Abstract
Background
The Multicentric Italian Lung Detection (MILD) trial demonstrated that prolonged low-dose computed tomography (LDCT) screening could achieve a 39% reduction in lung cancer (LC) mortality. We have here evaluated the long-term results of annual vs. biennial LDCT, and the impact of screening intensity on overall and LC specific mortality at 10 years.
Patients and methods
Between 2005 and 2018, the MILD trial prospectively randomized the 2,376 screening arm participants to annual (n=1190) or biennial (n=1186) LDCT, for a median screening period of 6.2 years and 23,083 person-years of follow-up. The primary outcomes were 10-year overall and LC specific mortality, and the secondary endpoints were the frequency of advanced stage and interval LCs.
Results
The biennial LDCT arm showed a similar overall mortality (HR 0.80, 95% CI 0.57-1.12) and LC specific mortality at 10 years (HR 1.10, 95% CI 0.59-2.05), as compared with annual LDCT arm. Biennial screening saved 44% of follow-up LDCTs in subjects with negative baseline LDCT, and 38% of LDCTs in all participants, with no increase in the occurrence of stage II-IV or interval LCs.
Conclusions
The MILD trial provides original evidence that prolonged screening beyond five years with biennial LDCT can achieve a LC mortality reduction comparable to annual LDCT, in subjects with a negative baseline examination.
Keywords: computed tomography, LDCT, screening, interval, biennial, early detection, lung cancer, mortality, overdiagnosis
INTRODUCTION
In 2011, the National Lung Screening Trial (NLST) proved the benefit of two-year LC screening with low-dose computed tomography (LDCT) on a population of 53,454 current and former smokers of ≥30 pack-years, aged 55–74 years, by achieving a 20% decrease in LC mortality as compared with annual chest radiography (1). Other European randomized trials testing annual LDCT versus observation on younger populations, with lower LC risk than NLST and a total screening period of 4-5 years, showed no mortality reductions, possibly due to small number of participants and short follow-up (2-4).
The 10-year results of prolonged LDCT screening in the Multicentric Italian Lung Detection (MILD) study showed a significant 39% reduction in lung cancer mortality (HR 0.61; 95% CI 0.39–0.95; P = 0.017), as well as a nonsignificant 20% decrease in all-cause mortality, in a population of 4,099 current and former smokers of ≥20 pack-years, aged 49–75 years, randomized to LDCT screening for a median period of 6.2 years or control without intervention (5). A recent meta-analysis of randomized LDCT screening trials, including preliminary report of the Nederlands-Leuvens Longkanker Screenings ONderzoek (NELSON) trial (6), has confirmed an overall LC mortality reduction of 20% (7).
According to the initial protocol, the screening arm of MILD trial was further randomized to an annual or biennial LDCT, and a preliminary analysis reported similar LC detection rates and interval cancers in the two LDCT interval arms at 7 years (8). We present here the 10-year results of the two MILD screening intensity arms, with a focus on overall and lung cancer mortality.
MATERIAL AND METHODS
Study population
The Multicentric Italian Lung Detection (MILD) project is a prospective randomized controlled screening trial launched in 2005 at the National Cancer Institute of Milan (ClinicalTrials.gov Identifier: ). Details of this program have been reported elsewhere (4). Briefly, the MILD project included 4,099 current or former smokers (within 10 years of recruitment) of ≥20 pack-years, aged from 49 to 75 years, without history of cancer in ≤ 5 years. The study was approved by our Institutional Review Board and Ethics Committee, and all eligible subjects provided written informed consent. Details about Ethics Committee approval, LDCT technique, diagnostic workup, baseline and early outcome of the MILD study were published elsewhere (4,5).
Among the 2,376 participants randomized to the screening arm, 1190 were further randomized to annual (LDCT every 12 months) and 1186 to biennial (LDCT every 24 months) screening (Table 1). Baseline LDCT was evaluated as negative for subjects without non-calcified nodules (NCN) or with NCN with volume <60mm3, indeterminate for NCN 60-250 mm3, and positive for NCN>250 mm3. In the biennial screening arm, subjects with positive or undetermined pulmonary nodules underwent diagnostic workup according to the general study protocol, with three-months and/or annual LDCT repeats, likewise the annual screening arm. Nonsolid or partly solid nodules were kept under active surveillance, by annual LDCT repeats in both interval arms, until development of a solid component >60mm3 in volume (9).
