Abstract
Over the last two decades there has been a broad paradigm shift in our understanding of gastric cancer (GC) and its premalignant states from gross histological models to increasingly precise molecular descriptions. In this review we reflect upon the historic approaches to describing premalignant lesions and GC, highlight the current molecular landscape and how this could inform future risk assessment prevention strategies.
Keywords: Helicobacter pylori, Correa cascade, Atrophic gastritis, Intestinal metaplasia, Point of no return, Dysplasia, Stem cells, Gastric cancer
Core tip: Despite recent advances in our understanding of the molecular and cellular events involved in gastric cancer, little is known about how gastric premalignant lesions actually lead to this usually lethal disease (5 years survival about 20% in most Western countries). It is still not clear whether some or all of these lesions are directly involved in the process of gastric carcinogenesis or whether they are simply bystanders. In this review, we attempt to shed some light into how our current understanding of premalignant lesions may be used to improve patient stratification and lead to better overall patient survival rates.
INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer worldwide and third highest cause of cancer-related death. In 2012, 950000 individuals were diagnosed with the disease and 723000 died. High incidence areas are Eastern Asia, particularly China, Japan and South Korea, Eastern Europe, Central and South America. Low incidence areas are Australia and New Zealand, North America, Western Europe, South Central Asia and most parts of Africa[1]. Risk factors for GC include male sex, age, high salt intake, including salt preserved foods, smoked or dried meat and fish, pickled food, low intake of fresh fruit and vegetables, smoking, radiation exposure, low levels of physical activity, obesity and low socioeconomic status[2-15].
HISTOLOGICAL AND MOLECULAR CLASSIFICATIONS OF GC
The majority of GC are adenocarcinomas and these can be subdivided by the Lauren histopathology system into intestinal and diffuse subtypes[16]. The intestinal subtype of GC (IGC) is characterised by tumour cells that form gland-like structures whereas the diffuse subtype (DGC) has single or groups of tumour cells that are poorly differentiated or undifferentiated infiltrating the gastric wall. GC with components of both DGC and IGC are referred to as mixed. Given all three subtypes are adenocarcinomas this raises questions regarding the pre-malignant pathways and aetiologies of each. Given they arise from the same gastric inflammatory milieu are they a spectrum of the same disease with overlapping molecular identities or do they represent unique entities with disparate causes and premalignant pathways?
The Cancer Genome Atlas (TCGA) Research Network published a landmark study into molecular classification of established GC in 2014. The study performed integrative genomic and epigenomic analysis of 295 gastric adenocarcinomas and reported on four major subclasses based on somatic copy number, mutation analysis, methylation and gene expression status. These were named: Epstein Barr virus positive, microsatellite unstable (MSI), genomically stable (GS) and chromosomal unstable subtypes[17]. While there was significant overlap regarding the molecular signatures between the IGC, DGC and mixed types consistent with common aspects of oncogenesis, 75% of the DGCs were of the GS subclass suggesting a divergent pathway. The TCGA analysis also demonstrates the potential limitation of histological systems such as the Lauren classification, with cellular phenotypes often not reflecting the heterogeneous nature of complex underlying molecular changes.
There are a number of inherited genetic conditions that predispose to GC such as somatic mismatch repair mutations in Lynch Syndrome and CDH1 mutations in Hereditary Diffuse GC. Although these are of interest in elucidating the molecular pathways of oncogenesis, discussion of these conditions is largely outside the scope of this review.
THE CORREA CASCADE
Chronic gastric inflammation
In 1975 Correa et al[18] described a stepwise progression of conditions within the stomach that were thought to result in GC. This was one of the first considerations of premalignant conditions in this disease and it was later found to be initiated by Helicobacter pylori (H. pylori). The initial step in the Correa cascade is the development of Chronic gastritis (ChG). H. pylori represents the archetypal cause of ChG, with infected patients in some studies having a greater than 10-fold higher chance of developing GC[19]. The effects of H. pylori on the gastric epithelium have been extensively studied, with one of the most important pathogenic factors being cytotoxin-associated gene A protein (CagA) positive strains. Virtually all of East Asian strains and 60% of Western strains of H. pylori strains are cagA+, with infected patients developing more distinct inflammation, gastric ulceration and higher risk of GC[20-22]. Bacterial CagA protein interacts with a series of host epithelial proteins including ASPP2, RUNX3, PI3K, SHP2 and E-cadherin, resulting in the degradation and inactivation of p53 and RUNX3, deregulation of the PI3K-AKT, Ras-ERK and Wnt pathways and disruption of adherens junctions[23]. CagA has also been shown to alter DNA methylation patterns further deregulating normal epithelial gene expression patterns[24]. Intestinal metaplasia (IM) samples show higher levels of methylation than Atrophic gastritis (AG) samples, suggesting that DNA methylation pattern changes may play a vital role in the Correa model of IGC[24,25].
Autoimmune gastritis and atrophic gastritis
Autoimmune gastritis is a common aetiology of ChG, which results in activation of the adaptive immune system against parietal cells and intrinsic factor, leading to the destruction of the oxyntic gastric mucosa. As with other forms of chronic inflammation, autoimmune gastritis is a risk factor for GC through progression to intestinal metaplasia[26]. In a meta-analysis the overall relative risk of GC in patients with autoimmune gastritis was 6.8 (95%CI: 2.6–18.1)[27].
ChG leads to AG which refers to the atrophy and loss of gastric mucosal glands. Loss of specialised cells has significant implications on gastric function, with hypochlorhyria being one of the most recognised. In this state the loss of peptic acid production and raised gastric pH has implications on nutrient absorption (such as iron) and has significant implications on the gastric microbiome[28]. There has been considerable interest in the relationship between the gastric microbiome and GC, with a recent study uncovering dysbiosis of bacterial taxa along the Correa cascade[29]. At this stage it is uncertain if this dysbiosis represents a pre-malignant factor contributing to carcinogenesis in its own right, or simply a reflection of the change in the gastric microenvironment.
A key risk factor of chronic inflammation is the release of large amounts of reactive oxygen and nitrogen free species (ROS and NOS respectively), which are associated with DNA damage and increased mutation rates. Previous studies have shown that ROS and NOS released by inflammatory and epithelial cells can cause oxidative and nitrative DNA damage including the production of 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG), a known mutagen and 8-nitroguanine[30,31]. The latter is formed by inducible nitric oxide synthase iNOS. Gene expression of iNOS is regulated by the NF-κB and STAT pathways among others[32]. These changes can result in DNA mutations thus promoting cellular changes and carcinogenesis.
