Abstract
Low-dose rivaroxaban was effective in secondary prevention of atherosclerotic cardiovascular disease (ASCVD) in the COMPASS trial. There is no established role, however, for oral anticoagulants in primary prevention. We evaluated whether coronary artery calcium (CAC) scoring identifies a high-risk primary prevention adult population who may benefit from low-dose rivaroxaban to prevent ASCVD events. We modeled expected outcomes of low-dose rivaroxaban in 5196 Multi-Ethnic Study of Atherosclerosis (MESA) cohort participants not already on antiplatelet or anticoagulant therapy. We applied relative risk ratios from COMPASS to absolute MESA event rates to estimate number needed to treat (NNT) to avoid a composite of cardiovascular death, non-fatal MI, or non-fatal stroke, as well as number needed to harm (NNH) to cause 1 hospitalized bleed; stratified by calculated ASCVD risk and by baseline CAC. MESA participants with CAC ≥300 had crude ASCVD event rate of 20 per 1000 patient-years, comparable to that observed in the COMPASS control-arm. CAC was independently associated with the composite ASCVD outcome [p < 0.001 for trend]. However, CAC was not independently associated with adjusted hazard ratio for hospitalized major bleeding. Predicted 5-year NNT (modeled from COMPASS risk ratio) was 75 among persons with CAC 100 to <300 and 45 with CAC ≥300 despite NNH values of 252 and 98, respectively. In conclusion, CAC helps to distinguish estimated ASCVD benefit from estimated bleeding harm, thereby identifying high-risk adults without established cardiovascular disease who may derive net-benefit from low-dose rivaroxaban.
Keywords: cardiac computer tomographic (CT) imaging, cardiac risk factors and prevention, acute coronary syndromes
INTRODUCTION
Myocardial infarction represents a thrombotic event on the background of unstable atherosclerotic plaque. Though most clinical trials have focused on antiplatelet therapy to prevent atherosclerotic cardiovascular disease (ASCVD), the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial suggested a role for low-dose anticoagulants in stable ischemic heart disease.1 Up to 70% of ST-elevation myocardial infarctions, however, occur in those without previously identified ASCVD.2 We propose that the COMPASS secondary prevention strategy could potentially be of benefit in the primary prevention setting if a population of comparable atherosclerotic risk is identified.3–5 Achieving clinical benefit in primary prevention centers around appropriate risk stratification, particularly when therapy is associated with potential for harm, such as bleeding on anticoagulant therapy. Coronary Artery Calcium (CAC) scoring provides a window into true atherosclerotic burden and offers refined risk stratification beyond traditional risk calculators.6–8 Our goal was to determine whether individualized assessment, using both CAC and traditional risk estimation, might identify a high-risk population who are predicted to achieve net benefit from low-dose oral antithrombotic therapy as primary ASCVD prevention.
METHODS
We modeled the effect of low dose rivaroxaban in the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective, multicenter, multi-ethnic population free of clinical ASCVD at baseline. The MESA cohort of 6814 individuals was recruited between July 2000 and September 2002. Full details regarding MESA study design were published previously.9 Six US communities were chosen as field centers based on broad ethnic diversity: New York, NY, Baltimore, MD, St. Paul, MN, Chicago, IL, Los Angeles, CA, and Forsyth County, NC. Participants were between ages 45 and 84 at enrollment and self-identified with 1 of 4 ethnic groups: white, black, Hispanic, and Chinese. All provided written informed consent. The institutional review board at all participating institutions approved the study protocol.
