but less than 45 years) and (ii) late-onset diabetes (n=677; age at diagnosis, 45 years or more). Data regarding age, sex, duration of disease, weight, height, and circumference of waist and hip (waist-to-hip ratio) of each patient were obtained from questionnaires. Blood samples for genomic DNA isolation were collected by venipuncture, and serological tests, including fasting glucose, hemoglobin A1c, and CRP, were performed at the time of enrollment. The study was approved by the Medical Ethics Committee of the China Medical University Hospital, Taichung, Taiwan (approval number: CMUH103-REC2-071) and performed according to the tenets of the Declaration of Helsinki for research involving human subjects.
SNP selection and genotyping. Five SNPs of CRP gene rs3093077, rs2808630, rs1800947, rs11265263, and rs11265265 (positions: 159709846, 159711078, 159713648, 159740727, and 159743766 bp, respectively) were selected from the Illumina Hap550K chip (12), which has been used previously for GWAS in the Han Chinese population of Taiwan. SNPs were selected by applying the following criteria: (i) a threshold minor allelic frequency in the HapMap CHB population of 0.10 for tag SNPs; and (ii) a genotyping score ≥0.6, as recommended by the manufacturer (Illumina, Inc., San Diego, CA, USA) to ensure a high genotyping success rate. In order to avoid redundancy, markers with pairwise r2 correlations ≥0.8 for any selected marker were not genotyped. Deviation from the Hardy–Weinberg equilibrium was not observed for any SNP. For genotyping, all blood samples were de-identified prior to analysis, and only the project investigator had access to the link for individual identities. Laboratory personnel involved in genotyping were blinded to the age at diabetic onset of the study patients. Genomic DNA was extracted from peripheral blood leukocytes using Genomic DNA kit (Qiagen, Valencia, CA, USA), and genotyping was performed using an allele-specific extension and ligation assay (Illumina) according to the manufacturer’s instructions.
Statistical analyses. The distributions of genotypic and allelic frequencies of the polymorphisms in patients with early-onset (age <45 years) and late-onset (age ≥45 years) T2DM ware analyzed using the chi-squared or Fisher exact test for differences in proportions. Odds ratios (OR) were calculated for associations with genotypic and allelic frequencies with a 95% confidence interval (CI) using unconditional logistical regression. The effect of the CRP gene SNP genotype on serum CRP level was analyzed using a general linear regression model after logarithmic transformation of CRP data. All statistical analyses were conducted using IBM SPSS Statistics 22 (IBM Co., Armonk, NY, USA), and p-values of less than 0.05 (two-sided) were considered significant.
Results
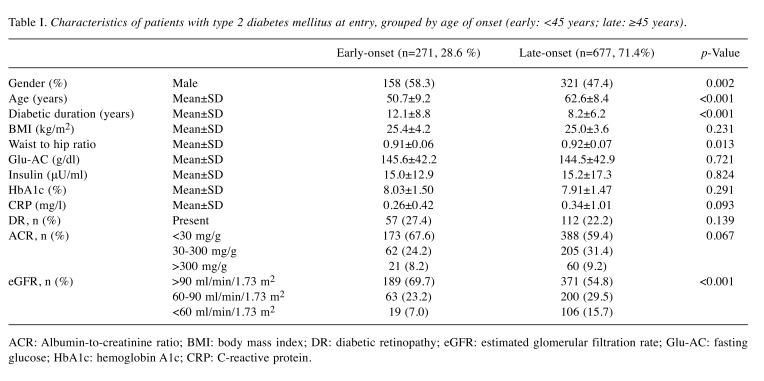
In our database, 28.6% (n=271) of patients had early-onset T2DM (mean age at diagnosis=38.6±4.7 years), and 71.4% (n=677) had late-onset T2DM (mean age at diagnosis= 54.47±6.4 years). Clinical and biomedical parameters of patients with early-onset and late-onset T2DM are compared in Table I. We recorded a higher number of men, younger individuals, longer disease duration, and lower percentage of those with an estimated glomerular filtration rate <60 ml/min/1.73 m2 in those with early-onset T2DM. None of the observed characteristics, including body mass index, waist-to-hip ratio, and serological marker levels (i.e. fasting glucose insulin, hemoglobin A1c, and CRP), in this study showed significant differences between these two groups. Furthermore, no significant differences in prevalence of diabetic retinopathy and albumin-to-creatinine ratio were observed.