GASTRIC STEM CELLS AND IM
In normal gastric epithelium stem cell populations give rise to nascent epithelial cells that mature and differentiate as they migrate to the apex of the gland[33]. Gastric and intestinal stem cells share an endodermal lineage, and through the process of chronic inflammation gastric stem cells may reprogram, producing metaplastic intestinal-type epithelium that replaces the normal gastric mucosa[34]. The continuing chronic inflammatory process results in further accumulation of genetic lesions in stem cells, ultimately resulting in dysplasia and cancer. As such IM can be thought of as a marker of stem cell stress and damage, with multiple inflammatory aetiologies converging to histologically identical metaplastic change. There have been multiple gastric stem cell populations characterised including Lgr5+ stem cells in the adult antrum and the neonatal corpus and antrum, Mist1+ stem cells found in the isthmus region of the corpus glands and Troy+ stem cells that are thought to reside in the base of the corpus glands[33,35,36]. The role each of these plays in oncogenesis is an area of ongoing research. However, it is notable that different regions of the stomach have different stems cells and based on epidemiological evidence histological and molecular subgroups are found in different anatomic distributions suggesting a possible predetermined pathway for conversion to specific GC subgroups. For instance, TCGA found different anatomic distribution of molecular subgroups of GC with MSI being more likely to occur in the gastric corpus and antrum but rarely in the cardia[17].
IM
IM is usually found incidentally in patients undergoing upper endoscopy and is usually asymptomatic. While IM is defined by intestinal differentiation it is molecularly heterogeneous but can be histologically categorised as complete or incomplete subtypes (Figure 1). Complete IM (type I) resembles the small intestine epithelium with goblet cells, Paneth cells, eosinophilic enterocytes and a brush border[37]. It is associated with loss of markers of gastric mucin (MUC1, MUC5AC, MUC6) and expression of the intestinal sialic mucin, MUC2[38]. Incomplete IM more closely resembles the large intestine epithelium, lacking absorptive cells, but with columnar cells resembling gastric foveolar cells. It does not have a brush border and maintains expression of gastric mucin markers (MUC1, MUC5AC, MUC6) usually together with gain of MUC2[38]. Incomplete IM is further subdivided into Type II IM, with cells expressing a mixture of neutral mucins and intestinal sialomucins and Type III IM, with cells expressing sulfomucins[37]. In practice histopathological classification between complete and incomplete IM is often not mutually exclusive, with segments of tissue containing elements of both subtypes. The distinction between complete and incomplete IM is clinically important as it appears incomplete harbours a higher risk of progression to cancer[39-42].
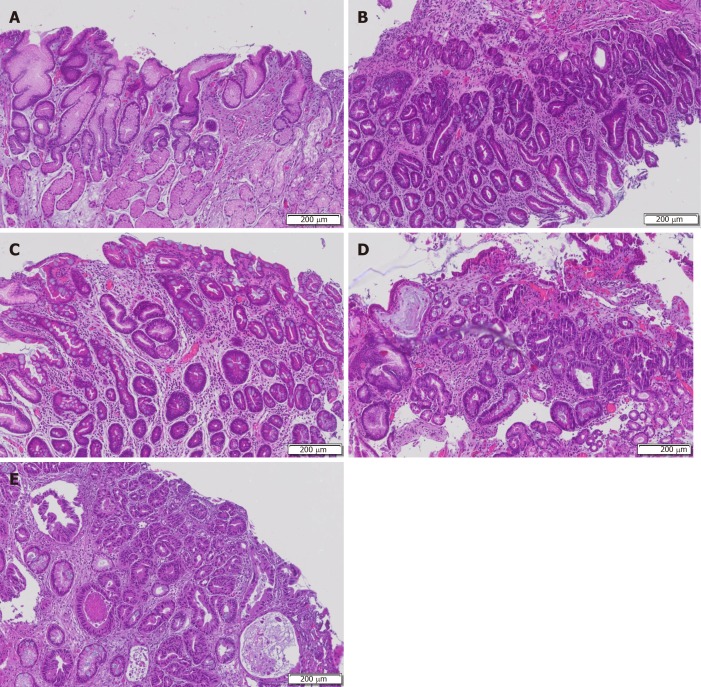
Figure 1.
Complete or incomplete subtypes. A: Chronic gastritis with mucosal atrophy and lymphocytic infiltrate (asterix); B: Incomplete intestinal metaplasia resembling the colonic-type epithelium with irregular mucin droplets (arrowheads) and absence of a brush border; C: Complete intestinal metaplasia resembling the small intestinal epithelium with goblet cells alternating with eosinophilic enterocytes, brush border and Paneth cells; D: Low-grade dysplasia characterized by crowded glands with columnar cells and preserved polarity and pseudostratified nuclei; E: High-grade dysplasia with cuboidal cells, mitotic activity, prominent nucleoi, and high nuclear-cytoplasmia ratio.
In the context of long-term H. pylori infection, IM possibly develops as an adaptive and protective lesion[43]. There has been extensive work into determining how H. pylori infection leads to IM with a number of genes implicated including SOX2 and CDX2. SOX2 is a transcription factor involved in gastric differentiation which negatively regulates intestinal differentiation, whereas CDX2 is a key intestinal transcription factor involved in establishing and maintaining IM[44]. SOX2 and CDX2 seem to be inversely regulated by H. pylori[45]. Complete IM has been shown to be predominantly SOX2 negative (93%) and incomplete IM mainly SOX2 positive (85%)[46]. Moreover CDX2 expression has been shown to be also induced in part through an NF-κB dependent mechanism following H. pylori infection[47].
Duodeno-gastric reflux is another proposed gastric insult contributing to ChG and IM formation, analogous to gastroesophageal acid reflux in Barrett’s oesophagus[48]. There has been an association of increased incidence of IM after exposure to bile acids reported in a large-scale study involving a total of 2283 patients[49]. In this context the development of IM may represent a protective mechanism, with a metaplasia to an intestinal phenotype more capable of resisting the effects of bile than the normal gastric mucosa.
Risk factors in IM
H. pylori is a significant risk factor in the establishment of IM, however there are other clinical and environmental exposures that have been shown to be important risk factors for IM progression to GC. In a large-scale US study (n = 810821 patients) IM was more common in men, was more prevalent with increasing age and East Asian ancestry. This suggests IM may occur due to environmental exposures but in the context of hereditary risk[50]. Hereditary risk is relevant in GC even excluding major genetic syndromes with several studies showing intestinal-type GC is associated with a strong family history of GC[51-53]. With respect to premalignant lesions, it was shown that among siblings with a family history of any precancerous change there is an increase in risk of subsequent non-cardia GC with a hazard ratio of 2.5 compared with siblings of index persons with “normal or minor mucosal changes”[54]. The availability of siblings' precancerous data to the clinician could be useful in assessing a patient’s risk of progressing to GC.
Once established, the degree of IM has been shown to be related to the risk of progression to cancer. Extensive IM with IM in the corpus, incomplete IM and IM located along the Maggenstrasse (along the lesser curve of the stomach) have been shown to increase the risk of progression towards cancer[40,42,55,56]. In one study of microsatellite instability (MSI), this molecular finding was enriched in GC and adjacent IM suggesting this may be an early event in MSI subtype GCs. It is notable that microsatellite unstable IM was of incomplete type in this study[57] which provides further evidence for the potential unique molecular pathways that begin in the premalignant context.
