Abstract
Background/Aim: As approximately 10% of individuals developing chronic myeloid leukemia (CML) are females aged 20-44 years, a considerable number will consider a planned pregnancy if disease is well controlled by pharmacological treatment. The management of these young patients during pregnancy represents a therapeutic dilemma due to the potential teratogen effects of several tyrosine kinase inhibitors (TKIs) and is a matter of continuous debate. Indeed, despite the existence of several studies, there is currently no consensus on how to manage different pregnancy situations in subjects with CML. Patients and Methods: We describe a female patient diagnosed with Ph-positive CML one month after her first delivery who achieved excellent hematological, cytogenetic and molecular responses while on imatinib mesylate (IM) treatment. Results: The excellent responses allowed the patient to suspend TKI treatment in order to plan a second pregnancy. Despite IM discontinuation, stringent molecular monitoring of her BCR-ABL1/ABL1 levels allowed the safe delivery of the child and, while the patient eventually developed a molecular relapse after four years of treatment discontinuation, upon restarting IM she quickly regained a deep molecular response that is still ongoing. Conclusion: Our case report demonstrates that, if the pregnancy is properly planned in CML patients, it can result in excellent management of the clinical therapeutic option for the benefit of both mother and child.
Keywords: Pregnancy, chronic myeloid leukemia, BCR-ABL1, imatinib mesylate
The Philadelphia (Ph) chromosome, generated by the reciprocal translocation of the BCR gene at 22q11 and the ABL1 gene at 9q34, is the cytogenetic culprit of chronic myeloid leukemia (CML) (1-3). At the molecular level, the Ph chromosome generates the BCR-ABL1 chimeric oncogene encoding for a protein with constitutive tyrosine kinase activity that alters the proliferation rate, survival signaling, immunological interactions and cytoskeleton dynamics of the hematopoietic stem cells (4-8). The development of BCR-ABL1 tyrosine kinase inhibitors (TKIs) over the past 20 years has significantly improved the outcomes for patients at every stage of Ph+ chromosome CML. Despite these achievements, the emergence of BCR-ABL1 TKI resistant clones represents a major hurdle for the successful treatment of Ph+ leukemias, requiring often alternative therapeutic approaches (9-13). To data the standard care for chronic-phase CML patients is imatinib mesylate (IM), a semi-specific BCR-ABL1 TKI. The introduction of IM in clinical practice has dramatically generated unprecedented rates of complete hematological (CHR), cytogenetic (CCyR) and molecular responses (MR) (14-18).
CML accounts for approximately 15% of all adult leukemias with an incidence of about 1 case per 100,000 individuals. Although the median age at diagnosis is 56 years, approximately 17% of cases occur in the range between 20 and 44 years. Therefore, young female CML patients are likely to consider the possibility of giving birth to one or more children during their lifetime (19,20). Nevertheless, to date there is still no consensus on how to properly manage pregnancy in female patients with CML. In general, TKI treatment is not recommended during pregnancy due to the teratogen effect of these drugs (21,22).
In the present report, we describe a patient diagnosed with chronic-phase Ph-positive CML one month after her first delivery who exhibited an optimal response to standard dose IM according to the 2013 European LeukemiaNet recommendations (23). Her excellent molecular response allowed IM discontinuation in order to plan a second pregnancy.
Case Report
In January 2006, a 30-year-old female was referred to our hospital one month after her first delivery with abnormal blood cell counts. At the time, her hemoglobin (Hgb) was 13.2 g/dl with 29.500 white blood cells (WBC) (64% neutrophils, 16% lymphocytes, 4% eosinophils, 3% basophils, 1% monocytes, 6% promyelocytes, 1% metamyelocytes, 4% myelocytes and 1% myeloblasts) and 797.000 platelets (Plt). The spleen was palpable 2 cm below the left costal margin while liver size was normal.
