Abstract
Lung cancer is one of the leading causes of tumor-associated mortality, and >75% of patients with lung cancer have non-small cell lung cancer (NSCLC). Pemetrexed, a folate antagonist, is a first-line chemotherapy drug for NSCLC that is administered alone or in combination with cisplatin. The present study established in vitro cell models of PTEN inhibition and overexpression, and the effects of the treatment with pemetrexed were investigated in these cell models. Result from the present study demonstrated that treatment with pemetrexed suppressed lung cancer cell proliferation, inhibited mRNA and protein expression levels of anti-apoptotic Bcl2, and increased the mRNA and the protein expression levels of pro-apoptotic p53 and apoptosis regulator BAX. The present study suggested that pemetrexed regulated apoptosis via the inhibition of the mTOR/PI3K/AKT signaling pathway. Additionally, cellular processes associated with the aerobic oxidation of carbohydrates were identified to be significantly inhibited. The present findings suggested that treatment with pemetrexed may exhibit synergistic effects with PTEN on lung cancer cells via the inhibition of the PI3K/AKT/mTOR signaling pathway and through carbohydrate metabolism, and treatment with pemetrexed combined with PTEN overexpression may represent a novel therapeutic strategy for the treatment of NSCLC.
Keywords: pemetrexed, non-small cell lung cancer, PTEN, carbohydrate metabolism
Introduction
Lung cancer is one of the leading causes of tumor-associated mortality worldwide (1). Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) are the most common types of lung cancer, and NSCLC affects ~80% of the total number of patients with lung cancer (2). NSCLC may be further classified into various subtypes, including squamous cell carcinoma, large cell carcinoma and adenocarcinoma, and each subtype exhibits particular characteristics. The majority of patients with NSCLC are diagnosed at late stages (primarily at stage IIIb and IV), and one-third of patients are diagnosed in the early stages of the disease (3). The prognosis of patients with NSCLC remains poor, and the average survival rate is 8–10 months, with a 5-year survival rate of ~15% (4). Numerous treatments are available to control NSCLC growth and metastasis, including surgery, radiation therapy, chemotherapy and targeted therapy, and various treatments exhibit positive results on NSCLC cells in vitro (5).
Pemetrexed is a first-line standard treatment for NSCLC (6). Previous studies demonstrated that treatment with pemetrexed alone or in combination with other chemotherapeutics may prolong the overall survival of patients with NSCLC, and pemetrexed has limited toxicity in humans (7). The toxic effects following treatment with pemetrexed were identified to primarily affect the immune, hematopoietic and digestive systems; however, overall toxicity is decreased compared with other chemotherapeutics (8). Pemetrexed was demonstrated to have antitumor activity primarily via the inhibition of thymidylate synthase, dihydrofolate reductase and glycinamide ribonucleotide formyl transferase (9). In addition, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase is involved in the antitumor activity of pemetrexed (9). PTEN, located on chromosome 10q23, is able to suppress the proliferation of multiple types of cancer (10). Although PTEN mediates the phosphorylation of various proteins, one of the most common substrates of PTEN is phosphatidylinositol-3,4,5-triphosphate (PIP3) (11). PIP3 is a second messenger involved in intracellular signaling pathways that, following phosphorylation by PTEN at position D3, is able to directly inhibit the activity of PI3K, thus negatively regulating the PI3K/AKT signaling pathway (12). The PIP3/PI3K/AKT signaling pathway regulates cellular metabolism, cell proliferation and migration (13), important processes involved in tumor development and progression. Human cancer may exhibit dysfunctions and mutations in PTEN, and its promoter was identified to be hypermethylated in various types of cancer, resulting in the silencing of PTEN and subsequent activation of the PI3K/AKT signaling pathway, thus promoting tumor growth and migration (14). In addition, a previous study identified that dysfunctions of PTEN were associated with drug resistance in human tumors (15). The invasive and metastatic ability of tumors increased significantly following dysregulation of PTEN (16). However, the detailed molecular mechanisms underlying the anti-tumor activity of PTEN and pemetrexed remain unclear, and whether the overexpression of PTEN is able to increase the anticancer activity of pemetrexed in NSCLC has not been previously investigated, to the best of the authors' knowledge.
In the present study, the antitumor activity of pemetrexed was demonstrated to increase following PTEN overexpression. The combination of pemetrexed with PTEN overexpression inhibited the AKT signaling pathway and activated the mTOR signaling pathway, thus promoting the upregulation of apoptosis-associated genes at the transcriptional and protein levels. In addition, treatment with pemetrexed combined with PTEN overexpression downregulated the expression of enzymes associated with the aerobic oxidation of carbohydrates.
