Abstract
Aim: We examined evidence on infective and non-infective endocarditis obtained from a database of 50,403 clinical autopsies performed at an Italian general hospital between January 1983 and December 2006. Materials and Methods: Out of 814 endocarditis cases, 409 were of infective endocarditis (IE) and 405 non-infective (NIE). The median age at the time of death was 78 years for those with IE and 83 for those with NIE. Data were collected on gender, clinical history, comorbidities, kind of affected valve (non-prosthetic/mechanical/biological), pathological features of endocarditis, endocarditis complications and microbiological agents. Results: The diagnosis of IE was frequently missed and these conditions were often complicated by cardiovascular events. IE was more common among patients with prior valve infection or cardiovascular surgery, while malignancies were frequent comorbidities of NIE. Conclusion: In general, we found several data that differ from those generally present in the scientific literature, and this could be explained by the fact that data on IE and NIE are generally obtained from surgical and clinical databases, while we analysed only autoptic cases.
Keywords: Infective endocarditis, non-infective endocarditis, nonbacterial thrombotic endocarditis, post mortem, autopsy, sudden death, forensic science
Endocarditis is a common clinical problem characterized by lesions known as vegetations, and is defined as inflammation of the endocardium that mainly, but not exclusively, affects the heart valve leaflets. Historically, endocarditis is classified as infective (IE) and non-infective (NIE) on the basis of whether or not it is caused by infections.
NIE can be caused by mechanical stress, chemical agents, immunological factors (Libman–Sacks, post-rheumatic disease, hypereosinophilic syndrome, or systemic lupus erythaematosus), or directly by turbulent blood flow that may mechanically damage the endocardium (non-bacterial thrombotic endocarditis). Moreover, it can be associated with hypercoagulable states or malignancies (nonbacterial thrombotic/marantic endocarditis).
From a clinical point of view, NIE and IE have similar signs and symptoms and it is difficult to differentiate between them. Furthermore, they are associated with a consistent risk of misdiagnosis: For example, it is not rare that the hypothesis of health-care associated IE is neglected among elderly patients with multiple comorbidities because of the low specificity of usual clinical presentation (1). The diagnosis can also be completely missed, and in these cases, cardiac and extra-cardiac complications of both IE and NIE can be responsible for unexpected sudden death (2-4).
These considerations help to explain why endocarditis is often diagnosed only at autopsy. However, a missed diagnosis of endocarditis is not always due to medical errors: For example, in several cases, the first clinical manifestation of NIE is a lethal stroke or severe systemic embolization, and thus the diagnosis is necessarily made only at autopsy (3).
In general, pathologists have a paramount role in this field; even when endocarditis is not the cause of death, it can provide crucial information and hints to the examiner. For example, the detection of undiagnosed NIE during an autopsy is a red flag that the possible presence of an advanced neoplasia should be carefully investigated (3). Another challenging aspect of endocarditis at autopsy is that, for example, a case of sudden death of a drug addict caused by IE can easily be misinterpreted as fatal substance intoxication if meticulous macroscopic and microscopic examination of endocardium and heart valves is not performed (4).
We, herein, present a post-mortem series of 409 cases of IE and 405 cases of NIE at the University of Trieste Hospital to address the similarities and differences between the two conditions. The epidemiology, pathophysiology, clinical presentation, diagnosis, treatment and pathological characterization are also discussed.
Materials and Methods
The data for this study were gathered from clinical autopsies performed at the Institute of Pathological Anatomy of the University of Trieste on adult patients who died in the general hospital of Trieste between January 1983 and December 2006.
All the autopsies were requested by the clinicians to ascertain the cause of the death when it was unknown or unclear.
Reviews of the pathology and clinical charts were performed for all cases, and data were abstracted for further analysis. When available, microbiology data were retrieved from the central laboratory repository to determine the aetiological agent.
The prevalence was measured as the number of cases with the diagnosis per the total number of autopsies performed, over the entire period or shorter periods. Out of 50,403 autopsies, 409 were IE and 405 were NIE.
IE and NIE cases were studied separately to determine their differences and similarities.
Data are presented as absolute numbers and percentages for discrete variables and as medians for continuous variables.
