Abstract
Background/Aim: Transient receptor potential vanilloid 1 (TRPV1)-expressing sensory nerves innervate the pancreatic islets. Sensory neuropeptides, including calcitonin gene-related peptide (CGRP) and substance P (SP), participate in insulin secretion. This study aimed to investigate the role of TRPV1 in glucose-induced insulin secretion. Materials and Methods: TRPV1–/– and wild-type (WT) mice were fed a normal diet for 24 weeks. Glucose tolerance and insulin secretion were measured at the end of the experiments. Results: TRPV1–/– mice had greater impairments in glucose tolerance and higher decrease in glucose-induced insulin secretion than WT mice. Capsaicin (a TRPV1 agonist) increased insulin secretion in WT, but not in TRPV1–/– mice. Glucose-induced insulin secretion was blunted in TRPV1–/– mice, and was attenuated by AMG9810 (a TRPV1 inhibitor), CGRP8-37 (a CGRP receptor antagonist), or RP67580 (a NK-1 receptor antagonist) in WT mice. Glucose-induced SP and CGRP release from WT pancreas was higher than that from TRPV1–/– pancreas. Conclusion: TRPV1 mediates glucose-induced insulin secretion likely through CGRP and SP release.
Keywords: TRPV1, pancreas, insulin, substance P, calcitonin generelated peptide
Transient receptor potential vanilloid 1 (TRPV1) is a non-selective cation channel and is predominantly expressed in sensory nerve fibers which innervate the pancreas (1). TRPV1-expressing pancreatic sensory nerves can release sensory neuropeptides, including calcitonin gene-related peptide (CGRP) and substance P (SP). Through sensation of surrounding glucose levels and subsequent release of CGRP and SP, sensory nerves may participate in the regulation of insulin secretion to maintain euglycemia. Although CGRP and SP have been linked to insulin secretion, the role of TRPV1 channels in glucose-induced insulin secretion is not clearly understood (2).
TRPV1 is activated by various stimuli and can be up-regulated by high glucose levels (3-5). Activation of TRPV1 in the sensory nerves causes release of a number of neuropeptides, including CGRP and SP, and these peptides exert direct effects on islet hormone secretion (2,6-8). In turn, insulin enhances TRPV1-mediated membrane currents, sensitizes TRPV1 function, and increases the release of CGRP (9). Capsaicin, a specific TRPV1 agonist, plays a role in glucose metabolism. However, the role of TRPV1 in insulin secretion is still controversial. Experiments with ablation of sensory nerves obtained various results. Karlsson et al. (10) have shown that depletion of capsaicin-sensitive sensory nerves increased glucose tolerance and glucose-induced early insulin secretion (10). However, Wall et al. (11) have reported that capsaicin-induced desensitization of TRPV1 decreased glucose-induced insulin secretion. Gram et al. (12) have reported that sensory nerve desensitization did not affect plasma insulin concentration in Zucker rats. Experiments with TRPV1 agonists obtained positive results. Akiba et al. (1) have shown that capsaicin dose-dependently increased insulin secretion and plasma insulin concentrations. Radu et al. have reported that a low dose of capsaicin reduced blood glucose levels in the late-phase of type 1 diabetes, with a partial reversal of the TRPV1 function by increasing the TRPV1 channel current density and recovery time (13). Tolan et al. (14) have shown that capsaicin enhanced glucose tolerance and elevated plasma insulin concentrations. Moreover, oral administration of N-oleoyldopamine, a selective TRPV1 agonist, elicited significant improvement in glucose tolerance (15). These findings suggest that TRPV1 may promote insulin secretion. However, the mechanism is still unknown.
In this study, we investigated the role of TRPV1 in glucose-induced insulin secretion in both in vivo and in vitro experiments by using the TRPV1 gene knockout (TRPV1–/–) mice.
