Abstract
Background/Aim: Blunt chest trauma is one of the major injuries in multiply injured patients and is associated with an increased risk of acute respiratory distress syndrome (ARDS) and ventilator-associated pneumonia (VAP). Accidental hypothermia is a common accompaniment of multiply injured patients. The objective of this study was to analyze the influence of accidental hypothermia on pulmonary complications in multiply injured patients with blunt chest trauma. Patients and Methods: Multiply injured patients [injury severity score (ISS) ≥16] with severe blunt chest trauma [abbreviated injury scale of the chest (AISchest) ≥3] were analyzed. Hypothermia was defined as body core temperature <35˚C. The primary endpoint was the development of ARDS and VAP. Propensity score matching was performed. Results: Data were analyzed for 238 patients, with a median ISS of 26 (interquartile range=12). A total of 67 patients (28%) were hypothermic on admission. Hypothermic patients were injured more severely (median ISS 34 vs. 24, p<0.001) and had a higher transfusion requirement (p<0.001). Their mortality rate was consequently increased (10% vs. 1%, p=0.002); After propensity score matching, the mortality rate was still higher (10% vs. 2%, p=0.046). However, hypothermia was not an independent predictor of mortality. Hypothermic patients had to be ventilated longer (p=0.02). However, there were no differences in occurrence of ARDS and VAP. Hypothermia was not identified as an independent predictor of ARDS and VAP. Conclusion: Among multiply injured patients with severe blunt chest trauma, accidental hypothermia is not an independent predictor of ARDS and VAP and is more likely to be an accompaniment of injury severity and hemorrhage.
Keywords: Blunt chest trauma, multiple trauma, adult respiratory distress syndrome, ventilator-associated pneumonia, hypothermia
Blunt chest trauma is one of the major injuries in multiply injured patients (1). It is associated with an increased risk of post-traumatic complications such as acute respiratory distress syndrome (ARDS), ventilator-associated pneumonia (VAP), sepsis, and multiple organ dysfunction syndrome (MODS) (2-5). Patients with severe thoracic trauma are susceptible to time-consuming pre-hospital treatment because of the increased probability of intubation and application of a chest drain. Thus, the prolonged pre-hospital phase entails the risk of accidental hypothermia. In general, multiply injured patients are particularly at risk for accidental hypothermia due to various reasons, one of which is exposure to the environment. In addition, shock with hypoperfusion and impaired thermoregulation, infusion of cold fluids, and therapy with anesthetics or muscle relaxants are assignable causes of accidental hypothermia (6-8). In multiply injured patients, the incidence of accidental hypothermia on admission may be up to 66% (8-10). Severe hypothermia induces cardiac dysfunction and is an inherent feature of trauma-induced coagulopathy (11-13). Furthermore, accidental hypothermia is assumed to be correlated with an increased risk of MODS and mortality (7,14). Various studies estimate a mortality of 30-80% in cases of concomitant hypothermia (14,15). However, whether accidental hypothermia is an independent predictor of mortality and post-traumatic complications or simply an accompanying symptom of injury severity and hemorrhage is still a controversial topic (7-10,16-18). In addition, hypothermia seems to induce immunosuppression due to a reduced release of pro-inflammatory cytokines (tumor necrosis factor α, interleukin 1β, interleukin 6) and increased secretion of anti-inflammatory mediators (15,19,20). Because of immunosuppression, hypothermia seems to be associated with an increased risk of infectious complications, e.g. pneumonia, among ventilated patients (21,22). On the other hand, it is unclear whether hypothermia-induced immunosuppression raises the risk of ARDS.
Therefore, the objective of this study was to analyze the impact of accidental hypothermia at the time of admission on the outcome of severely injured patients with a considerable risk profile owing to concomitant severe blunt chest trauma. We hypothesized that hypothermic patients are more likely to develop pulmonary post-traumatic complications (ARDS/VAP) and have higher mortality without hypothermia being an independent risk factor of the aforementioned adverse events.
Patients and Methods
Following Institutional Review Board approval (No. 3392-2016), we performed a retrospective cohort analysis. Between January 2005 and December 2012 multiply injured patients [injury severity score (ISS) ≥16] (23) with a minimum age of 16 years and admission within 6 hours of the trauma to our level I Trauma Center with severe thoracic trauma [Abbreviated Injury Scale (AIS)chest ≥3] (24) were included. Patients who suffered from severe traumatic brain injury (AIShead ≥3) or died within the first 48 hours were not eligible. All patients with penetrating injuries were excluded.