OLGA AND OLGIM
Both the Operative Link on Gastritis Assessment (OLGA) and on Gastric Intestinal Metaplasia (OLGIM) are based on histological assessment of random biopsies taken from designated areas of the stomach according to the Sydney protocol[58-60]. At least four sites are sampled from the stomach during upper gastroscopy (two antral and two corpus). Both OLGA and OLGIM are scoring standards used to grade and stage chronic gastric inflammation, gastric atrophy and intestinal metaplasia. They provide information with regards to topography and extent of atrophic gastritis and intestinal metaplasia, the latter being easier to assess and more consistent. Initially reported by Rugge et al[61,62] 2010 and 2011 for both OLGA and OLGIM and more recently by the meta-analysis carried out by Yue et al[63] 2018 OLGA and OLGIM stages Type III/IV are consistently associated with increased risk of progression to GC. These findings suggest that high risk patients with OLGA/OLGIM stages type III/IV would benefit from close and frequent monitoring to detect neoplastic lesions at the earliest possible stage.
POINT OF NO RETURN
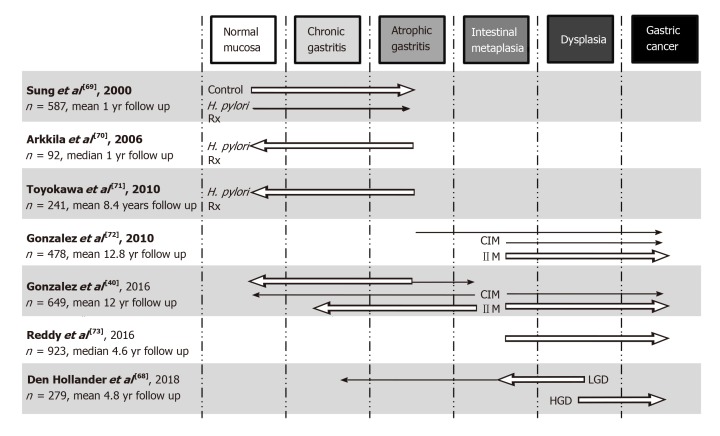
The Correa cascade is often referred to as a linear progression, however in the majority of patients there may be little to no change along the Cascade cascade over many years. In other patients it can be a dynamic process with regression and/or progression of lesions, perhaps even rapid progression bypassing some of the putative stages. It is clearly evident that H. pylori infection and chronic inflammation in selected individuals causes progression of the cascade and it has been observed that successful eradication of H. pylori can lead to regression of histological features. There has been speculation that there is a point at which eradication is less effective at causing regression and indeed does not change the risk of progression in certain individuals. This has been referred as the “point of no return”. H. pylori eradication results in complete resolution of histological inflammation and regression of atrophy in AG patients, with greater improvement seen in corpus AG compared to antral AG patients[64]. Unfortunately, the same effect is not seen in IM patients[64-67]. Once IM is established, eradication is only partially successful at reducing the risk of progression to GC. This suggests that IM may be the “point of no return” where genetic damage to gastric stem cells becomes irreversible. Although there is much evidence to support a point of no return, there has been evidence of regression from IM to AG or ChG in some cohorts[40,68]. A graphical summary of some of the larger IM progression studies is shown in Figure 2[40,68-73].
Figure 2.
Graphical representation of selected large studies investigating progression/regression of premalignant gastric lesions across the stages of the Correa cascade. Arrows represent the direction of effect findings, with the size of the arrow the strength of effect (not to scale between cohorts and only major findings of trials represented). H. pylori Rx: Helicobacter pylori antibiotic therapy; CIM: Complete IM; IIM: Incomplete IM; LGD: Low-grade dysplasia; HGD: High-grade dysplasia; IM: Intestinal metaplasia.
SPASMOLYTIC PEPTIDE EXPRESSING METAPLASIA
Work from animal models of GC has introduced the concept of Spasmolytic peptide expressing metaplasia (SPEM). This is a cell lineage shown to be strongly associated with chronic gastritis in the fundus and gastric adenocarcinoma in animals[74]. It is often thought as an alternative metaplastic lineage to IM. SPEM is morphologically similar to Brunner's glands of the duodenum and expresses the trefoil spasmolytic polypeptide (SP or TFF2)[75]. It has been hypothesised that SPEM is an alternative precursor to GC and is associated with increased risk compared to IM[76]. Although SPEM is not a defined stage in the Correa cascade, it has been useful for studying the process of metaplasia formation in mice. To avoid confusion, SPEM is identified in the corpus and the fundus but not in the antrum as its characteristics are very similar to those of the deep antral and pyloric glands which also express TFF2. In mice infected with Helicobacter felis, SPEM develops after 6 to 12 mo of infection in the presence of active inflammation. First parietal cells are lost (oxyntic atrophy) and then the normal gastric lineages are replaced with metaplastic cells[77]. In two acute drug-induced SPEM models, with DMP-777 protonophore (abrogated inflammation) and L635 (prominent inflammation) as well as with H. felis infection in mice (chronic inflammation), it is suggested SPEM arises from the transdifferentiation of chief cells[77,78]. However more recently a study by Kinoshita et al[79] suggested that SPEM is the result of a regenerative process initiated by neck progenitor cells after chief cell loss. In another mouse model, SPEM in INS-GAS mice progressed to dysplasia after 1 year[80]. Following H. pylori-infection, Mongolian gerbils progressively develop ChG, followed by loss of parietal cells and metaplasia[81]. After 1 year of infection, SPEM is observed and mixed glands expressing both SPEM and IM are also seen. In humans there is growing evidence that suggests SPEM can either progress directly to dysplasia or become IM in the presence of continuous chronic inflammation[82]. These animal systems have been useful in studying the natural history of these lesions but it remains to be seen whether they are reliable models of the human condition.
THE ROLE OF THE IMMUNE MICROENVIRONMENT
We have continually reiterated the role of chronic inflammation in the development of GC. In the context of a chronically inflamed microenvironment, there is some evidence that IM may arise due to the actions of specific immune cells. Using the murine model of L635-induced SPEM and following administration of clodronate, it was shown that macrophages are involved in the development of acute SPEM[83]. These macrophages were predominantly of the M2 subset (alternatively activated) and in the same study M2 macrophages were also shown to be increased in human SPEM and IM. Another prevalent immune cell in IM is neutrophils which were shown to be approximately 9-fold enriched compared to normal gastric tissue[84]. GC tissues were roughly 24 times enriched in neutrophils compared to normal gastric tissue. Thus macrophages and neutrophils may be vital immune cells required in the gastric microenvironment for SPEM and IM to develop and then to progress to GC. The role of the immune system in the process of gastric carcinogenesis has not yet been fully investigated.