Materials and methods
Reagents and materials
FBS (cat. no. 10100147), high glucose Dulbecco's Modified Eagle Medium (H-DMEM; cat. no. 11995065) and GlutaMAX™ (cat. no. 25030081) were purchased from Gibco® (Thermo Fisher Scientific, Inc.). Penicillin-streptomycin (cat. no. P1400) and the MTT Assay kit (cat. no. M1020) were purchased from Beijing Solarbio Science & Technology Co., Ltd. EcoRI (cat. no. R0101L) and XhoI (cat. no. R0146L) were purchased from New England BioLabs, Inc. Hieff Trans™ transfection reagent (cat. no. 40802ES01) and G418® (cat. no. 60220ES03) were purchased from Yeasen Biotechnology Co., Ltd. The chemical inhibitor of PTEN, SF1670 (cat. no. S7310), was purchased from Selleck Chemicals. Pemetrexed (cat. no. CDS024404) was purchased from Sigma-Aldrich (Merck KGaA). SuperRT One Step RT-PCR Kit (cat. no. CW0742), RNApure Tissue & Cell kit (cat. no. CW0584), HiFiScript cDNA Synthesis kit (cat. no. CW2569) and Super TaqMan OneStep reverse transcription-quantitative PCR (RT-qPCR) kit (cat. no. CW2695) were purchased from CoWin Biosciences Co., Ltd. The primary antibodies for malate dehydrogenase 1 (MDH1; cat. no. ab175455), succinate-Coenzyme A (CoA) ligase GDP-forming beta subunit (SUCLG2; cat. no. ab187996), aconitase 2 (ACO2; cat. no. ab110321), p53 (cat. no. ab26), proliferating cell nuclear antigen (PCNA; cat. no. ab29), ERK (cat. no. ab54230), phosphorylated (p-)ERK (cat. no. ab50011), AKT (cat. no. ab8805), p-AKT (cat. no. ab38449), mTOR (cat. no. ab2732), p-mTOR (cat. no. ab109268) and GAPDH (cat. no. ab181602); Donkey anti goat (cat. no. ab97120), goat anti rabbit (cat. no. ab97080) and goat anti-mouse (cat. no. ab97040) secondary antibodies were purchased from Abcam. A549 lung adenocarcinoma cells (cat. no. SCSP-503) were purchased from Cell library of typical culture preservation committee of the Chinese Academy of Science.
Vector construction
RNA of A549 cells were extracted according to the protocol of RNApure Tissue & Cell kit. The full-length coding sequence of PTEN was cloned following PCR from A549 cells using SuperRT One Step RT-PCR kit with the following primers: Forward, 5′-CGGAATTCGGATGTCCCGAAAGCAGG-3′, reverse 5′-CCGCTCGAGTCAGATGTTGAGCGG-3′. The reaction mixture was made up as recommended by the manufacturer of the kit, and the reaction steps were: Reverse transcription at 45°C for 30 min and pre-degeneration at 95°C for 2 min repeated for 40 cycles: Degeneration at 94°C for 30 sec, annealing at 58°C for 30 sec, extension at 72°C for 30 sec. Followed with final extension at 72°C for 5 min. The PCR product and the pcDNA3.1–3XFlag vector (MJ8001, Mingjing Biology) were digested with two restriction enzymes, EcoRI and XhoI. Following digestion, the DNA fragment containing the coding sequence was cloned into the vector to construct the expression plasmid pcDNA3.1–3XFlag-PTEN. A549 cells were transfected with 10 µg pcDNA3.1–3XFlag-PTEN or the empty plasmid at 37°C using Hieff Trans™ transfection reagent for 48 h, according to the manufacturer's protocol. Subsequently, cells with stable PTEN overexpression were selected by treating transfected cells with 1 mg/ml G418 at 37°C until stable expressed cells were successfully constructed.
MTT assay
MTT assay was performed according to a previous study (17). Cells were cultured in 96-well plates at a concentration of 1×104 cells/well. Cultures at 80–85% confluence were treated with pemetrexed at various concentrations (0, 5, 10 and 20 µg/ml). Following a 24-h incubation, cells were washed with sterile PBS to remove extra pemetrexed. MTT was diluted in the medium at a concentration of 5 mg/ml, and incubated with the cells for 4 h. Following incubation, DMSO was added to each well and the optical density (OD) at 560 nm was determined using a microplate reader. The viability of cells was calculated using the following formula: (ODExperiment-ODBlank)/ (ODControl-ODBlank) ×100%.
Cell culture and grouping
Cells were cultured in H-DMEM supplemented with 10% FBS and GlutaMAX™ (2 mmol/l) in a humid atmosphere with 5% CO2 at 37°C. Cells were divided into six groups: i) Negative control (NC) group; ii) treatment with 10 mg/ml pemetrexed for 24 h (NC + P) group; iii) PTEN inhibition (2 µmol/l for 36 h) (PI) group; iv) PTEN inhibition (2 µmol/l for 36 h) with treatment with 10 mg/ml pemetrexed for 24 h (PI + P) group; v) PTEN overexpression (PO) group; and vi) PTEN overexpression with treatment with 10 mg/ml pemetrexed for 24 h (PO+P) group. Following treatment, the cells were washed with sterile PBS to remove residual pemetrexed and collected for use in further experiments.
