Abstract
Hyperglycemia promotes podocyte apoptosis and contributes to the pathogenesis of diabetic nephropathy (DN). However, the mechanisms of hyperglycemia-induced podocyte apoptosis remain unknown. Recent studies have implicated Src-associated substrate during mitosis of 68 kDa (Sam68) in various cellular processes including RNA metabolism, apoptosis, signal transduction. This study sought to examine the effect of Sam68 on high glucose (HG)-induced podocytes apoptosis, and the mechanism underlying this effect. Immortalized mouse podocytes were exposed to medium containing normal glucose, or HG and Sam68 siRNA, respectively. The expression of Sam68 in podocytes was determined by fluorescence quantitative PCR (qPCR), immunofluorescence and immunoblotting. The role of Sam68 in HG-induced podocyte apoptosis was further evaluated by inhibiting Sam68 expression by Sam68 siRNA and performing flow cytometry. The mRNA and protein expression of pro-apoptosis gene Bax and anti-apoptotic gene Bcl-2 were assessed by qRCR and immunoblotting. In the present study, it was first demonstrated that Sam68 was upregulated in a time and dose-dependent manner in in vitro HG-treated podocytes. Pretreatment with Sam68 siRNA markedly decreased nuclear Sam68 expression. Moreover, the effects of HG-induced apoptosis were also abrogated by Sam68 knockdown in cultured podocytes. Furthermore, HG increased Bax and decreased Bcl-2 protein expression in cultured podocytes, and this effect was blocked by Sam68 knockdown. The results of the present study revealed that Sam68 mediated HG-induced podocyte apoptosis, probably through the Bax/Bcl-2 signaling pathway, and thus may be a potential therapeutic target for DN.
Keywords: Sam68, podocyte, apoptosis, high glucose, diabetic nephropathy
Introduction
The prevalence of diabetic nephropathy (DN) worldwide has markedly increased and DN has become a leading cause of end-stage renal disease (1). It is characterized by the appearance of albuminuria, hypertension and a gradual decline in the glomerular filtration rate (2,3). Despite emerging strategies, no treatment has been able to reverse disease progression (1). Therefore, much effort has been devoted to unraveling the mechanisms that promote glomerular damage in DN with the hope of identifying promising therapeutic targets.
Podocytes are terminally differentiated visceral epithelial cells located on the glomerular basement membrane outside the glomerular capillaries; their injury or loss leads to proteinuria and disease progression (4–6). A large body of evidence has demonstrated that podocyte apoptosis plays a vital role in the pathogenesis of DN (7–9) and inhibition of podocyte apoptosis is a promising therapeutic target for DN (10). However, the concert mechanisms involved in DN have not been fully elucidated.
Src-associated substrate during mitosis of 68 KDa (Sam68), also known as K homology (KH) domain-containing, RNA binding, signal transduction associated 1 (KHDRBS1), is a member of the signal transduction activator of RNA (STAR) family of RNA-binding proteins (11,12). Sam68 is a versatile protein with roles in various cellular processes including RNA metabolism, apoptosis, and signal transduction (13). Previous results have suggested that dysregulation of Sam68-regulated splicing events is a key event in tumor development and progression (14,15). In addition, some studies have also revealed that Sam68 modulates nuclear transcription factor kappa B (NF-κB) activation, thus inducing inflammation (16,17). Recently, increasing evidence has implicated Sam68 in cell apoptosis (17,18). However, Sam68 expression and its biological functions in podocytes are unclear. The aim of this study was to explore Sam68 expression in vitro in podocytes treated with high glucose (HG) and its function in HG-induced podocyte apoptosis.