Baseline examination included assessment of cardiovascular risk factors. Smoking status and family history of myocardial infarction or stroke were self-reported. Resting blood pressure was measured as the mean of the last 2 of 3 recordings after a minimum 5 minutes seated using a Dinamap Pro-100 automated oscillometric sphygmomanometer. Blood for laboratory testing was collected and processed at field centers and analyzed at the central MESA laboratory (University of Vermont, Burlington, VT). Diabetes mellitus was defined as either self-reported diagnosis, use of hypoglycemic medications, or fasting blood glucose ≥126 mg/dL. Fasting low-density lipoprotein cholesterol level (LDL-C) was calculated using the Friedewald equation. 10-year ASCVD risk was calculated using the Pooled Cohort Equations (PCE).10 Consistent with prior MESA analyses, Hispanic and Chinese participants were classified as white for the purpose of this calculator.10
Cardiac computed tomography (CT) was performed at baseline using either cardiac-gated electron-beam CT scanner (Chicago, Los Angles, New York) or multidetector CT system (Baltimore, Forsyth County, St. Paul). Full protocols for scanning and interpretation have been reported.11 Images were interpreted at the MESA CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA). All patients were scanned twice. CAC score was calculated by the Agatston method. The CAC score for each participant was recorded as the mean of both scores, with high intra- and inter-observer agreement (κ=0.93 and κ=0.90, respectively).6
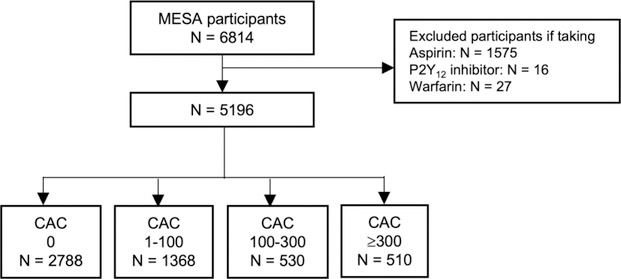
Our primary study population was the MESA primary prevention cohort. We excluded those who were prescribed anticoagulant or antiplatelet therapy at baseline (Figure 1). In a sensitivity analysis, we modeled the effect of adding low-dose rivaroxaban to aspirin monotherapy. We also identified a second exploratory study subsample of MESA participants who were on aspirin monotherapy at study enrollment. To facilitate comparison to COMPASS outcomes, we further limited this subsample to individuals who met all criteria for inclusion in the COMPASS trial with the exception of established atherosclerotic disease, given MESA is a primary prevention study (Supplemental Figure 1). Specifically, we included participants on aspirin monotherapy who also met any of the following criteria: (a) age >65 with 1 additional risk factor: CAC >0, carotid intimal medial thickness greater than population median, or ankle-brachial index ≤0.9 or ≥1.4; (b) age <65 and any 2 additional risk factors listed above; or (c) age <65 and 1 additional risk factor listed above, plus any of the following comorbid conditions: current smoking, diabetes mellitus, or estimated GFR (eGFR) <60 mL/min per 1.73 m2. Individuals with recent stroke or any history of hemorrhagic or lacunar stroke, severe heart failure (ejection fraction <30% or New York Heart Association class III or IV symptoms), evidence of liver dysfunction, or advanced stable kidney disease (eGFR <15 ml/min) were excluded from this subsample in accordance with COMPASS eligibility.1
Figure 1.
Patient selection from the MESA primary prevention cohort. CAC = Coronary Artery Calcium; MESA = Multi-Ethnic Study in Atherosclerosis.
Incidences of ASCVD events in MESA were documented at intervals of 9–12 months. Trained personnel called each subject or a family member to inquire about interim hospital admissions, outpatient diagnoses of ASCVD, and deaths. 92% of living participants completed telephone interviews. Medical records were successfully collected for 98% of hospital admissions and for 95% of reported outpatient ASCVD encounters. Two members of the MESA mortality and morbidity review committee independently reviewed each reported event with adjudication by the full committee in the event of disagreement.
To align with the primary COMPASS outcome, we defined ASCVD as a composite of cardiovascular death, non-fatal MI, or non-fatal stroke. To capture bleeding events, medical records of participants were searched for ICD-9 and ICD-10 codes indicative of fatal bleed or hospitalization for bleeding event (Supplemental Table 1).12,13 Additional details about MESA follow up methods and event reporting are available at http://www.mesa-nhlbi.org.
Count and percentage were calculated for categorical variables. For continuous variables, either mean ± standard deviation or median ± interquartile range were determined in the case of normal or non-normal distributions, respectively. Continuous variables were compared using ANOVA or Kruskal Wallis test as appropriate. Categorical variables were compared using the chi-squared test. Study participants were stratified based on CAC score, with subgroups delineated at CAC 0, CAC 1 to <100, CAC 100 to <300, and CAC ≥300.6 We further stratified based on calculated 10-year ASCVD risk using the PCE into 3 groups: those with 10-year estimated risk <10%, 10–20%, and ≥20%.
Absolute 2-year composite ASCVD outcome and major bleeding event rates were calculated for comparison to the COMPASS population event rates. Cox regression modeling was used to obtain multivariable-adjusted hazard ratios for the composite ASCVD outcome in each group with further determination of Kaplan-Meier estimates of cumulative event-free survival in each group. Models were adjusted for age, sex, BMI, race/ethnicity, MESA site, presence of diabetes mellitus, baseline systolic blood pressure, antihypertensive therapy, smoking status, creatinine, baseline LDL-C, lipid-lowering medication, high-sensitivity C-reactive protein, and family history of myocardial infarction or stroke.
The number-needed-to-treat (NNT) to prevent 1 ASCVD outcome at 5 years was calculated according to the Bland-Altman method using survival probability of ASCVD at 5 years in each MESA subgroup and expected relative risk reduction for ASCVD.1,14 Expected relative risk reduction was extrapolated from the independent effect of low-dose rivaroxaban in the COMPASS trial: 24%.1,14 We also reported NNT at 2 years to align with the COMPASS trial duration. Using the same methods, we performed sensitivity analyses in the COMPASS-eligible sub-sample.