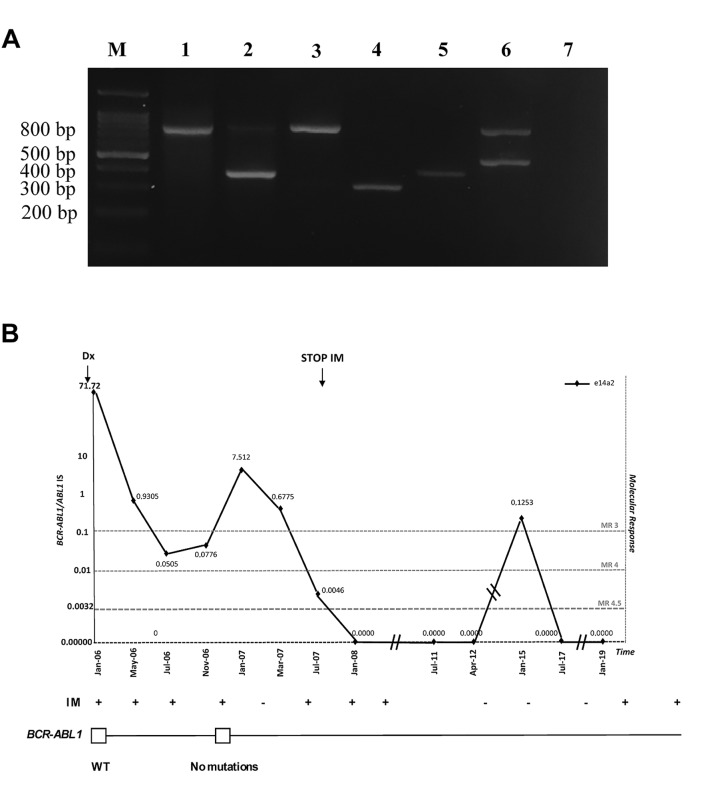
Conventional cytogenetics detected the Ph chromosome in all examined metaphases [karyotype 46, XX, t(9;22)(q34;q11)] and a FISH analysis showed the presence of BCR-ABL1 in 95% of interphase nuclei. Both conventional cytogenetics and FISH showed chromosome 9 deletion in 30% of the Ph-negative chromosomes examined. Multiplex reverse transcriptase (RT)-PCR detected the e14a2 BCR-ABL1 transcript (Figure 1A) with an e1a2 BCR-ABL1 variant barely noticeable by nested RT-PCR. At this time, amplification of the oncogenic transcripts was carried out by Real Time quantitative PCR (Q-PCR) and detected BCR-ABL1/ABL1 levels of 71.72% (Figure 1B).
Figure 1. A. Multiplex RT-PCR analysis of the different BCR-ABL1 fusion transcripts. Lane M: Molecular size marker (100-bp ladder); lane 1: patient negative for CML; lane 2: e14a2 (385 bp) from patient; lane 3: patient negative for CML; lane 4: e13a2 (310 pb) positive control; lane 5: e14a2 positive control; lane 6: e1a2 positive control; lane 7: negative control. B. Molecular response to IM. Monitoring of patient’s disease evolution indicates variations in e14a2 transcripts and drug treatment (top panel) or BCR-ABL1 mutant clones (middle panel). Dotted lines represent achievement of a major (MR3) or a deep molecular response (MR4; MR4.5). A white square indicates wild-type BCR-ABL1. IM: Imatinib.
Based on these clinical findings the patient was diagnosed with chronic phase CML and was classified as a low Sokal score (24) and low Hasford score (25). Soon thereafter, she began IM 400 mg/day achieving complete hematological and cytogenetic remissions within 3 months and a major molecular response (MR3) after 6 months of IM (BCR-ABL1/ABL1IS=0.05050; Figure 1B). Molecular follow up for the e14a2 transcript was continued every 3 months with Q-PCR as previously described (26), while nested RT-PCR was employed for the e1a2 variant with failure to amplify this variant after 6 months of therapy. However, in November 2006 the patient had to suspend IM because of grade II liver toxicity (Aspartate Aminotransferase< 200 mU/ml and Alanine Aminotransferase<300 mU/ml). Although, the patient maintained both her CHR and CCyR, we observed an increase of her p210 BCR-ABL1/ABL1IS levels (7.512%) (Figure 1B) and the nested RT-PCR successfully not amplified the e1a2 variant. Mutational analysis failed to detect any kinase domain mutations in their Ph-positive clones. Hence, in January 2007 she restarted IM 400 mg/day and after 6 months she achieved a deep molecular response MR4 (BCR-ABL1/ABL1IS=0.00465%) (Figure 1B).