Materials and methods
Podocyte culture and treatments
A conditionally immortalized mouse podocyte cell line was kindly provided by Professor Jochen Reiser (Rush University Medical Center, Chicago, USA). The mouse podocyte clone-5 (MPC-5) is characterized by expression of T antigen stimulated by γ-IFN and SV40 heat-sensitive variant gene (19). Cells were amplified at 33°C in RPMI-1640 medium (Gibco BRL; Thermo Fisher Scientific, Inc. Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco BRL; Thermo Fisher Scientific, Inc. Waltham, MA, USA) and recombinant IFN-γ (CYT-358, ProSpec-Tany Technogene Ltd., Rehovot, Israel). After passaging, the cells were cultured at 37°C under 5% CO2 for 10–14 days in RPMI-1640 medium without IFN-γ to induce differentiation. Differentiated podocytes were cultured for 24 h in serum-free DMEM basic (1X) medium (Thermo Fisher Scientific, Inc.) before being exposed to various experimental conditions. The cells were divided into the following groups: i) A normal glucose group (NG, 5.3 mM glucose); ii) a high glucose group (HG) incubated in basic DMEM (1X) containing various concentrations of glucose (20, 30 or 40 mM); and iii) a mannitol group (MA) incubated in NG (5.3 mM) medium supplemented with 24.7 mM D-mannitol (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as an osmotic control. The time-points of HG (30 mM) intervention were 6, 12, 24, 48 and 72 h. All the glucose used in this study was D(+)glucose (Sigma Aldrich; Merck KGaA). All experiments were performed in triplicate.
Transfection of small interfering RNA
Three pairs of siRNA sequences that targeted Sam68 and one pair of control-siRNA were designed and synthesized by RiboBio Co., Ltd. (Guangzhou, China). The siRNA sequences were as follows: Sam68-siRNA1: GAAAGAACGCGTGCTGATA; Sam68-siRNA2: GAGGAGAATTATTTGGATT; Sam68-siRNA3: TTACGAAGCCTACGGACAA; and the product no. of Con-siRNA: siN05815122147. Transfection was carried out in 6-well plates or 50 cm2 culture plates with siRNAs (50 nM) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) transfection reagent. The transfection efficiency was evaluated by assessing the mRNA expression of Sam68 using qPCR.
Immunofluorescence staining
Podocytes cultured under different conditions (NG, MA, HG, HG + Sam68-siRNA and Con-siRNA) were fixed with cold methanol for 20 min at −20°C, and then blocked with 5% bovine serum albumin for 30 min at room temperature. Then, the cells were incubated with rabbit anti-Sam68 antibody (1:100; cat. no. ab109197; Abcam, Cambridge, MA, USA) and goat anti-synaptopodin antibody (1:100; cat. no. sc21536; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Subsequently, the cells were incubated with donkey anti-goat Alexa Fluor 488 (1:250; cat. no. A-11055) and donkey anti-rabbit Alexa Fluor 546 (1:250; cat. no. A10040; both from Life Technologies; Thermo Fisher Scientific, Inc.) at room temperature. Cells were stained for 10 min with 4′-6-diamidino-2-phenylindole (DAPI; 1:1,000; Sigma Aldrich; Merck KGaA) for 10 min to visualize the nuclei. Images were captured with confocal microscopy (LCSM, Zeiss KS 400; Zeiss AG, Oberkochen, Germany).
RNA extraction and quantitative-PCR
Total RNA of cultured podocytes under different conditions (NG, MA, HG, HG + Sam68-siRNA and Con-siRNA) was extracted using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then, RNA (1 µg) was reverse-transcribed at 37°C for 30 min and 85°C for 5 sec using the PrimeScript™ RT reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China). Quantitative-PCR was performed using an ABI PRISM7900 Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.) with SYBR Green Real-Time PCR Master Mix (Takara Biotechnology Co., Ltd.). Samples were amplified at 95°C for 2 min, 40 cycles at 95°C for 30 sec, 95°C for 5 sec, 60°C for 5 sec, and 72°C for 10 min. The primers sequences were as follows: Sam68 upstream, 5′-TTATGGCCCATGCTATGGAAGA-3′ and downstream, 5′-AGGTACTCCGTTCAAGTAGGAC-3; GAPDH upstream 5′-AGGTCGGTGTGAACGGATTTG-3′ and downstream, 5′-TGTAGACCATGTAGTTGAGGTCA-3′. In order to confirm amplification specificity, the PCR products from each primer pair were subjected to melting curve analysis and subsequent agarose gel electrophoresis. All reactions were performed in triplicate and were normalized to GAPDH. Ratio results were calculated based on the 2−ΔΔCq method (20).