Demographic and baseline data were collected from the patient records. Injury pattern and severity were classified using the 2008 update of the AIS (24). Overall injury severity was calculated using the injury severity score (ISS) (23). Hypothermia was defined as a documented temperature <35˚C within the first 2 hours after admission, but at least prior to any operative interventions in order to exclude hypothermia resulting from a perioperative fall in body temperature. Body temperature was determined by bladder or esophageal probe, both representing reliable methods for the measurement of core temperature. Measurement was an integral part of initial resuscitation in the emergency room. Hypothermia was classified as mild (<35-34˚C), moderate (<34-32˚C) and severe (<32˚C) (25,26).
The primary endpoint of this study was the development of ARDS or VAP. Secondary endpoints were mortality, as well as systemic inflammatory response syndrome (SIRS), sepsis, and MODS. ARDS was diagnosed according to the 2012 Berlin definition i.e. ARDS was deemed present in cases where bilateral radiographic infiltration of the lung accompanied by reduced oxygenation (<300 mmHg) based on Horovitz score (PaO2/FiO2) was present during the first week after admission (27). Diagnosis of VAP was guided by the Center for Disease Control and Prevention recommendations (28). VAP was defined as pneumonia occurring >48 hours after intubation and mechanical ventilation. Diagnoses of SIRS and sepsis were made using the 2010 revised criteria of S-2k guidelines of the German Sepsis Society and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (29). In line with standard practice, SIRS or sepsis were deemed present if the criteria were met on at least two consecutive days (30). MODS was diagnosed on the basis of MODS score of Marshall et al., a generally accepted indicator: MODS was deemed present if the sum of single organ dysfunctions was >8 on at least one day (31-33).
Statistical analyses were performed with IBM SPSS (Version 22; IBM, Armonk, NY, USA). Propensity score matching was performed with a 1:3 nearest-neighbor matching model with the covariates age, sex, ISS, and AIS (head, face, chest, abdomen, extremities). For Gaussian-distributed data, statistical analysis included parametric tests (Student’s t-test). For non-Gaussian-distributed data, non-parametric tests (Mann–Whitney test for independent data and Wilcoxon test for dependent data). Fisher’s exact test (exact chi-squared test) was used in the analysis of contingency tables were used. Furthermore, logistic as well as linear regression analyses were performed. In the case of binomial logistic regression, variables were dichotomized when necessary. The odds ratio (OR) and 95% confidence interval (CI) were also calculated. Regression models were estimated by maximum likelihood estimation (R2; Nagelkerke’s pseudo-R2). Significance level was set to p<0.05.
Results
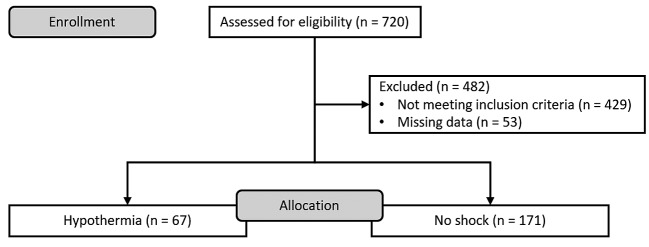
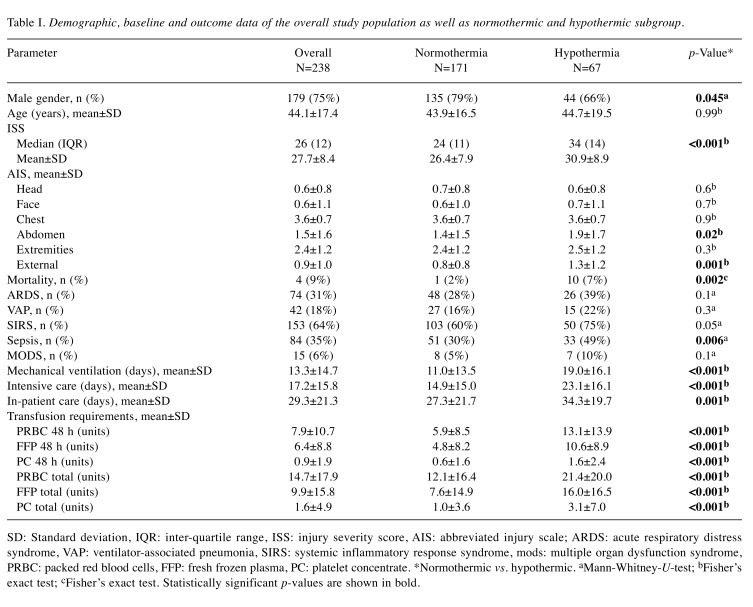