Over the next 4 years, the patient exhibited deep molecular responses varying between MR4 and MR4.5. In July 2011, she approached us wishing to plan a second pregnancy. After careful consideration, we agreed to discontinue IM as long as she would adhere to a strict monthly molecular follow-up as we feared a rapid rise in her oncogenic transcripts after TKI cessation. However, she maintained molecular responses between MR4 and MR4.5 even in the absence of IM (Figure 1B) and, at 38 weeks (April 2012), delivered via cesarean section a healthy baby girl (weight 2,92 kg; height 48 cm; APGAR 9). As her MR4 was stable after giving birth to her second child, she decided to prolong IM discontinuation and remained TKI-free until January 2015 when she lost her major molecular response (BCR-ABL1/ABL1IS=0.12534%; Figure 1B). At this time, she restarted IM and, after 6 months, again attained an MR4 that is ongoing at the present time.
Total RNA was extracted from peripheral blood or bone marrow leucocytes, using the RNeasy mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. cDNA synthesis was carried out using Moloney Murine Leukemia Virus Reserve Transcriptase (Life Technologies, Carlsbad, CA, USA). Cytogenetic analysis performed by G-banding (27) and FISH analysis carried out as previously reported (28). The detection of different BCR-ABL1 fusion transcripts was performed using multiplex reverse transcriptase protocol able to identify contemporary multiplex BCR-ABL1 isoforms (29,30).
The e14a2 BCR-ABL1 fusion transcripts were quantified by Q-PCR according to the suggested recommendations (31) and molecular analysis was performed as previously reported (32). Mutation analysis of the ABL kinase domain by clonal sequencing was performed as previously described (33).
Informed Consent
Informed consent was received from the patient for the publication of the report as specified in the Declaration of Helsinki.
Discussion and Conclusion
Pregnancy management in female patients diagnosed with chronic phase CML is a matter of debate and represents a therapeutic dilemma due to the potential teratogen effects of different TKIs. Indeed, several studies have highlighted the risk of embryo-fetal toxicity caused by some ABL1 kinase inhibitors resulting in skeletal malformations, spontaneous abortion, and fetal growth restriction (34,35).
At the present time, there are no consensus guidelines regarding the management of a pregnancy in CML patients. Experts in the field are only suggest that female subjects still in their reproductive age should be informed about the risks of an unintended pregnancy during TKI therapy and be encouraged to carefully discuss family planning with their physician. A pregnancy should be considered after achieving durable and complete cytogenetic responses followed by equally enduring major molecular responses for at least 18-24 months (20,34). Under these circumstances, TKI therapy should be discontinued shortly before ovulation and BCR-ABL1/ABL1 levels should be monitored on a monthly basis (34,35). In case of cytogenetic or hematological relapses occurring during pregnancy, each physician will have to evaluate patient clinical history, rapidity of disease relapse and pregnancy status (period of gestation) in order to advise the mother as to the most appropriate way to proceed.
In this study, we report the case of a female patient diagnosed with CML that rapidly achieved hematologic, cytogenetic, and molecular responses on IM treatment and therefore wanted to consider a second pregnancy. Several reports have shown that women with CML can successfully deliver healthy babies with careful planning and strict disease monitoring.
