Key Points
Increased mTNF and defect in M2-like macrophages are biomarkers specific to RA.
Increase in miR155 is responsible for this defect and could be a new target for therapeutic strategies.
Abstract
Proinflammatory macrophages and miR-155 are increased in patients with rheumatoid arthritis (RA). We studied membrane TNF (mTNF) expression on blood monocytes, polarization into macrophages, miR-155 expression, and the effect of anti-TNF on these biomarkers in RA patients. Sixty-seven RA patients and 109 controls (55 healthy, 54 with spondyloarthritis and connective tissue diseases) were studied. Monocytes were isolated and differentiated into macrophages with or without anti-TNF. mTNF expression was increased on monocytes from RA patients, but not from other inflammatory diseases, correlated with disease activity. Under human serum AB or M-CSF, only monocytes from RA had a defect of differentiation into M2-like macrophages and had a propensity for preferential maturation toward M1-like macrophages that contributed to synovial inflammation. This defect was correlated to mTNF expression and was partially reversed by monoclonal anti-TNF Abs but not by the TNF soluble receptor. miR-155 was increased in M2-macrophages except in adalimumab-treated patients. Transfection of healthy monocytes with miR-155 induced a decrease in M2-like markers, and transfection of RA monocytes with antagomir-155 allowed restoration of M2-like polarization. Defect in differentiation of monocytes into M2-like-macrophages linked to increased miR-155 and correlated with increased mTNF on monocytes could play a key role in RA pathogenesis. Monoclonal anti-TNF Abs but not the TNF soluble receptor partially restored this defect.
Introduction
Rheumatoid arthritis (RA) is the most frequent systemic autoimmune disease, affecting 0.5% of the general population, and is characterized by a chronic inflammatory that primarily affects multiple joints and causes irreversible bone and cartilage destruction in the absence of treatment (1). Monocytes/macrophages are key players in the pathogenesis of RA (1, 2). Indeed, a critical pathological feature of RA is the accumulation of monocytes/macrophages in the synovial tissue (2). Three types of monocytes subsets have been described based on their CD14 and CD16 expression profiles: classical (CD14high CD16dim), intermediate (CD14high CD16high), and nonclassical (CD14dim CD16high), the intermediate subpopulation being expanded in RA patients (3). RA monocytes are able to spontaneously produce inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (4, 5).
Monocytes can differentiate into the “classically activated macrophage phenotype” (M1-like), considered to be proinflammatory, or into the “alternatively activated macrophage phenotype” (M2-like) considered to be regulatory and anti-inflammatory. M1-like macrophages can be induced by IFN-γ, LPS, or cytokines as GM-CSF and exhibit an IL-12high, IL-23high, IL-1βhigh, TNFhigh, IL-6high, and IL-10low phenotype. M1-like macrophages are also characterized by a large amount of the transcription factor IFN regulatory factor 5 (IFR5) and by a high level of the inducible NO synthases isoform (iNOS) (6). M1-like macrophages contribute to RA pathogenesis by secreting proinflammatory cytokines (7). In contrast, the alternative M2-like macrophages share an IL-12low, IL-23low, and IL-10high phenotype, generally display high levels of macrophage mannose receptor (CD206) and scavenger receptor (CD163), and arginine metabolism is shifted to the production of ornithine and polyamines via the arginase activity (8–10). M2-like macrophages can be induced ex vivo by incubation in the presence of M-CSF supplemented or not with IL-4–IL-13 or of macrophage medium containing human serum AB (SAB) and have diverse functions, ranging from parasite control to immune suppression, wound repair, tissue remodeling, and angiogenesis (11). Some studies have clearly demonstrated that infiltrating synovial macrophages display a proinflammatory profile (2, 12–16).
MicroRNAs are modifiers of macrophage gene expression that contribute to regulating macrophage gene expression responses to polarization environmental conditions (17). Outside RA, published microarray demonstrated a dramatic differential expression of miRNAs in M1-like and M2-like macrophages (18). miR-155 was the most upregulated microRNA in M1-like macrophages as compared with M2-like macrophages (19–21). An upregulation of miR-155 expression has also been shown in RA synovial monocytes, macrophages, and fibroblasts but also in PBMCs and blood CD14+ monocytes associated with the production of proinflammatory mediators, including TNF-α (22–24).
The most important therapeutic advance in the past 20 years in RA has been the use of TNF inhibitors (TNFi) (25, 26). Two types of TNFi are available: mAbs and the TNFR2–Ig soluble receptor, etanercept (ETA). Monocytes/macrophages are one of the cells expressing and secreting the highest level of membrane TNF (mTNF) and soluble TNF (sTNF), respectively (14). With TNFi, but also with other biologics like rituximab, there is a correlation between the depletion of synovial macrophages and the efficacy of the drugs (27, 28). Both types of TNFi inhibit sTNF, secreted by all immune cells. Monoclonal anti-TNF Abs and the sTNF receptor can also bind mTNF, but the stability of the interaction is higher with monoclonal anti-TNF than with the sTNF receptor (25). This engagement of mTNF may induce regulatory T cells (29) or trigger a signaling event in TNF-producing cells, defined as “reverse” signaling to distinguish it from “forward” signaling induced by activation of TNF receptors. Although TNF revers” signaling has been emerging as an important phenomenon in the immune response, its molecular basis remains obscure (30).
In this study, we studied different monocyte/macrophage abnormalities in RA and in other systemic inflammatory diseases, the role of miR-155 on these abnormalities, and their modulation in vitro and in vivo by different TNFi.
Materials and Methods
Patients
Peripheral blood obtained from healthy donors (HD), connective tissue diseases (CTD), spondyloarthritis (SpA), and RA patients was used for mTNF expression on monocytes and for M1-like/M2-like phenotypes characterization. All RA patients fulfilled the 2010 American College of Rheumatology and European League Against Rheumatism RA criteria (31). The Disease Activity Score evaluated in 28 joints (DAS28-CRP) was used to assess the disease activity of RA patients. All patients were referred to the Department of Rheumatology of Hôpitaux Universitaires Paris-Sud between April 2017 and November 2018, with consecutive patients seen in consultation or in day-care unit. Because of the impact of corticosteroids on macrophages, patients treated with corticosteroid therapy ≥10 mg per day were excluded (32). CTD and SpA patients were included as controls.
The project conformed to the principals of the Declaration of Helsinki. Two institutional review boards gave their approval, one for the collection of blood from healthy people (centralized for all healthy blood donors at Etablissement Français du Sang in France) under the control of convention with the INSERM, the other for patients with autoimmune/inflammatory diseases followed in our reference center (protocol no. PP 15-004). Informed consent to use the cells for clinical research was obtained from each HD and patient. Supplemental Fig. 1 indicates the number of patients and controls used for each experiment.
Monocytes selection and differentiation into macrophages
Blood monocytes were isolated using a pan-monocyte negative selection according to the manufacturer’s instruction (Miltenyi Biotec) to achieve a purity of >90%. Then monocytes were cultured in serum-free RMPI 1640–Glutamax for 3 d and were treated by IVIg (10 μg/ml) as a control, adalimumab (ADA; 10 μg/ml), or ETA (10 μg/ml).
To generate M1-like phenotype macrophages or M2-like macrophages, purified blood monocytes were incubated in RMPI 1640–Glutamax medium supplemented with 100 IU/ml penicillin–streptomycin and with 10% FBS in the presence of 50 ng/ml recombinant human GM-CSF for M1-like or in the presence of 50 ng/ml recombinant human M-CSF for M2-like macrophages (PeproTech).
For M2-like differentiation with SAB, blood monocytes were cultured for 6 d in hydrophobic Teflon dishes (Lumox Sarstedt) in RMPI 1640–Glutamax medium supplemented with 100 IU/ml penicillin–streptomycin, 10 mM HEPES, 10 mM sodium pyruvate, 50 μM 2-ME, 1% MEM vitamins, 1% nonessential amino acids (Life Technologies) supplemented with 15% of heat inactivated SAB (Biowest) as a physiological source of M-CSF (620 pg/ml), IL-10 (10 pg/ml), IL-4 (11 pg/ml), and IL-13 (5.2 pg/ml).
After a 6-d culture, cells were harvested and washed, and supernatants were stored at −80°C until analysis.
miRNA mimic and antagomiR transfections
miR-155 (mimic or antagomir), control miR (mimic and antagomir), or fluorescent control mimic Cy3 (controls is a random sequence miRNA molecule that has been validated to not produce identifiable effects on miRNA function) were all purchased from Thermo Fisher Scientific. miRNA transfection was performed using AMAXA technology (Lonza). A total of 1 × 106 monocytes were diluted in Nucleofector solution and pulsed with Y-001 program with 100 nM of miRNA. Monocytes were transfected 18 h before and then incubated for 6 d in macrophages medium containing SAB or RPMI 1640 supplemented with 200 mM l-glutamine, 100 U of penicillin, 100 μg streptomycin, and 50 ng/ml recombinant human M-CSF (PeproTech).