Results
Infective endocarditis
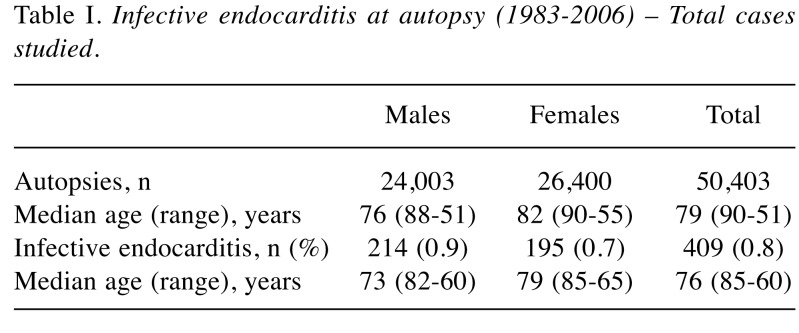
Epidemiological features. The prevalence of IE at post-mortem examination was 0.8%, with 409 cases diagnosed out of 50,403 autopsies performed (Table I). The overall incidence tended to increase over time.
Table I. Infective endocarditis at autopsy (1983-2006) – Total cases studied.
No significant difference in the gender distribution of IE was observed, although there was a slightly higher prevalence rate (+0.2%) in males.
The median age at the time of death was 76 years for IE cases compared to 79 years for all autopsied cases, with no significant differences between the groups. A significantly higher IE prevalence in younger patients (<59 years) was observed, possibly related to concomitant excess risks for immune-mediated disorders, hepatic cirrhosis, drug abuse/cardiac surgical interventions.
Prosthetic valve endocarditis occurred in 11.5% of cases (concerning biological valves in 6.8% of cases, mechanical valves in 2.9% and the Carpentier ring in 1.6%), without any gender-related differences.
In 46% of cases, patients had a surgical history, and 11.5% underwent valvular surgery.
In 40% of cases, patients over 70 years of age suffered from type 2 diabetes, 21.8% from chronic kidney disease, 16.1% from cirrhosis and 27.4% from neoplasms (23% of malignancies were adenocarcinomas).
A clinical diagnosis of IE was made in only 6.8% of cases.
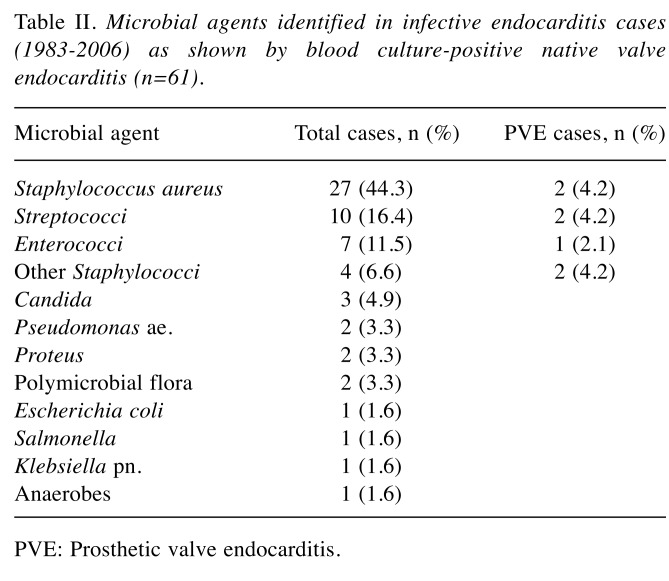
Microbiology. The results of blood cultures were available in 65 (16%) IE cases, which revealed a pathogen in 61 cases (94% of cases). In accordance with previous findings, the most frequently identified causative agent was Staphylococcus aureus (44%), followed by Streptococci spp (16%) and Enterococci spp (12%) (5). A similar frequency distribution of the three major pathogenic agents was found in prosthetic valve IE (Table II).
Table II. Microbial agents identified in infective endocarditis cases (1983-2006) as shown by blood culture-positive native valve endocarditis (n=61).
PVE: Prosthetic valve endocarditis.