In our case, the decision to discontinue TKI therapy was further supported by recent findings indicating that patients who achieve and maintain a deep molecular response (≥MR4) may be considered for TKI discontinuation as they could remain in treatment-free remission (TFR) even after drug cessation. Indeed, TFR is an attractive possibility for all CML patients as it often provides significant relief from TKI toxicities and general improvements in quality-of-life (36,37). TFR is now considered for many young patients affected by CML and also an end point for some clinical trials. We think that the optimal management of pregnancy in CML is another good reason for pursuing clinical studies aimed to find the best way to manage TFR.
In the last eighteen years, TKIs have radically transformed the management of CML that usually becomes a lifelong chronic condition. However, the management of a planned pregnancy in CML patients requires a thorough evaluation of both risks and benefits that should be carefully discussed between the patient and her physician. To avoid potential teratogenicity to the fetus, it is necessary to find a balance between the mother’s childbearing desire and the optimal pharmacological treatment required by the disease. Our case report demonstrated that, if the pregnancy is properly planned, it can result in excellent outcomes for both the mother and child.
Conflicts of Interest
The Authors declare that they have no competing interests regarding this study.
Authors’ Contributions
SS, ET and MM designed and performed the experiments; SS, ET, MM, SRV, MSP, AP CR and SDR analyzed and interpreted the data; SS wrote the paper; SR, FS, FDR, CM and LM made a critical revision of paper; LM conceived the original idea and supervised the project.
References
- 1.Radujkovic A, Topaly J, Fruehauf S, Zeller WJ. Combination treatment of imatinib-sensitive and -resistant bcr-abl-positive cml cells with imatinib and farnesyltransferase inhibitors. Anticancer Res. 2006;26(3A):2169–2177. PMID: 16827161. [PubMed] [Google Scholar]
- 2.Stagno F, Vigneri P, Del Fabro V, Stella S, Cupri A, Massimino M, Consoli C, Tambe L, Consoli ML, Antolino A, Di Raimondo F. Influence of complex variant chromosomal translocations in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Acta Oncol. 2010;49(4):506–508. doi: 10.3109/02841861003660031. PMID: 20331405. 10.3109/02841861003660031. [DOI] [PubMed] [Google Scholar]
- 3.Massimino M, Consoli ML, Mesuraca M, Stagno F, Tirro E, Stella S, Pennisi MS, Romano C, Buffa P, Bond HM, Morrone G, Sciacca L, Di Raimondo F, Manzella L, Vigneri P. Irf5 is a target of bcr-abl kinase activity and reduces cml cell proliferation. Carcinogenesis. 2014;35(5):1132–1143. doi: 10.1093/carcin/bgu013. PMID: 24445143. DOI: 10.1093/carcin/bgu013. [DOI] [PubMed] [Google Scholar]
- 4.Ren R. Mechanisms of bcr-abl in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5(3):172–183. doi: 10.1038/nrc1567. PMID: 15719031. DOI: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 5.Stella S, Tirro E, Conte E, Stagno F, Di Raimondo F, Manzella L, Vigneri P. Suppression of survivin induced by a bcr-abl/jak2/stat3 pathway sensitizes imatinib-resistant cml cells to different cytotoxic drugs. Mol Cancer Ther. 2013;12(6):1085–1098. doi: 10.1158/1535-7163.MCT-12-0550. PMID: 23536723. DOI: 10.1158/1535-7163.MCT-12-0550. [DOI] [PubMed] [Google Scholar]