Immunofluorescence microscopy and flow cytometry
Synovial biopsies from nine patients with active RA (seven on methotrexate [MTX], one on ETA, and one on ADA) and 10 with osteoarthritis (OA) were used. Four-micrometer sections were cut from the paraffin blocks of the fixed tissues from humans. Biopsies were deparaffinized, rehydrated, and subjected to high-temperature Ag retrieval (97°C for 20 min) in 10× citrate buffer (pH 6) (Sigma-Aldrich). Slides were permeabilized in 0.3% Triton X-100 for 5 min and saturated in PBS–FBS 20% BSA, 1 mg/ml for 1 h.
Coverslips with macrophages were fixed in 4% paraformaldehyde–PBS for 5 min, permeabilized for 5 min with 0.3% Triton X-100 (Sigma-Aldrich) in PBS, washed in PBS, and then blocked for 1 h at room temperature in PBS–FBS 20% BSA 1 mg/ml.
Biopsies and macrophages were incubated with anti-CD68 (1/100 dilution; Abcam or DAKO), anti-iNOS (1/200 dilution; Abcam), or anti-Arginase-1 (1/50 dilution; Invitrogen) overnight at 4°C. After three washings in PBS, the secondary Ab anti-rabbit and anti-mouse IgG conjugated to Alexa Fluor 488 or 546 fluorochromes (1/500 dilution; Life Technologies) supplemented with DAPI (1/1 000 dilution; Life Technologies) for nuclei detection in PBS–FBS 20% BSA 1 mg/ml were added to the biopsies or cells for 30 min at room temperature. Biopsies or cells were then mounted with Flouromount G medium (SouthernBiotech).
Biopsies and cells were imaged by an epifluorescence microscope (Olympus PROVIS) with 8-bit configuration and using 40× dry objective or 100× oil objective.
mTNF and CD115 expression on monocytes was determined on PBMCs at room temperature for 15 min with anti–HLA-DR (allophycocyanin-cy7), anti-CD2 (PerCPcy5.5), anti-CD19 (PerCPcy5.5), anti-CD56 (PerCPcy5.5), anti-CD14 (PE), anti-CD16 (allophycocyanin), and anti-TNF (FITC), or with HLA-DR (allophycocyanin-cy7), anti-CD2 (FITC), anti-CD19 (FITC), anti-CD56 (FITC), anti-CD14 (PE), anti-CD16 (allophycocyanin), and anti-CD115 (PerCPcy5.5). For macrophage phenotype, cells were harvested in FBS 100% and scratched, washed once with PBS, saturated at 4°C for 10 min with FcBlock (Becton Dickinson), and incubated 30 min with anti-CD11b (PerCP-cy5.5), anti-CD71(PE), anti-CD206 (Pe-cy7), and anti-CD163 (FITC) Abs, and death cells were excluded using live-death (allophycocyanin-cy7) (gating strategy in Supplemental Fig. 2). The indicated Abs and isotype-matched Abs used were obtained from Ozyme. Stained cells were acquired by a BD FACSCanto and analyzed using Flowjo V10 software.
ELISA and MULTIPLEX
Cell-free supernatants after a 6-d culture of monocytes polarization were collected and assayed for human IL-10 secretion by a commercially available ELISA kit (eBioscience) used according to the manufacturer’s instruction. The concentration of chemokines and cytokines in M2-like macrophages culture supernatants was quantified by immunofluorescence assay using the Bio-Plex 27 Plex on a Bio-Plex platform (Bio-Rad). The Bio-Plex 27 Plex was used in accordance with the manufacturer’s protocol to detect human MIP1-α, IL-8, IL-6, IL-1β, MCP-1, GM-CSF, and IL-7 on six HD and 27 RA patients (six on MTX, two on tocilizumab, one on rituximab, three on abatacept, seven on ETA, and eight on ADA).
Quantitative PCR
Total RNA was extracted with the GeneJET kit (Thermo Fisher Scientific) according to manufacturer specifications, at 6 d after monocytes polarization into M1-like or M2-like phenotype macrophages for IRF5, miR-155, CEBP/β, and suppressor of cytokine signaling-1 (SOCS-1) detection. The quantification of mRNA and miRNA expression were determined by TaqMan real-time PCR according to the manufacturer’s instructions. The amounts of IFR5, CEBP/β, and SOCS-1 were normalized to the endogenous GAPDH, and miR-155 expression was normalized to miR-25 and miR-223 (NormFinder analysis to find the best gene for normalization). Efficiency of PCR was performed using a serial dilution of amplicons. Calculations of RNA–miRNA expression levels were performed using the comparative cycle threshold method.
Statistical analysis
The data were analyzed by Graph Pad Prism V8. Data were tested by Mann–Whitney for two groups and Kruskal–Wallis test with Dunn multiple comparisons for multiple groups, expressed as mean ± SEM with plot individual values. Transfection data were tested by Wilcoxon matched pairs, and correlation between variables was tested using the two-tailed nonparametric Spearman with 95% of confidence interval; a p value < 0.05 was considered significant.
Results
Characteristics of patients and controls
Sixty-seven patients with RA were included, their characteristics being presented in Table I. We differentiated patients treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARs) alone (n = 20), monoclonal anti-TNF Abs (ADA or infliximab [IFX]) (n = 9), ETA (n = 15), and non-TNFi biologics (n = 14). Two types of controls were included: 55 HD and 54 patients with other inflammatory diseases; 6 systemic lupus erythematosus (SLE), 26 SS (SLE and SS patients can be grouped in the CTD group), and 22 SpA (Table I).
Table I. Demographic and clinical data for patients and HD.
| RA Patients (n = 67) | CTD Patients (n = 32) |
SpA Patients (n = 22) | HD (n = 55) | ||
|---|---|---|---|---|---|
| SLE Patients (n = 6) | SS Patients (n = 26) | ||||
| Female (%) | 52 (77) | 4 (66) | 23 (88) | 11 (50) | 8 (14) |
| Age (SD) | 56.9 (17) | 42.2 (13) | 62.0 (16) | 39.9 (14) | 45.1 (12) |
| Disease duration (SD) | 11.9 (10) | 12.2 (9) | 6.2 (6) | 4.8 (7) | — |
| Anti-CCP positivity (%) | 47 (70) | — | — | — | — |
| Anti-DNA (%) | — | 4 (66) | — | — | — |
| Anti-SSA (%) | — | — | 15 (57.7) | — | — |
| Anti-SSB (%) | — | — | 7 (26.9) | — | — |
| DAS28 for RA patients (SD) | 3.3 (1.5) | — | — | — | — |
| Treatment | — | ||||
| None | 9 | 1 | 16 | 4 | — |
| NSAIDS | — | — | — | 4 | — |
| Corticosteroid <10 mg | 8 | 2 | 2 | 1 | — |
| csDMARDs | 39 | 4 | 8 | 5 | — |
| ADA | 8 | — | — | 1 | — |
| IFX | 1 | — | — | 1 | — |
| ETA | 15 | — | — | 4 | — |
| Certolizumab | — | — | — | 2 | — |
| Non-TNF biologics | 14 | 2 | 1 | 1 | — |
Sixty-seven patients with RA were included and two types of controls were included. There were 55 HD and 54 patients with other inflammatory diseases: 6 SLE, 26 SS (SLE and SS patients can be grouped in the CTD group), and 22 SpA. To fulfill the 2010 American College of Rheumatology and European League Against Rheumatism requirements, anticyclic citrullinated peptide (anti-CCP) and DAS-28 were determined for RA patients, anti-DNA was determined for SLE patients, and anti-SS–related Ag A (anti-SSA) and anti-SS–related Ag B (anti-SSB) were determined for SS patients. We differentiated patients according to their treatment: nonsteroidal anti-inflammatory drugs (NSAIDS), csDMARs, ADA, IFX, ETA, certolizumab, or non-TNF biologics.