Between January 2005 and December 2012, 720 patients were checked for eligibility. A flowchart of excluded patients is shown in Figure 1. A total of 238 patients with a mean age of 44±17 years and a mean ISS of 27.7±8.4 (median ISS of 26, IQR=12) could be included. 179 patients (75%) were male and 59 patients (25%) female. 9 patients (4%) died within the treatment period. 67 patients (28%) were hypothermic at the time of admission. Female proportion was significantly higher in the hypothermic group (34% vs. 21%, p=0.045) without differences in age distribution. Hypothermic patients had a higher ISS (30.9±8.9 vs. 26.4±7.9, p<0.001) and abdominal AIS (1.9±1.7 vs. 1.4±1.5, p=0.02) compared with normothermic patients and had significantly increased transfusion requirements (see also Table I). Mortality rate was increased within hypothermic patients (10% vs. 1%, p=0.002). However, in a logistic regression analysis (Nagelkerke’s pseudo-R² 0.81, Hosmer–Lemeshow test p=1.0) with a model comprising hypothermia, age, ISS and transfusion requirements [packed red blood cells (PRBC), fresh frozen plasma (FFP), platelet concentrate (PC)] hypothermia was not identified as an independent predictor of mortality (p<0.05). Only total PRBC OR=1.13, 95% CI=1.01-1.27 per unit increase; p=0.04) as well as age (OR=1.39, 95% CI=1.06-1.83 per year increase; p=0.02) independently predicted mortality with statistical significance.
Figure 1. Flow diagram of study patients.
Table I. Demographic, baseline and outcome data of the overall study population as well as normothermic and hypothermic subgroup.
SD: Standard deviation, IQR: inter-quartile range, ISS: injury severity score, AIS: abbreviated injury scale; ARDS: acute respiratory distress syndrome, VAP: ventilator-associated pneumonia, SIRS: systemic inflammatory response syndrome, mods: multiple organ dysfunction syndrome, PRBC: packed red blood cells, FFP: fresh frozen plasma, PC: platelet concentrate. *Normothermic vs. hypothermic. aMann-Whitney-U-test; bFisher’s exact test; cFisher’s exact test. Statistically significant p-values are shown in bold.
The mean duration of mechanical ventilation was 13.3±14.7 days. The mean length of intensive care unit and hospital stay was 17±16 days and 29±21 days, respectively. Hypothermic patients had to be ventilated for a significantly longer period of time (19.0±16.1 vs. 11.0±13.5 days, p<0.001) and had a prolonged duration of ICU (23±16 vs. 15±15 days, p<0.001) and hospital stay (34±20 vs. 27±22 days, p<0.001). In linear regression (R2=0.43) with a model comprising hypothermia, injury severity (ISS≤26/>26), age (≤43/>43 years), AIShead (≤0/>0), AISabdomen (≤2/>2) as well as transfusion requirements by means of PRBC (≤9.5/>9.5 units), FFP (≤5/>5 units), and PC (≤0/>0 units) hypothermia was not identified as an independent prognostic factor for duration of mechanical ventilation (95% CI=33.0-134.3, p=0.2), ICU (95% CI=2.1-5.2, p=0.4), and overall hospital stay (95% CI=6.2-4.6, p=0.8). Table I gives an overview of demographic, baseline, and outcome data.