Materials and Methods
Animals. All experimental procedures were approved by the Michigan State University Animal Care and Use Committee and conform to NIH guidelines. TRPV1–/– mice (B6.129S4-TRPV1tm1Jul) and WT mice on the genetic background of C57BL/6J were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Twenty-four-week-old male mice were fasted (without food but with water) for 15 h, after which glucose (2 g/kg body weight) was administered to conscious mice by intraperitoneal injection (i.p.). Blood glucose levels at 0, 30, 60, 90, and 120 min after glucose administration were measured using an Accu-Chek glucose meter (Roche Diagnostics). Tail vein blood was also collected at 0, 30, 60, and 120 min for insulin determination. For measurement of acute insulin release, another group of mice were injected with glucose (3 g/kg, i.p.), and tail vein blood was collected at 2 to 14 min after glucose administration. Serum was immediately collected and stored at –80˚C for insulin determination. Insulin levels were measured using an ELISA kit (Crystal Chem, Downers Grove, IL, USA). The AUCs of insulin and glucose were calculated according to the trapezoidal rule. Glucose tolerance was defined as AUC versus time curve calculated with the trapezoidal rule. For studying the effects of capsaicin on insulin release and glucose tolerance test, another group of mice was fasted for 14 h, tail vein blood was collected for basal blood glucose and insulin levels measurement. Then, capsaicin was administered (0.25 mg/kg, i.p.), and tail vein blood was collected 1 h after capsaicin injection to measure insulin levels.
In Vitro insulin secretion. Mice were fasted for 15 h and anesthetized with pentobarbital sodium (50 mg/kg i.p.), and their pancreases were dissected out. The tissue was washed with cold Krebs-bicarbonate buffer (KRB, pH 7.4), containing (mmol/l) 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.0 KH2PO4, 2.5 CaCl2, 25.0 NaHCO3, supplemented with 1% (w/v) bovine-serum albumin, 10 μg/ml aprotinin, 2 mmol/l glucose, and saturated with air. The tissue was minced into small pieces (2 mm in diameter) and then pre-incubated in KRB for 60 min at 37˚C before being incubated with various substances or vehicle (16). To determine the effect of capsaicin on insulin release, capsaicin (10–9 mol/l), dissolved in vehicle consisting of Tween 80 (10%) and ethanol (10%) in 0.9% NaCl (80%), was added to KRB and incubated for 30 min. To determine the role of TRPV1, CGRP, and SP in glucose-induced insulin release, AMG 9810 (10–6 mol/l, a selective TRPV1 antagonist), CGRP8-37 (10–6 M, a CGRP receptor antagonist), or RP67580 (10–6 mol/l, a selective neurokinin 1 receptor antagonist) was added 5 min before adding glucose (8 mmol/l). The incubation was stopped by chilling the samples in ice-cold water; aliquots were removed from the medium and stored at -80˚C for insulin assay. A commercial mouse insulin ELISA kit (Crystal Chem Inc., Downers Grove, IL, USA) was used to determine insulin release that was normalized to the wet pancreas weight.
Measurement of CGRP and SP. The samples were prepared as described above, purified, and analyzed by radioimmunoassay. The assay was performed as recommended by the supplier. Commercially available rat CGRP and SP radioimmunoassay kits (Peninsula Laboratories Inc.) were used for determination of SP and CGRP release which was normalized to the wet pancreas weight.
Statistical analysis. All values are expressed as mean±SEM. Differences among groups were compared by one-way analysis of variance followed by the Tukey-Kramer multiple comparison test. Differences between two groups were analyzed by t-tests. The results were considered statistically significant at p<0.05.