Table I. Characteristics of patients with type 2 diabetes mellitus at entry, grouped by age of onset (early: <45 years; late: ≥45 years).
ACR: Albumin-to-creatinine ratio; BMI: body mass index; DR: diabetic retinopathy; eGFR: estimated glomerular filtration rate; Glu-AC: fasting glucose; HbA1c: hemoglobin A1c; CRP: C-reactive protein.
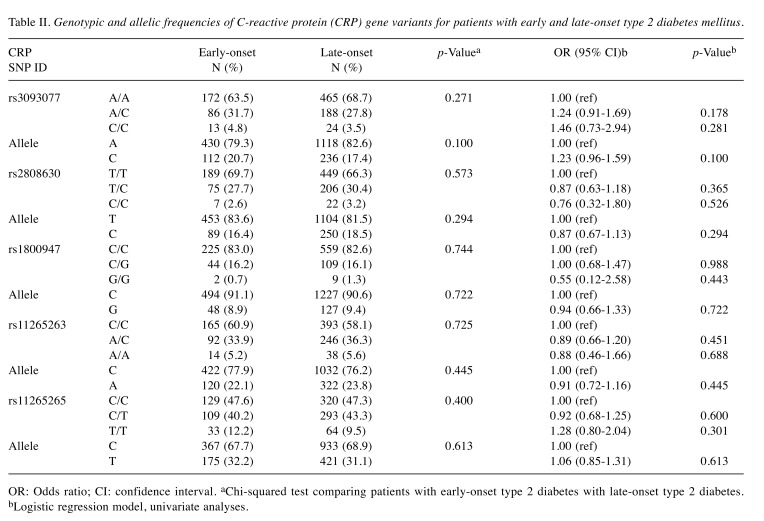
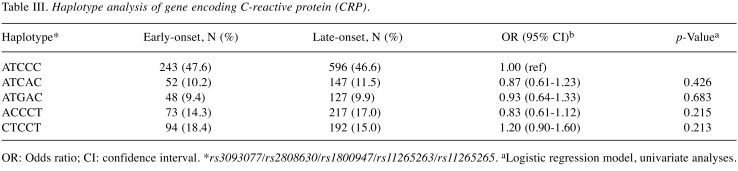
In order to identify SNPs associated with early-onset T2DM, five SNPs within CRP were genotyped. The genotypic and allelic frequencies of these five polymorphisms were not significantly different between patients with early-onset and late-onset T2DM (Table II). Furthermore, we investigated the association between the CRP haplotype and susceptibility to early-onset T2DM, and no significant difference was found (Table III).
Table II. Genotypic and allelic frequencies of C-reactive protein (CRP) gene variants for patients with early and late-onset type 2 diabetes mellitus.
OR: Odds ratio; CI: confidence interval. aChi-squared test comparing patients with early-onset type 2 diabetes with late-onset type 2 diabetes. bLogistic regression model, univariate analyses.
Table III. Haplotype analysis of gene encoding C-reactive protein (CRP).
OR: Odds ratio; CI: confidence interval. *rs3093077/rs2808630/rs1800947/rs11265263/rs11265265. aLogistic regression model, univariate analyses.