RNA extraction and RT
RNA extraction was performed using the RNApure Tissue & Cell kit according to the manufacturer's protocol. Cells were lysed, incubated for 5 min at room temperature, and centrifuged at 14,462 × g for 5 min. Ethanol was added and the mixture was loaded onto the adsorption columns provided in the kit. RNA was eluted using RNase-free water following washing with wash buffer provided in the kit. The concentration of extracted RNA was measured using the Nanodrop ND-2000 (Thermo Fisher Scientific, Inc.). An equal amount of RNA from each group was used as template to perform RT. The reaction solution was prepared according to the manufacturer's protocol. The reaction solution was mixed and incubated at 42°C for 15 min, followed by an incubation at 85°C for 5 min.
qPCR
Primers used for qPCR were: p53, forward 5′-ATTAGCGGCCGATGGAGGAGCCGC-3′, reverse 5′-ATCTCGAGTCAGTCTGAGTCAGGCCC-3′; Bcl, forward 5′-CGACGACTTCTCCCGCCGCTACCGC-3′, reverse 5′-CCGCATGCTGGGGCCGTACAGTTCC-3′; BAX, forward 5′-TGCAGAGGATGATTGCTGAC-3′, reverse 5′-GAGGACTCCAGCCACAAAGA-3′; GAPDH, forward 5′-GAATCTCACTCAGACGAGGACTT-3′, reverse 5′-GGTGTGTGGTTTAAGTGATGTCA-3′. qPCR was performed according to the manufacturer's protocol, and the thermocycling conditions were as follows: 40 cycles at 95°C for 15 sec and at 60°C for 40 sec. The threshold cycle value was calculated using the intensity of the fluorescence signal following PCR amplification (18). GAPDH was used as the internal reference, and the quantification result for each target gene was normalized to GAPDH.
Western blotting
Cells from each group were washed with PBS and were lysed with RIPA buffer supplied with protease inhibitor cocktail (CW2383, CWbio). Following lysis, the supernatant was collected following centrifugation at 14,462 × g for 10 min (4°C) and a bicinchoninic acid assay was used to measure the concentration of protein. Same amounts (60 µg) of protein from each group were used to perform 10% SDS-PAGE electrophoresis. Following electrophoresis, proteins were transferred onto 0.22-µm-thick nitrocellulose membranes using a semi-dry electro blotter. Membranes were blocked with 5% skimmed milk at room temperature for 1 h, and subsequently incubated with the primary antibody (1:1,000) at 4°C overnight, followed by incubation with the secondary antibody (1:5,000) for 1 h at room temperature. Chemiluminescence was performed using electrochemiluminescence reagent (WBKLS0500, Merck KGaA) to detect the protein expression levels. The densitometric analysis was performed using Scion Image (version 4.0.3.2; Scion Corporation) software and normalized to GAPDH.
Statistical analysis
Data are presented as the mean ± standard deviation. Each experiment was repeated three times. One-way ANOVA was performed to assess differences among groups followed by Tukey's post hoc test for multiple comparisons. GraphPad (version 7; GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the data. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of pemetrexed on the proliferation of A549 cells
MTT assay results suggested that the proliferation of A549 cells was inhibited with pemetrexed in a dose-dependent manner (Fig. 1A). Following treatment with 5 mg/ml pemetrexed for 24 h, the cell viability was 90.30±4.86%, with 10 mg/ml pemetrexed was 73.60±3.12% and with 20 mg/ml was 52.40±2.10%. Pemetrexed was able to significantly inhibit the viability of A549 cells at the concentrations of 10 mg/ml and 20 mg/ml. Therefore, 10 mg/ml was used as the working concentration for pemetrexed in the following experiments, since this concentration was the lowest to significantly inhibit the proliferation of A549 cells. PCNA regulates the replication of DNA, Notably, the protein expression level of PCNA decreased following treatment with pemetrexed (Fig. 1B); however, this decrease was not statistically significant in the single pemetrexed treatment group (Fig. 1C). The protein expression level of PCNA was significantly decreased in the PI + P group compared with the PI group. In addition, the protein expression levels of PCNA in the PO and in the PO + P groups were significantly decreased compared with the NC group.
Figure 1.
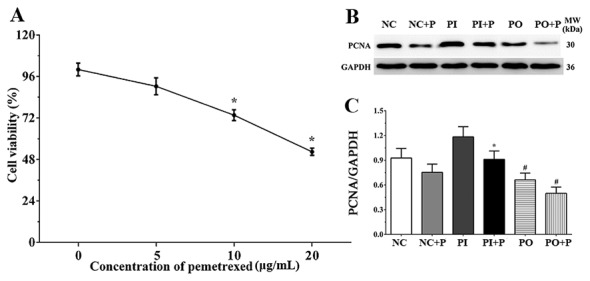
Effects of pemetrexed on the proliferation of A549 cells. (A) Dose-dependent effect of pemetrexed on the proliferation of A549 cells, as measured by MTT assay. *P<0.05 vs. untreated control group. (B) Protein expression level of PCNA in each group, as detected by western blotting. (C) Quantification analysis of western blotting. Data are presented as the mean ± standard deviation (n=3). GAPDH was used as the internal control. *P<0.05 vs. Inhibitor; #P<0.05 vs. NC group. MW, molecular weight; P, pemetrexed; PCNA, proliferating cell nuclear antigen.