Results
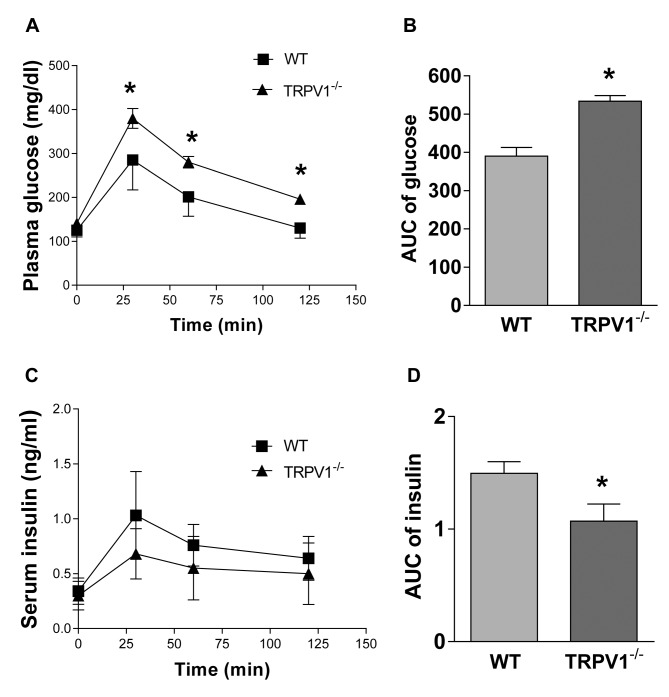
Impaired glucose tolerance in TRPV1–/– mice. The experiments were conducted in TRPV1–/– and wild-type (WT) mice at 24 weeks of age. No significant differences were observed between the two groups in body weight (WT 30.9±4.4 g vs. TRPV1–/– 32.6±4.7 g, p>0.05) and fasting glucose levels (WT 125±15 mg/dl vs. TRPV1–/– 141±18 mg/dl, p>0.05, Figure 1A). TRPV1–/– mice had impaired glucose tolerance, with significantly higher blood glucose values at 30, 60 and 120 min after glucose challenge, compared with WT mice (Figure 1A). The overall area under the curve (AUC) of glucose was higher in TRPV1–/– mice compared with WT mice (WT 390±69 mg-hour/dl vs. TRPV1–/– 534±45 mg-hour/dl, p<0.01, Figure 1B).
Figure 1. Glucose tolerance and insulin secretion of TRPV1–/– and WT mice. Plasma levels of glucose (A) and insulin (C) were measured in WT and TRPV1–/– mice before and after glucose injection (2 g/kg body weight, i.p.). Mean area under the curve (AUC) of glucose (B) and insulin (D) were calculated. Values are mean±SEM; n=8-9; *p<0.05 vs. WT.
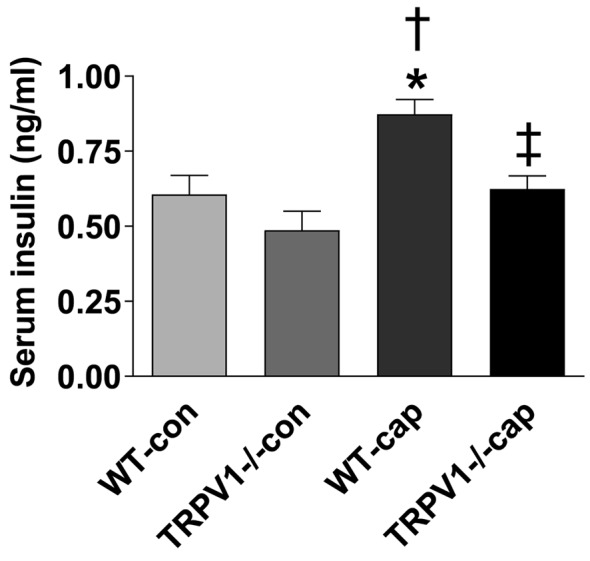
Decreased insulin secretion in TRPV1–/– mice. Fasting plasma insulin levels were similar between WT and TRPV1–/– mice (WT 0.34±0.12 ng/ml vs. TRPV1–/– 0.30±0.13 ng/ml, p>0.05, Figure 1C). Plasma insulin levels, after glucose challenge, were not significantly different between the two groups at the individual time points of 30, 60, and 120 min, but had a decreasing trend in TRPV1–/– mice (Figure 1C). Importantly, the AUC of insulin was lower in TRPV1–/– mice compared with WT mice (WT 1.50±0.30 ng-h/ml vs. TRPV1–/– 1.07±0.43 ng-h/ml, p<0.05, Figure 1D), indicating that glucose-induced insulin secretion was impaired in TRPV1–/– mice. Glucose-induced early-phase insulin secretion was observed at 4 minutes following glucose injection in WT mice but not in TRPV1–/– mice (Figure 2A), while insulin levels at 14 min after glucose injection were similar between WT and TRPV1–/– mice (Figure 2A). Plasma glucose levels were similar between the two groups at 2, 4, and 14 min after glucose administration (Figure 2B). These results suggested that the acute-phase insulin secretory response to glucose disappeared in TRPV1–/– mice. Furthermore, capsaicin (a TRPV1 agonist) caused a significant increase in insulin secretion in WT mice but not in TRPV1–/– mice (Figure 3).