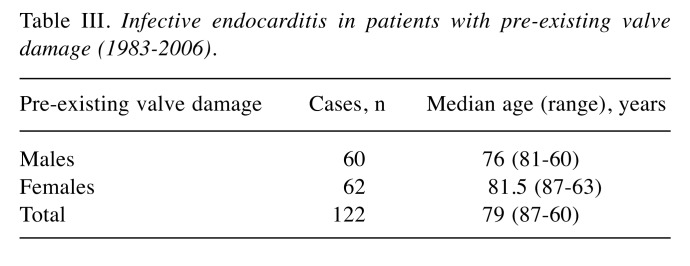
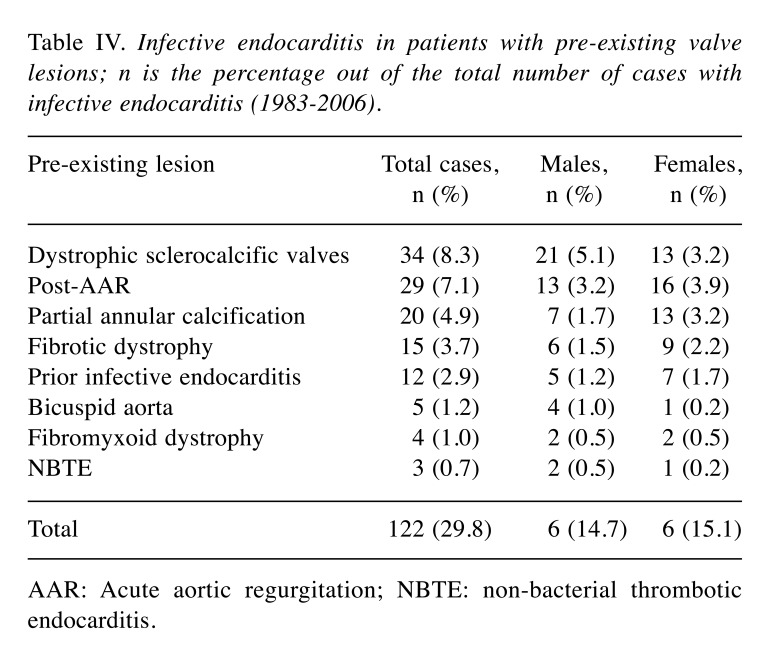
Pathogenesis. Pre-existing valve damage, the most commonly encountered predisposing factor, was found in 122 out of 409 cases (30%) with no substantial differences between genders (Table III). The most common valvular lesions found in males (5%) were cardiac valvular dystrophic sclerocalcifications, whereas post-rheumatic valve lesions were found most commonly in females (4%). This finding is consistent with the increased susceptibility of females to autoimmune diseases. Interestingly, IE occurred more frequently in patients who had prior valve infections (3% of IE) than in patients who had a previous history of NIE (0.7%), which suggests that the existence of background risk factors favours the relapse of the disease (Table IV).
Table III. Infective endocarditis in patients with pre-existing valve damage (1983-2006).
Table IV. Infective endocarditis in patients with pre-existing valve lesions; n is the percentage out of the total number of cases with infective endocarditis (1983-2006).
AAR: Acute aortic regurgitation; NBTE: non-bacterial thrombotic endocarditis.
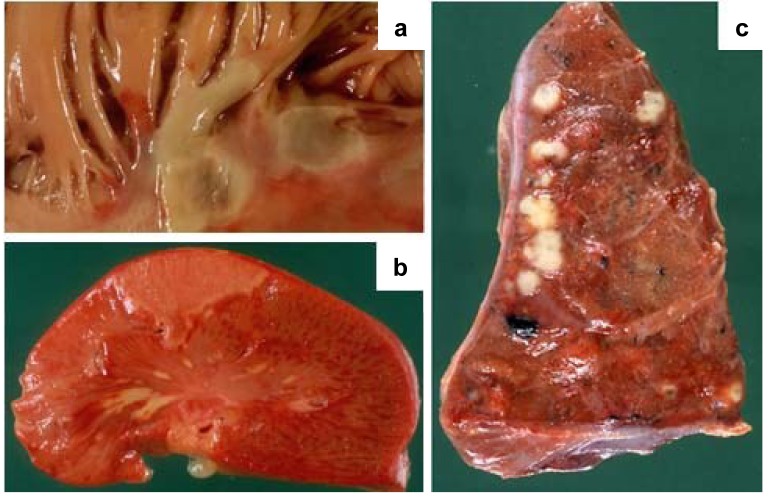
Pathology. In our cohort, cardiac complications were observed in 36% of cases, more commonly in females, whereas valvular and systemic complications occurred in 14% and 12% of cases, respectively, and were more frequent in males (Figures 1-3).
The main valvular complications were perforation (16.1%), ulceration (4.6%) and chordae tendineae rupture (2.5%).
The main cardiac and systemic complications were septic infarcts (10%), annular abscesses (8.8%) and embolic myocarditis (8.8%) and embolization of the brain (10%), spleen (7.3%) and kidney (2.3%), respectively.
Non-infective Endocarditis
Epidemiological features. Over a 6-year period (1983-2006), we diagnosed 405 NIE cases out of a total of 10,874 autopsies, representing 3.7% of cases (Table V). The prevalent trend of NIE at autopsy was rather constant over the period analysed. During the same time period, 124 IE cases were diagnosed, representing 1.1% of cases. The median age of patients with NIE was higher than that of patients with IE, 83 years versus 78 years, which was consistent with the higher prevalence of valvular lesions associated with older age.
Table V. Non-infectious endocarditis versus infective endocarditis at autopsy (1983-2006) – Total cases studied.
In cases over 70 years of age, 40% of the patients suffered from type 2 diabetes, 8% from chronic kidney disease, 5.4% from cirrhosis and 59% from neoplasms (62% of malignancies were gastric, pancreatic and colonic adenocarcinomas) (Table VI).