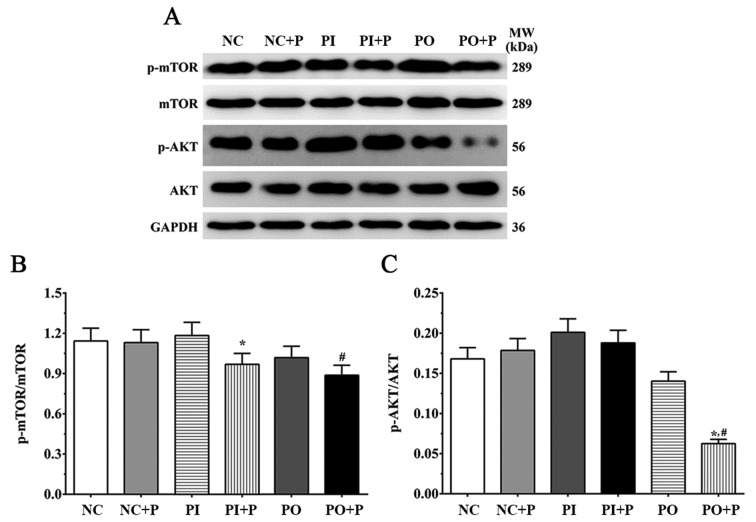
Protein expression levels of factors of the AKT/mTOR signaling pathway
The protein expression ratios of p-AKT/AKT and p-mTOR/mTOR were measured by western blotting and densitometric analysis (Fig. 2). The p-mTOR/mTOR ratio was slightly decreased following treatment with pemetrexed, and was significantly decreased in the PI + P and the PO + P groups compared with the PI and the NC groups, respectively (Fig. 2). The p-AKT/AKT ratio was also measured, and the protein expression levels presented a trend similar to the p-mTOR/mTOR ratio. Single inhibition or overexpression did not significantly change the ratio of p-AKT/AKT in these groups, and the p-AKT/AKT ratio was not significantly affected in the NC + P or PI + P groups compared with the corresponding control group without pemetrexed treatment; however, it was slightly decreased in the PO group and was significantly decreased in the PO + P group compared with the NC group (Fig. 2).
Figure 2.
Effects of pemetrexed on the AKT/mTOR signaling pathway. (A) Protein expression levels of p-mTOR, mTOR, p-AKT and AKT, as detected by western blotting. Quantification of (B) p-mTOR/mTOR and (C) p-AKT/AKT ratios. GAPDH was used as the internal control. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. corresponding control group without pemetrexed treatment; #P<0.05 vs. NC group. MW, molecular weight; P, pemetrexed; p-, phosphorylated.
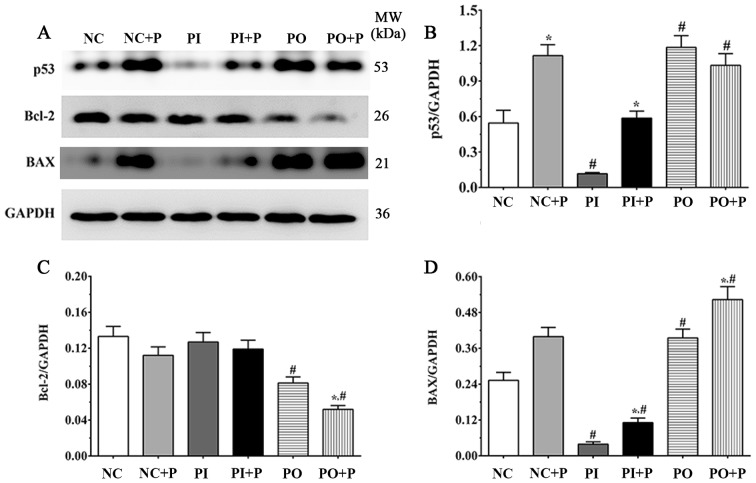
Alterations in the mRNA and the protein expression levels of apoptosis-associated factors
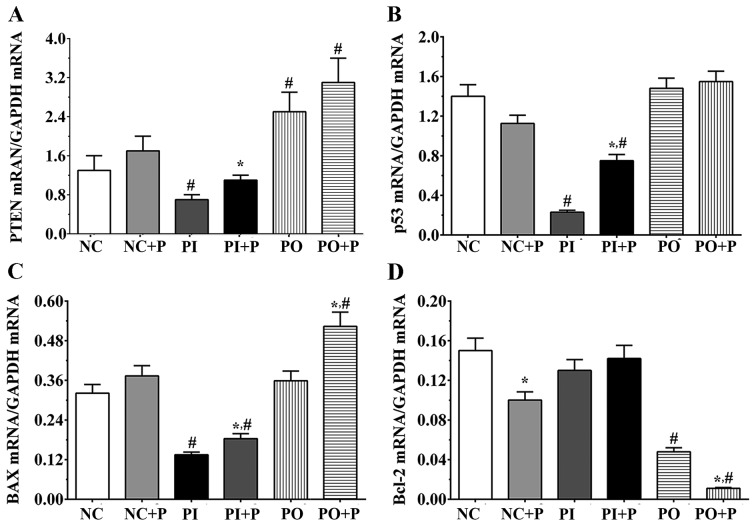
The protein expression level of p53 was significantly increased in the NC + P group compared with the NC group (Fig. 3A and B). Following treatment with PTEN inhibitor, the protein expression level of p53 in the PI group was significantly decreased compared with the NC group. The protein expression level of p53 in the PI + P group was significantly increased compared with the PI group, and significantly increased in the PO and PO + P groups compared with the NC groups. In addition, the protein expression levels of Bcl2 and BAX were detected in each group (Fig. 3A). The protein expression level of Bcl2 was slightly decreased following treatment with pemetrexed in the NC + P group (Fig. 3C); however, the protein expression level of Bcl2 was not significantly increased in PI + P group, and was significantly decreased in the PO + P group compared with the PO group. In addition the protein expression level of Bcl2 was significantly decreased in the PO group compared with the NC group. The protein expression level of BAX was slightly increased following treatment with pemetrexed and was significantly increased in the PI + P and PO + P groups compared with the PI and the PO groups, respectively (Fig. 3D). Compared with NC group, the expression level of BAX was significantly decreased following inhibition of PTEN. The expression of PTEN was detected using PCR. Pemetrexed significantly increased the expression of PTEN compared with inhibitor group. The expression of PTEN was significantly decreased in inhibitor group and significantly increased in PO and PO+P group compared with NC group. The mRNA expression levels of p53, BAX and Bcl2 were also measured. The expression of p53 was significantly decreased in inhibitor group and PI+P group compared with the NC group, and pemetrexed significantly increased the expression of PTEN compared with the inhibitor group. Pemetrexed significantly increased the expression of BAX in PO+P and PI+P group compared with the PO and PI groups, and significantly decreased in inhibitor and PI+P group compared with the NC group, and significantly increased in PO+P group compared with the NC group. Pemetrexed significantly decreased the expression of Bcl-2 in NC+P and PO+P group compared with NC and PO groups, and significantly decreased in PO and PO+P groups compared with the NC group (Fig. 4). The present qPCR and western blot results suggested that the expression levels of apoptosis-associated factors were altered at the transcriptional and the protein levels.