CELLULAR AND MOLECULAR PATHWAYS OF PROGRESSION
IM progression to dysplasia and subsequently cancer occurs infrequently and the molecular mechanisms responsible for this progression are still not well understood. There are a number of challenges with studying this paradigm in view of the long duration over which these conditions progress, thus limiting prospective studies. This is compounded by the low rates of progression from each of the Correa stages and the potential confounder of tissue sampling when undertaking endoscopic follow up. Although the exact genomic or epigenomic pathways for IM progression to dysplasia are still being investigated, it is possible to postulate how certain events are necessary for progression by combining available data from a small number of key studies. It is known that: (1) IM is clonally derived from within the gastric mucosa[34,85]; (2) Gastric and IM glands divide by fission to form clonal patches[34,85,86]; (3) Over time, different gastric stem cells with accumulated genomic events (somatic mutations/ chromosomal copy number gains and/or losses) can give rise to unique IM glands; (4) Further genomic changes may drive IM glands to proliferate or persist over a long period of time; (5) Dysplastic glands are formed that are genetically related to IM glands; entire dysplastic fields can share a foundation mutation[86] from which multiple subclones can result; and (6) This event can happen simultaneously in multiple regions of the stomach leading to an increased risk of GC developing across several locations and therefore providing a field cancerization effect.
To better understand how this process may unfold, studies on gastric adenoma (GA) and Barrett’s oesophagus (BO) progression to GC and oesophageal adenocarcinoma (OAC), respectively, can be used as examples. In a recent study of gastric adenoma and paired GC from the same patients were used to determine clonal evolution. Clonal structure analyses showed that most GA/GC pairs exhibit parallel evolution with early divergence instead of a linear sequence of GA to GC progression[87]. Additionally, a small number of GC cases were clonally unrelated from paired GA suggesting the synchronous evolution of multiple clones that may progress to GC. BO is a premalignant intestinal metaplastic lesion that’s often associated with gastro-oesophageal disease and predisposes patients to develop OAC[88]. Although exact values differ between studies, two population-based BO follow-up studies showed that the annual risk of progression of BO is 0.12%-0.14%[89,90]. BO has a higher mutation load (6.76 SNVs/Mb) than gastric IM but still lower than OAC (10.02 SNVs/Mb) and was shown to be polyclonal[91]. In one patient with BO, high grade dysplasia was shown to arise from multiple clones suggesting that the severity of intestinal metaplasia (a result of clonal expansion and cumulative molecular aberrations) may also play a key role in synchronous progression to GC[91].
Overall a holistic molecular approach is needed to elucidate the crucial events of how premalignant lesions actually cross the bridge to malignancy, a so-called "Pre-Cancer Genome Atlas"[92]. Using whole genome MBD-seq and RRBS analyses Kim et al showed that hypermethylation of gastrointestinal hormone receptors may play a key role in early gastric carcinogenesis[93]. Both gastrin and gastric acid secretion are thought to play important roles in cell differentiation and may play a part in creating a permissive environment or even directly involved in the process of carcinogenesis. A good summary review on the potential cellular and molecular pathways of gastric carcinogenesis was written by Rivas-Ortiz which added useful insight to this area[94]. It is very likely that GC is the result of multiple events co-occurring over time and space leading to various subtypes of GC (see TCGA molecular subtypes). If this is true, then it is also likely that differing sets of pre-cancerous events contribute to gastric carcinogenesis. Although some events may overlap across all GC subtypes e.g., chronic gastritis, others may be specific for a particular GC subtype e.g., the breakdown of cellular mechanisms that keep diploidy intact leading to the chromosomal instability subtype.
HIGH RISK GENOMIC AND EPIGENOMIC ALTERATIONS IN IM
The median time for gastric intestinal metaplasia to progress to GC has been estimated to be 6.1 years, in contrast to low grade dysplasia which is only 2.6 years[95]. A recent study of genomic and epigenomic profiling of IM showed that IM has a low mutational burden compared to non-hypermutated GC (2.6 vs 6.9 mutations/Mb) and harbour recurrent mutations in certain tumour suppressor genes like FBXW7 (6/108 IM cases) but less in others, specifically TP53 and ARID1A (2/108 and 3/108 IM cases)[96]. However the presence of low frequency TP53 mutations in IM patients is in contrast to previous work within our group that showed an absence of TP53 mutations in IM samples paired with GC samples from the same patients[97]. A second finding of our study was that overexpression of p53 protein using immunohistochemistry has limited correlation to TP53 mutations. In the Huang et al[96] 2018 study, patients that progressed to dysplasia and GC had previously chromosome 8q amplifications and shortened telomeres. Interestingly, patients with IM that regressed had normal epigenomic patterns. DNA methylation profiling showed that the majority of IM patients in the high methylation group had relatively high mutational load, frequent chromosomal copy number variations and FBXW7 mutations and occurred mainly in the antrum.
LOW AND HIGH-GRADE DYSPLASIA DIFFER IN MUTATIONAL PATTERNS
The Padova classification was developed in 2000 to standardise histopathological reporting, which identifies five main categories for dysplastic lesions: (1) Negative for dysplasia; (2) Indefinite for dysplasia; (3) Non-invasive neoplasia; (4) Suspicious for invasive carcinoma; and (5) Invasive adenocarcinoma[98]. In practice, pathologists use categories 1 and 2 and subdivide category 3 as low (LGD) and high grade (HGD), the latter being associated with a higher risk of progression. A recent study using targeted deep DNA sequencing of 67 GC-related genes detected APC mutations in all LGD and also in some HGD cases[99]. However, APC and TP53 appeared to be mutually exclusive, the latter being present only in HGD and diminutive intramucosal GC (diameter < 10 mm). Analysis of tumor variant allele frequency suggested TP53 mutation is the initial event in TP53-mutated intramucosal GC. Importantly, this study suggested that linear evolution of LGD to HGD is rare and that early mutational events determine the evolution of dysplastic lesions. Early APC mutations lead to LGD whereas TP53 mutations lead to HGD which, following other genomic aberrations, subsequently evolve into early GC.
THE CORREA CASCADE AND DIFFUSE GC
Although there is considerable evidence of IM progressing to dysplasia and then to IGC, it is still debated whether any of the premalignant lesions that are part of the Correa cascade actually play a role in diffuse gastric carcinogenesis. In a prospective Japanese study, a proportion of patients that developed DGC had pangastritis (9/13), moderate to severe atrophy (9/13 and 1/13 respectively) and IM (8/13) at base line[100], suggesting a significant association between IM and DGC development[100]. A more recent study in South Korea showed that OLGA and OLGIM may have clinical utility in patients at risk of developing DGC[101]. Multivariate logistic regression analysis showed family history of GC, H. pylori infection, and OLGA/OLGIM stages III/IV were independent risk factors for both IGC and DGC[101]. Thus, atrophy and particularly IM likely play a dual role in gastric carcinogenesis dependent on context. If the right conditions are met, cellular and molecular changes within the stem cell compartment of these lesions lead to GC; if not, their presence creates a permissive environment for progression to GC to occur, possibly through hypochlorhydria and dysbiosis, and is also an indicator of increased patient progression risk. DGC may be the result of an "alternative" route to carcinogenesis, where a CDH1 mutation within the glandular stem cell compartment of a gastric gland or atrophic gland or even a metaplastic gland/crypt produces a parent tumour cell.