Figure 2. Acute phase insulin secretory response. Plasma levels of insulin (A) and glucose (B) were measured in WT and TRPV1–/– mice before (pre) and at 2, 4, and 14 min after glucose injection (3 g/kg body weight, i.p.). Values are mean±SEM; n=5-8; * p<0.05 vs. WT-pre, TRPV1–/–-pre, WT-2 min, TRPV1–/–-2 min; †p<0.05 vs. WT-4 min; ‡p<0.05 vs. TRPV1–/–-4 min.
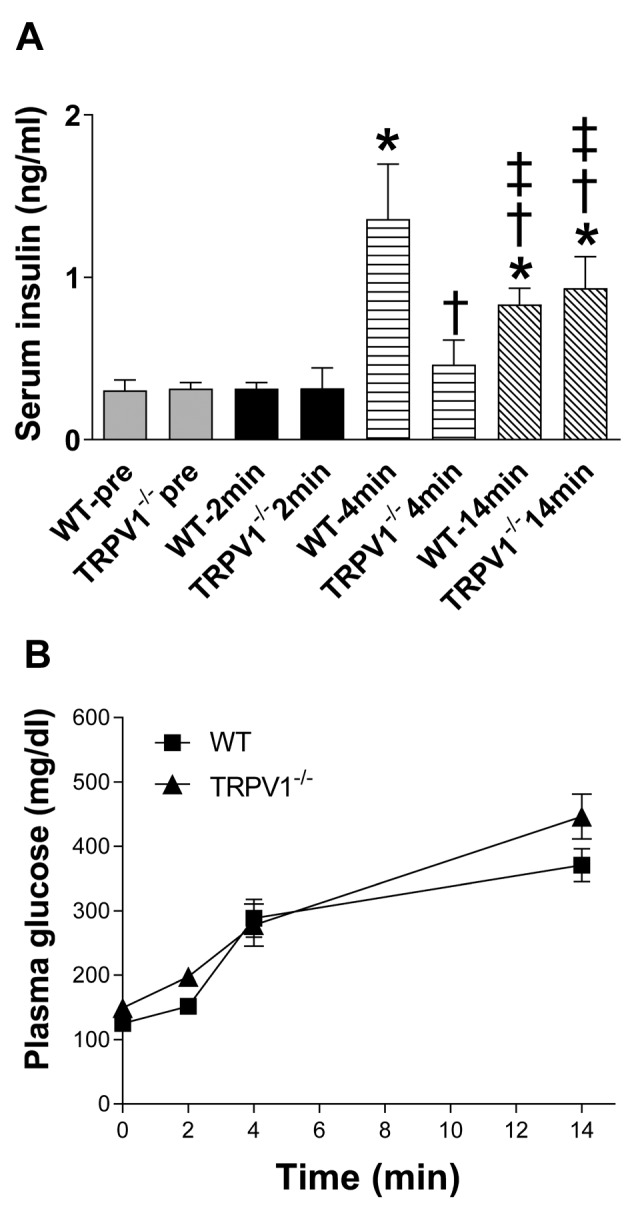
Figure 3. Effects of capsaicin on insulin secretory response. Plasma levels of insulin were measured in WT and TRPV1–/– mice before and after i.p. injection of capsaicin (0.25 mg/kg body weight). Values are mean±SEM; n=8; *p<0.05 vs. WT-CON; †p<0.05 vs. TRPV1–/–-CON; ‡p<0.05 vs. WT-cap.