Table 1.
Selected characteristics of 2,376 MILD participants by randomization arm.
| Total | Group [N(%)] | ||
|---|---|---|---|
| (N=2,376) | Annual (N=1,190) | Biennial (N=1,186) | |
| Age (years) | |||
| <55 | 773 (32.5%) | 394 (33.1%) | 379 (32.0%) |
| 55-64 | 1235 (52.0%) | 611 (51.3%) | 624 (52.6%) |
| ≥65 | 368 (15.5%) | 185 (15.5%) | 183 (15.4%) |
| Females | 750 (31.6%) | 376 (31.6%) | 374 (31.5%) |
| Pack-years | |||
| <30 | 521 (21.9%) | 251 (21.1%) | 270 (22.8%) |
| ≥30 | 1855 (78.1%) | 939 (78.9%) | 916 (77.2%) |
| Smoking Status at randomization | |||
| Former | 747 (31.4%) | 370 (31.1%) | 377 (31.8%) |
| Current | 1629 (68.6%) | 820 (68.9%) | 809 (68.2%) |
| Quitters on screening | 464 (19.5%) | 236 (19.8%) | 228 (19.2%) |
Comparison between the control arm and the screening arm at 10-year of follow-up has been reported (4). In the present study we restricted the analysis on the 2,376 participants of the screening arms, with the aim of testing the performance of low intensity biennial rounds.
Data collection and follow-up
All participants underwent LDCT scan and a program of primary prevention (smoking cessation) with pulmonary function test evaluation and blood sample collection. Furthermore, outcome information on stage, resectability, histology of disease was collected during follow-up. Each member of the study cohort accumulated person-years of follow-up from baseline (i.e., at the date of the randomization) until the date of death or the date of last follow-up (June 2018). The vital status of participants was collected through the platform SIATEL 2.0 and the causes of death were retrieved from the Istituto Nazionale di Statistica (ISTAT). Cause of death was missing in 3 cases, 1 of the annual arm and 2 in the biennial arm.
Of the 2,376 randomized participants, 216 (9%) withdrew from the study (94 in the Annual arm and 122 in the Biennial arm), among them 20 within two years from the baseline and 100 within 5 years, for different reasons, including health problems, distance problems or loss of interest in the study. A total of 23,083 person years were accumulated, 11,521 in the annual arm and 11,562 in the biennial arm.
Statistical Analysis
According with the intention to treat principle, we considered each subject in the study arm at which he/she was initially assigned. Primary analysis included all the 2,376 subjects, as follows: 1,190 of the annual arm and 1,186 of the biennial arm. The analysis was also restricted to 1,974 subjects with negative baseline LDCT, 981 in the annual arm and 993 in the biennial arm, respectively. Descriptive statistics were reported as numbers and percentages comparing the annual LDCT arm and the biennial LDCT arm. Comparisons were made by Chi-square test or Fisher test, as appropriate.
Primary outcomes were lung cancer incidence at 10 years, overall 10-year mortality, lung cancer 10-year specific-mortality and other causes mortality at 10 years, other cancers mortality and non-neoplastic mortality.
Cumulative incidence and cumulative mortality were evaluated using Kaplan-Meier estimator and differences among groups were tested using Log-rank test. The diagnostic and prognostic value of the assigned arm was investigated by hazard ratio (HR) and 95% confidence interval (CI) estimated by Cox’s proportional hazard regression models with Annual arm as reference. Analyses were obtained using Statistical Analysis System Software (version 9.4; SAS Institute, Cary, North Carolina, USA).