mTNF expression is increased in RA monocytes, influenced by disease activity but not by in vitro TNFi treatment
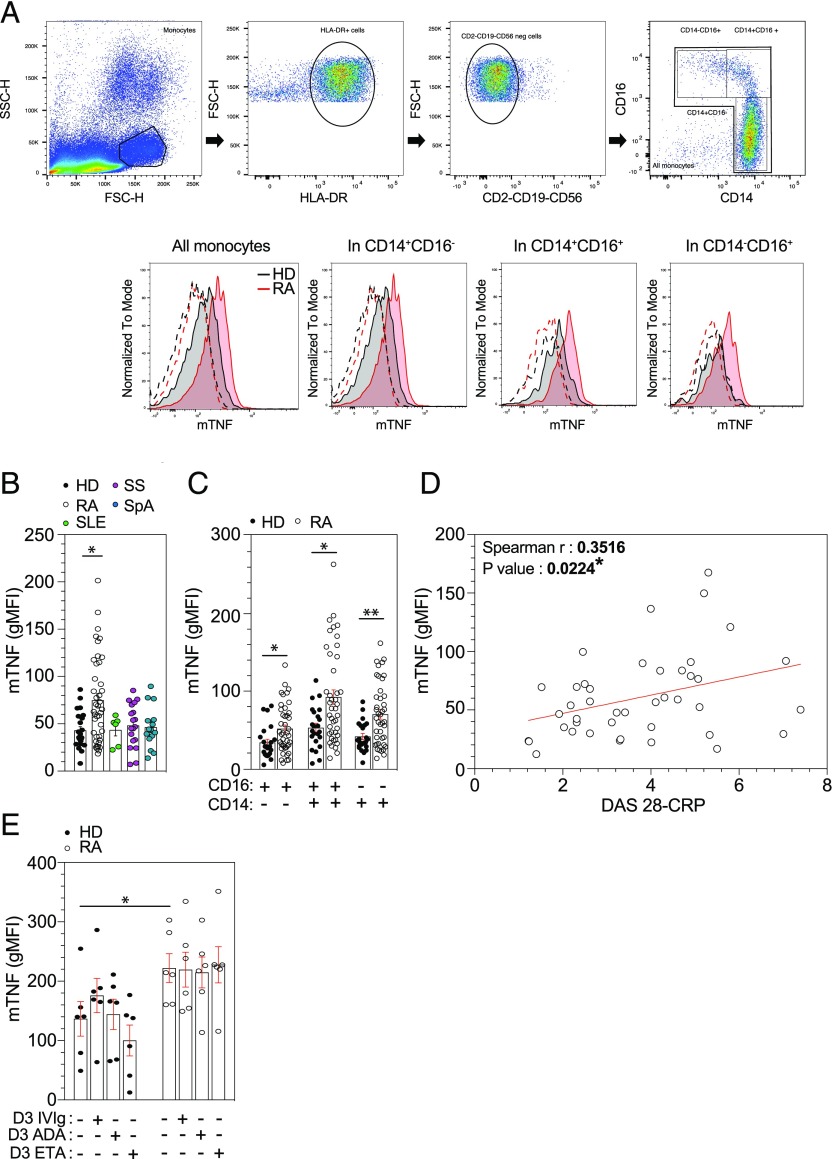
mTNF expression was significantly higher only in blood monocytes from RA patients and not from other inflammatory diseases or from HD (p = 0.016) (Fig. 1B). In addition, mTNF was significantly increased in the three monocytes subsets in RA patients (Fig. 1C). The mTNF expression on monocytes was positively correlated with disease activity assessed by DAS28-CRP (r = 0.3516 et p = 0.0224) (Fig. 1D). To search for a potential confounding impact of treatment, age, and sex on mTNF expression, we compared mTNF expression according treatment group (csDMARDs versus anti-TNF versus other biologics), sex, and age. None of these parameters was associated with mTNF expression (data not shown). As there is a strong association between mTNF expression and activity of the disease, and because TNFi decrease activity of the disease, the only way for exploring this issue was an in vitro study. We investigated in vitro the impact of TNFi on isolated monocytes from HD or RA patients (four were on MTX, one on rituximab, and one on tocilizumab) cultured for 3 d with polyvalent IVIg, ADA, or ETA and found no effect of both TNFi on mTNF expression (Fig. 1E). Therefore, monocytes from RA patients have an increased expression of mTNF linked to activity of the disease and not influenced by in vitro treatment with different TNFi.
FIGURE 1.
mTNF expression is increased in RA monocytes. (A) Gating strategy used to determined expression of mTNF on monocytes subpopulation on PBMCs. (B and C) Ex vivo monocyte mTNF expression on HD (n = 23), RA (n = 42), SS (n = 18), SLE (n = 6), and SpA (n = 15) patients was determined in flow cytometry analyses after PBMCs isolation by Pancoll separation with anti–HLA-DR, anti-CD2, anti-CD19, anti-CD56, anti-CD14, anti-CD16, and anti-mTNF Ab. (D) Scatterplot showed a significant positive correlation between monocyte mTNF expression (geometric mean fluorescence intensity [gMFI]) and the DAS28-CRP in RA patients (n = 42). (E) Purified blood monocytes treated with TNFi (ETA or ADA) or with IVIg were harvested after 3 d and analyzed for monocytes mTNF expression with anti-CD14, anti-mTNF, and live death (HD n = 6 and RA n = 6). Results are shown as mean ± SEM. Kruskal–Wallis test with Dunn multiple comparisons and the two-tailed nonparametric Spearman for correlation. *p ≤ 0.05, **p ≤ 0.01.
A specific defect in M2-like polarization of RA monocytes is modulated by ADA but not by ETA
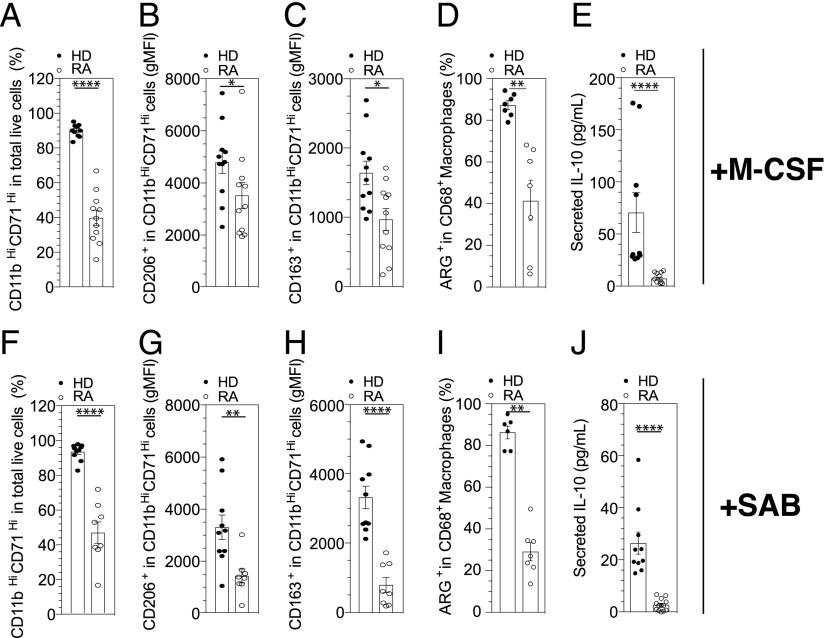
We next investigated the possible link between the increased expression of mTNF on monocyte and macrophage polarization. To assess monocyte polarization into M1-like or M2-like macrophages, purified monocytes from HD, Sjögren syndrome (SS), SpA, or RA patients were cultured for 6 d with GM-CSF for M1-like polarization, and for M2-like polarization we used M-CSF or human SAB methods. No difference was observed concerning monocyte polarization into M1-like phenotype in RA and HD (Supplemental Fig. 3, A–C). Conversely, when polarization into M2-like phenotype was studied by a 6-d culture of monocytes in the presence of M-CSF or human SAB, a significant decrease of the pan-macrophage markers CD11b and CD71 was observed in RA patients compared with HD (Fig. 2A, 2F), and this defect was specific to RA, because it was not found in SpA or SS (Fig. 3A). This defect concerns the polarization into M2-like macrophages, as we found a decrease in macrophages expressing CD206+ (Fig. 2B, 2G) or CD163+ (Fig. 2C, 2H), a decrease of IL-10 secretion (Fig. 2E, 2J), and Arg1 expression (Fig. 2D, 2I).
FIGURE 2.
A specific M2-like polarization of RA monocytes with M-CSF and SAB methods. (A–E) The differentiation of blood monocytes into M2-like macrophages by M-CSF was assessed in HD (n = 11) and in patients with RA treated with MTX (n = 11) by flow cytometry analysis using anti-CD11b and anti-CD71 Abs (A). Specifics markers of M2-like macrophage polarization were assessed, CD206 (B) and CD163 (C). Frequencies of CD68+ Arg+ (HD = 7 and RA = 7) (D) and concentrations of IL-10 in cell culture supernatant were detected by ELISA after 6 d (HD = 11 and RA = 11) (E). (F–J) Differentiation of blood monocytes by SAB into M2-like macrophages was assessed in HD (n = 10) and in patients with RA treated with MTX (n = 8), for CD11b–CD71 (F), CD206 (G), and CD163 (H). Frequencies of CD68+ Arg+ (HD = 6 and RA = 4) (I) and concentrations of IL-10 in cell culture supernatant were detected by ELISA after 6 d (HD = 11 and RA = 11) (J). Results are shown as mean ± SEM. Mann–Whitney t test *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001.