- 6.Giallongo C, Tibullo D, La Cava P, Branca A, Parrinello N, Spina P, Stagno F, Conticello C, Chiarenza A, Vigneri P, Palumbo GA, Di Raimondo F. Brit1/mcph1 expression in chronic myeloid leukemia and its regulation of the g2/m checkpoint. Acta Haematol. 2011;126(4):205–210. doi: 10.1159/000329911. PMID: 21934293. DOI: 10.1159/000329911. [DOI] [PubMed] [Google Scholar]
- 7.Preyer M, Vigneri P, Wang JY. Interplay between kinase domain autophosphorylation and f-actin binding domain in regulating imatinib sensitivity and nuclear import of bcr-abl. PLoS One. 2011;6(2):e17020–e17020. doi: 10.1371/journal.pone.0017020. PMID: 21347248. DOI: 10.1371/ journal.pone.0017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzella L, Tirro E, Pennisi MS, Massimino M, Stella S, Romano C, Vitale SR, Vigneri P. Roles of interferon regulatory factors in chronic myeloid leukemia. Curr Cancer Drug Targets. 2016;16(7):594–605. doi: 10.2174/1568009616666160105105857. PMID: 26728039. [DOI] [PubMed] [Google Scholar]
- 9.Rosti G, Castagnetti F, Gugliotta G, Baccarani M. Tyrosine kinase inhibitors in chronic myeloid leukaemia: Which, when, for whom. Nat Rev Clin Oncol. 2017;14(3):141–154. doi: 10.1038/nrclinonc.2016.139. PMID: 27752053. DOI: 10.1038/nrclinonc.2016.139. [DOI] [PubMed] [Google Scholar]
- 10.Buffa P, Romano C, Pandini A, Massimino M, Tirro E, Di Raimondo F, Manzella L, Fraternali F, Vigneri PG. Bcr-abl residues interacting with ponatinib are critical to preserve the tumorigenic potential of the oncoprotein. FASEB J. 2014;28(3):1221–1236. doi: 10.1096/fj.13-236992. PMID: 24297701. DOI: 10.1096/fj.13-236992. [DOI] [PubMed] [Google Scholar]
- 11.Stagno F, Vigneri P, Del Fabro V, Stella S, Restuccia N, Giallongo C, Massimino M, Berretta S, Pennisi MS, Tibullo D, Tirro E, Buscarino C, Messina A, Di Raimondo F. Concomitant and feasible treatment with dasatinib and the anti-egfr antibody cetuximab plus radiotherapy in a CML patient with multiple squamous neoplasias. Acta Oncol. 2010;49(1):109–110. doi: 10.3109/02841860903302913. PMID: 19842797. DOI: 10.3109/02841860903302913. [DOI] [PubMed] [Google Scholar]
- 12.Stagno F, Vigneri P, Del Fabro V, Stella S, Massimino M, Berretta S, Cupri A, Consoli C, Messina L, Tirro E, Messina A, Di Raimondo F. Successful nilotinib therapy in an imatinib-resistant chronic myeloid leukemia patient displaying an intron-derived insertion/truncation mutation in the bcr-abl kinase domain. Leuk Res. 2009;33(9):e157–e158. doi: 10.1016/j.leukres.2009.03.040. PMID: 19406471. DOI: 10.1016/j.leukres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Massimino M, Stella S, Tirro E, Romano C, Pennisi MS, Puma A, Manzella L, Zanghi A, Stagno F, Di Raimondo F, Vigneri P. Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia. Mol Cancer. 2018;17(1):56–56. doi: 10.1186/s12943-018-0805-1. PMID: 29455672. DOI: 10.1186/s12943-018-0805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O’Brien SG, Druker BJ, Investigators I. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–927. doi: 10.1056/NEJMoa1609324. PMID: 28273028. DOI: 10.1056/NEJM oa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagno F, Stella S, Spitaleri A, Pennisi MS, Di Raimondo F, Vigneri P. Imatinib mesylate in chronic myeloid leukemia: Frontline treatment and long-term outcomes. Expert Rev Anticancer Ther. 2016;16(3):273–278. doi: 10.1586/14737140.2016.1151356. PMID: 26852913. DOI: 10.1586/14737140.2016.1151356. [DOI] [PubMed] [Google Scholar]