Western blotting
Differentiated podocytes were collected with a cold plastic cell scraper and lysed with lysis buffer (Beyotime Institute of Biotechnology) containing 20% SDS, glycerol and 1mM PMSF. A nuclear and cytoplasmic extraction kit (Nanjing KeyGEN Biotech Co., Ltd., Nanjing, China) was used to obtain nuclear proteins from the cultured podocytes. Protein concentrations were measured with a Pierce bicinchoninic protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Cellular proteins (40 µg) were separated by 10% SDS-polyacrylamide denaturing gel and transferred to nitrocellulose membranes, which were then blocked in 5% non-fat dry milk for 1 h, then, incubated overnight at 4°C with primary antibodies against Sam68 (1:10,000; cat. no. ab109197; Abcam), Histone H3 (1:1,000; cat. no. 9715S; Cell Signaling Technology, Inc., Danvers, MA, USA), Bax (1:500; cat. no. sc7480; Santa Cruz Biotechnology, Inc.), Bcl-2 (1:1,000; cat. no. ab59348; Abcam) and GAPDH (1:1,000; cat. no. AP0063; Biogot Technology Co., Ltd., Nanjing, China). After being washed in TBS-Tween buffer, the membranes were incubated with anti-rabbit IgG (1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h at room temperature. Protein bands were visualized with ECL reagent (Advansta, Inc., Menlo Park, CA, USA). The intensities of the identified bands were quantified with ImageJ 1.47 software (National Institutes of Health, Bethesda, MD, USA). GAPDH or Histone H3 was used as the internal control to standardize protein expression.
Analysis of apoptosis by flow cytometry
Apoptosis of the podocytes was assessed 48 h after exposure to various treatments (NG, MA, HG, HG + Sam68-siRNA and Con-siRNA). Apoptosis was determined with an Annexin V-FITC/propidium iodide (PI) apoptosis detection kit according to the manufacturer's instructions (Nanjing KeyGEN Biotech. Co., Ltd.). Briefly, podocytes were trypsinized and centrifuged at 878 × g for 3 min at room temperature. The cells were then suspended in 1X binding buffer and incubated with 5 µl Annexin V-FITC and 5 µl PI in the dark for 10 min. Cell fluorescence was then assessed by using a Cell Lab Quanta™ SC flow cytometer (Beckman Coulter, Inc., Brea, CA, USA). Cells that stained positive for Annexin V-FITC and negative for PI were considered apoptotic.
Statistical analysis
The data were analyzed with SPSS17.0 (SPSS, Inc., Chicago, IL, USA). All values are presented as the means ± SEM. Multiple comparisons among the groups were performed with one-way analysis of variance (ANOVA) with a Bonferroni/Tukey's test or Dunnett's T3-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Sam68 is increased in HG-treated podocytes in vitro