Within each risk subgroup, we used identical methods to those used for NNT to determine number-needed-to-harm (NNH) for 1 hospitalized major bleeding event. We extrapolated absolute risk increase by multiplying the observed absolute risk for bleeding within each subgroup by the relative risk increase for major bleeding attributable to the addition of low-dose rivaroxaban reported in COMPASS: 70%.1,7 We then used the same method to calculate net clinical benefit within each risk subgroup, defined as the avoidance of composite ASCVD outcome or hospitalized major bleeding event. This number was derived from absolute baseline ischemic and bleeding risks and relative change in net clinical benefit with addition of rivaroxaban therapy in the COMPASS trial: 20%.1,7
RESULTS
Baseline characteristics of the overall MESA study sample are displayed in Table 1. There was a crude association between higher CAC scores and other known comorbid ASCVD risk factors: older age, male sex, white race, need for antihypertensive or lipid-lowering medications, and family history of myocardial infarction [p <0.001 for each].
Table 1.
Baseline demographic and clinical characteristics of the primary study population
| Variable | Study population (N = 5196) | CAC 0 (N = 2788) | CAC 1 to <100 (N = 1368) | CAC 100 to <300 (N = 530) | CAC ≥300 (N = 510) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 61 ± 10 | 57 ± 9 | 63 ± 10 | 67 ± 9 | 70 ± 8 | <0.001 |
| Men | 2337 (45%) | 992 (36%) | 709 (52%) | 296 (56%) | 340 (67%) | <0.001 |
| Race/Ethnicity | <0.001 | |||||
| White | 1770 (34%) | 823 (30%) | 478 (35%) | 224 (42%) | 245 (48%) | |
| Chinese | 682 (13%) | 358 (13%) | 199 (15%) | 79 (15%) | 46 (9%) | |
| African American | 1499 (29%) | 888 (32%) | 372 (27%) | 124 (23%) | 115 (23%) | |
| Hispanic | 1245 (24%) | 719 (26%) | 319 (23%) | 103 (19%) | 104 (20%) | |
| Diabetes mellitus | 589 (11%) | 241 (9%) | 174 (13%) | 83 (16%) | 91 (18%) | <0.001 |
| Body mass index (kg/m2) | 28.3 ± 5.6 | 28.3 ± 5.7 | 28.2 ± 5.4 | 28.3 ± 5.7 | 28.5 ± 5.1 | 0.82 |
| Systolic blood pressure (mm Hg) | 126 ± 21 | 122 ± 20 | 128 ± 21 | 132 ± 22 | 135 ± 22 | <0.001 |
| Antihypertensive medication use | 1731 (33%) | 737 (26%) | 512 (37%) | 219 (41%) | 263 (52%) | <0.001 |
| Current smoker | 714 (14%) | 373 (13%) | 199 (15%) | 73 (14%) | 69 (14%) | <0.001 |
| Creatinine (mg/dL)* | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 1.0 (0.9–1.1) | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 118 ± 32 | 117 ± 31 | 121 ± 33 | 121 ± 32 | 118 ± 33 | 0.001 |
| Lipid-lowering medication use | 665 (13%) | 246 (9%) | 203 (15%) | 100 (19%) | 116 (23%) | <0.001 |
| High-sensitivity C-reactive protein (mg/dL)* | 1.9 (0.8 – 4.3) | 1.9 (0.8 – 4.4) | 2.0 (0.9 – 4.3) | 1.9 (0.9 – 4.5) | 1.9 (0.8 – 4.1) | 0.69 |
| Family history of myocardial infarction | 1991 (41%) | 960 (36%) | 555 (43%) | 221 (45%) | 255 (55%) | <0.001 |
Results are stratified by CAC. Categorical variables are summarized as count (percentage). Continuous variables are summarized as mean (standard deviation) or median (interquartile range)* depending on the normality of the data. CAC = Coronary Artery Calcium.