- 16.Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, Clark RE, Apperley JF, Milojkovic D, Bua M, Pavlu J, Paliompeis C, Reid A, Rezvani K, Goldman JM, Foroni L. Assessment of bcr-abl1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30(3):232–238. doi: 10.1200/JCO.2011.38.6565. PMID: 22067393. DOI: 10.1200/ JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stella S, Zammit V, Vitale SR, Pennisi MS, Massimino M, Tirro E, Forte S, Spitaleri A, Antolino A, Siracusa S, Accurso V, Mannina D, Impera S, Musolino C, Russo S, Malato A, Mineo G, Musso M, Porretto F, Martino B, Di Raimondo F, Manzella L, Vigneri P, Stagno F. Clinical implications of discordant early molecular responses in cml patients treated with imatinib. Int J Mol Sci. 2019;20(9) doi: 10.3390/ijms20092226. PMID: 31064152. DOI: 10.3390/ijms20092226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stagno F, Vigneri P, Stagno F, Vigneri P, Del Fabro V, Stella S, Berretta S, Massimino M, Tirro E, Messina A, Di Raimondo F. Uncommon long-term survival in a patient with chronic myeloid leukemia. Acta Oncol. 2009;48(8):1215–1216. doi: 10.3109/02841860903156475. PMID: 19863235. DOI: 10.3109/02841860903156475. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari A, Rolen K, Shah BK. Management of chronic myelogenous leukemia in pregnancy. Anticancer Res. 2015;35(1):1–11. PMID: 25550528. [PubMed] [Google Scholar]
- 20.Apperley J. Issues of imatinib and pregnancy outcome. J Natl Compr Canc Netw. 2009;7(10):1050–1058. doi: 10.6004/jnccn.2009.0069. PMID: 19930974. [DOI] [PubMed] [Google Scholar]
- 21.Pye SM, Cortes J, Ault P, Hatfield A, Kantarjian H, Pilot R, Rosti G, Apperley JF. The effects of imatinib on pregnancy outcome. Blood. 2008;111(12):5505–5508. doi: 10.1182/blood-2007-10-114900. PMID: 18322153. DOI: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alizadeh H, Jaafar H, Rajnics P, Khan MI, Kajtar B. Outcome of pregnancy in chronic myeloid leukaemia patients treated with tyrosine kinase inhibitors: Short report from a single centre. Leuk Res. 2015;39(1):47–51. doi: 10.1016/j.leukres.2014.10.002. PMID: 25455655. DOI: 10.1016/j.leukres.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, Hjorth-Hansen H, Hughes TP, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Martinelli G, Mayer J, Muller MC, Niederwieser D, Pane F, Radich JP, Rousselot P, Saglio G, Saussele S, Schiffer C, Silver R, Simonsson B, Steegmann JL, Goldman JM, Hehlmann R. European leukemianet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. PMID: 23803709. DOI: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, Tso CY, Braun TJ, Clarkson BD, Cervantes F. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984;63(4):789–799. PMID: 6584184. [PubMed] [Google Scholar]
- 25.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, Alimena G, Steegmann JL, Ansari H. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing committee for the collaborative cml prognostic factors project group. J Natl Cancer Inst. 1998;90(11):850–858. doi: 10.1093/jnci/90.11.850. PMID: 9625174. DOI: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 26.Vigneri P, Stagno F, Stella S, Cupri A, Forte S, Massimino M, Antolino A, Siragusa S, Mannina D, Impera SS, Musolino C, Malato A, Mineo G, Tomaselli C, Murgano P, Musso M, Morabito F, Molica S, Martino B, Manzella L, Muller MC, Hochhaus A, Raimondo FD. High bcr-abl/gus(is) levels at diagnosis of chronic phase CML are associated with unfavorable responses to standard-dose imatinib. Clin Cancer Res. 2017;23(23):7189–7198. doi: 10.1158/1078-0432.CCR-17-0962. PMID: 28928163. DOI: 10.1158/1078-0432.CCR-17-0962. [DOI] [PubMed] [Google Scholar]