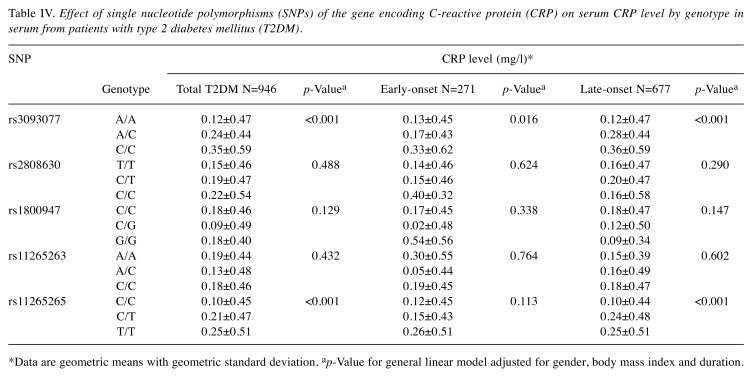
The effect of genotype on serum CRP level was also investigated (Table IV). A significant effect (both p<0.001) of the C-allele of SNP rs3093077 and T-allele of SNP rs11265265 on elevation of serum CRP level was observed in multivariate regression analysis after adjusting for gender, DM duration, and BMI. In addition, we stratified the patients with T2DM according to early- or late-onset, and the C-allele of rs3093077 was significantly associated with elevation of serum CRP level in both the early- and late-onset groups (p=0.016 and p<0.001, respectively). However, the T-allele of rs1126265 was only significantly associated with elevation of CRP level in the group with late-onset T2DM (p<0.001).
Table IV. Effect of single nucleotide polymorphisms (SNPs) of the gene encoding C-reactive protein (CRP) on serum CRP level by genotype in serum from patients with type 2 diabetes mellitus (T2DM).
*Data are geometric means with geometric standard deviation. ap-Value for general linear model adjusted for gender, body mass index and duration.
Discussion
Polymorphisms of several inflammation-related candidate genes such as those for toll-like receptor 4, ILα, IL6 and TNFα have been reported to contribute to T2DM (17,25). However, only a few studies have examined the influence of genetics on age at T2DM diagnosis, and no high impact genes have been directly linked to T2DM onset. This study investigated five SNPs (rs3093077, rs2808630, rs1800947, rs11265263, and rs11265265) of the CRP gene and their association with early-onset T2DM. None of the selected SNPs were significantly associated with early-onset T2DM. It is possible that the SNPs examined in this study may not play important roles in T2DM development at an early age or might not capture all possible genetic variations in the CRP gene. Further studies exploring other CPR variants within this population, as well as experiments addressing genetic changes and their relationship to protein function, will help to identify the true causal variants of T2DM. In addition, the non-significant results of the association between the CRP gene and early-onset T2DM may be due to the sample size. Thus, the study may not have been sufficiently powered to detect a weak association between CRP genotype and early-onset T2DM.
Several SNPs, including rs1800947 (G1059C) (26-28), rs2794521 (A1009G) (29), rs3091244, and rs1205 (26), of CRP gene have been associated with concentrations of circulating CRP in patients of different ethnicities with different diseases. The relationship between CRP variants and serum CRP level was also explored in our T2DM study population. A significant genetic effect on serum CRP level was observed in this study. SNPs rs3093077 and rs11265265 were associated with CRP level in patients with T2DM. Among these SNPs, rs3093077 was previously reported to be associated with CRP level in different populations, including in Denmark (30), the Framingham Heart Study cohort in America (31), the United Kingdom (32), and Taiwan (33), but not in Italians (34). SNP rs3093077 is located in the 3’ flanking region of the CRP gene. It often contains a transcription unit to regulate formation of the 3’ end of the message and may also contain enhancers or other protein-binding sites. SNPs in this region of CRP have been shown to affect the serum CRP level (29).
Furthermore, studies have shown that obesity can influence CRP concentration by stimulating overexpression of cytokines such as IL6 and TNFα (26). In addition, the effect of gender on CRP level has been shown in previous studies. Sheu et al. found that the CRP rs2794521 GG genotype was associated with lower serum CRP concentrations in a group of elderly men (29). Hence, we adjusted for the effect of BMI and gender in a multivariate model, and the results showed that the serum CRP level was affected by the CRP gene independently. The results highlight the importance of the interplay between genetic and lifestyle factors in phenotypic development of complex traits.
Conclusion
In conclusion, this study provides evidence for the association of serum CRP level and CRP gene polymorphisms in patients with T2DM, although a significant association between polymorphisms of the CRP gene and age at T2DM diagnosis was not observed.
Conflicts of Interest
None of the Authors have any financial interests to disclose.
Authors’ Contributions
FJ Tsai and WL Liao conceived and supervised all works; YC Huang and WL Liao designed, analyzed and drafted the article; CC Chen, TY Wang, TTH Nguyen, YH Chen, CM Wu, and YW Chang participated interpretation the data. All Authors read and approved the final article.