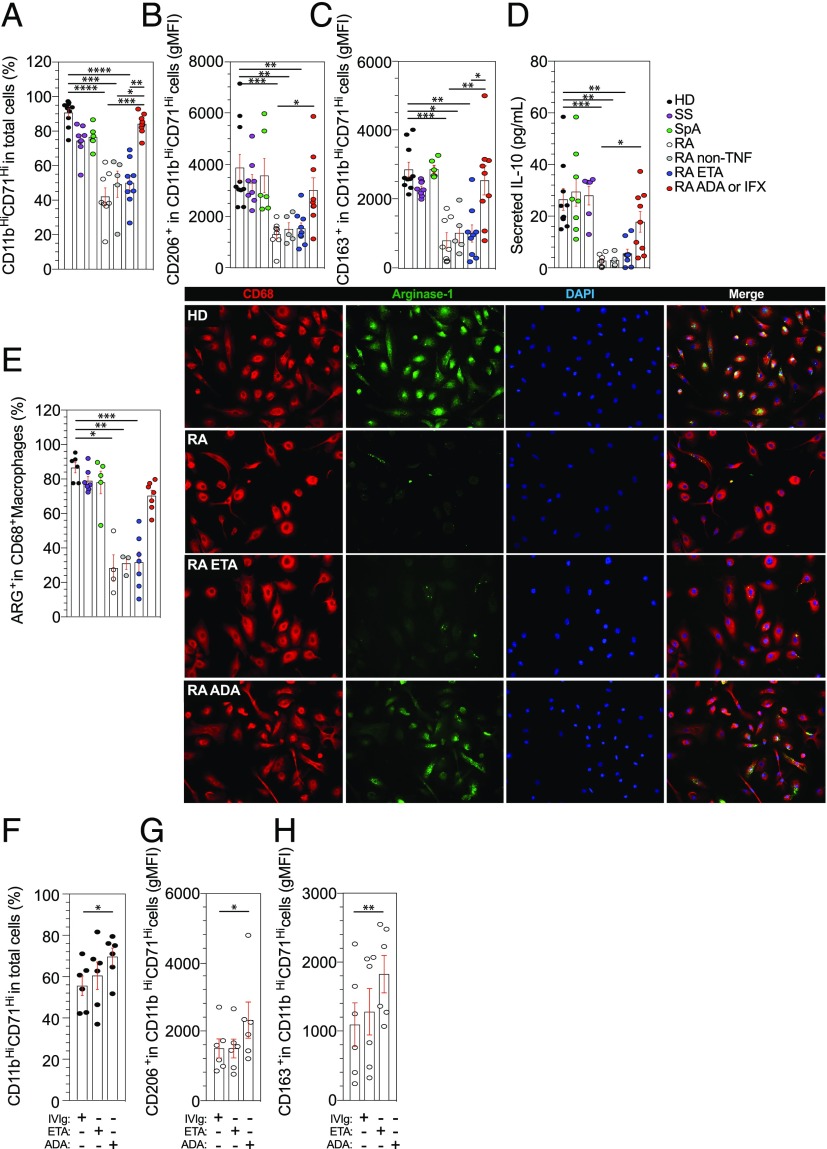
FIGURE 3.
A specific defect in M2-like polarization of RA monocytes as compared with SS and SpA patients. (A–C) The differentiation of blood monocytes into M2-like macrophages by SAB was assessed in HD (n = 10) and in patients with SS (n = 8), SpA (n = 6), RA treated with MTX (n = 8), RA treated with MTX + ETA (n = 9), and RA treated with MTX + ADA or IFX (n = 9) and RA treated with MTX + non-TNFi biologic (n = 5) by flow cytometry analysis using anti-CD11b and anti-CD71 Abs (A). Specifics markers of M2-like macrophages polarization were assessed, CD206 (B) and CD163 (C). (D) Concentrations of IL-10 in cell culture supernatant were detected by ELISA after 6 d. (E) Representative images and frequencies of CD68+ Arg+ detected in HD (n = 6) and in patients with SS (n = 7), SpA (n = 5), RA treated with MTX (n = 4), RA treated with MTX + ETA (n = 7), and RA treated with MTX + ADA or IFX (n = 7) and RA treated with MTX + non-TNFi biologic (n = 3). Scale bar, 10 μm. (F–H) TNFi or control treated purified monocytes were harvested after 6 d in macrophage SAB medium and analyzed for M2-like polarization with anti-CD11b, anti-CD71, anti-CD206, anti-CD163, and live-death (HD n = 6 and RA n = 6 [four were on MTX, one on rituximab, and one on tocilizimab]). Results are shown as mean ± SEM. Kruskal–Wallis test with Dunn multiple comparisons *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
We found that M2-like macrophages in ADA-treated RA patients, and not in ETA-treated RA patients or non-TNFi-treated RA patients, partially restored CD11b and CD71 expression levels (Fig. 3A) but also CD206 (p = 0.02) (Fig. 3B), CD163 (p = 0.006) (Fig. 3C), IL-10 secretion (p = 0.04) (Fig. 3D), and Arg1 expression (Fig. 3E). To confirm this result in vitro, we isolated RA monocytes and cultured them in macrophage medium containing human SAB in the presence of TNFi (ADA or ETA) or IVIg as controls for 6 d. At the end of the culture, no difference was detected between control and ETA group on CD11b–CD71 (Fig. 3F), CD206 (Fig. 3G), and CD163 (Fig. 3H) expression levels. Conversely, ADA was able to partially restore M2-like polarization defect (Fig. 3F–H), consistent with ex vivo findings in ADA-treated patients.
Thus, RA patients had a specific impaired maturation of monocytes into M2-like macrophages while the differentiation to M1-like phenotype was maintained. The use of ADA partially restored M2-like polarization, whereas ETA or non-TNFi drugs did not restore M2-like polarization.
The defect in M2-like polarization leads to an M1-like phenotype modulated by ADA but not by ETA
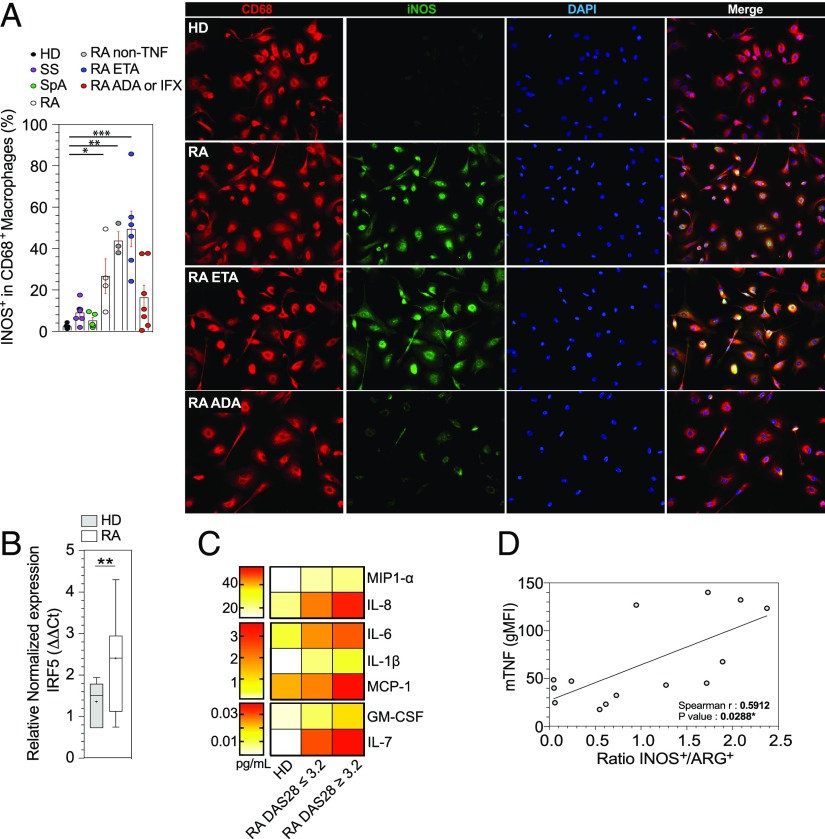
On the basis of our observation that RA patients had a defect in M2-like polarization, we aimed to determine whether such defect could generate M1-like macrophages. Indeed, M2-like macrophages from RA patients produced iNOS (p = 0.04) (Fig. 4A). In contrast, we did not observe any difference in iNOS expression between HD and patients with SS and SpA (Fig. 4A).
FIGURE 4.