Figure 3.
Effects of pemetrexed on the protein expression level of apoptosis-associated factors. (A) Protein expression levels of p53, Bcl2 and BAX, as detected by western blotting. Quantification of the protein expression levels of (B) p53, (C) Bcl2 and (D) BAX. GAPDH was used as the internal control. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. corresponding control group without pemetrexed treatment; #P<0.05 vs. NC group. MW, molecular weight; P, pemetrexed.
Figure 4.
Effects of pemetrexed on the expression levels of apoptosis-associated factors. Quantification of the mRNA expression levels of (A) PTEN, (B) p53, (C) Bcl2 and (D) BAX, as measured by reverse transcription-quantitative polymerase chain reaction. GAPDH was used as the internal control. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. corresponding control group without pemetrexed treatment; #P<0.05 vs. NC group. P, pemetrexed.
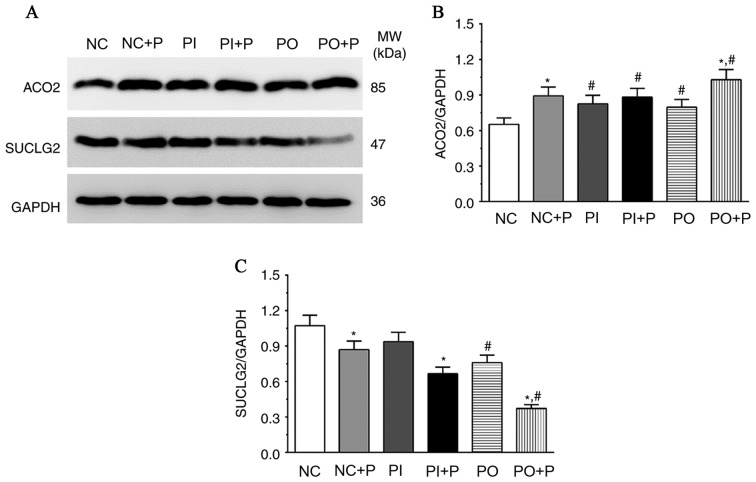
Protein expression levels of proteins associated with carbohydrate metabolism following treatment with pemetrexed
The protein expression levels of ACO2 and SUCLG2 were significantly altered following treatment with pemetrexed (Fig. 5). The protein expression level of ACO2 was significantly increased in the NC + P and OP + P groups compared with the NC and OP groups, respectively, and was significantly increased in the PI, PI+P and PO groups compared with the NC group. However, the protein expression level of SUCLG2 exhibited an opposite trend. Pemetrexed significantly decreased the protein expression level of SUCLG2 in NC + P, PI + P and PO + P groups compared with the NC, PI and PO groups, respectively. In addition, the protein expression level of SUCLG2 was significantly decreased in the PO and PO + P group compared with the NC group.
Figure 5.
Effects of pemetrexed on the protein expression levels of enzymes involved in the tricarboxylic acid cycle. (A) Protein expression levels of ACO2 and SUCLG2 in each group, as measured by western blotting. Quantification of the protein expression level of (B) ACO2 and (C) SUCLG2. GAPDH was used as the internal control. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. corresponding control group without pemetrexed treatment; #P<0.05 vs. NC group. ACO2, aconitase 2; MW, molecular weight; P, pemetrexed; SUCLG2 succinate-Coenzyme A ligase GDP-forming beta subunit.