Neuroendocrine cell dedifferentiation: An alternative route to GC?
An alternative paradigm to the stem cell theory as the tumour cell of origin has been gaining ground in recent years. In certain circumstances mature neuroendocrine cells may dedifferentiate, accumulate mutations and other genomic events and become tumour cells themselves[102]. Neuroendocrine tumours are the most likely results of such an event but also gastric adenocarcinomas. The enterochromafin like cell (ECL) is the main neuroendocrine cell in the oxyntic stomach, it produces and releases histamine and has gastrin receptors. Although the exact molecular and cellular mechanisms of this pathway to GC are not known, it is thought that loss of parietal cells and atrophy precedes cellular dedifferentiation of ECL cells. Thus, this pathway to GC may not fully follow the Correa cascade, going directly from an atrophic state to a hyperplastic, then dysplastic and then to either a neuroendocrine tumour or a gastric adenocarcinoma.
CONCLUSION
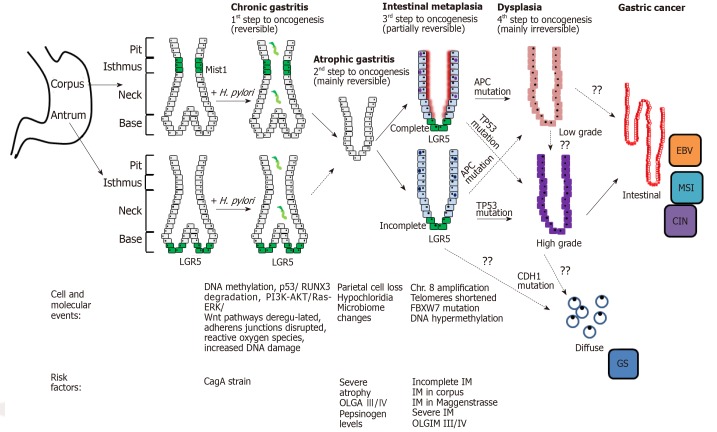
Our understanding of the molecular basis of GC and its premalignant lesions is accumulating rapidly, providing useful insights into the natural history of the disease (Figure 3). Knowledge remains lacking in many domains however, including the relationship between premalignant lesions and TCGA subtypes of GC. It could be the molecular changes characterising the TCGA GC subtypes represent disparate insults predisposing to initiation of the Correa cascade. Alternatively, the subtypes could represent accumulated “hits” following initiation with H. pylori infection. Insights into this pathway would stratify those at risk as well as inform prognosis and surveillance guidelines.
Figure 3.
Summary of the cellular and molecular events associated with progression to cancer. H. pylori: Helicobacter pylori; EBV: Epstein Barr virus positive; MSI: Microsatellite unstable; CagA: Cytotoxin-associated gene A protein; IM: Intestinal metaplasia; GS: Genomically stable.
The observation that IM appears a relative point of no return along the Correa cascade, with only a small fraction of patients progressing to dysplasia and GC raises questions of the molecular determinants of progression. In the first study of its type, Huang et al laid the foundation for understanding this process, following an IM cohort for a minimum of 5 years and describing the molecular changes associated with progression in a large Chinese cohort. Validation of these findings in alternative cohorts is required for its use clinically.
In high-risk populations screening and surveillance has been successful in the early detection of GCs and improvements in 5-year survivals. However further work is required in low-risk populations to make strong evidence-based decisions regarding clinical screening or surveillance. In those known to have IM the risk factors discussed above should prompt the clinician to carefully consider surveillance including incomplete IM, dysplasia, extensive IM involving the corpus, male gender and those from high-risk ethnicities. The addition of molecular data such as TP53 mutation data, methylation patterns and chromosome 8 status would further improve risk-assessment algorithms, however larger population-based data is required for this to be accurate and practical.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: February 26, 2019
First decision: May 24, 2019
Article in press: August 21, 2019
Specialty type: Oncology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Silva AP, Gassler N, Park WS S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Qi LL
Contributor Information
Athanasios Koulis, Upper Gastrointestinal Translational Laboratory, Peter MacCallum Cancer Centre, Melbourne 3000, Australia; the Sir Peter MacCallum Department of Surgical Oncology, the University of Melbourne, Melbourne 3010, Australia.
Andrew Buckle, Upper Gastrointestinal Translational Laboratory, Peter MacCallum Cancer Centre, Melbourne 3000, Australia; the Sir Peter MacCallum Department of Surgical Oncology, the University of Melbourne, Melbourne 3010, Australia.