RESULTS
Patients’ characteristics stratified by intensity arm are summarized in Table 1. The two LDCT arms were similar for age, sex, smoking status and number of pack-years. The smoking quit rate during the whole screening period was 19.8% in annual and 19.2% in biennial arm, with the same proportion of permanent smokers (49.1%). The frequency of positive or indeterminate baseline LCDT was 14.4% in annual, and 13.3% in biennial arm. Participants underwent a total of 12,375 chest LDCT scans, 7,369 in the annual and 5,006 in the biennial arm respectively. A median of 7 LDCT scans was recorded in the annual arm and a median of 4 LDCT scans in the biennial arm. A total of 73 participants (3.1%) did not receive any LDCT (38 and 35 respectively). Baseline LDCT results were positive or indeterminate for 329 participants (171 and 158 respectively), while 1974 were negative (981 and 993 respectively). Subjects who returned within 3 months from the date of baseline evaluation were 308 (158 and 150 respectively), at 1 year from the baseline 1235 (1082 and 153 respectively), and at 2 years from the baseline 2149 (1087 and 1062 respectively). Extended screening interval prevented 86% of first year LDCT repeats and 38% of all repeats in the biennial arm, allowing a 32% reduction in the total number of LDCT, and 37% reduction among subjects with negative baseline LDCT.
Lung cancer detection
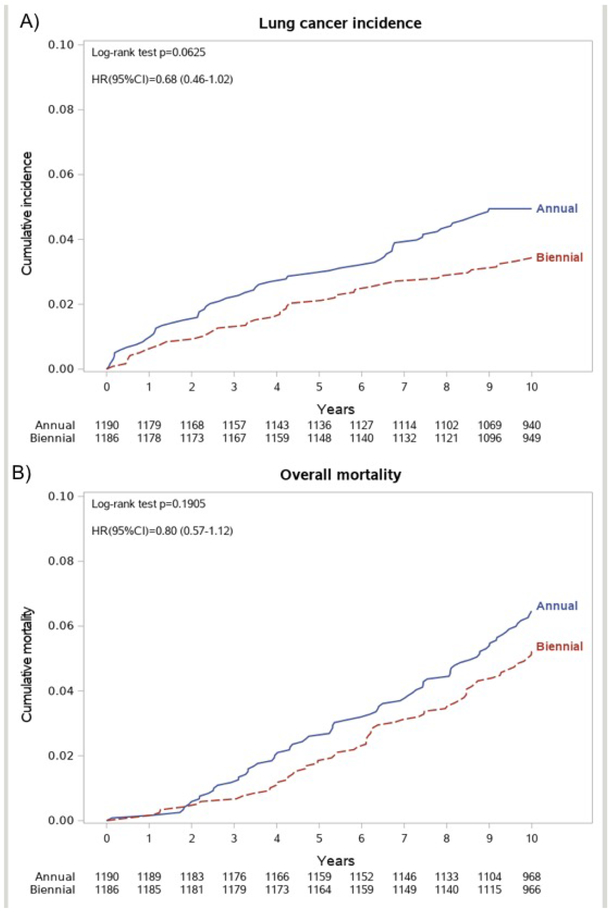
Lung cancer (LC) was diagnosed in 58 participants (514/100 000 person-years) of the annual arm and 40 (350/100 000 person-years) of the biennial arm (Table 2), but the difference did not reach statistical significance (HR = 0.68, 95% CI 0.46 to 1.02, Figure 1a). There were no differences between the two arms in the LC stage frequency (p=0.4110), LC histology (p=0.0998), or proportion of interval LC (not screen detected, p=0.3625). Interestingly, the number of LC detected in stage II-IV was higher in the annual arm (27 vs. 22 cases). Furthermore, the prevalence of LC resections was significantly higher in the annual arm compared with the biennial arm (74% vs. 53%, p=0.0004). The absolute excess of LC resections in the annual arm (22 cases) was consistent with the excess of LC diagnoses (18 cases). The frequency of subsolid nodules was 17% in annual and 15,8% in biennial arm (Table 3), with a non-significant excess of LC in the annual arm (22 vs. 11 cases, p=0.0765).
Table 2.
Lung cancer and mortality at 10 years by randomization arm.