Table VI. Major diseases associated with thrombotic non-infective endocarditis (NIE) and infective endocarditis (1983-2006).
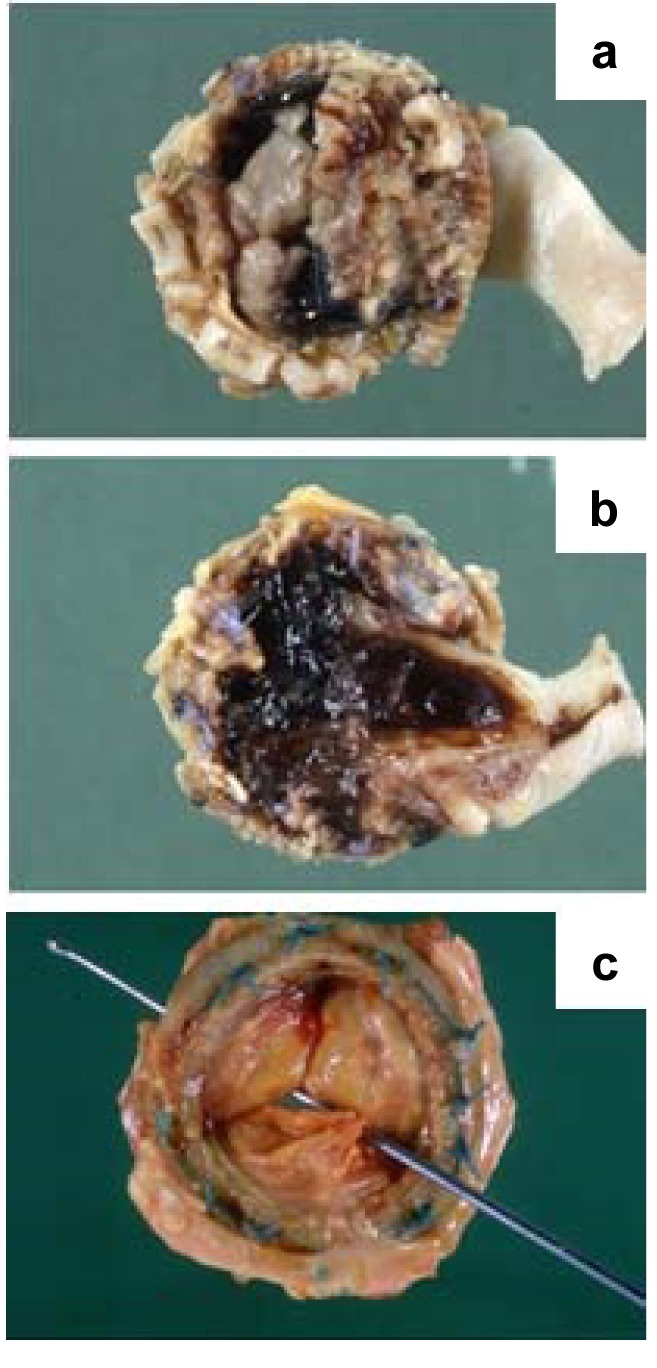
Pathogenesis. In our cohort, NIE developed on pre-existing valvular lesions more frequently than IE (35% vs. 13%). Post-rheumatic (10%), sclerocalcific (8%) and fibrous lesions (7%) along with valve ring calcification (4%) were commonly associated with NIE (Figure 4 and Figure 5; Table VII).
Figure 4. A: Mitral valve orifice reduced to a narrow slit in a case of chronic rheumatic heart disease. B: Numerous small vegetations on the line of closure of the mitral valve in a case of rheumatic endocarditis. C: Several small vegetations on the line of closure of the mitral valve causing fusion of the chordae in a case of rheumatic endocarditis. D: Rheumatic pericarditis: the epicardial surface is covered by a fibrinous inflammatory exudate (‘bread and butter’ pericarditis). E: Several small vegetations on the line of closure of the mitral valve causing fusion and thickening of the chordae in a case of rheumatic endocarditis. F: Valve leaflets appear thickened, fibrotic and shrunken, with fusion and deposition of calcium in a case of rheumatic endocarditis.