The defect in M2-like polarization leads to an M1-like phenotype. (A) Representative images and frequencies of CD68+ iNOS+ macrophages detected in HD (n = 6) and in patients with SS (n = 7), SpA (n = 5), RA treated with MTX (n = 4), RA treated with MTX + ETA (n = 7), and RA treated with MTX + ADA or IFX (n = 7) and RA treated with MTX + non-TNFi biologic (n = 3). Scale bar, 10 μm. (B) IRF5 mRNA expression on M2-like macrophages was determined by quantitative real-time PCR (HD n = 5 and RA n = 7). Results are show as box and whiskers (min to max). (C) Spontaneous 6-d cytokine production by M2-like macrophages from HD (n = 6), RA DAS28-CRP ≤ 3.2 (n = 14), and RA DAS28-CRP ≥ 3.2 (n = 13) (27 patients included; 6 were on MTX, 2 on tocilizumab, 1 on rituximab, 3 on abatacept, 7 on ETA, and 8 on ADA). (D) Regression analysis showing a significant positive correlation between monocytes mTNF expression (geometric mean fluorescence intensity [gMFI]) and the ratio iNOS+/ARG+ in monocyte-derived M2-like macrophages from RA treated with MTX or non-TNF biologics or RA treated with MTX + ETA. Results are shown as mean ± SEM. Mann–Whitney t test, Kruskal–Wallis test with Dunn multiple comparisons, and the two-tailed nonparametric Spearman for correlation *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
M2-like macrophages from ADA-treated patients had a decreased expression of iNOS, whereas ETA had no effect (Fig. 4A). Likewise, M2-like macrophages from active (mean DAS28 = 5.15) RA patients (five on MTX, one on rituximab, and one on tocilizumab) had an increased expression of IRF5 (p = 0.026) (Fig. 4B). Finally, levels of the proinflammatory cytokines MIP1-α, IL-8, IL-6, IL-1β, MCP-1, GM-CSF, and IL-7 were increased in supernatants from RA M2-like macrophages, mainly in cases of moderate activity of the disease (Fig. 4C).
Thus, under human SAB treatment, RA monocytes, instead of differentiating into an M2-like phenotype, have a propensity for preferential maturation toward M1-like phenotype.
Correlation between mTNF expression and the M2-like defect in RA
The iNOS/Arg ratio in monocyte-derived M2-like macrophages from RA patients can be considered as a good reflection of the defect of the polarization of monocytes into M2-like macrophages in favor of the M1-like macrophages. We found a significant correlation between monocytes mTNF expression and this iNOS/Arg ratio in in monocyte-derived M2-like macrophages from RA patients. We excluded patients treated with ADA because it is the only drug that impacted the iNOS/ARG ratio. Neither other types of treatment nor age impacted this ratio (data not shown). Interestingly, this correlation was lost in patients treated with ADA (Fig. 4D).
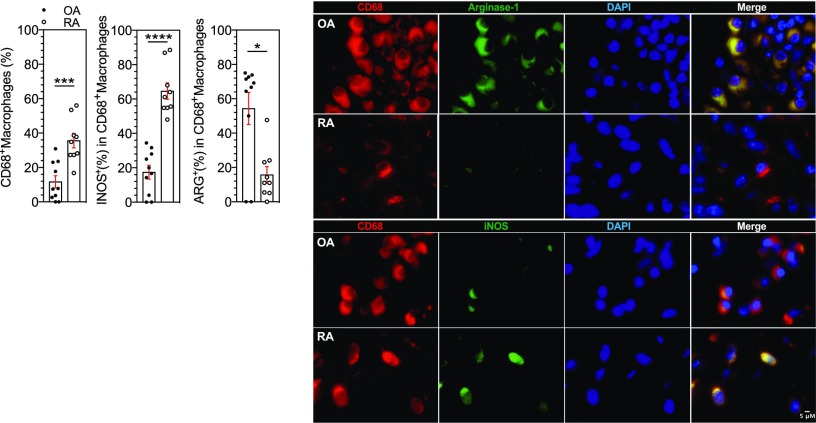
Synovium macrophages is skewed to the M1-like subtype in RA
We therefore examined the phenotype of macrophages in the synovial tissue of patients with RA compared with patients with OA. We found higher infiltration of CD68+ macrophages in RA patients (p = 0.0009) (Fig. 5). Double immunofluorescence staining revealed that Arg1 expression (an M2-like marker) was more present in CD68+ macrophages in OA than in RA synovium (p = 0.0206). Furthermore, as in our ex vivo experiments, iNOS (an M1-like marker) was expressed in CD68+ macrophages in RA synovium but not in OA (p < 0.0001) (Fig. 5).
FIGURE 5.
Synovium macrophages is skewed to the M1-like subtype in RA. Representative images and frequencies of CD68+ iNOS+ or CD68+ Arg+ detected in OA (n = 10) and RA (n = 9). Scale bar, 5 μm. Results are shown as mean ± SEM. Mann–Whitney t test *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001.
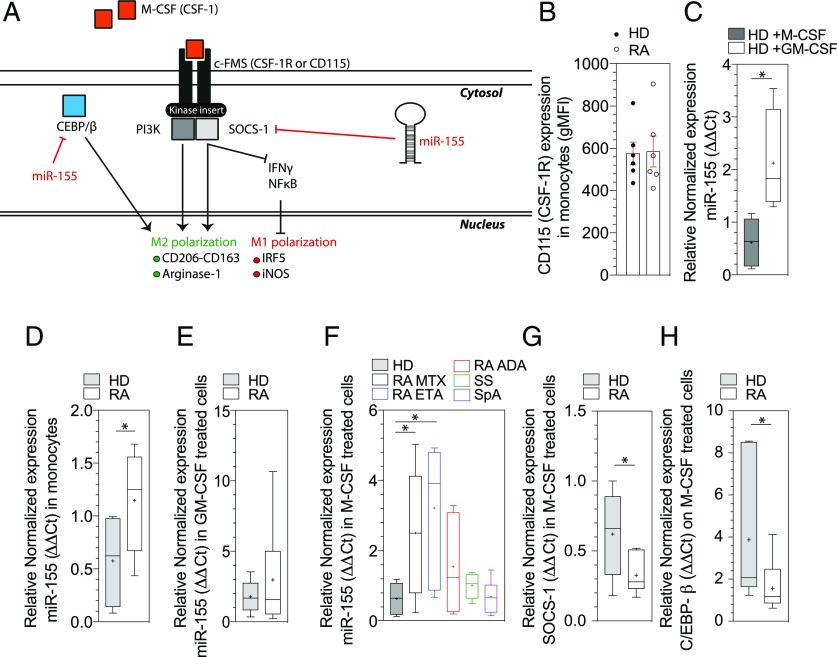
M-CSF signaling pathway is impaired in RA patients
M-CSF recruits monocytes to inflammatory or injury sites and binds to its receptor CSF-1 (or CD115), which leads to activation of the SOCS-1 necessary to maintain the Arg/iNOS balance in M2-like macrophages (33). Arg1 is also regulated by the transcription factors C/EBP-β (34). Finally, miR-155 inhibits both SOCS-1 and C/EBP-β expression, which may explain its proinflammatory effect and its inhibition of the M2-like differentiation (33, 35) (Fig. 6A). We thus analyzed this pathway in monocytes from RA patients and controls.
FIGURE 6.
M-CSF signaling pathway and miR-155 involvement. (A) The figure represents the interaction between CSF-1 and its receptor CSF-1R, downstream signaling pathway, and involvement of miR-155. (B) Ex vivo monocyte CD115 expression on HD (n = 6) and on RA patients (n = 6) was determined in flow cytometry analyses after PBMCs isolation by Pancoll separation with anti–HLA-DR, anti-CD2, anti-CD19, anti-CD56, anti-CD14, anti-CD16, and anti-CD115 Ab. Results are shown as mean ± SEM. (C) miR-155 expression on M1-like and M2-like HD macrophages (n = 6) was determined by quantitative real-time PCR. (D) miR-155 mRNA expression in monocytes (HD n = 6, RA n = 6). (E) miR-155 mRNA expression in M1-like macrophages (HD n = 5 and RA n = 6) were determined by qPCR. (F) miR-155 expression in M2-like macrophages (HD n = 5, RA n = 9, RA+ETA n = 7, RA+ADA n = 6, SS n = 7, and SpA n = 7). (G and H) Target mRNA SOCS-1 (G) and C/EBP-β (H) expression on HD (n = 7) and RA (n = 11). Results are show as box and whiskers (min to max). Mann–Whitney t test and Kruskal–Wallis test with Dunn multiple comparisons *p ≤ 0.05.