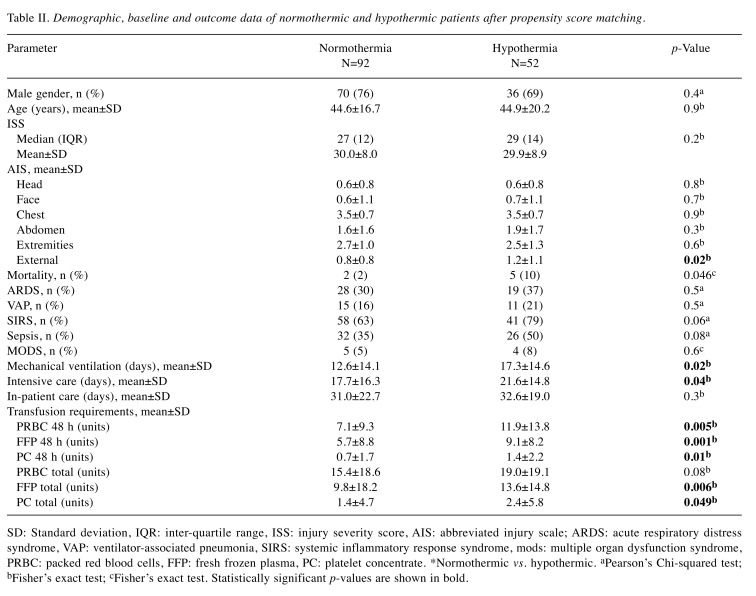
After propensity score matching with nearest-neighbor matching and a 1:3 match ratio, 144 patients remained (52 hypothermic, 92 normothermic). Table II gives an overview of demographic, baseline, and outcome data after propensity score matching. Mortality rate was higher in the hypothermic group. However, hypothermia was not identified as an independent predictor of mortality (OR=3.6, 95% CI=0.6-22.2; p=0.2). Transfusion requirements were also increased in hypothermic patients, but there was only moderate correlation between transfusion requirements and hypothermia (Spearman rho: PBRC 48 h 0.24, p=0.004; FFP 48 h 0.27, p=0.001; PC 48 h 0.21, p=0.01). Again, hypothermic patients had a prolonged duration of mechanical ventilation 17.3±14.6 vs. 12.6±14.1 days, p=0.02) and intensive care (21.6±14.8 vs. 17.7±16.3 days, p=0.04). Nevertheless, hypothermia was not identified as an independent predictor. Although being ventilated significantly longer, there were no differences in incidence of ARDS [26 (39%) vs. 48 (28%), p=0.1] and VAP [15 (22%) vs. 27 (16%), p=0.3] between hypothermic and normothermic patients.
Table II. Demographic, baseline and outcome data of normothermic and hypothermic patients after propensity score matching.
SD: Standard deviation, IQR: inter-quartile range, ISS: injury severity score, AIS: abbreviated injury scale; ARDS: acute respiratory distress syndrome, VAP: ventilator-associated pneumonia, SIRS: systemic inflammatory response syndrome, mods: multiple organ dysfunction syndrome, PRBC: packed red blood cells, FFP: fresh frozen plasma, PC: platelet concentrate. *Normothermic vs. hypothermic. aPearson’s Chi-squared test; bFisher’s exact test; cFisher’s exact test. Statistically significant p-values are shown in bold.
Discussion
The present study aimed to analyze the influence of accidental hypothermia on pulmonary post-traumatic complications and mortality in multiply injured patients with severe thoracic trauma. Hypothermic patients showed considerable differences in mortality compared to normothermic patients without hypothermia being an independent predictor of mortality. Furthermore, despite a prolonged mechanical ventilation, hypothermia was not associated with an increased risk of ARDS and VAP.
In the present study, 28% of patients were hypothermic at the time of admission. This is in line with the current literature. The incidence of accidental hypothermia ranges between 12% and 66% (8-10). With increasing injury severity and pre-hospital duration of treatment, incidence of accidental hypothermia also increases (7,8,10,14,16). The mean ISS of 27.7 reflects the above-average injury severity of the present study population in view of the fact that patients with a severe traumatic brain injury were excluded. The chest was by far the most severely injured organ. One can assume that thoracic injury severity affects the outcome in terms of mortality and post-traumatic complications most in this particular study population. Besides ISS, the increased transfusion requirement of hypothermic patients can be assumed to be an indicator of injury severity with subsequent hemorrhage. The results of Beilman and colleagues in a comparable study population confirm this assumption (7). In contrast, Ireland and colleagues reported no difference in transfusion requirements in hypothermic and normothermic patients. However, overall injury severity, as well as injury severity of the hypothermic and normothermic subgroups, was perceptibly lower compared with our study population, which might be an explanation for this finding (8).