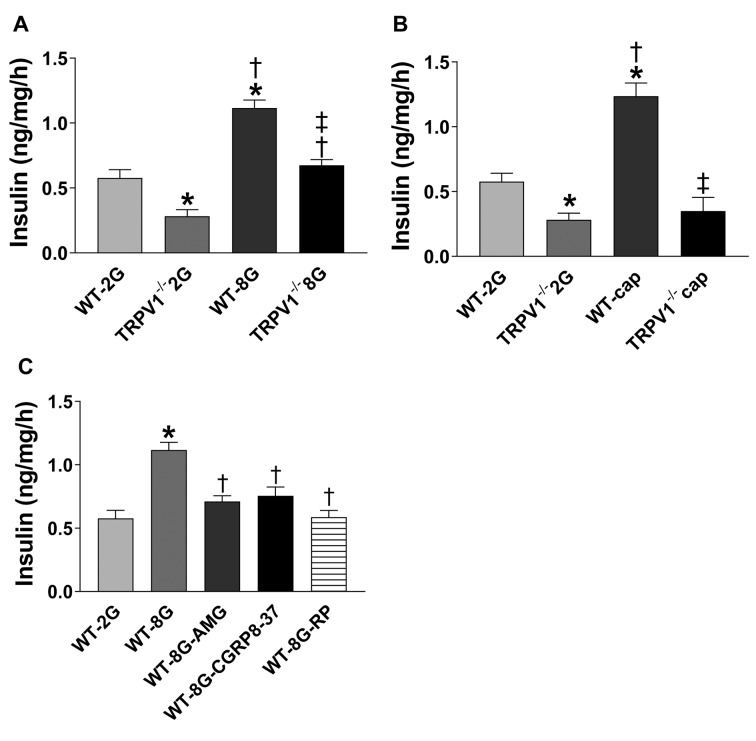
Decreased insulin secretion from TRPV1–/– pancreases. Next, insulin release from isolated pancreases was measured in vitro. Upon high glucose stimulation, insulin secretion was increased in both WT and TRPV1–/– pancreases, with higher levels in WT compared to TRPV1–/– pancreases (p<0.05, Figure 4A). Insulin release was increased in response to capsaicin stimulation in WT but not TRPV1–/– pancreases (Figure 4B). Inhibition of TRPV1, CGRP, or SP with AMG9810 (10–6 M, a selective TRPV1 antagonist), CGRP8-37 (10–6 M, a selective CGRP receptor antagonist), or RP67580 (10–6 M, a selective NK1 receptor antagonist), respectively, abolished glucose-induced insulin secretion in WT pancreases (Figure 4C), indicating that TRPV1, CGRP, and SP mediate glucose-induced insulin secretion.
Figure 4. Glucose- and capsaicin-induced insulin secretion by pancreatic minces. Samples incubated in KRB medium containing 2 mmol/l glucose (2G) or 8 mmol/l glucose (8G) (A), or capsaicin (cap) (B). (C) AMG9810 (AMG, a selective TRPV1 antagonist), CGRP8-37 (a selective CGRP receptor antagonist), or RP67580 (RP, a selective NK1 receptor antagonist) was added 5 min before adding glucose (8 mmol/l). Values are mean±SEM; n=4; *p<0.05 vs. WT-2G; †p<0.05 vs. WT-8G (or TRPV1–/– 2G); ‡p<0.05 vs. WT-8G (or WT-cap).
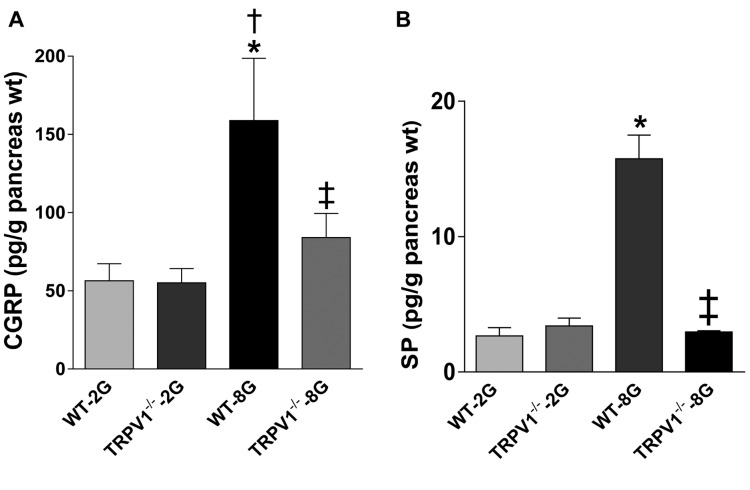
Impaired SP and CGRP release by TRPV1–/– pancreases. The release of CGRP and SP at baseline was not different between WT and TRPV1–/– pancreases (Figure 5A and B). Upon high glucose stimulation, CGRP and SP release was remarkably increased compare to baseline in WT pancreas (p<0.05), but was not increased in TRPV1–/– pancreases (Figure 5A and B).