Discussion
Lung cancer is one of the leading causes of tumor-associated mortalities in men and women worldwide (19). In 2015, the guidelines of The American Society of Clinical Oncology recommended two drugs for the treatment of NSCLC, docetaxel and pemetrexed (20). The present study established in vitro models of PTEN inhibition and overexpression, and the present results suggested that pemetrexed was able to suppress the proliferation of A549 cells by inhibiting the PI3K/AKT/mTOR signaling pathway and the carbohydrate metabolism, inducing apoptosis in A549 cells and exerting anti-tumor activities. In the present study, PTEN overexpression was identified to increase the effects of pemetrexed on lung cancer cells, enhance the anti-tumor effect of pemetrexed. A previous studies demonstrated that pemetrexed regulated the activity of the PTEN/PI3K/AKT/mTOR pathway indirectly (21), and further experiments are required to confirm the mechanism observed in the present study.
PTEN is a tumor suppressor gene, and is able to limit the aggressiveness of kidney, breast and prostate cancers by regulating cell proliferation exerting lipid phosphatase activity (22). The phosphatase activity of PTEN was identified in the C-terminal region of the protein (23), and the phosphorylation state of PTEN affects the intramolecular partners of PTEN, altering its subcellular localization and phosphatase activity (24). Mutations in the active site of PTEN are associated with the loss of its lipid phosphatase activity, and are identified in various types of human cancer (25). The PI3K/PTEN/AKT signaling axis is associated with cell proliferation, and dysfunctions or mutations of its components may lead to abnormal cell growth and tumor development (26). Activation of the PI3K/AKT pathway was identified in various types of cancer and is associated with tumor progression and with poor prognosis of patients with cancer. As a downstream molecular factor of the PI3K/AKT pathway, the activity of mTOR is associated with cancer (27). A previous study identified that inhibition of mTOR was able to increase the anti-tumor effect of pemetrexed by inhibiting autophagy in NSCLC cells (28). In the present study, the activity of the PI3K/mTOR signaling pathway was not significantly changed following single pemetrexed treatment, although the effect increased following PTEN overexpression. The present results suggested that pemetrexed may inhibit the growth of A549 cells by inhibiting the PI3K/AKT/mTOR signaling pathway, and inhibition of PTEN may increase the effect of pemetrexed, whereas, PTEN overexpression may decrease pemetrexed activity.
BAX, Bcl2 and p53 are downstream molecules of the PI3K/AKT/mTOR pathway, and serve important role in apoptosis. In normal tissue, p53 is involved in various biological processes, including the regulation of cell cycle, homeostasis and apoptosis (29). Notably, mutations in p53 lead to cancer development, and the transcription factor p53 was identified to be mutated in >50% of patients with cancer (30). Additionally, a previous study demonstrated that the expression of p53 is silenced in human cancer tissues (31). Therefore, p53 is considered the ‘guardian of the genome’ (32). PTEN was identified to directly interact with p53, and decreases the degradation of p53 by regulating MDM2 proto-oncogene, thus increasing the protein expression level of p53 (33). The expression level of Bcl2 binding component 3 (BBC3) was identified to be promoted by p53, and BBC3 may be able to interact with Bcl2 or Bcl2 like 1 via its Bcl2 homology 3 domain, thus inducing the expression of BAX (34). Additionally, p53 was demonstrated to be able to upregulate the expression level of BAX via the TNF receptor superfamily member 10b/Fas-associated via death domain pathway (35). The present results suggested that pemetrexed may increase the expression level of p53 following inhibition of PTEN, thus suggesting that pemetrexed may inhibit the proliferation of A549 cells by regulating p53 at the protein and transcriptional levels. BAX, a tumor suppressor protein, belongs to the Bcl2 family and serves an important role in the intrinsic apoptosis pathway (36). Upon activation, BAX translocates on the outer membrane of mitochondria following apoptotic stimuli, and this translocation is a crucial step in the initiation of apoptosis (37). In addition, BAX may be directly activated by p53 (38). BAX promotes the release of cytochrome c from the mitochondria and activate multiple caspases, thus inducing apoptosis in cancer cells (39). In the present study, treatment with pemetrexed was able to increase the expression of BAX at the protein and transcriptional levels, and overexpression of PTEN increased this effect, whereas, inhibition of PTEN led to the opposite effect.
Bcl2 is an anti-apoptotic member of the Bcl2 family (40), and overexpression of anti-apoptotic molecules of the Bcl2 family including Bcl2 and Bcl2-like 1 is frequently observed in human tumor tissues (41). Overexpression of Bcl2 was identified to be associated with cancer occurrence and progression, and chemotherapy resistance (42). Various small molecule inhibitors of Bcl2 have been investigated in the treatment of hematological cancer (43). In contrast with the effects of pemetrexed on BAX, pemetrexed decreased the expression level of Bcl2 at the protein and transcriptional levels, and PTEN overexpression increased this effect. Collectively, treatment with pemetrexed was able to promote apoptosis in cancer cells, inhibiting the anti-apoptotic factors BAX and p53, thus exerting anti-tumor activity. Notably, the expression level of PTEN was associated with pemetrexed activity, and overexpression of PTEN increased the anti-tumor effects of pemetrexed.