In the present study population, 31% of the patients developed ARDS. This is above average compared with different studies either on patients with combined/isolated blunt chest trauma or multiply injured patients with or without blunt chest trauma. Recent studies report an incidence of ARDS of 5-18% (1,3). Our study population predominantly comprised patients with blunt severe thoracic trauma as the leading injury due to our inclusion (AISchest≥3) and exclusion criteria (AIShead≥3). In our opinion, this is the main reason for the higher incidence of ARDS. For analysis of the additional effect of risk factors on outcome, we tried to level the influence of the severity of thoracic injury by including patients with severe thoracic injuries only. In this case, hypothermia was not identified as an independent risk factor for ARDS and VAP. Comparable data in the literature are scarce. Shell-Chaple and colleagues reported that hypothermia at the time of appearance of ARDS was correlated with increased mortality (34). However, the study population comprised patients all of whom had ARDS independent of the cause and hypothermia was defined as body temperature at the time of ARDS appearance. Therefore, comparability is limited. Royon and colleagues reported that patients with pulmonary contusion and concomitant hypothermia on admission had a 2.6-fold risk of bacterial infection (22). However, bacterial infection as the primary outcome is not congruent with VAP. Furthermore, incidence of ARDS was markedly lower at 12% compared with 31% in our study population. Thus, comparability of the study results is also limited. In any case, even the aforementioned limitations cannot explain the contrary results. In the present study population, only blood transfusion was found to be an independent predictor. This is in line with the current literature (3,4). However, Miller and colleagues reported that injury severity (ISS>25) was an independent risk factor for ARDS. This is in contrast to our own results, but is mainly attributable to the different study populations. Whereas Miller included all patients with a blunt thoracic trauma (AISchest≥1), we only included multiply injured patients with a severe thoracic trauma (AISchest≥3) (3). Since sicker patients (identified by means of APACHE II score, SAPS II score or SOFA score) are more susceptible to developing ARDS (35), it is comprehensible that multiply injured patients are more at risk of developing ARDS than less severely injured patients. In any case, our own results suggest that the overall injury severity has no additional and independent influence on ARDS development, if the two most important influencing injuries (severe blunt thoracic trauma and traumatic brain injury) are excluded (36,37). Although hypothermic patients displayed significantly increased mortality in the present study population (10% vs. 1%, p=0.002), hypothermia was not identified as an independent predictor of mortality. This is in accordance with the results of Beilman et al. (7), Steinemann et al. (9), and Mommsen et al. (17), but in contrast to several other studies that identified pre-hospital hypothermia as an independent risk factor for mortality (8,10,16,18). Mommsen attributed this to blood transfusion as a potential confounding factor, among others, since a reduced multivariate analytic model without transfused blood products demonstrated hypothermia to be an independent predictor of mortality (17). With reference to the increased use of transfused blood products in hypothermic patients and the results of the regression analysis, it appears more likely that hypothermia is primarily an accompaniment of injury severity and hemorrhage than vice versa.
Besides the inherent limitations of retrospective cohort studies, there might be a potential bias because of missing data, since 53 patients (18.2%) had to be excluded from our analysis because of missing temperature data. However, this is in accordance with comparable data from the literature (38). The decision to exclude patients with a severe traumatic brain injury was based on the consideration that traumatic brain injury itself affects the necessity for and duration of mechanical ventilation and the risk of iatrogenic ARDS and VAP (36,37). Certainly, it is possible to consider this in a regression analysis. However, the fit of regression models decreases with an increasing variable. By excluding patients with considerable traumatic brain injuries this could be avoided. Nevertheless, the multifactorially influenced outcome of severely injured patients cannot be predicted reliably by only a few factors such as injury severity or hypothermia. The maximum likelihood estimates of some of our logistic regression analyses indicate that there must be unknown confounding factors that influence the outcome. Nevertheless, in a complex disease pattern like multiple trauma, this probably has to be accepted.
This matched-pair analysis investigated multiply injured patients with leading severe blunt thoracic trauma with precisely defined outcome on admission without any loss to follow-up at a level-I trauma center in a high-income country with a sophisticated trauma system. Data analyses comprise validated methods only. For this reason, our results may be generalized to countries with comparable medical standards.