Figure 5. The release of CGRP (A) and SP (B) from isolated pancreases. Samples incubated in KRB medium containing 2 mmol/l glucose (2G) or 8 mmol/l glucose (8G). Values are mean±SEM; n=4; *p<0.05 vs. WT-2G; †p<0.05 vs. TRPV1–/– 2G; ‡p<0.05 vs. WT-8G.
Discussion
This study provides evidence that TRPV1 plays an important regulatory role in glucose-induced insulin secretion. Genetic ablation of TRPV1 resulted in impaired glucose-induced insulin secretion and glucose intolerance. It is interesting to highlight that the metabolic changes observed in TRPV1–/– mice were different from previous reports obtained in experiments with sensory nerve desensitization by using resiniferatoxin or high doses of capsaicin (10). This inconsistency may be due to the fact that chemical denervation did not completely ablate the sensory nerves. The remaining sensory nerves may be sufficient to compensate through upregulating and sensitizing TRPV1 channels. This hypothesis is supported by previous studies showing that capsaicin pretreatment resulted in a significant upregulation of TRPV1 (17).
The present study showed that the first phase of insulin secretory response to glucose disappeared in TRPV1–/– mice. Glucose induces a biphasic insulin secretion (18). Previous studies have shown that TRPV1-expressing sensory nerves innervate the pancreatic islets (19,20). TRPV1 expression and function were decreased in high-glucose conditions and in obesity and diabetes (21). The first phase of insulin secretion is lost in obesity and diabetes (22). Present data showed that both WT and TRPV1–/– pancreases had increased insulin secretion when glucose concentration was increased from 2 to 8 mmol/l, while insulin secretion was higher in WT compared to TRPV1–/– pancreases. Inhibition of TRPV1 with AMG 9810 inhibited glucose-induced insulin secretion in WT pancreases, indicating that TRPV1 mediates glucose-induced insulin secretion. The mechanism underlying the effects of TRPV1 on glucose-induced insulin secretion is largely unknown. A previous study has demonstrated that TRPV1 regulated glucose homeostasis through the induction of glucagon-like peptide-1 secretion from the gut (23). The present study focused on the role of neuropeptides released from pancreatic TRPV1-expressing sensory nerves.
CGRP has been identified in sensory nerve endings which innervate the pancreas (24). CGRP-like immunoreactivity has been demonstrated in pancreatic perivascular nerve fibers and in the islet insulin cells (25). However, the results on the effect of CGRP on insulin secretion are inconsistent (6,26). Studies conducted by Rasmussen et al. have shown that CGRP at 10–10 and 10–9 mol/l increased insulin and glucagon secretion from isolated, perfused porcine pancreas, whereas significant decreases were observed with 10–8 mol/l (26). In contrast, Hermansen et al. have shown that CGRP inhibited insulin secretion at 10–10 mol/l, whereas stimulated insulin secretion at 10–9 mol/l, and the stimulation was marked and sustained at 10–8 mol/l in the perfused dog pancreas (6). In many studies, CGRP was infused directly into the superior pancreatic artery at a low dose rate. Given that CGRP is the most potent vasodilator agonist (27), infused CGRP might cause a severe fall in blood pressure. That might be the reason why insulin release was decreased. Our studies showed that glucose (8 mmol/l) increased insulin secretion from pancreases, and this effect was inhibited by the CGRP receptor antagonist. Radioimmunoassay also showed that the release of CGRP was higher in WT than in TRPV1–/– pancreases after stimulation with glucose, indicating that CGRP participates in glucose-induced insulin secretion.
SP may also be important in insulin secretion (28). Brown et al. (29) have shown that SP caused hypoinsulinemia, hyperglucagonemia, and hyperglycemia in rats. Fu et al. (30) have studied the effects of SP on insulin release from cultured rat islets, indicating that SP stimulated insulin secretion in a dose-dependent manner. Hermansen et al. (8) have shown that SP increased insulin release in a dose-dependent manner at high glucose conditions, but not at low glucose conditions. Moreover, Kaneto et al. have reported that endogenously released SP may stimulate insulin secretion (7). Chiba et al. (31) have studied the effects of SP on insulin release in rats and dogs under the same conditions. In rats, SP inhibited insulin secretion in a dose-dependent manner. By contrast, in dogs, SP increased insulin secretion. These results demonstrated that the effects of SP on insulin secretion are dependent on species and glucose concentrations. Our studies showed that glucose increased insulin secretion from the pancreas, and this effect was attenuated by the NK1 receptor antagonist. Radioimmunoassay also showed that the release of SP was higher in WT than in TRPV1–/– pancreases after glucose stimulation. It is well established that activation of TRPV1 in sensory nerve endings stimulates SP release. Taken together, these findings suggest that SP mediates the effects of TRPV1 on glucose-induced insulin secretion.