Expression of CD115 was equal between HD and RA patients (Fig. 6B). We confirmed that miR-155 was significantly upregulated in HD in response to M1 stimulation conditions (GM-CSF) (p = 0.016); in contrast, miR-155 was not upregulated in HD M2 (differentiated with human SAB) (Fig. 6C). RA monocytes overexpressed miR-155 (p = 0.048) (Fig. 6D). We did not observe any significantly difference of miR-155 expression in RA M1-like macrophages as compared with HD (Fig. 6E). Conversely, M2-like macrophages from RA patients overexpressed miR-155 as compared with HD (p = 0.04) and patients with SS and SpA (Fig. 6F). The only RA patients who did not harbor increased miR155 in M2-like macrophages were those treated with ADA, whereas those treated with ETA or other biologics had a miR-155 increase (Fig. 6F).
Finally, SOCS-1 and C/EBP-β mRNA were downregulated in M2-like macrophages from RA patients compared with HD (p = 0.04 and p = 0.02, respectively) (Fig. 6G, 6H).
Altogether, these results suggest that overexpression of miR-155 in RA monocytes and M2-like macrophages could promote the defect of monocytes transformation into M2-like phenotype seen in RA patients and that expression of miR-155 is influenced by the use of ADA and not by ETA.
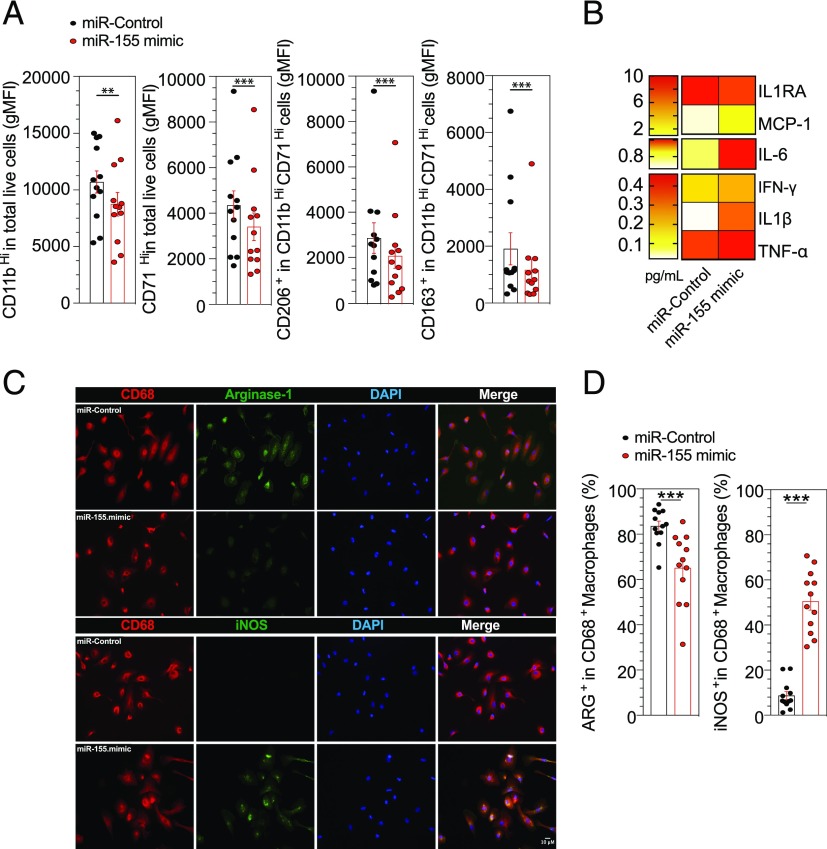
miR-155 is involved in the defect of M2 polarization
To address the effect of miR-155 on the defect of M2-like macrophage polarization, a miR-155 mimic was used in HD monocytes by transfection. Effective transfection was confirmed with a nontargeting negative control Cy3-labeled and showed that a mean of 64% of monocytes were transfected (Supplemental Fig. 4A). A specific overexpression of miR-155 was confirmed by quantitative PCR (qPCR) after a 6-d culture in human SAB medium (p = 0.007) (Supplemental Fig. 4B). Overexpression of miR-155 in HD monocytes significantly decreased the geometric mean fluorescence intensity of the pan-macrophage markers, CD11b (p = 0.0005) and CD71 (p = 0.001), but also the M2-like markers, CD206 (p = 0.001) and CD163 (p = 0.0005), after 6 d of culture with human SAB (Fig. 7A). Likewise, levels of the proinflammatory cytokines MCP-1, IL-6, IFN-γ, IL-1β, and TNF-α were increased and the IL-1 receptor antagonist (IL-1RA) was decreased in supernatants from HD monocytes transfected by miR-155 mimic (Fig. 7B). Finally, we demonstrated a significant reduction of Arg1 expression (p = 0.0005) and increase in iNOS expression (p = 0.0005) in M2-like macrophages after miR-155 transfection of HD monocytes (Fig. 7C, 7D).
FIGURE 7.
miR-155 is involved in the defect of M2-like polarization. (A) HD monocytes were transfected with miR-155 mimic or control for 18 h and then cultured with human SAB or with M-CSF for 6 d. Cells were assessed for quantification of geometric mean fluorescence intensity (gMFI) of CD11b–CD71–CD206 and CD163 (n = 12). (B) Spontaneous 6-d cytokine production by M2-like macrophages from HD (n = 12) after miR-155 mimic or miR-control transfection. (C and D) Representative images and frequencies of CD68+ Arg+ or CD68+ iNOS+ (n = 12). Scale bar, 10 μm. Results are shown as mean ± SEM. Wilcoxon matched-pairs signed rank test. **p ≤ 0.01, ***p ≤ 0.001.
This suggests a potential direct role of miR-155 in monocyte reprogramming of macrophage phenotype in favor of the M1-like type subset.
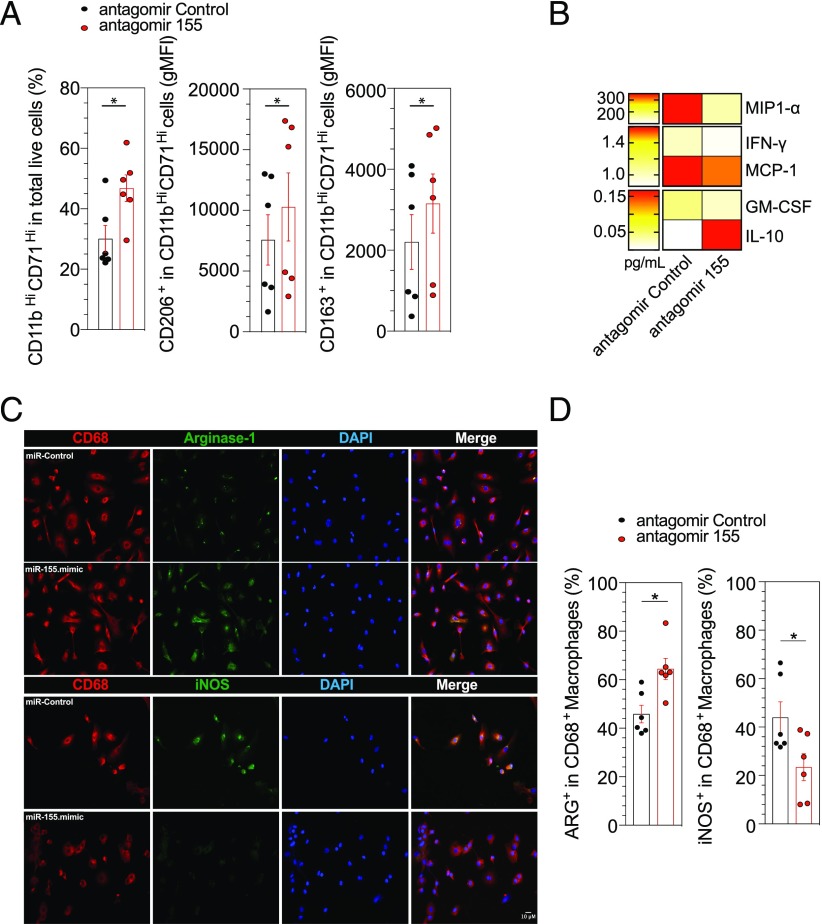
Antagomir-155 restores the defect of M2 polarization
We next investigated whether ex vivo silencing of miR-155 in RA monocytes could restore the defect of M2-like phenotype macrophages. For this purpose, we transfected RA monocytes with either an antagomir control or antagomir-155. The inhibition of miR-155 and overexpression of SOCS-1 was confirmed by qPCR after a 6-d culture with human SAB (Supplemental Fig. 4C, 4D). miR-155 antagomir treatment significantly increased the percentage of pan-macrophage markers CD11b and CD71 (p = 0.03) and M2-like macrophages markers CD206 (p = 0.03) and CD163 (p = 0.03) after a 6-d culture in human SAB (Fig. 8A). Moreover, levels of the proinflammatory cytokines MIP1-α, IFN-γ, MCP-1, and GM-CSF were decreased and IL-10, an anti-inflammatory cytokine, was increased in supernatants from RA monocytes transfected by antagomiR-155 (Fig. 8B). Furthermore, miR-155 antagomir increased significantly the Arg1 expression (p = 0.03) and reduced iNOS expression in M2-like macrophages (p = 0.03) (Fig. 8C, 8D).