In conclusion, accidental hypothermia was not identified as an independent predictor of ARDS and VAP in multiply injured patients with a leading severe blunt chest trauma and is more likely to accompany injury severity and hemorrhage.
Conflicts of Interest
The Authors declare that there is no conflict of interest.
Authors’ Contributions
Marcel Winkelmann: Concept and design of the study, data acquisition, analysis and interpretation of the data, draft of the manuscript, critical revision of the manuscript. Jan-Dierk Clausen: Data acquisition, analysis and interpretation of the data, critical revision of the manuscript. Pascal Graeff: Analysis and interpretation of the data, draft of the manuscript, critical revision of the manuscript. Christian Schröter: Analysis and interpretation of the data, critical revision of the manuscript. Christian Zeckey: Concept and design of the study, critical revision of the manuscript. Sanjay Weber-Spickschen: Critical revision of the manuscript. Philipp Mommsen: Concept and design of the study, analysis and interpretation of the data, draft of the manuscript, critical revision of the manuscript.
References
- 1.Probst C, Pape HC, Hildebrand F, Regel G, Mahlke L, Giannoudis P, Krettek C, Grotz MR. 30 years of polytrauma care: An analysis of the change in strategies and results of 4849 cases treated at a single institution. Injury. 2009;40(1):77–83. doi: 10.1016/j.injury.2008.10.004. PMID: 19117558. DOI: 10.1016/j.injury.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Trupka A, Nast-Kolb D, Schweiberer L. Thoracic trauma. Unfallchirurg. 1998;101(4):244–258. doi: 10.1007/s001130050265. PMID: 9613209. [DOI] [PubMed] [Google Scholar]
- 3.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: Identification of independent risk factors. Am Surg. 2002;68(10):845–850. PMID: 12412708. [PubMed] [Google Scholar]
- 4.Wu J, Sheng L, Ma Y, Gu J, Zhang M, Gan J, Xu S, Jiang G. The analysis of risk factors of impacting mortality rate in severe multiple trauma patients with post-traumatic acute respiratory distress syndrome. Am J Emerg Med. 2008;26(4):419–424. doi: 10.1016/j.ajem.2007.06.032. PMID: 18410809. DOI: 10.1016/j.ajem.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Daurat A, Millet I, Roustan JP, Maury C, Taourel P, Jaber S, Capdevila X, Charbit J. Thoracic trauma severity score on admission allows to determine the risk of delayed ARDS in trauma patients with pulmonary contusion. Injury. 2016;47(1):147–153. doi: 10.1016/j.injury.2015.08.031. PMID: 26358517. DOI: 10.1016/j.injury.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Tisherman SA, Rodriguez A, Safar P. Therapeutic hypothermia in traumatology. Surg Clin North Am. 1999;79(6):1269–1289. doi: 10.1016/s0039-6109(05)70077-3. PMID: 10625978. [DOI] [PubMed] [Google Scholar]
- 7.Beilman GJ, Blondet JJ, Nelson TR, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, Cohn SM. Early hypothermia in severely injured trauma patients is a significant risk factor for multiple organ dysfunction syndrome but not mortality. Ann Surg. 2009;249(5):845–850. doi: 10.1097/SLA.0b013e3181a41f6f. PMID: 19387315. DOI: 10.1097/SLA. 0b013e3181a41f6f. [DOI] [PubMed] [Google Scholar]
- 8.Ireland S, Endacott R, Cameron P, Fitzgerald M, Paul E. The incidence and significance of accidental hypothermia in major trauma–a prospective observational study. Resuscitation. 2011;82(3):300–306. doi: 10.1016/j.resuscitation.2010.10.016. PMID: 21074927. DOI: 10.1016/j.resuscitation. 2010.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Steinemann S, Shackford SR, Davis JW. Implications of admission hypothermia in trauma patients. J Trauma. 1990;30(2):200–202. doi: 10.1097/00005373-199002000-00011. PMID: 2304115. [DOI] [PubMed] [Google Scholar]