Acknowledgements
The Authors thank the National Center for Genome Medicine, Taipei, Taiwan for providing support services for data coordination, subject recruitment, and project management.
Grant support: This work was supported in part by research grants from China Medical University Hospital (DMR-107-056 and DMR-108-036), Taichung City, Taiwan; and from Academia Sinica (AS-BD-108-9), Taipei City, Taiwan.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. PMID: 19896746. DOI: 10.1016/ j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Koopman RJ, Mainous AG 3rd, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med. 2005;3(1):60–63. doi: 10.1370/afm.214. PMID: 15671192. DOI: 10.1370/afm.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng CH, Tseng CP, Chong CK, Huang TP, Song YM, Chou CW, Lai SM, Tai TY, Cheng JC. Increasing incidence of diagnosed type 2 diabetes in Taiwan: Analysis of data from a national cohort. Diabetologia. 2006;49(8):1755–1760. doi: 10.1007/s00125-006-0314-4. PMID: 16788802. DOI: 10.1007/s00125-006-0314-4. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Shau WY, Jiang YD, Li HY, Chang TJ, Sheu WH, Kwok CF, Ho LT, Chuang LM. Type 2 diabetes prevalence and incidence among adults in Taiwan during 1999-2004: A National Health Insurance data set study. Diabet Med. 2010;27(6):636–643. doi: 10.1111/j.1464-5491.2010.03007.x. PMID: 20546280. DOI: 10.1111/j.1464-5491. 2010.03007.x. [DOI] [PubMed] [Google Scholar]
- 5.Shield JP, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94(3):206–209. doi: 10.1136/adc.2008.143313. PMID: 18838418. DOI: 10.1136/adc.2008.143313. [DOI] [PubMed] [Google Scholar]
- 6.Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: Results from prescription data from a UK general practice database. Br J Clin Pharmacol. 2009;67(2):242–249. doi: 10.1111/j.1365-2125.2008.03347.x. PMID: 19260863. DOI: 10.1111/j.1365-2125.2008. 03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao WL, Chen CC, Chang CT, Wu JY, Chen CH, Huang YC, Tsai CH, Tsai FJ. Gene polymorphisms of adiponectin and leptin receptor are associated with early onset of type 2 diabetes mellitus in the Taiwanese population. Int J Obes. 2012;36(6):790–796. doi: 10.1038/ijo.2011.174. PMID: 21931325. DOI: 10.1038/ijo.2011.174. [DOI] [PubMed] [Google Scholar]
- 8.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99(3):1193–1204. doi: 10.1152/japplphysiol.00160.2005. PMID: 16103522. DOI: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 9.Sifnaios E, Mastorakos G, Psarra K, Panagopoulos ND, Panoulis K, Vitoratos N, Rizos D, Creatsas G. Gestational diabetes and T-cell (Th1/Th2/Th17/Treg) immune profile. In Vivo. 2019;33(1):31–40. doi: 10.21873/invivo.11435. PMID: 30587599. DOI: 10.21873/invivo.11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy MI, Zeggini E. Genome-wide association studies in type 2 diabetes. Curr Diab Rep. 2009;9(2):164–171. doi: 10.1007/s11892-009-0027-4. PMID: 19323962. DOI: 10.1007/s11892-009-0027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lango H, Palmer CN, Morris AD, Zeggini E, Hattersley AT, McCarthy MI, Frayling TM, Weedon MN. Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes. 2008;57(11):3129–3135. doi: 10.2337/db08-0504. PMID: 18591388. DOI: 10.2337/db08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai FJ, Yang CF, Chen CC, Chuang LM, Lu CH, Chang CT, Wang TY, Chen RH, Shiu CF, Liu YM, Chang CC, Chen P, Chen CH, Fann CS, Chen YT, Wu JY. A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLoS Genet. 