Conclusion
In conclusion, our findings indicate that TRPV1 mediates glucose-induced insulin secretion likely through the release of CGRP and SP.
Conflicts of Interest
There are no conflicts of interest to declare regarding this study.
Authors’ Contributions
BZ, DHW: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; SM: data interpretation and manuscript revision; BZ, SM, DHW: final approval of manuscript.
Acknowledgements
This work was supported in part by the National Institutes of Health (grants HL-57853, HL-73287, and DK67620).
References
- 1.Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–225. doi: 10.1016/j.bbrc.2004.06.149. PMID: 15358238. [DOI] [PubMed] [Google Scholar]
- 2.Hiriart M, Aguilar-Bryan L. Channel regulation of glucose sensing in the pancreatic beta-cell. Am J Physiol Endocrinol Metab. 2008;295:E1298–E1306. doi: 10.1152/ajpendo.90493.2008. PMID: 18940941. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. PMID: 10764638. DOI: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 4.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. PMID: 11557989. DOI: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 5.Lam D, Momeni Z, Theaker M, Jagadeeshan S, Yamamoto Y, Ianowski JP, Campanucci VA. RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS One. 2018;13:e0193312–e0193312. doi: 10.1371/journal.pone.0193312. PMID: 29474476. DOI: 10.1371/journal.pone.0193312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermansen K, Ahren B. Dual effects of calcitonin gene-related peptide on insulin secretion in the perfused dog pancreas. Regul Pept. 1990;27:149–157. doi: 10.1016/0167-0115(90)90213-g. PMID: 1968674. [DOI] [PubMed] [Google Scholar]
- 7.Kaneto A, Kaneko T, Kajinuma H, Kosaka K. Effects of substance P and neurotensin infused intrapancreatically on glucagon and insulin secretion. Endocrinology. 1978;102:393–401. doi: 10.1210/endo-102-2-393. PMID: 743963. [DOI] [PubMed] [Google Scholar]
- 8.Hermansen K. Effects of substance P and other peptides on the release of somatostatin, insulin, and glucagon in vitro. Endocrinology. 1980;107:256–261. doi: 10.1210/endo-107-1-256. PMID: 6155261. [DOI] [PubMed] [Google Scholar]
- 9.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17–17. doi: 10.1186/1744-8069-1-17. PMID: 15857517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson S, Scheurink AJ, Steffens AB, Ahren B. Involvement of capsaicin-sensitive nerves in regulation of insulin secretion and glucose tolerance in conscious mice. Am J Physiol. 1994;267:R1071–R1077. doi: 10.1152/ajpregu.1994.267.4.R1071. PMID: 7943418. [DOI] [PubMed] [Google Scholar]
- 11.van de Wall EH, Gram DX, Strubbe JH, Scheurink AJ, Koolhaas JM. Ablation of capsaicin-sensitive afferent nerves affects insulin response during an intravenous glucose tolerance test. Life Sci. 2005;77:1283–1292. doi: 10.1016/j.lfs.2005.03.011. PMID: 15939440. [DOI] [PubMed] [Google Scholar]
- 12.Gram DX, Hansen AJ, Wilken M, Elm T, Svendsen O, Carr RD, Ahren B, Brand CL. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol. 2005;153:963–969. doi: 10.1530/eje.1.02046. PMID: 16322403. [DOI] [PubMed] [Google Scholar]
- 13.Radu BM, Iancu AD, Dumitrescu DI, Flonta ML, Radu M. TRPV1 properties in thoracic dorsal root ganglia neurons are modulated by intraperitoneal capsaicin administration in the late phase of type-1 autoimmune diabetes. Cell Mol Neurobiol. 33:187–196. doi: 10.1007/s10571-012-9883-6. PMID: 23111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolan I, Ragoobirsingh D, Morrison EY. Isolation and purification of the hypoglycaemic principle present in Capsicum frutescens. Phytother Res. 2004;18:95–96. doi: 10.1002/ptr.1328. PMID: 14750210. [DOI] [PubMed] [Google Scholar]