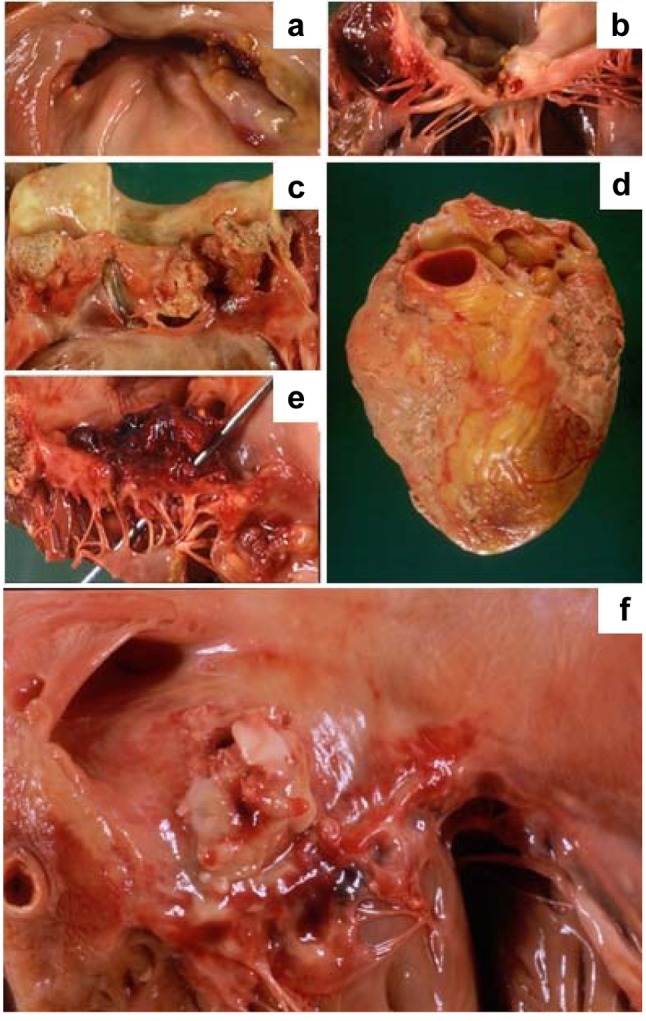
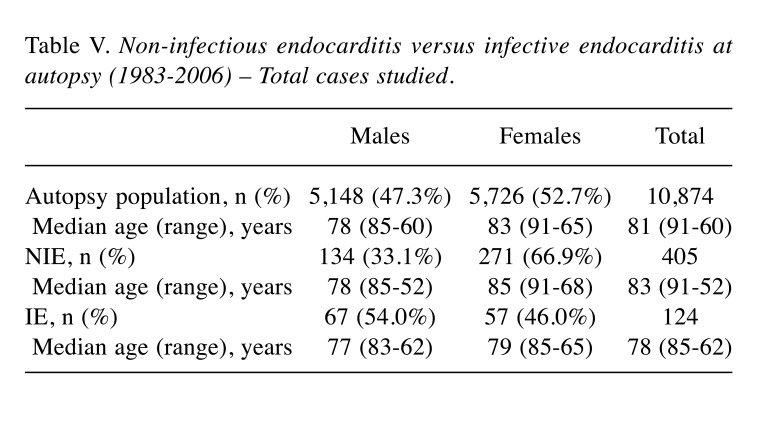
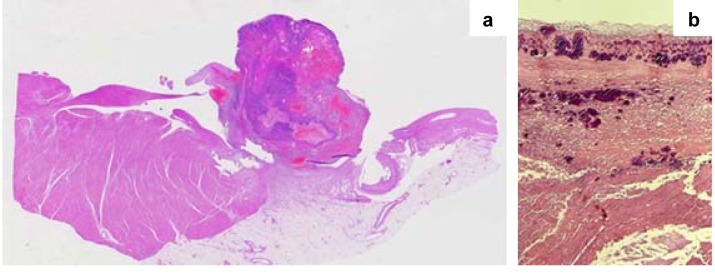
Figure 5. A: Thrombotic, non-bacterial endocarditis of the mitral valve in a case of marantic endocarditis. B: Multiple vegetation of the tricuspid valve in a case of NEI in a patient with a hypercoagulable state caused by a malignant neoplasm. C: Histological appearance of the valve vegetation presented in panel A; the thrombotic mass was only loosely attached to the underlying non-inflamed valve (original magnification, ×10).
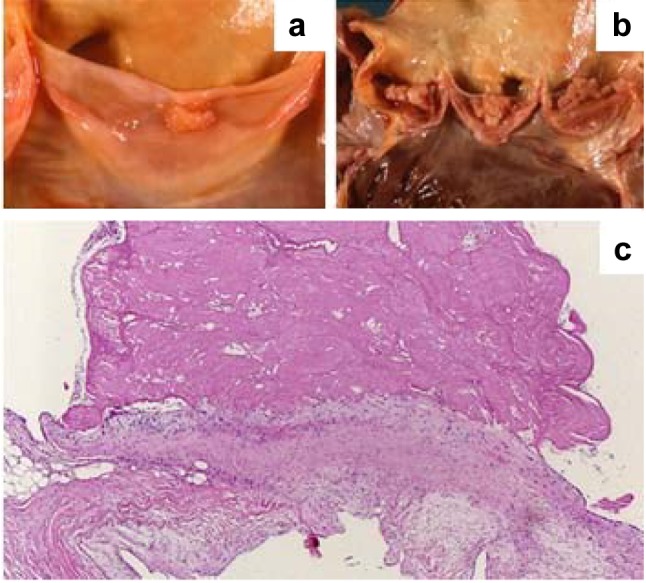
Table VII. Pre-existing valvular lesions in patients with non-infective endocarditis (NIE) versus infective endocarditis (IE); n is the percentage of cases out of the total number of cases with infective endocarditis (1983-2006).