FIGURE 8.
AntagomiR-155 restored the defect of M2-like polarization. (A) RA Monocytes were transfected with antagomir-155 or control for 18 h and then cultured with human SAB for 6 d. Percentage of CD11HiCD71Hi and geometric mean fluorescence intensity (gMFI) of CD206 and CD163 were determined (n = 6). (B) Spontaneous 6-d cytokine production by M2-like macrophages from RA (n = 6) after AntagomiR-155 or control transfection. (C and D) Representative images and frequencies of CD68+ Arg+ or CD68+ iNOS+ (n = 6). Scale bar, 10 μm. Results are shown as mean ± SEM. Wilcoxon matched-pairs signed rank test. *p ≤ 0.05.
Thus, miR-155 inhibition may reverse the M2-like polarization defect in RA patients.
Discussion
We found in RA patients specific abnormalities of monocytes/macrophages not found in patients with other rheumatic inflammatory diseases, including CTD and SpA. Increased mTNF on monocytes was associated with disease activity. RA patients had an impaired maturation of monocytes into M2-like anti-inflammatory macrophages with increased differentiation into an M1-like proinflammatory phenotype. This abnormal differentiation into M1-like macrophages under M2 stimulation was correlated with the expression of mTNF on monocytes. M2-like macrophages were also absent in synovium from patients with active RA. Inhibiting miR-155 in RA monocytes with an antagomir reversed this defect of M2 polarization, whereas transfecting miR-155 in HD monocytes reproduced this defect, suggesting that excessive miR-155 in monocytes and M2 macrophages could play a key role in this phenomenon. Finally, patients treated by ADA and not by ETA or other biologics had a correction of the defect of monocytes differentiation into anti-inflammatory M2 macrophages paralleled with an absence of increased miR-155 in M2 macrophages.
Even though TNFi have demonstrated great effectiveness in RA for 20 y, it is, to our knowledge, the first time that increased mTNF expression on blood monocytes was found to be a specific biomarker of RA not present in HD or in patients with other inflammatory diseases. This mTNF expression was found linked to disease activity in RA patients. We confirmed the increased level of blood-intermediate monocytes found in RA (3), but increased mTNF expression was present in the three subsets of blood monocytes. Expression of mTNF was not influenced by TNFi in vitro treatment. Conversely to a recent publication, we did not observe an increase of mTNF in monocytes treated with ADA (29), which could be due to different methods of extraction of monocytes. Because no simple blood biomarker has been found predictive of response to TNFi treatments in RA, and because synovium TNF expression has been considered predictive of response to TNFi in some reports (2, 27, 28), the predictive value of blood monocytes mTNF expression in response to TNFi needs to be evaluated.
One of the major functions of monocytes is to differentiate into macrophages. Depending on the cytokine environment, they can differentiate into M1-like proinflammatory macrophages or M2-like anti-inflammatory macrophages. In this study, first, we used GM-CSF and M-CSF without any other cytokines to polarize monocytes into M1-like or M2-like macrophages. Jaguin et al. (36) have demonstrated that blood monocyte polarization into M2-like macrophages with M-CSF or with M-CSF + IL-4 showed no difference in CD80, CD206, IL-10, and other cytokines or transcription factor expression involved in macrophage polarization. Because macrophage activation cannot be explained by a dichotomous model (37, 38), and to mimic tissue environment, we used the SAB as a physiological source of M-CSF, IL-4, and IL-10 to obtain an heterogeneous M2-like populations. We demonstrated that RA patients had an impaired differentiation of blood monocytes into M2-like macrophages, both in phenotype (CD206 and CD163) and in function (IL-10 and Arg1). Conversely, the differentiation into M1-like macrophages was maintained. Actually, RA monocytes transformation into M2-like macrophages by SAB induced a phenotype mimicking an M1-like phenotype, secreting proinflammatory cytokines, and expressing iNOS and IRF5. Interestingly, this preferred M1-like differentiation of monocytes after M2 stimulation was linked to high mTNF expression. A better characterization of these RA SAB-derived differentiated macrophages must be done by a global transcriptomic analysis. Finally, as others (12, 13, 15), we confirmed the defect of M2-like polarization of blood monocytes in the synovial tissue from patients with active RA contained much more macrophages than patients with OA but much less anti-inflammatory M2-like macrophages.
Increased expression of miR-155 has been demonstrated in the context of several autoimmune diseases, such as in whole peripheral blood in lupus patients (39) and in blood mononuclear cells in patients with primary Sjögren syndrome (40). In RA, miR-155 has been found elevated in PBMCs (41) and blood monocytes (42, 43) but also in synovial tissue both in synovial B cells (44) and in synovial monocytes and macrophages (22). In the latter study, it was shown that cells overexpressing miR-155 produce proinflammatory cytokines. In this study, we demonstrate, in patients with RA, that increase in miR-155 is mainly located in blood monocytes and in monocyte-derived M2-like macrophages and is associated with the defect of polarization of monocytes into M2-like macrophages. This increase in miR-155 was specific to RA because it was not found in patients with Sjögren disease or SpA. However, we cannot determine if miR-155–increased expression is a primary event or secondary to another RA-specific monocyte/macrophage abnormality. Nonetheless, increasing miR-155 with a miR-155 mimic in normal HD monocytes was sufficient to reproduce the defect of monocytes polarization into M2-like macrophages, and conversely, transfection of RA monocytes with antagomir-155 corrected this lack of M2-like polarization. Outside RA, previous studies demonstrated that depletion of miR-155 in M1-like macrophages using an antagomir allowed an upregulation of M2 markers and a downregulation of M1 markers (17–21).
The defect in polarization into M2-like anti-inflammatory macrophages was partially reversed in vitro and in vivo by treatment with ADA but not with ETA. This should be confirmed in a longitudinal study. In this study, we hypothesize that the use of monoclonal anti-TNF Abs, but not ETA, binding strongly to mTNF on activated RA monocytes leads to reverse signaling, which carries on to the modification of gene activity pattern during monocyte transformation into M2-like macrophages. Interestingly, M. Erhenstein’s group also found a differential effect between the different types of TNFi regarding the activation of regulatory T cells as being much more efficient with ADA than with ETA (29). Thus, different types of TNFi treatment may work by different mechanisms. Monoclonal anti-TNF treatments may bind mTNF on monocytes and restore M2-like polarization, but ETA, even if it does not reverse this abnormality, may reduce inflammation just by inhibiting sTNF and proinflammatory cytokines as found in our Bio-Plex assay (data not shown). In parallel to what is observed for defect of polarization into M2 macrophages, RA patients treated with ADA and not by ETA or other biologics were the only ones with an absence of increased miR-155 in M2-like macrophages, suggesting a link between the two phenomena and a role of monoclonal anti-TNF Abs for inhibiting the defect of monocyte polarization in M2-like macrophages.
In conclusion, we showed that increased mTNF expression on monocytes and defect of monocyte capacity of differentiation into M2-like anti-inflammatory macrophages were biomarkers specific of RA and of its clinical activity. Our data suggest that an increased expression of miR-155 in monocytes/macrophages could be responsible for this defect that could be partially reversed by monoclonal anti-TNF Abs and not by the soluble receptor. Targeting miR-155 in monocytes/macrophages with an a miR-155 antagomiR could represent a new therapeutic strategy in RA.
Supplementary Material
Acknowledgments
We are grateful to T. Lazure and L. Amazi (UMS-32) for their assistance regarding histology experiments, to C. Bourgeois for assistance regarding cytometry, and to A. Dupré.
This work was supported by the Laboratoire d'Excellence en Recherche sur le Médicament et l'Innovation Thérapeutique (ANR10) and Fondation pour la Recherche Médicale DEQ20150934719.
The online version of this article contains supplemental material.