- 15.Chu ZL, Carroll C, Chen R, Alfonso J, Gutierrez V, He H, Lucman A, Xing C, Sebring K, Zhou J, Wagner B, Unett D, Jones RM, Behan DP, Leonard J. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol. 24:161–170. doi: 10.1210/me.2009-0239. 19901198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coore HG, Randle PJ. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964;93:66–78. doi: 10.1042/bj0930066. PMID: 5320084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zvara A, Bencsik P, Fodor G, Csont T, Hackler L Jr., Dux M, Furst S, Jancso G, Puskas LG, Ferdinandy P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. Faseb J. 2006;20:160–162. doi: 10.1096/fj.05-4060fje. PMID: 16278290. [DOI] [PubMed] [Google Scholar]
- 18.Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. The Cell Physiology of Biphasic Insulin Secretion. News Physiol Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. PMID: 11390882. [DOI] [PubMed] [Google Scholar]
- 19.Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. PMID: 24855942. DOI: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. PMID: 17174891. DOI: 10.1016/j.cell.2006. 10.038. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Pu Y, Wang P, Chen S, Zhao Y, Liu C, Shang Q, Zhu Z, Liu D. TRPV1-mediated UCP2 upregulation ameliorates hyperglycemia-induced endothelial dysfunction. Cardiovasc Diabetol. 2013;12:69–69. doi: 10.1186/1475-2840-12-69. PMID: 23607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164–174. doi: 10.1002/dmrr.198. PMID: 11424229. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, Ma L, Zhao Z, Liu D, Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–2165. doi: 10.2337/db11-1503. PMID: 22664955. DOI: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sternini C, De Giorgio R, Brunicardi FC, Widdison AL, Reber HA, Go VL. CGRP immunoreactivity in the mammalian pancreas. Ann NY Acad Sci. 1992;657:487–490. doi: 10.1111/j.1749-6632.1992.tb22808.x. PMID: 1637106. [DOI] [PubMed] [Google Scholar]
- 25.Ahren B, Sundler F. Localization of calcitonin gene-related peptide and islet amyloid polypeptide in the rat and mouse pancreas. Cell Tissue Res. 1992;269:315–322. doi: 10.1007/BF00319623. PMID: 1423499. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen TN, Bersani M, Schmidt P, Thim L, Kofod H, Jorgensen PN, Poulsen SS, Holst JJ. Isolation and molecular characterization of porcine calcitonin gene-related peptide (CGRP) and its endocrine effects in the porcine pancreas. Pancreas. 1998;16:195–204. doi: 10.1097/00006676-199803000-00014. PMID: 9510144. [DOI] [PubMed] [Google Scholar]
- 27.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. PMID: 15269340. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt PT, Tornoe K, Poulsen SS, Rasmussen TN, Holst JJ. Tachykinins in the porcine pancreas: potent exocrine and endocrine effects via NK-1 receptors. Pancreas. 2000;20:241–247. doi: 10.1097/00006676-200004000-00004. PMID: 10766449. [DOI] [PubMed] [Google Scholar]
- 29.Brown M, Vale W. Effects of neurotensin and substance P on plasma insulin, glucagon and glucose levels. Endocrinology. 1976;98:819–822. doi: 10.1210/endo-98-3-819. PMID: 1261503. [DOI] [PubMed] [Google Scholar]
- 30.Fu XW, Sun AM. Stimulative effect of substance P on insulin secretion from isolated rat islets under normobaric oxygen incubation. Zhongguo Yao Li Xue Bao. 1989;10:69–73. PMID: 2479220. [PubMed] [Google Scholar]
- 31.Chiba Y, Kawai K, Okuda Y, Munekata E, Yamashita K. Effects of substance P and substance P-(6-11) on hormone release from isolated perfused pancreas: their opposite actions on rat and canine islets. Endocrinology. 1985;117:1996–2000. doi: 10.1210/endo-117-5-1996. PMID: 2412802. [DOI] [PubMed] [Google Scholar]