AAR: Acute aortic regurgitation; NBTE: non-bacterial thrombotic endocarditis.
Malignant neoplasms were found in 59% of NIE cases and only 27% of IE cases. Thrombosis (deep vein thrombosis or intracardiac) was found in 38% of NIE cases and was consistent with the hypercoagulable state in the pathogenesis of thrombotic vegetations (Tables VI and VII).
Pathology. Valves were affected in the following order of frequency: Mitral valve 43%, aortic valve 36%, tricuspid valve 4%, pulmonic valve 1%, and aortic and mitral valve juncture 13%.
Discussion
Endocarditis involves the formation of vegetations on cardiac valves. Endocarditis occurs due to either direct infection of the valve structure generally associated with bacteraemia in a patient with a pre-existing valve injury (IE) or a chronic low-grade thrombotic event in a patient with acquired thrombophilia associated with cancer, sepsis or autoimmune conditions (NIE) (6-9). While it is highly difficult to distinguish IE from NIE at the macroscopic level, the two forms of endocarditis present with quite distinct clinical features, and are easily distinguished with microscopic pathology.
Clinically, a patient is much more likely to be diagnosed with IE than NIE. IE is characterized by recurrent fever and often causes the patient to seek medical attention. NIE is generally diagnosed either incidentally on imaging tests ordered for another reason or at post-mortem. Several post-mortem case series have documented the presence of NIE in patients with various clinical conditions, primarily cancer (10-14).
We present the largest post-mortem series including 409 cases of IE and 405 cases of NIE. The prevalence of NIE in this large cohort was 3.7%, which is in accordance with previous reports (15). No prior autopsy case series has compared the prevalence of NIE and IE in the same cohort, to the best of our knowledge. In our cohort, NIE was diagnosed three times more often than IE; moreover, although the majority of IE cases had been diagnosed clinically, none of the 405 NIE cases had been diagnosed pre-mortem. This finding suggests that while the incidence of IE is known to be between two and six new cases per 100,000 per year (10-14,16-18), the true clinical incidence/prevalence of NIE is unknown.
It is important to underline that we found some epidemiological data that highly differ from the majority of evidence in scientific literature: for example, we underlined that in our study population, gender did not influence the prevalence of IE, while this disease is usually considered to have male predilection (19,20).
This consideration can be explained by the fact that evidence on IE and NIE is normally obtained from clinical and surgical databases, while we expose a vast number of exclusively autoptic cases.
The location and characteristics of the vegetations in NIE and IE largely overlap, which suggests that rheological factors or prior valve injury predispose to the formation of lesions in specific areas. In the case of NIE, the prothrombotic milieu of the blood allows for growth of these lesions. In IE, the bacteria circulating in the blood likely find easy attachment and growing sites for the same reasons. NIE was first described by Zeigler in 1888 as fibrin deposition on cardiac valves and was further studied by several authors (16,21,22). Their studies used different terminologies for the disease: thromboendocarditis, terminal or marantic endocarditis, non-bacterial thrombotic endocarditis, and degenerative verrucous endocarditis. The most commonly used term is non-bacterial thromboembolic endocarditis, which suggests that emboli are a typical clinical feature of the disease. However, this term is also not completely accurate because it excludes non-fungal aetiologies, and therefore the term NIE is preferred. Fungal IE is rare, however, we encountered no cases of fungal IE in the 409 autopsied cases.
Not surprisingly, patients with NIE were older at their time of death and were much more often diagnosed with concomitant cancer, often in a disseminated phase. In contrast, IE was more often seen in patients with history of prior cardiac and valvular surgery.
The use of autopsy case series has inherent limitations related to the obvious selection bias. The bias is related not only to the inclusion of only fatal cases but also referral biases regarding which cases are considered for autopsy. However, while we cannot overcome the limitations of selection biases related to death, we stress that the referral bias at the University of Trieste likely plays a smaller role because all patients are automatically referred for and usually undergo autopsy. The approximate 100-fold higher rate of IE observed in this study than the clinical incidence and prevalence of the disease highlight the selection of an enriched population with unfavourable prognosis and is in accordance with the increased risk of death associated with IE. The rate of NIE was three-fold higher than IE, and we were unable to estimate the true incidence and prevalence in the population. Considering the advanced median age of the individuals, diseases and conditions related to ageing would be expected (i.e. cancer) and would be over-represented in this series compared to the general population.