- 10.Luna GK, Maier RV, Pavlin EG, Anardi D, Copass MK, Oreskovich MR. Incidence and effect of hypothermia in seriously injured patients. J Trauma. 1987;27(9):1014–1018. doi: 10.1097/00005373-198709000-00010. PMID: 3656463. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand F, Probst C, Frink M, Huber-Wagner S, Krettek C. Importance of hypothermia in multiple trauma patients. Unfallchirurg. 2009;112(11):959–964. doi: 10.1007/s00113-009-1683-1. PMID: 19816669. DOI: 10.1007/s00113-009-1683-1. [DOI] [PubMed] [Google Scholar]
- 12.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. PMID: 12813 333. DOI: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 13.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B. The coagulopathy of trauma: A review of mechanisms. J Trauma. 2008;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. PMID: 18849786. DOI: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 14.Tsuei BJ, Kearney PA. Hypothermia in the trauma patient. Injury. 2004;35(1):7–15. doi: 10.1016/s0020-1383(03)00309-7. PMID: 14728949. [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand F, Giannoudis PV, van Griensven M, Chawda M, Pape HC. Pathophysiologic changes and effects of hypothermia on outcome in elective surgery and trauma patients. Am J Surg. 2004;187(3):363–371. doi: 10.1016/j.amjsurg.2003.12.016. PMID: 15006564. DOI: 10.1016/ j.amjsurg.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Martin RS, Kilgo PD, Miller PR, Hoth JJ, Meredith JW, Chang MC. Injury-associated hypothermia: An analysis of the 2004 National Trauma Data Bank. Shock. 2005;24(2):114–118. doi: 10.1097/01.shk.0000169726.25189.b1. PMID: 16044080. [DOI] [PubMed] [Google Scholar]
- 17.Mommsen P, Andruszkow H, Fromke C, Zeckey C, Wagner U, van Griensven M, Frink M, Krettek C, Hildebrand F. Effects of accidental hypothermia on post-traumatic complications and outcome in multiple trauma patients. Injury. 2013;44(1):86–90. doi: 10.1016/j.injury.2011.10.013. PMID: 22040695. DOI: 10.1016/j.injury.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Shafi S, Elliott AC, Gentilello L. Is hypothermia simply a marker of shock and injury severity or an independent risk factor for mortality in trauma patients? Analysis of a large national trauma registry. J Trauma. 2005;59(5):1081–1085. doi: 10.1097/01.ta.0000188647.03665.fd. PMID: 16385283. [DOI] [PubMed] [Google Scholar]
- 19.Lee SL, Battistella FD, Go K. Hypothermia induces T-cell production of immunosuppressive cytokines. J Surg Res. 2001;100(2):150–153. doi: 10.1006/jsre.2001.6230. PMID: 11592784. DOI: 10.1006/jsre.2001.6230. [DOI] [PubMed] [Google Scholar]
- 20.Schneider A, Popp E, Teschendorf P, Bottiger BW. Therapeutic hypothermia. Anaesthesist. 2008;57(2):197–206. doi: 10.1007/s00101-008-1311-4. PMID: 18246320. DOI: 10.1007/s00101-008-1311-4. [DOI] [PubMed] [Google Scholar]
- 21.Henderson WR, Dhingra VK, Chittock DR, Fenwick JC, Ronco JJ. Hypothermia in the management of traumatic brain injury. A systematic review and meta-analysis. Intensive Care Med. 2003;29(10):1637–1644. doi: 10.1007/s00134-003-1848-2. PMID: 12915937. DOI: 10.1007/ s00134-003-1848-2. [DOI] [PubMed] [Google Scholar]
- 22.Royon V, Guitard PG, Abriou C, Frebourg N, Menard JF, Clavier T, Dureuil B, Veber B. Hypothermia at admission increases the risk of pulmonary contusion's infection in intubated trauma patients. Ann Fr Anesth Reanim. 2012;31(11):870–875. doi: 10.1016/j.annfar.2012.08.014. PMID: 23044347 DOI: 10.1016/j.annfar.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Baker SP, O'Neill B, Haddon W Jr., Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. PMID: 4814394. [PubMed] [Google Scholar]
- 24.Gennarelli TA, Wodzin E, Association for the Advancement of Automotive Medicine . Association for the Advancement of Automotive Barrington. 2008. Abbreviated injury scale 2005: Update 2008. ISBN: 000000202X 97800000 02020. [Google Scholar]
- 25.Segers MJ, Diephuis JC, van Kesteren RG, van der Werken C. Hypothermia in trauma patients. Unfallchirurg. 1998;101(10):742–749. PMID: 9847700. [PubMed] [Google Scholar]