| Total (N=2,376) |
Annual (N=1,190) |
Biennial (N=1,186) |
P-values | |
|---|---|---|---|---|
| Lung cancer incidence | 98 (4.1%) | 58 (4.9%) | 40 (3.4%) | 0.0658 |
| Stage I | 49 (50.0%) | 31 (53.4%) | 18 (45%) | 0.4110 |
| Resected cancers | 64 (65.3%) | 43 (74.1%) | 21 (52.5%) | 0.0004 |
| Adenocarcinoma | 55 (56.1%) | 33 (56.9%) | 22 (55.0%) | 0.8525 |
| Interval cancers | 27 (27.6%) | 14 (24.1%) | 13 (32.5%) | 0.3625 |
| Lung cancers beyond 5 years | 39 (39.8%) | 23 (39.7%) | 16 (40.0%) | 0.9727 |
| Total deaths a | 137 (5.8%) | 76 | 61 | 0.1936 |
| Overall mortality rate (per 100,000) | 593.5 | 659.7 | 527.6 | |
| Lung cancer deaths | 40 (1.7%) | 19 | 21 | 0.7417 |
| Lung cancer mortality rate (per 100,000) | 173.3 | 164.9 | 181.6 | |
| Other causes of deaths | 94 | 56 | 38 | 0.0604 |
| Other causes mortality rate (per 100,000) | 407.2 | 486.1 | 328.7 | |
| Number of LDCTs performed | 12,375 | 7,369 | 5,006 | |
| Number of LDCTs performed after baseline | 10,072 | 6,217 | 3,855 | |
| Number of PETs performed | 51 | 37 | 14 | |
| Benign lung resection | 3 | 0 | 3 |
3 missing causes of death (1 Annual arm and 2 Biennial arm).
Figure 1.
(A) Lung cancer incidence at 10 years by randomization arm.
(B) Overall mortality at 10 years by randomization arm.
Table 3.
Active surveillance of subsolid nodules by randomization arm.
| Total (N=2,376) |
Annual (N=1,190) |
Biennial (N=1,186) |
P-values | |
|---|---|---|---|---|
| Subsolid Nodules | 389 (16.4%) | 202 (17.0%) | 187 (15.8%) | 0.4264 |
| Lung cancer incidence | 33 (8.5%) | 22 (10.9%) | 11 (5.9%) | 0.0765 |
| Total deaths | 29 (7.5%) | 16 (7.9%) | 13 (7.0%) | 0.7162 |
| Lung cancer deaths | 10 (2.6%) | 5 (2.5%) | 5 (2.7%) | 0.9016 |
| Other causes deaths | 19 (4.9%) | 11 (5.4%) | 8 (4.3%) | 0.5935 |
Ten-year mortality
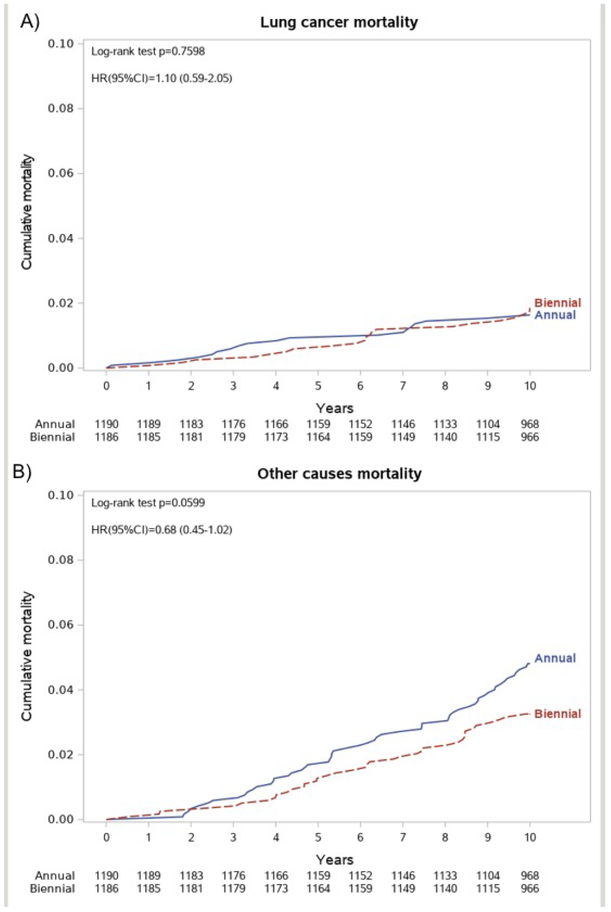
At 10-year follow-up, a total of 137 deaths were recorded, 76 in the annual arm (660/100 000 person-years) and 61 in the biennial arm (528/100 000 person-years), with a non-significant decrease of the overall 10-years mortality in the biennial arm (HR 0.80, 95% CI 0.57-1.12, Figure 1b). LC specific mortality was 165/100 000 person-years (19 deaths) in the annual arm and 182/100 000 (21 deaths) in the biennial arm, and cumulative lung cancer mortality curves showed no difference among the annual and the biennial arm (HR 1.10, 95% CI 0.59-2.05, Figure 2a). Of interest, in the subset of individuals kept on active surveillance for subsolid nodules, we observed a similar overall mortality and the same number of LC deaths (5 in each arm, Table 3). Deaths for other causes were 56 in the annual arm (486/100 000 person-years) and 38 in the biennial arm (329/100 000 person-years), showing a non-significant difference between the two arms (HR 0.72, 95% CI 0.46 to 1.13, Figure 2b).