- 27.Stagno F, Vigneri P, Consoli ML, Cupri A, Stella S, Tambe L, Massimino M, Manzella L, Di Raimondo F. Hyperdiploidy associated with a high bcr-abl transcript level may identify patients at risk of progression in chronic myeloid leukemia. Acta Haematol. 2012;127(1):7–9. doi: 10.1159/000330607. PMID: 21986290. DOI: 10.1159/ 000330607. [DOI] [PubMed] [Google Scholar]
- 28.Douet-Guilbert N, Morel F, Le Charpentier T, Le Bris MJ, Herry A, Morice P, Bourquard P, Abgrall JF, Berthou C, De Braekeleer M. Interphase fish for follow-up of philadelphia chromosome-positive chronic myeloid leukemia treatment. Anticancer Res. 2004;24(4):2535–2539. PMID: 15330210. [PubMed] [Google Scholar]
- 29.Cross NC. Detection of bcr-abl in hematological malignancies by rt-pcr. Methods Mol Med. 1996;6:25–36. doi: 10.1385/0-89603-341-4:25. PMID: 21380694. DOI: 10.1385/0-89603-341-4:25. [DOI] [PubMed] [Google Scholar]
- 30.Stella S, Massimino M, Tirro E, Vitale SR, Scalise L, Leotta S, Pennisi MS, Puma A, Romano C, Stagno F, Sapienza G, Milone G, Manzella L. B-all relapses after autologous stem cell transplantation associated with a shift from e1a2 to e14a2 bcr-abl transcripts: A case report. Anticancer Res. 2019;39(1):431–435. doi: 10.21873/anticanres.13130. PMID: 30591491. DOI: 10.21873/anticanres.13130. [DOI] [PubMed] [Google Scholar]
- 31.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ, Gabert J, Grimwade D, Hehlmann R, Kamel-Reid S, Lipton JH, Longtine J, Martinelli G, Saglio G, Soverini S, Stock W, Goldman JM. Monitoring cml patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting bcr-abl transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. PMID: 16522812. DOI: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vella V, Puppin C, Damante G, Vigneri R, Sanfilippo M, Vigneri P, Tell G, Frasca F. Deltanp73alpha inhibits pten expression in thyroid cancer cells. Int J Cancer. 2009;124(11):2539–2548. doi: 10.1002/ijc.24221. PMID: 19173293. DOI: 10.1002/ijc.24221. [DOI] [PubMed] [Google Scholar]
- 33.Pirosa MC, Leotta S, Cupri A, Stella S, Martino EA, Scalise L, Sapienza G, Calafiore V, Mauro E, Spadaro A, Vigneri P, Di Raimondo F, Milone G. Long-term molecular remission achieved by antibody anti-cd22 and ponatinib in a patient affected by ph’+ acute lymphoblastic leukemia relapsed after second allogeneic hematopoietic stem cell transplantation: A case report. Chemotherapy. 2018;63(4):220–224. doi: 10.1159/000492941. PMID: 30372691. DOI: 10.1159/000492941. [DOI] [PubMed] [Google Scholar]
- 34.Abruzzese E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis. 2014;6(1):e2014028–e2014028. doi: 10.4084/MJHID.2014.028. PMID: 24804001. DOI: 10.4084/MJHID.2014.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Apostolova M, Woolley PV. A case of chronic myelogenous leukemia in pregnancy characterized by a complex translocation t(9;22;11)(q34;q11.2;q13) Hematol Rep. 2011;3(3):e27. doi: 10.4081/hr.2011.e27. PMID: 22593818. DOI: 10.4081/hr.2011.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in cml. Blood. 2016;128(1):17–23. doi: 10.1182/blood-2016-01-694265. PMID: 27013442. DOI: 10.1182/blood-2016-01-694265. [DOI] [PubMed] [Google Scholar]
- 37.Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, Janssen J, Mayer J, Koskenvesa P, Panayiotidis P, Olsson-Stromberg U, Martinez-Lopez J, Rousselot P, Vestergaard H, Ehrencrona H, Kairisto V, Machova Polakova K, Muller MC, Mustjoki S, Berger MG, Fabarius A, Hofmann WK, Hochhaus A, Pfirrmann M, Mahon FX, Investigators E-S Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (euro-ski): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–757. doi: 10.1016/S1470-2045(18)30192-X. PMID: 29735299. DOI: 10.1016/S1470-2045(18)30192-X. [DOI] [PubMed] [Google Scholar]