- 26.Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: Effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44(5):846–854. doi: 10.1097/00005373-199805000-00017. PMID: 9603087. [DOI] [PubMed] [Google Scholar]
- 27.ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. PMID: 22797452. DOI: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 28.Pneumonia (Ventilatorassociated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event [Internet]. Device-associated Module PNEU. Centers for Disease Control and Prevention. 2016 Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/6pscVAPcurrent.pdf Accessed 2016/02/03. [Google Scholar]
- 29.Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Forst H, Gastmeier P, Gerlach H, Grundling M, John S, Kern W, Kreymann G, Kruger W, Kujath P, Marggraf G, Martin J, Mayer K, Meier-Hellmann A, Oppert M, Putensen C, Quintel M, Ragaller M, Rossaint R, Seifert H, Spies C, Stuber F, Weiler N, Weimann A, Werdan K, Welte T, German Sepsis Society , German Interdisciplinary Association of Intensive Care and Emergency Medicine Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinare Vereinigung fur Intensiv-und Notfallmedizin (DIVI)) Ger Med Sci. 2010;8 Doc14 doi: 10.3205/000103. PMID: 20628653. DOI: 10.3205/000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talmor M, Hydo L, Barie PS. Relationship of systemic inflammatory response syndrome to organ dysfunction, length of stay, and mortality in critical surgical illness: Effect of intensive care unit resuscitation. Arch Surg. 1999;134(1):81–87. doi: 10.1001/archsurg.134.1.81. PMID: 9927137. [DOI] [PubMed] [Google Scholar]
- 31.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. PMID: 7587228. [DOI] [PubMed] [Google Scholar]
- 32.Grotz M, von Griensven M, Stalp M, Kaufmann U, Hildebrand F, Pape HC. Scoring multiple organ failure after severe trauma. Comparison of the Goris, Marshall and Moore scores. Chirurg. 2001;72(6):723–730. doi: 10.1007/s001040170130. PMID: 11469095. [DOI] [PubMed] [Google Scholar]
- 33.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009;31(5):438–447. doi: 10.1097/SHK.0b013e31818ba4c6. PMID: 18838942. DOI: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schell-Chaple HM, Puntillo KA, Matthay MA, Liu KD, National Heart, Lung , Blood Institute Acute Respiratory Distress Syndrome Network Body temperature and mortality in patients with acute respiratory distress syndrome. Am J Crit Care. 2015;24(1):15–23. doi: 10.4037/ajcc2015320. PMID: 25554550. DOI: 10.4037/ajcc 2015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alisha C, Gajanan G, Jyothi H. Risk factors affecting the prognosis in patients with pulmonary contusion following chest trauma. J Clin Diagn Res. 2015;9(8):OC17–19. doi: 10.7860/JCDR/2015/13285.6375. PMID: 26435 984. DOI: 10.7860/JCDR/2015/13285.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aisiku IP, Yamal JM, Doshi P, Rubin ML, Benoit JS, Hannay J, Tilley BC, Gopinath S, Robertson CS. The incidence of ards and associated mortality in severe TBI using the Berlin definition. J Trauma Acute Care Surg. 2016;80(2):308–312. doi: 10.1097/TA.0000000000000903. PMID: 26491799. DOI: 10.1097/TA.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendrickson CM, Howard BM, Kornblith LZ, Conroy AS, Nelson MF, Zhuo H, Liu KD, Manley GT, Matthay MA, Calfee CS, Cohen MJ. The acute respiratory distress syndrome following isolated severe traumatic brain injury. J Trauma Acute Care Surg. 2016;80(6):989–997. doi: 10.1097/TA.0000000000000982. PMID: 26881489. DOI: 10.1097/TA.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HE, Callaway CW, Peitzman AB, Tisherman SA. Admission hypothermia and outcome after major trauma. Crit Care Med. 2005;33(6):1296–1301. doi: 10.1097/01.ccm.0000165965.31895.80. PMID: 15942347. [DOI] [PubMed] [Google Scholar]