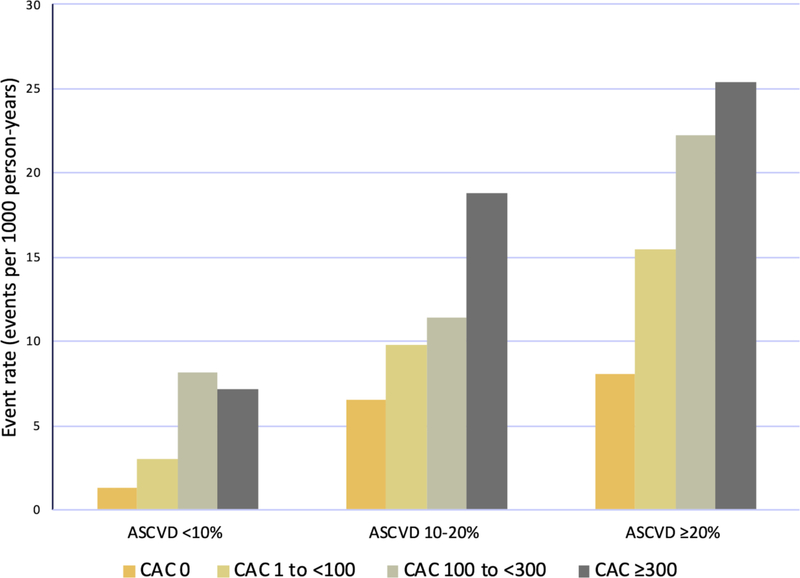
In our sample, CAC score was independently associated with observed ASCVD events (Table 2). The composite ischemic outcome occurred at a rate of 20.1 events / 1000 person-years with CAC ≥300, compared with 2.7 / 1000 person-years with CAC 0, resulting in a nearly 3-fold adjusted hazard ratio in the highest CAC subset. Within each PCE-estimated ASCVD risk subgroup, CAC provided further risk information (Figure 2). For example, in the MESA subgroup with estimated ASCVD risk of ≥20% but CAC 0, ischemic events were less frequent than would have been predicted, compared with the substantial ASCVD event rate in the subgroup with calculated risk ≥20% and CAC ≥300.
Table 2.
Composite atherosclerotic cardiovascular disease outcomes and hospitalized major bleeding events in the Multi-Ethnic Study in Atherosclerosis study population.
| ASCVD event | Major bleeding event | ||||
|---|---|---|---|---|---|
| CAC | n (%) | Event Rate | HR (95% CI)* | Event Rate | HR (95% CI)* |
| Overall Study Population (N = 5196) | |||||
| 0 | 2788 (54%) | 2.7 | 1 (ref) | 1.8 | 1 (ref) |
| 1 to <100 | 1368 (26%) | 7.7 | 1.8 (1.29,2.43) | 3.3 | 1.4 (0.91,2.02) |
| 100 to <300 | 530 (10%) | 14.5 | 2.7 (1.88,3.86) | 3.6 | 1.2 (0.70,2.08) |
| ≥300 | 510 (10%) | 20.1 | 2.9 (2.03,4.25) | 5.4 | 1.5 (0.88,2.56) |
| p-value | – | <0.001 | <0.001 | <0.001 | 0.16 |
| ASCVD <10% (N = 2908) | |||||
| 0 | 2047 (70%) | 1.3 | 1 (ref) | 1.3 | 1 (ref) |
| 1 to <100 | 634 (22%) | 3.0 | 1.7 (0.91,3.04) | 2.0 | 1.2 (0.64,2.22) |
| 100 to <300 | 143 (5%) | 8.1 | 3.9 (1.89,8.15) | 3.8 | 1.9 (0.77,4.79) |
| ≥300 | 84 (3%) | 7.2 | 3.3 (1.28,8.48) | 0.9 | 0.5 (0.06,3.74) |
| p-value | – | <0.001 | 0.048 | 0.64 | |
| ASCVD 10–20% (N = 1159) | |||||
| 0 | 451 (39%) | 6.6 | 1 (ref) | 2.5 | 1 (ref) |
| 1 to <100 | 393 (34%) | 9.8 | 1.5 (0.89,2.46) | 3.9 | 1.6 (0.74,3.31) |
| 100 to <300 | 174 (15%) | 11.4 | 2.0 (1.05,3.72) | 1.0 | 0.5 (0.10,2.05) |
| ≥300 | 141 (12%) | 18.8 | 2.3 (1.19,4.39) | 2.6 | 0.9 (0.24,3.55) |
| p-value | – | 0.001 | 0.01 | 0.21 | 0.65 |
| ASCVD ≥20% (N = 1129) | |||||
| 0 | 290 (26%) | 8.1 | 1 (ref) | 4.4 | 1 (ref) |
| 1 to <100 | 341 (30%) | 15.5 | 1.7 (0.97,2.97) | 5.6 | 1.4 (0.65,3.03) |
| 100 to <300 | 213 (19%) | 22.2 | 2.7 (1.50,4.72) | 5.8 | 1.5 (0.62,3.42) |
| ≥300 | 285 (25%) | 25.4 | 3.2 (1.83,5.53) | 8.6 | 2.0 (0.90,4.24) |
| p-value | – | <0.001 | <0.001 | 0.18 | 0.09 |
Results are reported for the primary study population (not on aspirin or anticoagulant at baseline). Results are stratified by CAC and 10-year ASCVD risk calculated by the Pooled Cohort Equations. Outcomes are expressed as event rates (per 1000 person-years) and hazard ratios (95% CI). Model is adjusted for age, sex, body mass index, race/ethnicity, education, MESA site, diabetes status, baseline systolic blood pressure, antihypertensive therapy use, tobacco use, creatinine, baseline low-density lipoprotein cholesterol, lipid-lowering medication, high-sensitivity C-reactive protein, and family history of myocardial infarction. ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; HR = hazard ratio; MESA = Multi-Ethnic Study in Atherosclerosis
Figure 2.