Alex Boussioutas, Upper Gastrointestinal Translational Laboratory, Peter MacCallum Cancer Centre, Melbourne 3000, Australia; the Sir Peter MacCallum Department of Surgical Oncology, the University of Melbourne, Melbourne 3010, Australia; Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, 3050, Australia. alex.boussioutas@petermac.org.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, Nichols R, Kesteloot H. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 3.Haenszel W, Kurihara M, Segi M, Lee RK. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49:969–988. [PubMed] [Google Scholar]
- 4.Strumylaite L, Zickute J, Dudzevicius J, Dregval L. Salt-preserved foods and risk of gastric cancer. Medicina (Kaunas) 2006;42:164–170. [PubMed] [Google Scholar]
- 5.Lin SH, Li YH, Leung K, Huang CY, Wang XR. Salt processed food and gastric cancer in a Chinese population. Asian Pac J Cancer Prev. 2014;15:5293–5298. doi: 10.7314/apjcp.2014.15.13.5293. [DOI] [PubMed] [Google Scholar]
- 6.Ren JS, Kamangar F, Forman D, Islami F. Pickled food and risk of gastric cancer--a systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev. 2012;21:905–915. doi: 10.1158/1055-9965.EPI-12-0202. [DOI] [PubMed] [Google Scholar]
- 7.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 8.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjønneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Touvier M, Boeing H, Schulz M, Linseisen J, Nagel G, Trichopoulou A, Naska A, Oikonomou E, Krogh V, Panico S, Masala G, Sacerdote C, Tumino R, Peeters PH, Numans ME, Bueno-de-Mesquita HB, Büchner FL, Lund E, Pera G, Sanchez CN, Sánchez MJ, Arriola L, Barricarte A, Quirós JR, Hallmans G, Stenling R, Berglund G, Bingham S, Khaw KT, Key T, Allen N, Carneiro F, Mahlke U, Del Giudice G, Palli D, Kaaks R, Gonzalez CA. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27:2250–2257. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 9.González CA, Pera G, Agudo A, Palli D, Krogh V, Vineis P, Tumino R, Panico S, Berglund G, Simán H, Nyrén O, Agren A, Martinez C, Dorronsoro M, Barricarte A, Tormo MJ, Quiros JR, Allen N, Bingham S, Day N, Miller A, Nagel G, Boeing H, Overvad K, Tjonneland A, Bueno-De-Mesquita HB, Boshuizen HC, Peeters P, Numans M, Clavel-Chapelon F, Helen I, Agapitos E, Lund E, Fahey M, Saracci R, Kaaks R, Riboli E. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Int J Cancer. 2003;107:629–634. doi: 10.1002/ijc.11426. [DOI] [PubMed] [Google Scholar]
- 10.Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, Zhang ZF, Matsuo K, Ito H, Hu J, Johnson KC, Yu GP, Palli D, Ferraroni M, Muscat J, Lunet N, Peleteiro B, Malekzadeh R, Ye W, Song H, Zaridze D, Maximovitch D, Aragonés N, Castaño-Vinyals G, Vioque J, Navarrete-Muñoz EM, Pakseresht M, Pourfarzi F, Wolk A, Orsini N, Bellavia A, Håkansson N, Mu L, Pastorino R, Kurtz RC, Derakhshan MH, Lagiou A, Lagiou P, Boffetta P, Boccia S, Negri E, La Vecchia C. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev. 2018;27:124–133. doi: 10.1097/CEJ.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 11.Han MA, Kim YW, Choi IJ, Oh MG, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Ryu KW. Association of smoking history with cancer recurrence and survival in stage III-IV male gastric cancer patients. Cancer Epidemiol Biomarkers Prev. 2013;22:1805–1812. doi: 10.1158/1055-9965.EPI-13-0385. [DOI] [PubMed] [Google Scholar]
- 12.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 13.Abioye AI, Odesanya MO, Abioye AI, Ibrahim NA. Physical activity and risk of gastric cancer: a meta-analysis of observational studies. Br J Sports Med. 2015;49:224–229. doi: 10.1136/bjsports-2013-092778. [DOI] [PubMed] [Google Scholar]
- 14.O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. 2012;61:1261–1268. doi: 10.1136/gutjnl-2011-300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uthman OA, Jadidi E, Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health. 2013;67:854–860. doi: 10.1136/jech-2012-201108. [DOI] [PubMed] [Google Scholar]
- 16.Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim N, Park RY, Cho SI, Lim SH, Lee KH, Lee W, Kang HM, Lee HS, Jung HC, Song IS. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol. 2008;42:448–454. doi: 10.1097/MCG.0b013e318046eac3. [DOI] [PubMed] [Google Scholar]
- 20.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 21.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata-Kamiya N. Pathophysiological functions of the CagA oncoprotein during infection by Helicobacter pylori. Microbes Infect. 2011;13:799–807. doi: 10.1016/j.micinf.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:196–219. doi: 10.2183/pjab.93.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Na HK, Woo JH. Helicobacter pylori Induces Hypermethylation of CpG Islands Through Upregulation of DNA Methyltransferase: Possible Involvement of Reactive Oxygen/Nitrogen Species. J Cancer Prev. 2014;19:259–264. doi: 10.15430/JCP.2014.19.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider BG, Piazuelo MB, Sicinschi LA, Mera R, Peng DF, Roa JC, Romero-Gallo J, Delgado AG, de Sablet T, Bravo LE, Wilson KT, El-Rifai W, Peek RM, Jr, Correa P. Virulence of infecting Helicobacter pylori strains and intensity of mononuclear cell infiltration are associated with levels of DNA hypermethylation in gastric mucosae. Epigenetics. 2013;8:1153–1161. doi: 10.4161/epi.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coati I, Fassan M, Farinati F, Graham DY, Genta RM, Rugge M. Autoimmune gastritis: Pathologist's viewpoint. World J Gastroenterol. 2015;21:12179–12189. doi: 10.3748/wjg.v21.i42.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37:375–382. doi: 10.1111/apt.12177. [DOI] [PubMed] [Google Scholar]
- 28.Parsons BN, Ijaz UZ, D'Amore R, Burkitt MD, Eccles R, Lenzi L, Duckworth CA, Moore AR, Tiszlavicz L, Varro A, Hall N, Pritchard DM. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13:e1006653. doi: 10.1371/journal.ppat.1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, Yu J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67:1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001;488:65–76. doi: 10.1016/s1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 31.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 32.Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G626–G634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- 33.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Waddingham W, Graham D, Banks M, Jansen M. The evolving role of endoscopy in the diagnosis of premalignant gastric lesions. F1000Res. 2018;7 doi: 10.12688/f1000research.12087.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V, Wang TC. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jass JR, Filipe MI. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology. 1979;3:191–199. doi: 10.1111/j.1365-2559.1979.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 38.Reis CA, David L, Correa P, Carneiro F, de Bolós C, Garcia E, Mandel U, Clausen H, Sobrinho-Simões M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 39.González CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer. 2013;133:1023–1032. doi: 10.1002/ijc.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González CA, Sanz-Anquela JM, Companioni O, Bonet C, Berdasco M, López C, Mendoza J, Martín-Arranz MD, Rey E, Poves E, Espinosa L, Barrio J, Torres MÁ, Cuatrecasas M, Elizalde I, Bujanda L, Garmendia M, Ferrández Á, Muñoz G, Andreu V, Paules MJ, Lario S, Ramírez MJ, Barkun A. Study group, Gisbert JP. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol. 2016;31:953–958. doi: 10.1111/jgh.13249. [DOI] [PubMed] [Google Scholar]