From a pathology standpoint, this study highlighted that NIE is three times more common than IE at autopsy evaluation. The data also suggest that in the presence of NIE, co-existing cancer or other diseases, such as sepsis or autoimmune illnesses, should be diligently investigated.
From a pathophysiological aspect, the similarities of the vegetations in the two syndromes suggest that a common mechanism may be involved that may favour the formation of both IE and NIE vegetations. This observation also highlights why cancer and other chronic inflammatory conditions may not only cause NIE, but also predispose to IE.
From a clinical perspective, this study may suggest that the incidental finding of a vegetation in a patient with symptoms of infection is rather unlikely to represent IE and more likely to be NIE, but may also carry an unfavourable prognosis that is likely due to its association with advanced cancer.
In conclusion, we suggest that during forensic and clinical autopsies, an accurate examination of endocardium and heart valves should always be performed to obtain as much information about clinical conditions and the cause of death of the deceased person as possible.
Conflicts of Interest
None declared.
Authors’ Contributions
RB, GP, LZ and FS performed the autopsies. All Authors participated in the interpretation of the results and in writing the article.
Figure 1. A: Histological appearance of a vegetation of the left heart valve caused by bacterial endocarditis: the vegetation can be seen to be made up of fibrinous-haemorrhagic material with a lymphocytic infiltrate (original magnification, ×5). B: At higher magnification, the bacterial colonies are clearly visible (original magnification, ×20).
Figure 2. A: Inflammation and thrombus deposition as an irregular warty growth causing damage to the papillary muscles in a case of bacterial endocarditis. B: Spleen infarction caused by a small embolus of infected thrombotic material in a case of bacterial endocarditis. C: Several abscesses of the lung caused by superinfection of the necrotic areas in a clinical setting of multiple occluding thrombi generated by small emboli from endocardial vegetations in a case of bacterial endocarditis.
Figure 3. A: Thrombotic left heart valve vegetations, caused by bacterial endocarditis, with valve ulceration. B: Thrombotic left heart valve vegetations caused by bacterial endocarditis, with valve ulceration and chordae tendinae rupture. C: Calcification of tricuspid valve with thickening and fusion of the commissures that lead to stenosis.
References
- 1.Fernandez Guerrero ML, Alvarez B, Manzarbeitia F, Renedo G. Infective endocarditis at autopsy. A review of pathologic manifestations and clinical correlates. Medicine. 2012;91:152–164. doi: 10.1097/MD.0b013e31825631ea. PMID: 22543628. DOI: 10.1097/MD.0b013e31825631ea. [DOI] [PubMed] [Google Scholar]
- 2.Byramji A, Gilbert JD, Byard RW. Sudden death as a complication of bacterial endocarditis. Am J Forensic Med Pathol. 2011;32:140–142. doi: 10.1097/PAF.0b013e31821984fb. PMID: 21512391. DOI: 10.1097/ PAF.0b013e31821984fb. [DOI] [PubMed] [Google Scholar]
- 3.Lee V, Gilbert JD, Byard RW. Marantic endocarditis – A not-so-benign entity. J Forensic Leg Med. 2012;19(6):312–315. doi: 10.1016/j.jflm.2012.02.021. PMID: 22847046. DOI: 10.1016/j.jflm.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Geisenberger D, Huppertz LM, Büchsel M, Kramer L, Pollak S, Grosse Perdekamp M. Non-traumatic subdural hematoma secondary to septic brain embolism: A rare cause of unexpected death in a drug addict suffering from undiagnosed bacterial endocarditis. Forensic Sci Int. 2015;257:e1–e5. doi: 10.1016/j.forsciint.2015.07.055. PMID: 26296471. DOI: 10.1016/j.forsciint.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. PMID: 26341945. DOI: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 6.Vincent LL, Otto CM. Infective endocarditis: Update on epidemiology, outcomes, and management. Curr Cardiol Rep. 2018;20(10):86–86. doi: 10.1007/s11886-018-1043-2. PMID: 30117004. DOI: 10.1007/s11886-018-1043-2. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Cruz A, Muñoz P, Sandoval C, Fariñas C, Gutiérrez-Cuadra M, Pericás Pulido JM, Miró JM, Goenaga-Sánchez MÁ, de Alarcón A, Bonache-Bernal F, Rodríguez M, Noureddine M, Bouza Santiago E, Spanish Collaboration on Endocarditis (GAMES) Infective endocarditis in patients with cancer: A consequence of invasive procedures or a harbinger of neoplasm? A prospective, multicenter cohort. Medicine. 2017;96(38):e7913–e7913. doi: 10.1097/MD.0000000000007913. PMID: 28930826. DOI: 10.1097/MD.0000000000007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Albéniz X, Hsu J, Lipsitch M, Logan RW, Hernández-Díaz S, Hernán MA. Infective endocarditis and cancer in the elderly. Eur J Epidemiol. 2016;31(1):41–49. doi: 10.1007/s10654-015-0111-9. PMID: 26683995. DOI: 10.1007/s10654-015-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Kim D, Lee SE, Cho IJ, Shim CY, Hong GR, Ha JW. Infective Endocarditis in cancer patients – causative organisms, predisposing procedures, and prognosis differ from infective endocarditis in non-cancer patients. Circ J. 2019;83(2):452–460. doi: 10.1253/circj.CJ-18-0609. PMID: 30555101. DOI: 10.1253/circj.CJ-18-0609. [DOI] [PubMed] [Google Scholar]
- 10.Chino F, Kodama A, Otake M, Dock DS. Nonbacterial thrombotic endocarditis in a Japanese autopsy sample. A review of eighty cases. Am Heart J. 1975;90:190–198. doi: 10.1016/0002-8703(75)90119-2. PMID: 168760. DOI: 10.1016/0002-8703(75)90119-2. [DOI] [PubMed] [Google Scholar]
- 11.Chomette G, Auriol M, Baubion D, de Frejacques C. Non-bacterial thrombotic endocarditis. Autopsy study, clinico-pathological correlations. Ann Med Interne. 1980;131:443–447. PMID: 7224455. [PubMed] [Google Scholar]
- 12.Dickens P, Chan AC. Nonbacterial thrombotic endocarditis in Hong Kong Chinese. Arch Pathol Lab Med. 1991;115:359–361. PMID: 2012496. [PubMed] [Google Scholar]
- 13.Llenas-García J, Guerra-Vales JM, Montes-Moreno S, López-Ríos F, Castelbón-Fernández FJ, Chimeno-García J. Nonbacterial thrombotic endocarditis: clinicopathologic study of a necropsy series. Rev Esp Cardiol. 2007;60:493–500. PMID: 17535760. [PubMed] [Google Scholar]
- 14.Maté del Tío M, Gómez Cerezo J, Garcés Jiménez MC, Mate Valdezate A, Gamallo C, Barbado Hernández FJ, Vázquez Rodríguez JJ. Nonbacterial thrombotic endocarditis: A review of a necropsy series. Rev Clin Esp. 1997;197:9–13. PMID: 9102684 (In Spanish). [PubMed] [Google Scholar]
- 15.Buja LM, Butany J. Cardiovascular Pathology. Fourth Edition. Academic Press, London. 2016 DOI: 10.1016/C2013-0-12761-4. [Google Scholar]
- 16.Allen AC, Sirota JH. The morphogenesis and significance of degenerative verrucal endocardiosis (terminal endocarditis, endocarditis simplex, nonbacterial thrombotic endocarditis) Am J Pathol. 1994;20:1025–1055. PMID: 19970792. [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard DG, Ross RS, Dittrich HC. Nonbacterial thrombotic endocarditis. Assessment by transesophageal echocardiography. Chest. 1992;102:954–956. doi: 10.1378/chest.102.3.954. PMID: 1516432. DOI: 10.1378/chest.102.3.954. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Suzuki M, Lie JT, Titus JL. Nonbacterial thrombotic endocarditis (NBTE) and disseminated intravascular coagulation (DIC): Autopsy study of 36 patients. Arch Pathol Lab Med. 1977;101:65–68. PMID: 576391. [PubMed] [Google Scholar]
- 19.Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363(9403):139–149. doi: 10.1016/S0140-6736(03)15266-X. PMID: 14726169. DOI: 10.1016/ S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 20.Hoen B, Duval X. Clinical practice. Infective endocarditis. N Engl J Med. 2013;368(15):1425–1433. doi: 10.1056/NEJMcp1206782. PMID: 23574121. DOI: 10.1056/NEJMcp1206782. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Fausto N, Abbas A, Abbas A. Elsevier Saunders, Philadelphia. 2005. Robbins and Cotran. Pathologic Basis of Disease. Seventh Edition. DOI: 10.1080/00313020500059191. [Google Scholar]
- 22.Libman E, Sacks B. A hitherto undescribed form of valvular and mural endocarditis. Arch Int Med. 1924;33:701–737. DOI: 10.1001/archinte.1924.00110300044002. [Google Scholar]