Rapid cell proliferation is a characteristic of cancer cells; in order to sustain their metabolic requirements, multiple metabolic alterations occur in cancer cells (44), including the increased uptake of nutrients and the preferential use of glucose as carbon source (45), resulting in an increased metabolic rate. Carbohydrate metabolism is an important pathway used by cancer cells to sustain their proliferation during tumor development (46). The tricarboxylic acid (TCA) cycle is a central hub in the catabolism of carbohydrates, and it is involved in various physiological processes. Previous studies identified that the TCA cycle in cancer cells may be uncoupled to glycolysis to allow the use of additional carbon sources, including glutamine, to fulfill their metabolic requirements (45,47). Therefore, in the present study, it was hypothesized that the expression level of factors associated to the aerobic oxidation of glucose, including enzymes of the TCA cycle, may be altered following treatment with pemetrexed. ACO2 and SUCLG2 are important enzymes of the TCA cycle. ACO2 is able to reversibly catalyze citrate into isocitrate forming a cis-aconitate intermediate, and SUCLG2 is a subunit of the succinyl-CoA synthetase, which is able to reversibly catalyze the formation of succinyl-CoA into succinate. Treatment with pemetrexed led to inverse effects on ACO and SUCLG2, the protein expression level of ACO2 increased and the protein expression level of SUCLG2 decreased. This effect was increased following PTEN overexpression and decreased following PTEN inhibition. The present results suggested that pemetrexed may exert an anti-tumor activity via the inhibition of the TCA cycle, decreasing the energy supply of cancer cells and inducing apoptosis. The effects of pemetrexed were altered following PTEN inhibition or overexpression, suggesting that PTEN overexpression may enhance the anti-tumor effect of pemetrexed, and may contribute to the development of a novel therapeutic strategy in the future.
In the present study, pemetrexed was demonstrated to inhibit the proliferation of lung cancer cells via the inhibition of the PI3K/AKT/mTOR signaling pathway and the upregulation of pro-apoptotic factors at the protein and transcriptional levels. Furthermore, the results suggested that pemetrexed may inhibit the aerobic oxidation of glucose, decreasing the energy supply of cancer cells, leading to apoptosis. In the present in vitro model, and PTEN overexpression was able to increase the effect of pemetrexed on lung cancer cells. The modulation of PTEN, in combination with pemetrexed, may represent a therapeutic strategy against NSCLC. However, the present study presents certain limitations, since only one cell line was examined, and further in vitro and clinical experiments are required to be performed in future studies, including knockdown of PTEN via small interfering RNA technology.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Tianjin Science and Technology Planning Project (grant no. 16ZXHLSY00120).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BL, JKZ and YS performed the experiment and wrote the manuscript. YLH and ZGW designed the experiment. LZ, HF and SKS revised the manuscript and analyzed the data. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Park S, Keam B, Kim SH, Kim KH, Kim YJ, Kim JS, Kim TM, Lee SH, Kim DW, Lee JS, Heo DS. Pemetrexed singlet versus nonpemetrexed-based platinum doublet as second-line chemotherapy following first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor failure in non-small cell lung cancer patients with EGFR mutations. Cancer Res Treat. 2015;47:630–637. doi: 10.4143/crt.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masuda T, Imai H, Kuwako T, Miura Y, Yoshino R, Kaira K, Shimizu K, Sunaga N, Tomizawa Y, Ishihara S, et al. Effcacy of platinum combination chemotherapy following frst-line geftinib treatment in non-small cell lung cancer patients harboring sensitive EGFR mutations. Clin Transl Oncol. 2015;17:702–709. doi: 10.1007/s12094-015-1297-8. [DOI] [PubMed] [Google Scholar]
- 3.Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:1023–1028. doi: 10.2147/OTT.S100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H, Fan HX, Song LH, Xie JC, Fan SF. Relationship between contrast-enhanced CT and clinicopathological characteristics and prognosis of non-small cell lung cancer. Oncol Res Treat. 2017;40:516–522. doi: 10.1159/000472256. [DOI] [PubMed] [Google Scholar]
- 5.Facchinetti F, Pilotto S, Metro G, Baldini E, Bertolaccini L, Cappuzzo F, Delmonte A, Gasparini S, Inno A, Marchetti A, et al. Treatment of metastatic non-small cell lung cancer: 2018 guidelines of the Italian association of medical oncology (AIOM) Tumori 105 (5 Suppl) 2019:S3–S14. doi: 10.1177/0300891619857418. [DOI] [PubMed] [Google Scholar]
- 6.Stinchcombe TE, Borghaei H, Barker SS, Treat JA, Obasaju C. Pemetrexed with platinum combination as a backbone for targeted therapy in non-small-cell lung cancer. Clin Lung Cancer. 2016;17:1–9. doi: 10.1016/j.cllc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Al-Saleh K, Quinton C, Ellis PM. Role of pemetrexed in advanced non-small-cell lung cancer: Meta-analysis of randomized controlled trials, with histology subgroup analysis. Curr Oncol. 2012;19:e9–e15. doi: 10.3747/co.19.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 9.Fuld AD, Dragnev KH, Rigas JR. Pemetrexed in advanced non-small-cell lung cancer. Expert Opin Pharmacother. 2010;11:1387–1402. doi: 10.1517/14656566.2010.482560. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 11.Carrera AC, Anderson R. The cell biology behind the oncogenic PIP3 lipids. J Cell Sci. 2019;132(pii):jcs228395. doi: 10.1242/jcs.228395. [DOI] [PubMed] [Google Scholar]