- ADA
- adalimumab
- csDMAR
- conventional synthetic disease-modifying antirheumatic drug
- CTD
- connective tissue disease
- DAS28-CRP
- Disease Activity Score evaluated in 28 joints
- ETA
- etanercept
- HD
- healthy donor
- IFX
- infliximab
- iNOS
- inducible NO synthase isoform
- M1-like
- classically activated macrophage phenotype
- M2-like
- alternatively activated macrophage phenotype
- mTNF
- membrane TNF
- MTX
- methotrexate
- OA
- osteoarthritis
- qPCR
- quantitative PCR
- RA
- rheumatoid arthritis
- SAB
- human serum AB
- SLE
- systemic lupus erythematosus
- SOCS-1
- suppressor of cytokine signaling-1
- SpA
- spondyloarthritis
- SS
- Sjögren syndrome
- sTNF
- soluble TNF
- TNFi
- TNF inhibitor.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.McInnes I. B., Schett G. 2017. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389: 2328–2337. [DOI] [PubMed] [Google Scholar]
- 2.Bresnihan B., Gerlag D. M., Rooney T., Smeets T. J., Wijbrandts C. A., Boyle D., Fitzgerald O., Kirkham B. W., McInnes I. B., Smith M., et al. 2007. Synovial macrophages as a biomarker of response to therapeutic intervention in rheumatoid arthritis: standardization and consistency across centers. J. Rheumatol. 34: 620–622. [PubMed] [Google Scholar]
- 3.Kawanaka N., Yamamura M., Aita T., Morita Y., Okamoto A., Kawashima M., Iwahashi M., Ueno A., Ohmoto Y., Makino H. 2002. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 46: 2578–2586. [DOI] [PubMed] [Google Scholar]
- 4.Lioté F., Boval-Boizard B., Weill D., Kuntz D., Wautier J. L. 1996. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin. Exp. Immunol. 106: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shore A., Jaglal S., Keystone E. C. 1986. Enhanced interleukin 1 generation by monocytes in vitro is temporally linked to an early event in the onset or exacerbation of rheumatoid arthritis. Clin. Exp. Immunol. 65: 293–302. [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss M., Blazek K., Byrne A. J., Perocheau D. P., Udalova I. A. 2013. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators Inflamm. 2013: 245804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabaud M., Lubberts E., Joosten L., van Den Berg W., Miossec P. 2001. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 3: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 9.Munder M., Eichmann K., Modolell M. 1998. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 160: 5347–5354. [PubMed] [Google Scholar]
- 10.Martinez F. O., Helming L., Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27: 451–483. [DOI] [PubMed] [Google Scholar]
- 11.Sica A., Mantovani A. 2012. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambarus C. A., Noordenbos T., de Hair M. J., Tak P. P., Baeten D. L. 2012. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 14: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soler Palacios B., Estrada-Capetillo L., Izquierdo E., Criado G., Nieto C., Municio C., González-Alvaro I., Sánchez-Mateos P., Pablos J. L., Corbí A. L., Puig-Kröger A. 2015. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile. J. Pathol. 235: 515–526. [DOI] [PubMed] [Google Scholar]
- 14.Davignon J. L., Hayder M., Baron M., Boyer J. F., Constantin A., Apparailly F., Poupot R., Cantagrel A. 2013. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 52: 590–598. [DOI] [PubMed] [Google Scholar]
- 15.Kurowska-Stolarska M., Alivernini S. 2017. Synovial tissue macrophages: friend or foe? RMD Open 3: e000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udalova I. A., Mantovani A., Feldmann M. 2016. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 12: 472–485. [DOI] [PubMed] [Google Scholar]
- 17.Graff J. W., Dickson A. M., Clay G., McCaffrey A. P., Wilson M. E. 2012. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 287: 21816–21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai X., Yin Y., Li N., Zhu D., Zhang J., Zhang C. Y., Zen K. 2012. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 4: 341–343. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Mei H., Chang X., Chen F., Zhu Y., Han X. 2016. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 8: 505–517. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Zhang J., Guo H., Yang S., Fan W., Ye N., Tian Z., Yu T., Ai G., Shen Z., et al. 2018. Critical role of alternative M2 skewing in miR-155 deletion-mediated protection of colitis. Front. Immunol. 9: 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie L., Cai S. Y., Sun J., Chen J. 2019. MicroRNA-155 promotes pro-inflammatory functions and augments apoptosis of monocytes/macrophages during Vibrio anguillarum infection in ayu, Plecoglossus altivelis. Fish Shellfish Immunol. 86: 70–81. [DOI] [PubMed] [Google Scholar]
- 22.Kurowska-Stolarska M., Alivernini S., Ballantine L. E., Asquith D. L., Millar N. L., Gilchrist D. S., Reilly J., Ierna M., Fraser A. R., Stolarski B., et al. 2011. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 108: 11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmesmari A., Fraser A. R., Wood C., Gilchrist D., Vaughan D., Stewart L., McSharry C., McInnes I. B., Kurowska-Stolarska M. 2016. MicroRNA-155 regulates monocyte chemokine and chemokine receptor expression in Rheumatoid Arthritis. Rheumatology (Oxford) 55: 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanczyk J., Pedrioli D. M., Brentano F., Sanchez-Pernaute O., Kolling C., Gay R. E., Detmar M., Gay S., Kyburz D. 2008. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 58: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 25.Tracey D., Klareskog L., Sasso E. H., Salfeld J. G., Tak P. P. 2008. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117: 244–279. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi T., Mitoma H., Harashima S., Tsukamoto H., Shimoda T. 2010. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 49: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijbrandts C. A., Dijkgraaf M. G., Kraan M. C., Vinkenoog M., Smeets T. J., Dinant H., Vos K., Lems W. F., Wolbink G. J., Sijpkens D., et al. 2008. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann. Rheum. Dis. 67: 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toubi E., Kessel A., Slobodin G., Boulman N., Pavlotzky E., Zisman D., Rozenbaum M., Rosner I. 2007. Changes in macrophage function after rituximab treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 66: 818–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen D. X., Ehrenstein M. R. 2016. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. J. Exp. Med. 213: 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyer J. F., Baron M., Constantin A., Degboé Y., Cantagrel A., Davignon J. L. 2016. Anti-TNF certolizumab pegol induces antioxidant response in human monocytes via reverse signaling. Arthritis Res. Ther. 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O., III, Birnbaum N. S., Burmester G. R., Bykerk V. P., Cohen M. D., et al. 2010. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 32.Chinenov Y., Coppo M., Gupte R., Sacta M. A., Rogatsky I. 2014. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genomics 15: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyte C. S., Bishop E. T., Rückerl D., Gaspar-Pereira S., Barker R. N., Allen J. E., Rees A. J., Wilson H. M. 2011. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J. Leukoc. Biol. 90: 845–854. [DOI] [PubMed] [Google Scholar]
- 34.Albina J. E., Mahoney E. J., Daley J. M., Wesche D. E., Morris S. M., Jr., Reichner J. S. 2005. Macrophage arginase regulation by CCAAT/enhancer-binding protein β. Shock 23: 168–172. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Zhang M., Li X., Tang Z., Wang X., Zhong M., Suo Q., Zhang Y., Lv K. 2016. Silencing microRNA-155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci. Rep. 6: 22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaguin M., Houlbert N., Fardel O., Lecureur V. 2013. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell. Immunol. 281: 51–61. [DOI] [PubMed] [Google Scholar]
- 37.Xue J., Schmidt S. V., Sander J., Draffehn A., Krebs W., Quester I., De Nardo D., Gohel T. D., Emde M., Schmidleithner L., et al. 2014. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40: 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginhoux F., Schultze J. L., Murray P. J., Ochando J., Biswas S. K. 2016. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 17: 34–40. [DOI] [PubMed] [Google Scholar]
- 39.Shumnalieva R., Kachakova D., Shoumnalieva-Ivanova V., Miteva P., Kaneva R., Monov S. 2018. Whole peripheral blood miR-146a and miR-155 expression levels in systemic lupus erythematosus patients. ACTA Reumatol. Port. 43: 217–225. [PubMed] [Google Scholar]
- 40.Chen J. Q., Zilahi E., Papp G., Sipka S., Zeher M. 2017. Simultaneously increased expression of microRNA-155 and suppressor of cytokine signaling 1 (SOCS1) gene in the peripheral blood mononuclear cells of patients with primary Sjögren’s syndrome. Int. J. Rheum. Dis. 20: 609–613. [DOI] [PubMed] [Google Scholar]
- 41.Li X., Tian F., Wang F. 2013. Rheumatoid arthritis-associated microRNA-155 targets SOCS1 and upregulates TNF-α and IL-1β in PBMCs. Int. J. Mol. Sci. 14: 23910–23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajasekhar M., Olsson A. M., Steel K. J., Georgouli M., Ranasinghe U., Brender Read C., Frederiksen K. S., Taams L. S. 2017. MicroRNA-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis. J. Autoimmun. 79: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alivernini S., Gremese E., McSharry C., Tolusso B., Ferraccioli G., McInnes I. B., Kurowska-Stolarska M. 2018. MicroRNA-155-at the critical interface of innate and adaptive immunity in arthritis. Front. Immunol. 8: 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alivernini S., Kurowska-Stolarska M., Tolusso B., Benvenuto R., Elmesmari A., Canestri S., Petricca L., Mangoni A., Fedele A. L., Di Mario C., et al. 2016. MicroRNA-155 influences B-cell function through PU.1 in rheumatoid arthritis. Nat. Commun. 7: 12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.