Event rate for composite ASCVD outcome in MESA participants stratified by ASCVD risk prediction score by PCE and further stratified by CAC. ASCVD = Atherosclerotic Cardiovascular Disease; CAC = Coronary Artery Calcium; MESA = Multi-Ethnic Study in Atherosclerosis; PCE= Pooled Cohort Equations.
Table 3 demonstrates the 2-year and 5-year NNT predicted by our modelling to prevent 1 composite ASCVD event if low-dose rivaroxaban were used as antithrombotic therapy in the MESA population. Higher CAC was correlated with lower 5-year NNT to prevent the composite ischemic outcome of cardiovascular death, non-fatal MI, or non-fatal stroke. Notably, estimated 5-year NNT to avoid 1 ASCVD event reached 75 with CAC 100 to <300 and 45 with CAC ≥300.
Table 3.
Estimated number needed to treat for composite atherosclerotic cardiovascular disease outcome and number needed to harm for hospitalized major bleeding event with very low dose rivaroxaban as primary prevention in Multi-Ethnic Study in Atherosclerosis participants
| CAC | Estimated 5-Year NNT | Estimated 5-Year NNH | Estimated 2-Year NNT | Estimated 2-Year NNH |
|---|---|---|---|---|
| Overall Study Population (N = 5196) | ||||
| 0 | 387 | 388 | 1043 | 1356 |
| 1 to <100 | 118 | 170 | 370 | 470 |
| 100 to <300 | 75 | 252 | 219 | 643 |
| ≥300 | 45 | 98 | 84 | 174 |
| ASCVD <10% (N = 2908) | ||||
| 0 | 1390 | 610 | 8335 | 2440 |
| 1 to <100 | 317 | 298 | 870 | 763 |
| 100 to <300 | 119 | 172 | 198 | 349 |
| ≥300 | 87 | – | 117 | – |
| ASCVD 10–20% (N = 1159) | ||||
| 0 | 180 | 263 | 614 | 1109 |
| 1 to <100 | 79 | 117 | 229 | 317 |
| 100 to <300 | 89 | – | 240 | – |
| ≥300 | 63 | 326 | 143 | 326 |
| ASCVD ≥20% (N = 1129) | ||||
| 0 | 88 | 136 | 168 | 354 |
| 1 to <100 | 74 | 131 | 272 | 414 |
| 100 to <300 | 55 | 169 | 220 | 509 |
| ≥300 | 35 | 60 | 65 | 114 |
Results are stratified by baseline CAC and further stratified by 10-year ASCVD risk calculated using the Pooled Cohort Equations. Calculations were based on baseline absolute ASCVD risk and bleeding risk and relative risk derived from the COMPASS trial outcome. Participants in the primary analysis were not on aspirin or anticoagulants at baseline. ASCVD = Atherosclerotic Cardiovascular Disease; CAC = Coronary Artery Calcium; NNH = number needed to harm; NNT = number needed to treat.
While higher CAC was associated with hospitalized major bleeding in crude analyses, after adjustment there was no independent association between CAC level and the bleeding outcome (Table 2). When modelling the effect of low-dose rivaroxaban therapy, higher CAC was also correlated with estimated 5-year NNH (an unadjusted parameter) for bleeding risk (Table 3). However, there was a greater relative gain in estimated ischemic benefit compared to concurrent bleeding risk for low-dose rivaroxaban at higher CAC scores. Indeed, when considering the ratio between estimated NNT and NNH at each CAC level, the ischemic benefit and hemorrhagic risk were essentially equivalent with CAC 0, compared to a 2–3-fold ratio of higher predicted NNH compared to NNT with CAC ≥100. Risk stratification by CAC led to greater separation of observed risk curves in the high-risk subset when compared to risk stratification by PCE (Figure 3).
Figure 3.
Comparison between event rate for composite ASCVD outcome and event rate for hospitalized major bleeding event (right axis) in MESA participants and comparison between predicted 5-year NNT to prevent 1 ischemic event and 5-year NNH to cause 1 bleeding event with rivaroxaban (left axis), stratified by (a) baseline CAC and (b) ASCVD risk prediction score by PCE. ASCVD = atherosclerotic cardiovascular disease; CAC = Coronary Artery Calcium; MESA = Multi-Ethnic Study in Atherosclerosis; NNH = number needed to harm; NNT = number needed to treat; PCE = Pooled Cohort Equations.
Using the relative risk reduction of 20% for net-clinical-benefit achieved by addition of low-dose rivaroxaban therapy in the COMPASS trial, the greatest 2-year and 5-year net benefit in the MESA population was predicted in those with CAC ≥100 (Table 4). Estimated 5-year net benefit reached 85 with CAC 100 to <300 and 49 with CAC ≥300.
Table 4.