2010;6(2):e1000847–e1000847. doi: 10.1371/journal.pgen.1000847. PMID: 20174558. DOI: 10.1371/journal.pgen.1000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao WL, Tsai FJ. Personalized medicine in type 2 diabetes. Biomedicine. 2014;4:8–8. doi: 10.7603/s40681-014-0008-z. PMID: 25520921. DOI: 10.7603/ s40681-014-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Q, Song Y, Wang X, Won S, Cui Y, Elston RC. The effect of multiple genetic variants in predicting the risk of type 2 diabetes. BMC Proc. 2009;3(Suppl 7):S49–S49. doi: 10.1186/1753-6561-3-s7-s49. PMID: 20018041. DOI: 10.1186/1753-6561-3-s7-s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang K, Wang Y, Zheng T, Jia W, Li J, Chen L, Shen K, Wu S, Lin X, Zhang G, Wang C, Wang S, Lu H, Fang Q, Shi Y, Zhang R, Xu J, Weng Q. Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the chinese: Significant linkage to chromosome 6q21-q23 and chromosome 1q21-q24. Diabetes. 2004;53(1):228–234. doi: 10.2337/diabetes.53.1.228. PMID: 14693720. DOI: 10.2337/diabetes.53.1.228. [DOI] [PubMed] [Google Scholar]
- 16.Ng MC, So WY, Cox NJ, Lam VK, Cockram CS, Critchley JA, Bell GI, Chan JC. Genome-wide scan for type 2 diabetes loci in Hong Kong Chinese and confirmation of a susceptibility locus on chromosome 1q21-q25. Diabetes. 2004;53(6):1609–1613. doi: 10.2337/diabetes.53.6.1609. PMID: 15161769. DOI: 10.2337/diabetes.53.6.1609. [DOI] [PubMed] [Google Scholar]
- 17.Arora P, Garcia-Bailo B, Dastani Z, Brenner D, Villegas A, Malik S, Spector TD, Richards B, El-Sohemy A, Karmali M, Badawi A. Genetic polymorphisms of innate immunity-related inflammatory pathways and their association with factors related to type 2 diabetes. BMC Med Genet. 2011;12:95–95. doi: 10.1186/1471-2350-12-95. PMID: 21756351. DOI: 10.1186/1471-2350-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfutzner A, Standl E, Strotmann HJ, Schulze J, Hohberg C, Lubben G, Pahler S, Schondorf T, Forst T. Association of high-sensitive C-reactive protein with advanced stage beta-cell dysfunction and insulin resistance in patients with type 2 diabetes mellitus. Clin Chem Lab Med. 2006;44(5):556–560. doi: 10.1515/CCLM.2006.108. PMID: 16681424. DOI: 10.1515/cclm.2006.108. [DOI] [PubMed] [Google Scholar]
- 19.Chou HH, Hsu LA, Liu CJ, Teng MS, Wu S, Ko YL. Insulin resistance is associated with C-reactive protein independent of abdominal obesity in nondiabetic Taiwanese. Metabolism. 2010;59(6):824–830. doi: 10.1016/j.metabol.2009.09.030. PMID: 20004425. DOI: 10.1016/j.metabol. 2009.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Pfutzner A, Schondorf T, Hanefeld M, Forst T. High-sensitivity c-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: Effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol. 2010;4(3):706–716. doi: 10.1177/193229681000400326. PMID: 20513338. DOI: 10.1177/193229 68100040 0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anan F, Takahashi N, Nakagawa M, Ooie T, Saikawa T, Yoshimatsu H. High-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Metabolism. 2005;54(4):552–558. doi: 10.1016/j.metabol.2004.11.012. PMID: 15798966. DOI: 10.1016/j.metabol.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Mugabo Y, Li L, Renier G. The connection between C-reactive protein (CRP) and diabetic vasculopathy. Focus on preclinical findings. Curr Diabetes Rev. 2010;6(1):27–34. doi: 10.2174/157339910790442628. PMID: 20034371. DOI: 10.2174/157339910790442628. [DOI] [PubMed] [Google Scholar]