- 41.Pittayanon R, Rerknimitr R, Klaikaew N, Sanpavat A, Chaithongrat S, Mahachai V, Kullavanijaya P, Barkun A. The risk of gastric cancer in patients with gastric intestinal metaplasia in 5-year follow-up. Aliment Pharmacol Ther. 2017;46:40–45. doi: 10.1111/apt.14082. [DOI] [PubMed] [Google Scholar]
- 42.Shao L, Li P, Ye J, Chen J, Han Y, Cai J, Lu X. Risk of gastric cancer among patients with gastric intestinal metaplasia. Int J Cancer. 2018 doi: 10.1002/ijc.31571. [DOI] [PubMed] [Google Scholar]
- 43.Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17:594–604. doi: 10.1038/nrc.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, Tomiyama T, Sato Y, Yamamoto H, Isoda N, Yoshida T, Ido K, Sugano K. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740–7747. doi: 10.1158/0008-5472.CAN-04-1617. [DOI] [PubMed] [Google Scholar]
- 45.Camilo V, Barros R, Sousa S, Magalhães AM, Lopes T, Mário Santos A, Pereira T, Figueiredo C, David L, Almeida R. Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis. 2012;33:1985–1992. doi: 10.1093/carcin/bgs233. [DOI] [PubMed] [Google Scholar]
- 46.Camilo V, Garrido M, Valente P, Ricardo S, Amaral AL, Barros R, Chaves P, Carneiro F, David L, Almeida R. Differentiation reprogramming in gastric intestinal metaplasia and dysplasia: role of SOX2 and CDX2. Histopathology. 2015;66:343–350. doi: 10.1111/his.12544. [DOI] [PubMed] [Google Scholar]
- 47.Asano N, Imatani A, Watanabe T, Fushiya J, Kondo Y, Jin X, Ara N, Uno K, Iijima K, Koike T, Strober W, Shimosegawa T. Cdx2 Expression and Intestinal Metaplasia Induced by H. pylori Infection of Gastric Cells Is Regulated by NOD1-Mediated Innate Immune Responses. Cancer Res. 2016;76:1135–1145. doi: 10.1158/0008-5472.CAN-15-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sobala GM, O'Connor HJ, Dewar EP, King RF, Axon AT, Dixon MF. Bile reflux and intestinal metaplasia in gastric mucosa. J Clin Pathol. 1993;46:235–240. doi: 10.1136/jcp.46.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuhisa T, Arakawa T, Watanabe T, Tokutomi T, Sakurai K, Okamura S, Chono S, Kamada T, Sugiyama A, Fujimura Y, Matsuzawa K, Ito M, Yasuda M, Ota H, Haruma K. Relation between bile acid reflux into the stomach and the risk of atrophic gastritis and intestinal metaplasia: a multicenter study of 2283 cases. Dig Endosc. 2013;25:519–525. doi: 10.1111/den.12030. [DOI] [PubMed] [Google Scholar]
- 50.Genta RM, Sonnenberg A. Characteristics of the gastric mucosa in patients with intestinal metaplasia. Am J Surg Pathol. 2015;39:700–704. doi: 10.1097/PAS.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 51.Lissowska J, Groves FD, Sobin LH, Fraumeni JF, Jr, Nasierowska-Guttmejer A, Radziszewski J, Regula J, Hsing AW, Zatonski W, Blot WJ, Chow WH. Family history and risk of stomach cancer in Warsaw, Poland. Eur J Cancer Prev. 1999;8:223–227. doi: 10.1097/00008469-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Eto K, Ohyama S, Yamaguchi T, Wada T, Suzuki Y, Mitsumori N, Kashiwagi H, Anazawa S, Yanaga K, Urashima M. Familial clustering in subgroups of gastric cancer stratified by histology, age group and location. Eur J Surg Oncol. 2006;32:743–748. doi: 10.1016/j.ejso.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Shin CM, Kim N, Lee HS, Lee DH, Kim JS, Jung HC, Song IS. Intrafamilial aggregation of gastric cancer: a comprehensive approach including environmental factors, Helicobacter pylori virulence, and genetic susceptibility. Eur J Gastroenterol Hepatol. 2011;23:411–417. doi: 10.1097/MEG.0b013e328343b7f5. [DOI] [PubMed] [Google Scholar]
- 54.Song H, Ekheden IG, Ploner A, Ericsson J, Nyren O, Ye W. Family history of gastric mucosal abnormality and the risk of gastric cancer: a population-based observational study. Int J Epidemiol. 2018;47:440–449. doi: 10.1093/ije/dyx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cassaro M, Rugge M, Gutierrez O, Leandro G, Graham DY, Genta RM. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431–1438. doi: 10.1111/j.1572-0241.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 56.Sakitani K, Hirata Y, Watabe H, Yamada A, Sugimoto T, Yamaji Y, Yoshida H, Maeda S, Omata M, Koike K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570–1575. doi: 10.1111/j.1440-1746.2011.06767.x. [DOI] [PubMed] [Google Scholar]
- 57.Hamamoto T, Yokozaki H, Semba S, Yasui W, Yunotani S, Miyazaki K, Tahara E. Altered microsatellites in incomplete-type intestinal metaplasia adjacent to primary gastric cancers. J Clin Pathol. 1997;50:841–846. doi: 10.1136/jcp.50.10.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, Genta RM, Graham DY, Hattori T, Malfertheiner P, Nakajima S, Sipponen P, Sung J, Weinstein W, Vieth M. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650–658. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 60.Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 61.Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fassan M, Basso D, Plebani M, Graham DY. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104–1111. doi: 10.1111/j.1365-2036.2010.04277.x. [DOI] [PubMed] [Google Scholar]
- 62.Rugge M, Fassan M, Pizzi M, Farinati F, Sturniolo GC, Plebani M, Graham DY. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol. 2011;17:4596–4601. doi: 10.3748/wjg.v17.i41.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579–587. doi: 10.1007/s10120-018-0812-3. [DOI] [PubMed] [Google Scholar]
- 64.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12 Suppl 2:32–38. doi: 10.1111/j.1523-5378.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, Zhang G. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253–260. doi: 10.1159/000280318. [DOI] [PubMed] [Google Scholar]
- 66.Shin CM, Kim N, Chang H, Kim JS, Lee DH, Jung HC. Follow-Up Study on CDX1 and CDX2 mRNA Expression in Noncancerous Gastric Mucosae After Helicobacter pylori Eradication. Dig Dis Sci. 2016;61:1051–1059. doi: 10.1007/s10620-016-4048-y. [DOI] [PubMed] [Google Scholar]
- 67.Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166–175. doi: 10.1007/s10120-015-0462-7. [DOI] [PubMed] [Google Scholar]
- 68.den Hollander WJ, Holster IL, den Hoed CM, Capelle LG, Tang TJ, Anten MP, Prytz-Berset I, Witteman EM, Ter Borg F, Hartog GD, Bruno MJ, Peppelenbosch MP, Lesterhuis W, Doukas M, Kuipers EJ, Spaander MCW. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut. 2019;68:585–593. doi: 10.1136/gutjnl-2017-314498. [DOI] [PubMed] [Google Scholar]