- 12.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 13.Bufu T, Di X, Yilin Z, Gege L, Xi C, Ling W. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs. 2018;29:530–538. doi: 10.1097/CAD.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 14.Kechagioglou P, Papi RM, Provatopoulou X, Kalogera E, Papadimitriou E, Grigoropoulos P, Nonni A, Zografos G, Kyriakidis DA, Gounaris A. Tumor suppressor PTEN in breast cancer: Heterozygosity, mutations and protein expression. Anticancer Res. 2014;34:1387–1400. [PubMed] [Google Scholar]
- 15.Pérez-Ramírez C, Cañadas-Garre M, Molina MÁ, Faus-Dáder MJ, Calleja-Hernández MÁ. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics. 2015;16:1843–1862. doi: 10.2217/pgs.15.122. [DOI] [PubMed] [Google Scholar]
- 16.Ni S, Wang H, Zhu X, Wan C, Xu J, Lu C, Xiao L, He J, Jiang C, Wang W, He Z. CBX7 suppresses cell proliferation, migration, and invasion through the inhibition of PTEN/Akt signaling in pancreatic cancer. Oncotarget. 2017;8:8010–8021. doi: 10.18632/oncotarget.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X, Xu Z, Fan R, Wang C, Ji W, Ma Y, Cai W, Zhang Y, Yang N, Zou S, et al. HO-1 alleviates cholesterol-induced oxidative stress through activation of Nrf2/ERK and inhibition of PI3K/AKT pathways in endothelial cells. Mol Med Rep. 2017;16:3519–3527. doi: 10.3892/mmr.2017.6962. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Acheampong E, Spencer I, Lin W, Ziman M, Millward M, Gray E. Is the blood an alternative for programmed cell death ligand 1 assessment in non-small cell lung cancer? Cancers (Basel) 2019;11(pii):E920. doi: 10.3390/cancers11070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S, Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, et al. Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33:3488–3515. doi: 10.1200/JCO.2015.62.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothbart SB, Racanelli AC, Moran RG. Pemetrexed indirectly activates the metabolic kinase AMPK in human carcinomas. Cancer Res. 2010;70:10299–10309. doi: 10.1158/0008-5472.CAN-10-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 23.Tibarewal P, Zilidis G, Spinelli L, Schurch N, Maccario H, Gray A, Perera NM, Davidson L, Barton GJ, Leslie NR. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal. 2012;5:ra18. doi: 10.1126/scisignal.2002138. [DOI] [PubMed] [Google Scholar]
- 24.Andrés-Pons A, Gil A, Oliver MD, Sotelo NS, Pulido R. Cytoplasmic p27Kip1 counteracts the pro-apoptotic function of the open conformation of PTEN by retention and destabilization of PTEN outside of the nucleus. Cell Signal. 2012;24:577–587. doi: 10.1016/j.cellsig.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Luna S, Mingo J, Aurtenetxe O, Blanco L, Amo L, Schepens J, Hendriks WJ, Pulido R. Tailor-made protein tyrosine phosphatases: In vitro site-directed mutagenesis of PTEN and PTPRZ-B. Methods Mol Biol. 2016;1447:79–93. doi: 10.1007/978-1-4939-3746-2_5. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Qiu Y, Pang X, Li J, Wu S, Yin S, Han L, Zhang Y, Jin C, Gao X, et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma. Mol Cancer Ther. 2017;16:2711–2723. doi: 10.1158/1535-7163.MCT-17-0498. [DOI] [PubMed] [Google Scholar]
- 28.Hwang KE, Kim YS, Jung JW, Kwon SJ, Park DS, Cha BK, Oh SH, Yoon KH, Jeong ET, Kim HR. Inhibition of autophagy potentiates pemetrexed and simvastatin-induced apoptotic cell death in malignant mesothelioma and non-small cell lung cancer cells. Oncotarget. 2015;6:29482–29496. doi: 10.18632/oncotarget.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Simpson ER, Brown KA. p53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 2015;75:5001–5007. doi: 10.1158/0008-5472.CAN-15-0563. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda S, Nakagawa Y, Kitagishi Y, Nakanishi A, Murai T. Reactive oxygen species, superoxide dimutases, and PTEN-p53-AKT-MDM2 signaling loop network in mesenchymal stem/stromal cells regulation. Cells. 2018;7(pii):E36. doi: 10.3390/cells7050036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 32.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Guessous F, Kwon S, Kumar M, Ibidapo O, Fuller L, Johnson E, Lal B, Hussaini I, Bao Y, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res. 2008;68:1723–1731. doi: 10.1158/0008-5472.CAN-07-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/S1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr, el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- 36.Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 37.Cosentino K, García-Sáez AJ. BAX and Bak pores: Are we closing the circle? Trends Cell Biol. 2017;27:266–275. doi: 10.1016/j.tcb.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 39.Ugarte-Uribe B, García-Sáez AJ. Apoptotic foci at mitochondria: In and around Bax pores. Philos Trans R Soc Lond B Biol Sci. 2017;372(pii):20160217. doi: 10.1098/rstb.2016.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villanova L, Careccia S, De Maria R, Fiori ME. Micro-economics of apoptosis in cancer: ncRNAs modulation of BCL-2 family members. Int J Mol Sci. 2018;19(pii):E958. doi: 10.3390/ijms19040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ. Anti-apoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6:241–249. doi: 10.1016/j.ccr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 43.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 44.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 45.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 47.Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta. 2012;1826:370–384. doi: 10.1016/j.bbcan.2012.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.