Estimated net clinical benefit with use of very low dose rivaroxaban as primary prevention in Multi-Ethnic Study in Atherosclerosis participants.
| CAC | Estimated 5-year NCB | Estimated 2-year NCB |
|---|---|---|
| Overall Study Population (N = 5196) | ||
| 0 | 338 | 1066 |
| 1 to <100 | 114 | 354 |
| 100 to <300 | 85 | 262 |
| ≥300 | 49 | 97 |
| ASCVD <10% (N = 2908) | ||
| 0 | 835 | 3335 |
| 1 to <100 | 278 | 783 |
| 100 to <300 | 103 | 179 |
| ≥300 | 138 | 210 |
| ASCVD 10–20% (N = 1159) | ||
| 0 | 167 | 737 |
| 1 to <100 | 76 | 241 |
| 100 to <300 | 106 | 288 |
| ≥300 | 69 | 138 |
| ASCVD ≥20% (N = 1129) | ||
| 0 | 92 | 203 |
| 1 to <100 | 74 | 236 |
| 100 to <300 | 66 | 349 |
| ≥300 | 37 | 75 |
Results are stratified by baseline CAC and further stratified by 10-year ASCVD risk calculated by the Pooled Cohort Equations. Net clinical benefit is defined as the avoidance of composite ASCVD outcome or hospitalized major bleeding event; number is derived from absolute baseline ischemic and bleeding risks and relative change in net clinical benefit with addition of rivaroxaban therapy in the COMPASS trial. Participants in the primary analysis were not on aspirin or anticoagulants at baseline. ASCVD = Atherosclerotic Cardiovascular Disease; CAC = Coronary Artery Calcium; NCB = net clinical benefit.
In a sensitivity analysis, CAC score also predicted observed event rate for the primary ischemic outcome in the MESA subsample prescribed aspirin for primary prevention and who meet the modified COMPASS eligibility criteria (Table 5). There were 19.8 events / 1000 person-years with CAC ≥ 300 compared with 4.8 events / 1000 person-years with CAC 0. The use of both CAC and PCE risk stratification provided further differentiation of observed risk in this subsample just as in the primary study group. Similar to the primary study sample, 5-year NNT and 5-year NNH predicted by our modelling for the addition of low-dose rivaroxaban were each progressively lower at higher CAC. A more favorable NNH:NNT ratio was also predicted at higher CAC, as described above, with estimated NNH:NNT ratio of 2–2.5 for those with non-zero CAC.
Table 5.
Estimated number needed to treat for composite atherosclerotic cardiovascular disease outcome and number needed to harm for hospitalized major bleeding event with the addition of very low dose rivaroxaban to aspirin monotherapy in the sub-population of Multi-Ethnic Study in Atherosclerosis participants prescribed aspirin at baseline and meeting modified COMPASS trial eligibility criteria.
| CAC | n | Event Rate | HR (95% CI)* | Estimated 5-Year NNT | Estimated 5-Year NNH | Estimated 2-Year NNT | Estimated 2-Year NNH |
|---|---|---|---|---|---|---|---|
| 0 | 223 | 4.8 | 1 (ref) | 459 | 176 | 928 | 543 |
| 1 to <100 | 350 | 6.9 | 1.2 (0.57,2.66) | 103 | 207 | 161 | 842 |
| 100 to <300 | 197 | 15.6 | 2.3 (1.09,5.00) | 62 | 153 | 270 | 461 |
| ≥300 | 286 | 19.8 | 2.5 (1.17,5.17) | 56 | 107 | 163 | 678 |
| p-value | <0.001 | 0.003 |
Results are stratified by baseline CAC. ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; HR = hazard ratio; NNH = number needed to harm; NNT = number needed to treat.
DISCUSSION
Using observed primary prevention MESA cohort outcomes and modeling the potential effect of low-dose rivaroxaban therapy, we demonstrate that combined risk stratification using CAC and PCE can identify a sub-population at sufficiently high ASCVD risk to consider further investigation of low-dose thromboprophylaxis as primary prevention.
Modeling outcome data from MESA participants with CAC ≥100 and an expected 24% ASCVD relative risk reduction extrapolated from the COMPASS trial, we estimate that treating <75 high-risk individuals (e.g., those with CAC ≥100 and calculated ASCVD risk ≥10%) with low-dose antithrombotic therapy for 5 years would prevent 1 ischemic event. In the highest CAC category (score ≥300), estimated NNT-5 reached as low as 45 with a favorable ratio of benefit compared to attributable hospitalized major bleeding events.