Figure 2.
(A) Lung cancer mortality at 10 years by randomization arm.
(B) Other causes mortality at 10 years by randomization arm.
Negative baseline LDCT
When the analysis was restricted to the 1,974 participants with negative baseline LDCT, biennial screening prevented 44% of all repeats, allowing a 37% reduction in the total number of LDCT scans (Table S1). The LC incidence was higher in the annual arm (Figure S1a), with an excess of LC diagnosis in the annual arm of 15 cases (37 LC, 3.8% in the annual arm vs. 22 LC, 2.2%, in the biennial arm, HR 0.58, 95% CI 0.34-0.98). Nonetheless, the stage and histology distribution, as well as resection rate, were not significantly different in the two arms. In particular, the number of LC detected in stage II-IV and of interval cancers were similar (16 vs. 12, and 13 vs. 8 cases respectively). Indeed, no difference was observed in 10-year overall mortality (HR 0.84, 95%CI 0.57-1.24, Figure S1b), lung cancer mortality (HR 1.16, 95%CI 0.52-2.59, Figure S2a) or other causes of death (HR 0.72, 95%CI 0.46-1.13, Figure S2b).
DISCUSSION
MILD is the only randomized LC screening trial designed to compare the performance of two different LDCT intervals. After a median active LDCT screening period of 6.2 years, MILD trial results showed a statistically significant 39% reduction of LC mortality at 10 years in the LDCT arm (5), providing a strong confirmation of the 20% LC mortality reduction shown by NLST with 2 years of annual LDCT screening (10).
The novel results of the present study indicate that biennial intensity of LC screening can achieve a clinical outcome similar to annual intensity, in subjects with negative baseline LDCT, that represent the vast majority (83%) of MILD screening population. In particular, the low-intensity screening algorithm allowed a 38% reduction of LDCT burden and did not incur in detrimental effect on survival.
A study from the U.S. Preventive Service task Force (USPSTF) modelled several scenarios for lung cancer screening and reported that annual intensity is expected to outperform biennial and triennial approach (11). Nonetheless, several post-hoc analyses of NLST data showed that biennial intensity could be pursued by post-test risk stratification, namely by stratification of lung cancer risk by nodule categories, suggesting that biennial repeats could be safe in case of negative baseline LDCT (12-14). Indeed, personalized stratification of LC risk by nodule size seems to be the most accurate option, especially when volumetric assessment is applied (15,16). Noteworthy, a prospective evaluation of longer-than-annual approach comes from the two positive European trials – NELSON and MILD – where long-term survival was maintained by screening algorithms with lower intensity in subjects with nodule volume below predefined thresholds (17, 5). Only the MILD trial prospectively randomized screening participants in two arms with different LDCT intensity, namely annual or biennial round. Such differentiation was adopted to assess the best screening strategy in terms of health care resources and radiation exposure, that were uncertain when randomized trials were initiated. Biennial screening saved about one third of LDCTs, maintaining similar performance and mortality rates (4,8). In fact, while the 2.5-year timeframe in the fourth round of NELSON resulted in a significant increase in interval cancers and more cancers detected at a later stage (18), the individually selective design of MILD randomization granted a similar proportion of stage II-IV, and interval cancers in the two arms, with lower costs and radiation exposure in the biennial arm.
Recently, Robbins et al. showed that many, but not all, screen-negatives (e.g. 57.8% of the NLST screen-negatives) might reasonably lengthen their CT screening interval by using a risk-based approach (14). Cost-effectiveness analyses currently support biennial screening (19), reflecting an increasing attention of North American stakeholders towards this approach for lung cancer screening (10). Such low-intensity approach is also convenient to minimize medical risk (e.g. procedure-related morbidity and mortality) (20), psychological stress (21), economic burden (22), and added oncologic risk from radiation exposure (23) while granting prolonged screening (5), which outstands as a pivotal strategy for continuous and incremental control of lung cancer and overall mortality (7).