- 69.Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, Leung WK, Ng EK, Lau JY, Lee YT, Yeung CK, Chao W, Chung SC. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7–14. doi: 10.1053/gast.2000.8550. [DOI] [PubMed] [Google Scholar]
- 70.Arkkila PE, Seppälä K, Färkkilä MA, Veijola L, Sipponen P. Helicobacter pylori eradication in the healing of atrophic gastritis: a one-year prospective study. Scand J Gastroenterol. 2006;41:782–790. doi: 10.1080/00365520500463175. [DOI] [PubMed] [Google Scholar]
- 71.Toyokawa T, Suwaki K, Miyake Y, Nakatsu M, Ando M. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J Gastroenterol Hepatol. 2010;25:544–547. doi: 10.1111/j.1440-1746.2009.05995.x. [DOI] [PubMed] [Google Scholar]
- 72.González CA, Pardo ML, Liso JM, Alonso P, Bonet C, Garcia RM, Sala N, Capella G, Sanz-Anquela JM. Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer. 2010;127:2654–2660. doi: 10.1002/ijc.25273. [DOI] [PubMed] [Google Scholar]
- 73.Reddy KM, Chang JI, Shi JM, Wu BU. Risk of Gastric Cancer Among Patients With Intestinal Metaplasia of the Stomach in a US Integrated Health Care System. Clin Gastroenterol Hepatol. 2016;14:1420–1425. doi: 10.1016/j.cgh.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PubMed] [Google Scholar]
- 75.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 76.Halldórsdóttir AM, Sigurdardóttrir M, Jónasson JG, Oddsdóttir M, Magnússon J, Lee JR, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 77.Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037.e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weis VG, Petersen CP, Weis JA, Meyer AR, Choi E, Mills JC, Goldenring JR. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol. 2017;312:G67–G76. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinoshita H, Hayakawa Y, Niu Z, Konishi M, Hata M, Tsuboi M, Hayata Y, Hikiba Y, Ihara S, Nakagawa H, Hirata Y, Wang TC, Koike K. Mature gastric chief cells are not required for the development of metaplasia. Am J Physiol Gastrointest Liver Physiol. 2018;314:G583–G596. doi: 10.1152/ajpgi.00351.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu T, Choi E, Petersen CP, Noto JM, Romero-Gallo J, Piazuelo MB, Washington MK, Peek RM, Jr, Goldenring JR. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J Pathol. 2016;239:399–410. doi: 10.1002/path.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210, 2210.e1. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146:1727–38.e8. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu H, Ma Y, Yang M, Zhang C, Huang H, Xia Y, Lu L, Jin W, Cui D. Persisting and Increasing Neutrophil Infiltration Associates with Gastric Carcinogenesis and E-cadherin Downregulation. Sci Rep. 2016;6:29762. doi: 10.1038/srep29762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, Hunt T, Elia G, Oukrif D, Harrison R, Novelli MR, Mitchell I, Stoker DL, Turnbull DM, Jankowski JA, Wright NA. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 86.Gutierrez-Gonzalez L, Graham TA, Rodriguez-Justo M, Leedham SJ, Novelli MR, Gay LJ, Ventayol-Garcia T, Green A, Mitchell I, Stoker DL, Preston SL, Bamba S, Yamada E, Kishi Y, Harrison R, Jankowski JA, Wright NA, McDonald SA. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology. 2011;140:1251–1260.e1-6. doi: 10.1053/j.gastro.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 87.Jung SH, Kim SY, An CH, Lee SH, Jung ES, Park HC, Kim MS, Chung YJ, Lee SH. Clonal Structures of Regionally Synchronous Gastric Adenomas and Carcinomas. Clin Cancer Res. 2018;24:4715–4725. doi: 10.1158/1078-0432.CCR-18-0345. [DOI] [PubMed] [Google Scholar]
- 88.Schoofs N, Bisschops R, Prenen H. Progression of Barrett's esophagus toward esophageal adenocarcinoma: an overview. Ann Gastroenterol. 2017;30:1–6. doi: 10.20524/aog.2016.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–1036. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 90.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 91.Ross-Innes CS, Becq J, Warren A, Cheetham RK, Northen H, O'Donovan M, Malhotra S, di Pietro M, Ivakhno S, He M, Weaver JMJ, Lynch AG, Kingsbury Z, Ross M, Humphray S, Bentley D, Fitzgerald RC. Whole-genome sequencing provides new insights into the clonal architecture of Barrett's esophagus and esophageal adenocarcinoma. Nat Genet. 2015;47:1038–1046. doi: 10.1038/ng.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Campbell JD, Mazzilli SA, Reid ME, Dhillon SS, Platero S, Beane J, Spira AE. The Case for a Pre-Cancer Genome Atlas (PCGA) Cancer Prev Res (Phila) 2016;9:119–124. doi: 10.1158/1940-6207.CAPR-16-0024. [DOI] [PubMed] [Google Scholar]
- 93.Kim HJ, Kang TW, Haam K, Kim M, Kim SK, Kim SY, Lee SI, Song KS, Jeong HY, Kim YS. Whole genome MBD-seq and RRBS analyses reveal that hypermethylation of gastrointestinal hormone receptors is associated with gastric carcinogenesis. Exp Mol Med. 2018;50:156. doi: 10.1038/s12276-018-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rivas-Ortiz CI, Lopez-Vidal Y, Arredondo-Hernandez LJR, Castillo-Rojas G. Genetic Alterations in Gastric Cancer Associated with <i>Helicobacter pylori</i> Infection. Front Med (Lausanne) 2017;4:47. doi: 10.3389/fmed.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li D, Bautista MC, Jiang SF, Daryani P, Brackett M, Armstrong MA, Hung YY, Postlethwaite D, Ladabaum U. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am J Gastroenterol. 2016;111:1104–1113. doi: 10.1038/ajg.2016.188. [DOI] [PubMed] [Google Scholar]
- 96.Huang KK, Ramnarayanan K, Zhu F, Srivastava S, Xu C, Tan ALK, Lee M, Tay S, Das K, Xing M, Fatehullah A, Alkaff SMF, Lim TKH, Lee J, Ho KY, Rozen SG, Teh BT, Barker N, Chia CK, Khor C, Ooi CJ, Fock KM, So J, Lim WC, Ling KL, Ang TL, Wong A, Rao J, Rajnakova A, Lim LG, Yap WM, Teh M, Yeoh KG, Tan P. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell. 2018;33:137–150.e5. doi: 10.1016/j.ccell.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 97.Busuttil RA, Zapparoli GV, Haupt S, Fennell C, Wong SQ, Pang JM, Takeno EA, Mitchell C, Di Costanzo N, Fox S, Haupt Y, Dobrovic A, Boussioutas A. Role of p53 in the progression of gastric cancer. Oncotarget. 2014;5:12016–12026. doi: 10.18632/oncotarget.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 99.Rokutan H, Abe H, Nakamura H, Ushiku T, Arakawa E, Hosoda F, Yachida S, Tsuji Y, Fujishiro M, Koike K, Totoki Y, Fukayama M, Shibata T. Initial and crucial genetic events in intestinal-type gastric intramucosal neoplasia. J Pathol. 2019;247:494–504. doi: 10.1002/path.5208. [DOI] [PubMed] [Google Scholar]
- 100.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 101.Yun CY, Kim N, Lee J, Lee JY, Hwang YJ, Lee HS, Yoon H, Shin CM, Park YS, Kim JW, Lee DH. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter. 2018;23:e12542. doi: 10.1111/hel.12542. [DOI] [PubMed] [Google Scholar]
- 102.Waldum HL, Öberg K, Sørdal ØF, Sandvik AK, Gustafsson BI, Mjønes P, Fossmark R. Not only stem cells, but also mature cells, particularly neuroendocrine cells, may develop into tumours: time for a paradigm shift. Therap Adv Gastroenterol. 2018;11:1756284818775054. doi: 10.1177/1756284818775054. [DOI] [PMC free article] [PubMed] [Google Scholar]