Event rates in MESA participants with either CAC ≥300 (irrespective of ASCVD risk level), or in participants with both CAC ≥100 and ASCVD risk ≥10%, both approached those observed in the COMPASS control group (5.4% over mean 23 months of follow up, equivalent to 28.4 events / 1000 person-years). Consequently, the estimated 2-year NNT of 84 to prevent ischemic event with addition of low-dose rivaroxaban in participants with CAC ≥300 is comparable to the 2-year NNT for the addition of low-dose rivaroxaban to aspirin in the COMPASS secondary prevention population: 76.9.1
A noteworthy finding of our analysis was that the margin of modeled ASCVD benefit to modeled bleeding harm with low-dose rivaroxaban improved among higher CAC groups. Furthermore, CAC was not independently associated with major bleeding. Therfore, CAC appeared superior to the PCE in identifying those more likely to benefit from low-dose rivaroxaban than to experience harm in the form of major bleeding. A potential explanation is that age is a key driver of risk in the PCE, a factor also associated with increased incidence and morbidity of bleeding events.15 Though age-based modeling is outside the scope of this study, further refining traditional scores by measuring subclinical disease (i.e., CAC) may identify a population most likely to gain net benefit from interventions with bleeding risk.
Currently, antithrombotic therapy does not have a widely established clinical role for either primary or secondary prevention, with early secondary prevention trials limited by high rates of major bleeding with use of either full-dose anticoagulants or low-dose anticoagulants added to dual antiplatelet therapy.16–19 The COMPASS trial used low-dose rivaroxaban added to low dose aspirin in an effort to more appropriately balance competing ischemic and hemorrhagic outcomes.1 This lower-dose strategy has, in fact, been previously evaluated in those without established heart disease. In the Thrombosis Prevention Trial, combined aspirin and low dose warfarin (mean INR 1.47) led to a 34% reduction in ischemic events compared with placebo, with a 21% relative risk reduction attributed to warfarin in the factorial design, similar to the 24% risk reduction observed with addition of warfarin to aspirin monotherapy in COMPASS.20 However, high absolute bleeding risk was felt to be unacceptable in the Thrombosis Prevention Trial in the setting of lower baseline event rates.
The utility of CAC for refining ASCVD risk stratification has been previously established.21–23 Our results add evidence that CAC appears useful for identifying those with the highest ASCVD event rates but who do not also have excessively high bleeding rates-thereby potentially enabling the identification of persons who have more to gain from low-dose rivaroxaban than have to lose in terms of absolute bleeding risk. Furthermore, CAC can also identify persons at lower risk for bleeding on low-dose rivaroxaban than the typical NNH reported for aspirin in primary prevention. The estimated NNH-2 to cause a major bleed in the high-risk subgroups of our MESA analysis (e.g., CAC ≥100 and calculated ASCVD risk ≥10%) ranged from 114–509. For indirect comparison, in a decision analysis study for the U.S. Preventive Services Task Force, the NNH to cause 1 non-fatal GI bleed or intra-cranial hemorrhage for individuals recommended aspirin therapy ranged from as low as 32 to 47.24
If the clinical benefit predicted by our modeling data is confirmed in a trial setting, low-dose rivaroxaban may find a role in primary prevention of ASCVD events in those felt to be at very high risk despite addressing other risk factors. The newest American College of Cardiology / American Heart Association prevention guidelines feature stepwise risk stratification to determine statin eligibility.25 It may be possible that, using traditional atherosclerotic and bleeding risk profiles in combination with CAC, those estimated to have very high ASCVD risk with favorable risk:benefit ratio will in the future be recommended low-dose rivaroxaban in addition to statin for primary prevention.
LIMITATIONS
We assumed relative risk reduction from therapeutic intervention (in this case low-dose rivaroxaban) to be preserved across the categories of baseline CAC. Nonetheless, it is well established that relative risk is stable across different categories of baseline absolute risk,14,26 and prior studies in MESA using this assumption have demonstrated that CAC may inform NNT estimation for primary prevention therapies.21–23 Furthermore, COMPASS subgroup analyses revealed that relative risk reduction achieved with rivaroxaban was consistent across demographic subgroups, irrespective of baseline risk.1,27,28 Nonetheless, the next logical step after this hypothesis-generating study is design of a controlled clinical trial evaluating the effect of low-dose antithrombotic therapy as high-risk primary prevention.
Our relative risks were derived from the relative risks associated with addition of rivaroxaban to aspirin monotherapy. We interpreted this as the additional benefit gained exclusively from the rivaroxaban component of combined therapy, though we cannot exclude the possibility of effect modification.
As MESA was designed to capture ASCVD events, major bleeding events were not adjudicated. Therefore, potential exists for incomplete recording of bleeding events, which would overestimate net benefit of intervention. However, use of ICD codes to track bleeding on anticoagulant therapy has been shown to have high negative predictive value at the expense of potential over-reporting.12,13 Indeed, our rate of 4.7 events / 1000 person-years is slightly higher than rates in other primary prevention trial control groups (aspirin trials).29,30
CONCLUSION
In individuals without known ASCVD, assessment of subclinical atherosclerosis using CAC, in combination with traditional risk estimation, identified a high-risk population who may gain net benefit from low-dose oral antithrombotic therapy in the primary prevention setting. This study contributes evidence to suggest that, as investigation into the role of preventive antithrombotic therapy progresses, the high-risk primary preventive population should be given consideration in addition to those with known prior atherosclerotic events.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
SOURCES OF FUNDING: The MESA study is supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Research Resources.