Even though the screening intensity issue will require further validation by a multicentric randomized trial with larger sample size (24,25), MILD results at 10 years provide substantial evidence that tailored biennial LDCT did not hamper the efficacy of prolonged screening (5), notwithstanding the implementation of an active surveillance program to reduce the frequency of unnecessary resection for subsolid pulmonary lesion (26). In this respect, even the absolute excess of LC cases and resections, without favorable stage shift or decrease of LC mortality in the annual arm, may be the effect of overdiagnosis. We recognize that MILD trial sample size is clearly insufficient for a proper non-inferiority efficacy assessment, that may require ten times more subjects. Nonetheless, the randomized design with two very balanced populations, total length of intervention, quality of follow-up (93% at 9 years), as well as the non-significant mortality trend in favor of biennial arm, provide a reliable estimate of long-term safety and efficacy, to be confirmed by a new randomized trial with adequate sample size.
Individual risk stratification by radiomic and artificial intelligence analysis of baseline LDCT findings represents a promising development for future screening programs, to improve the efficacy of limited healthcare resources and reduce costs (25).
Furthermore, circulating biomarkers could help the individual risk stratification, and optimize LDCT intensity. We are currently testing the value of blood microRNAs (26) in the prospective bioMILD trial, which scheduled triennial rounds for subjects with double negative baseline LDCT and microRNAs (27,28).
Conclusions
The MILD trial provides new evidence that prolonged biennial LDCT screening is safe and effective when compared to annual screening, in subjects with negative baseline LDCT. Biennial LDCT has achieved a similar mortality reduction at 10 years, notwithstanding the active surveillance protocol for subsolid pulmonary nodules.
Supplementary Material
HIGHLIGHTS.
MILD is the only randomized lung cancer screening trial comparing two different LDCT intervals.
10-year results of MILD demonstrate that biennial screening is safe in subjects with a negative baseline LDCT.
low-intensity screening algorithm allowed a 38% reduction of LDCT burden, without increasing advanced stage or interval cancers.
biennial design is a model for personalized screening, based on individual risk profile.
Acknowledgments
The authors thank Associazione Italiana Registri Tumori (AIRTUM) for data retrieval, Elena Bertocchi for project management, Claudio Jacomelli for data management, Paola Suatoni for MILD biobanking, and the MILD staff (Chiara Banfi, Annamaria Calanca, and Carolina Ninni).
Funding sources
The MILD trial was supported by grants from the Italian Ministry of Health (RF 2004), the Italian Association for Cancer Research (AIRC 2004 IG 1227 and AIRC 5xmille IG 12162), Fondazione Cariplo (2004-1560), and the National Cancer Institute (EDRN UO1 CA166905). The sponsors had no role in conducting and interpreting the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared.