- 23.Wolford JK, Gruber JD, Ossowski VM, Vozarova B, Antonio Tataranni P, Bogardus C, Hanson RL. A C-reactive protein promoter polymorphism is associated with type 2 diabetes mellitus in Pima Indians. Mol Genet Metab. 2003;78(2):136–144. doi: 10.1016/s1096-7192(02)00230-5. PMID: 12618085. DOI: 10.1016/S1096-7192(02)00230-5. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. PMID: 23264425. DOI: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, Karmali M. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–186. doi: 10.2147/dmsott.s9089. PMID: 21437087. DOI: 10.2147/DMSO.S9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng MS, Hsu LA, Wu S, Chang HH, Chou HH, Ko YL. Association between c-reactive protein gene haplotypes and C-reactive protein levels in Taiwanese: Interaction with obesity. Atherosclerosis. 2009;204(2):e64–e69. doi: 10.1016/j.atherosclerosis.2008.10.034. PMID: 19101671. DOI: 10.1016/j.atherosclerosis.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Dai DF, Chiang FT, Lin JL, Huang LY, Chen CL, Chang CJ, Lai LP, Hsu KL, Tseng CD, Tseng YZ, Hwang JJ. Human C-reactive protein (CRP) gene 1059g>c polymorphism is associated with plasma CRP concentration in patients receiving coronary angiography. J Formos Med Assoc. 2007;106(5):347–354. doi: 10.1016/S0929-6646(09)60319-3. PMID: 17561469. DOI: 10.1016/s0929-6646(09)60319-3. [DOI] [PubMed] [Google Scholar]
- 28.Thalmaier D, Dambacher J, Seiderer J, Konrad A, Schachinger V, Pfennig S, Otte JM, Crispin A, Goke B, Ochsenkuhn T, Lohse P, Brand S. The +1059G/C polymorphism in the C-reactive protein (CRP) gene is associated with involvement of the terminal ileum and decreased serum crp levels in patients with crohn’s disease. Aliment Pharmacol Ther. 2006;24(7):1105–1115. doi: 10.1111/j.1365-2036.2006.03093.x. PMID: 16984505. DOI: 10.1111/j.1365-2036.2006. 03093.x. [DOI] [PubMed] [Google Scholar]
- 29.Sheu WH, Chen YD, Yu CY, Guo X, Lee TC, Lee WJ, Chen YT, Rotter JI. C-reactive protein gene polymorphism 1009A>G is associated with serum CRP levels in Chinese men: A TCVGHAGE study. Clin Chim Acta. 2007;382(1-2):117–123. doi: 10.1016/j.cca.2007.04.013. PMID: 17511977. DOI: 10.1016/j.cca.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359(18):1897–1908. doi: 10.1056/NEJMoa0707402. PMID: 18971492. DOI: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 31.Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney JF Jr., Wilson PW, Newton-Cheh C, Musone SL, Camargo AL, Drake JA, Levy D, O’Donnell CJ, Hirschhorn JN, Benjamin EJ. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum c-reactive protein level. Circulation. 2006;113(11):1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. PMID: 16534007. DOI: 10.1161/circulationaha.105.591271. [DOI] [PubMed] [Google Scholar]
- 32.Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, Shah T, Humphries SE, Hingorani AD, Marmot MG, Timpson NJ, Kumari M. Inflammation, insulin resistance, and diabetes--mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5(8):e155–e155. doi: 10.1371/journal.pmed.0050155. PMID: 18700811. DOI: 10.1371/ journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CC, Chung CM, Leu HB, Lin TH, Hung SI, Wu TC, Huang PH, Lin SJ, Pan WH, Chen JW. Genetic variation in C-reactive protein in ethnic Chinese population in Taiwan. Eur J Clin Invest. 2013;43(5):449–456. doi: 10.1111/eci.12067. PMID: 23496329. DOI: 10.1111/eci.12067. [DOI] [PubMed] [Google Scholar]
- 34.Ottaviani S, Gorrini M, Scabini R, Kadija Z, Paracchini E, Mariani F, Ferrarotti I, Luisetti M. C-Reactive protein and alpha1-antitrypsin: Relationship between levels and gene variants. Transl Res. 2011;157(6):332–338. doi: 10.1016/j.trsl.2010.12.014. PMID: 21575917. DOI: 10.1016/j.trsl.2010.12.014. [DOI] [PubMed] [Google Scholar]