Footnotes
COMPETING INTERESTS: The authors state that there are no relevant financial relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S, COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 2.Miedema MD, Cohn JN, Garberich RF, Knickelbine T, Graham KJ, Henry TD. Underuse of cardiovascular preventive pharmacotherapy in patients presenting with ST-elevation myocardial infarction. Am Heart J 2012;164:259–67. [DOI] [PubMed] [Google Scholar]
- 3.Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;6736:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The ASCEND Study Collaborative, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. The ASCEND Study Collaborative Group. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 5.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, Storey E, Shah RC, Lockery JE, Tonkin AM, Newman AB, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Donnan GA, Gibbs P, Johnston CI, Ryan J, Radziszewska B, Grimm R, Murray AM, ASPREE Investigator Group. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N Engl J Med 2018;379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 7.Blankstein R, Budoff MJ, Shaw LJ, Goff DC, Polak JF, Lima J, Blumenthal RS, Nasir K. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2011;58:364–74. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary Artery Calcium Score and Risk Classification for Coronary Heart Disease Prediction. JAMA 2010;303:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV., Folsom AR, Greenland P, Jacobs DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 12.Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K. Chronic Kidney Disease and Risk for Gastrointestinal Bleeding in the Community: The Atherosclerosis Risk in Communities (ARIC) Study. Clin J Am Soc Nephrol 2016;11:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delate T, Jones AE, Clark NP, Witt DM. Assessment of the coding accuracy of warfarin-related bleeding events. Thromb Res 2017;159:86–90. [DOI] [PubMed] [Google Scholar]
- 14.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Geraghty OC, Mehta Z, Rothwell PM, Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017;390:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, Tijssen JGP, Van de Werf F, Wallentin L, RE-DEEM Investigators. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J 2011;32:2781–2789. [DOI] [PubMed] [Google Scholar]
- 17.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update. J Am Coll Cardiol 2011;58:2432–2446. [DOI] [PubMed] [Google Scholar]
- 18.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L, APPRAISE-2 Investigators. Apixaban with Antiplatelet Therapy after Acute Coronary Syndrome. N Engl J Med 2011;365:699–708. [DOI] [PubMed] [Google Scholar]
- 19.Mega JL, Braunwald E, Wiviott SD, Bassand J-P, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM, ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 20.The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998;351:233–41. [PubMed] [Google Scholar]
- 21.McEvoy JW, Martin SS, Dardari ZA, Miedema MD, Sandfort V, Yeboah J, Budoff MJ, Goff DC, Psaty BM, Post WS, Nasir K, Blumenthal RS, Blaha MJ. Coronary Artery Calcium to Guide a Personalized Risk-Based Approach to Initiation and Intensification of Antihypertensive Therapy. Circulation 2017;135:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014;7:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O’Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehmer S, Maciosek M, Flottemesch T, LaFrance A, Whitlock E. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:777–86. [DOI] [PubMed] [Google Scholar]
- 25.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B, Ziaeian B, MEMBERS ATF, O’Gara PT, Beckman JA, Levine GN, Chair IP, Al-Khatib SM, Hlatky MA, Birtcher KK, Ikonomidis J, Cigarroa JE, Joglar JA, Deswal A, Mauri L, Fleisher LA, Piano MR, Gentile F, Riegel B, Goldberger ZD, Wijeysundera DN. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Coll Cardiol 2019:26029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furukawa TA, Guyatt H, Griffith LE. Can we individualize the ‘number needed to treat’? An empirical study of summary effect measures in meta-analyses. Int J Epidemiol 2002;31:72–76. [DOI] [PubMed] [Google Scholar]
- 27.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O’Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S, COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, Metsarinne K, O’Donnell M, Dans AL, Ha J-W, Parkhomenko AN, Avezum AA, Lonn E, Lisheng L, Torp-Pedersen C, Widimsky P, Maggioni AP, Felix C, Keltai K, Hori M, Yusoff K, Guzik TJ, Bhatt DL, Branch KRH, Cook Bruns N, Berkowitz SD, Anand SS, Varigos JD, Fox KAA, Yusuf S, COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:205–218. [DOI] [PubMed] [Google Scholar]
- 29.Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding Risks With Aspirin Use for Primary Prevention in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:826. [DOI] [PubMed] [Google Scholar]
- 30.DeBerardis G, Lucisano G, D’Ettorre A, Pellegrini F, Lepore V, Tognoni G, Nicolucci A. Association of Aspirin Use With Major Bleeding in Patients With and Without Diabetes. JAMA 2012;307:2286–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.