REFERENCES
- 1.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. ; DANTE Study Group. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med. 2015;191:1166–75. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 3.Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med. 2016;193:542–51. doi: 10.1164/rccm.201505-10400C. [DOI] [PubMed] [Google Scholar]
- 4.Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21:308–15. doi: 10.1097/CEJ.0b013e328351e1b6. [DOI] [PubMed] [Google Scholar]
- 5.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged Lung Cancer Screening Reduced 10-year Mortality in the MILD Trial. Ann Oncol. 2019. doi: 10.1093/annonc/mdz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholten ET, Horeweg N, de Koning HJ, Vliegenthart R, Oudkerk M, Mali WP, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25:81–8. doi: 10.1007/s00330-014-3394-4. [DOI] [PubMed] [Google Scholar]
- 7.Rota M, Pizzato M, La Vecchia C, Boffetta P. Efficacy of lung cancer screening appears to increase with prolonged intervention: results from the MILD trial and a meta-analysis. Ann Oncol. 2019. doi: 10.1093/annonc/mdz145. [DOI] [PubMed] [Google Scholar]
- 8.Sverzellati N, Silva M, Calareso G, Galeone C, Marchianò A, Sestini S, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol. 2016;26:3821–3829. [DOI] [PubMed] [Google Scholar]
- 9.Silva M, Prokop M, Jacobs C, Capretti G, Sverzellati N, Ciompi F, et al. Long-Term Active Surveillance of Screening Detected Subsolid Nodules is a Safe Strategy to Reduce Overtreatment. J Thorac Oncol. 2018;13:1454–1463. doi: 10.1016/j.jtho.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Schabath MB, Aberle DR. MILD trial, strong confirmation of lung cancer screening efficacy. Nat Rev Clin Oncol. 2019. doi: 10.1038/s41571-019-0231-3. [DOI] [PubMed] [Google Scholar]
- 11.de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–20. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patz EF Jr, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;17:590–9. doi: 10.1016/S1470-2045(15)00621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreuder A, Schaefer-Prokop CM, Scholten ET, Jacobs C, Prokop M, van Ginneken B. Lung cancer risk to personalise annual and biennial follow-up computed tomography screening. Thorax. 2018. doi: 10.1136/thoraxjnl-2017-211107. [DOI] [PubMed] [Google Scholar]
- 14.Robbins HA, Berg CD, Cheung LC, Chaturvedi AK, Katki HA. Identification of candidates for longer lung cancer screening intervals following a negative low-dose computed tomography result. J Natl Cancer Inst. 2019. doi: 10.1093/jnci/djz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15:1332–41. doi: 10.1016/S1470-2045(14)70389-4. [DOI] [PubMed] [Google Scholar]
- 16.Heuvelmans MA, Walter JE, Vliegenthart R, van Ooijen PMA, De Bock GH, de Koning HJ, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax. 2018;73:779–781. doi: 10.1136/thoraxjnl-2017-210770. [DOI] [PubMed] [Google Scholar]
- 17.de Koning H, van der Aalst C, Ten Haaf K, Oudkerk M. Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. World Congress of Lung Cancer; September 25, 2018; Toronto (CA-ON) 2018. p. 2. [Google Scholar]
- 18.Yousaf-Khan U, van der Aalst C, de Jong PA, Heuvelmans M, Scholten E, Lammers JW, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax. 2017;72:48–56. doi: 10.1136/thoraxjnl-2016-208655. [DOI] [PubMed] [Google Scholar]
- 19.Goffin JR, Flanagan WM, Miller AB, Fitzgerald NR, Memon S, Wolfson MC, et al. Biennial lung cancer screening in Canada with smoking cessation-outcomes and cost-effectiveness. Lung Cancer. 2016;101:98–103. doi: 10.1016/j.lungcan.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750–6. [DOI] [PubMed] [Google Scholar]
- 21.Freiman MR, Clark JA, Slatore CG, Gould MK, Woloshin S, Schwartz LM, et al. Patients' Knowledge, Beliefs, and Distress Associated with Detection and Evaluation of Incidental Pulmonary Nodules for Cancer: Results from a Multicenter Survey. J Thorac Oncol. 2016;11:700–708. doi: 10.1016/j.jtho.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer F, Kauczor HU, Stargardt T. Cost-utility analysis of a potential lung cancer screening program for a high-risk population in Germany: A modelling approach. Lung Cancer. 2018;124:189–198. doi: 10.1016/j.lungcan.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yousaf-Khan U, van der Aalst C, de Jong PA, Heuvelmans M, Scholten E, Walter J, et al. Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax. 2017;72:819–824. doi: 10.1136/thoraxjnl-2016-209892. [DOI] [PubMed] [Google Scholar]
- 25.van der Aalst CM, Ten Haaf K, de Koning HJ. Lung cancer screening: latest developments and unanswered questions. Lancet Respir Med. 2016;4:749–761. doi: 10.1016/S2213-2600(16)30200-4. [DOI] [PubMed] [Google Scholar]
- 26.Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014;32:768–73. doi: 10.1200/JCO.2013.50.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastorino U, Sestini S. https://clinicaltrials.gov/ct2/show/study/NCT02247453 on August 17 2016.
- 28.Seijo LM, Peled N, Ajona D, Boeri M, Field JK, Sozzi G, Pio R, Zulueta JJ, Spira A, Massion PP, Mazzone PJ, Montuenga LM. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol. 2019;14:343–357. doi: 10.1016/j.jtho.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.