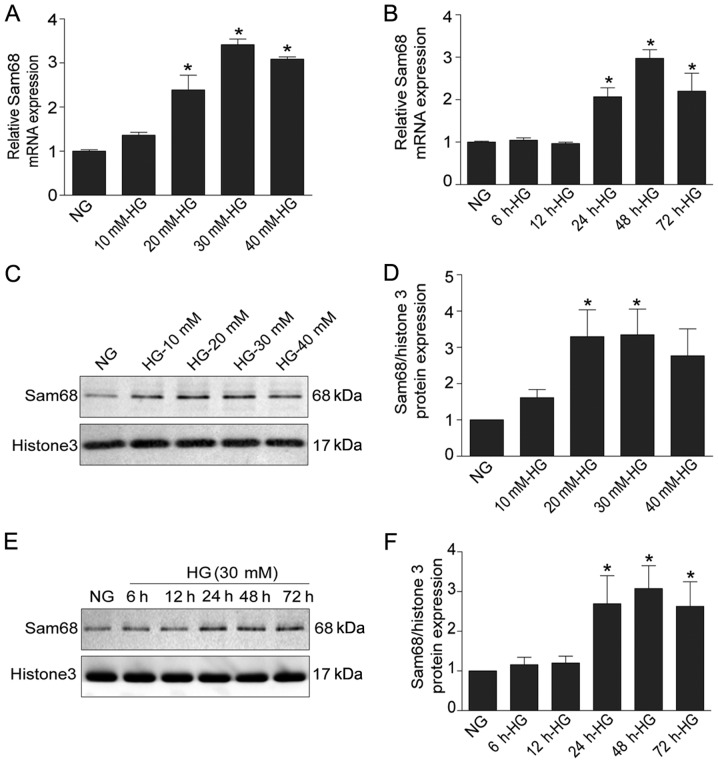
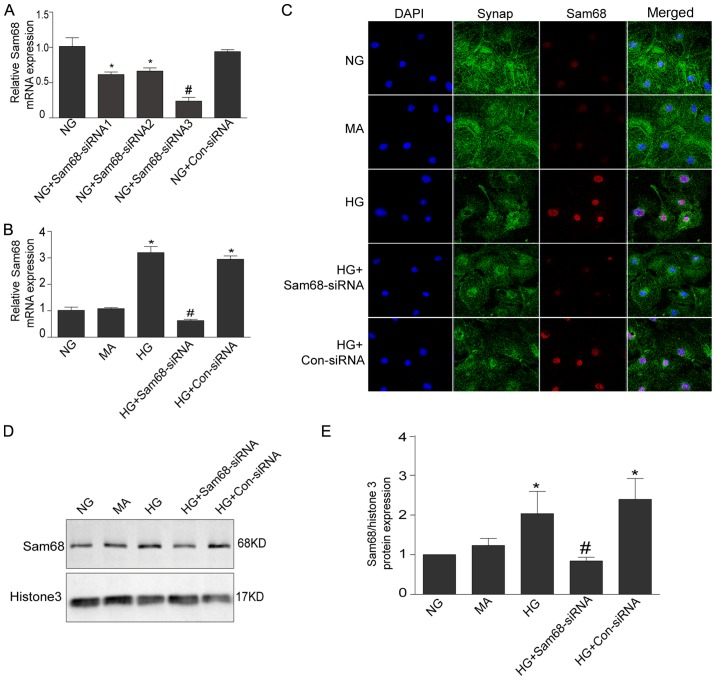
To evaluate the effect of HG on Sam68 expression, Sam68 expression was assessed under different treatments (NG, MA, HG, HG + Sam68-siRNA and Con-siRNA) by using real-time PCR, immunoblotting and immunofluorescence staining. As revealed in Fig. 1A and B, real-time PCR revealed that HG stimulation significantly increased the mRNA levels of Sam68 in a dose- and time-dependent manner (P<0.05). Western blot analysis also demonstrated that the protein expression of Sam68 was increased in HG-cultured podocytes after 48 h in a dose-dependent manner (Fig. 1C). In a time-response experiment, higher expression of Sam68 protein was observed in the nuclear extracts of podocytes treated with HG (30 mM) for 48 h (Fig. 1D). Densitometric analysis of the immunoblots is presented in Fig. 1E and F. Similar results from confocal microscopy also demonstrated that Sam68 protein expression was markedly increased in cultured podocytes treated with 30 mM HG for 48 h, and this effect was blocked by concurrent treatment with Sam68 siRNA (Fig. 2). Collectively, these data indicated that HG treatment increased Sam68 expression in differentiated mouse podocytes, and this effect was prevented by concurrent treatment with Sam68 knockdown.
Figure 1.
Sam68 is increased in HG-treated podocytes in vitro. (A) The effects of HG with different concentrations on the mRNA expression of Sam68. (B) The effects of HG at different time-points on the mRNA expression of Sam68. The mRNA level of Sam68 was detected using real-time-quantitative PCR, and was calculated with the 2−ΔΔCq method. (C) The protein level of Sam68 was detected by immunoblotting in the nuclear extract of podocytes treated with different concentrations of glucose. Histone H3 was used as a nuclear protein loading control. (D) Densitometric analysis of three independent experiments displayed in C. Data are expressed as the mean ± SEM. *P<0.05 HG (20 mM, 30 mM, 40 mM) vs. NG. (E) The protein expression of Sam68 was detected by immunoblotting in the nuclear extract of podocytes incubated with HG at different time-points. Histone H3 was used as the nuclear protein loading control. (F) Densitometric analysis of three repetitions of the experiment displayed in E. Data are expressed as the mean ± SEM. *P<0.05, 24 HG (30 mM) or 48 h HG (30 mM) or 72 h HG (30 mM) vs. NG. Sam68, Src-associated substrate during mitosis of 68 kDa; HG, high glucose; PCR, polymerase chain reaction.
Figure 2.
Increased Sam68 expression in cultured podocytes with HG is inhibited by Sam68 knockdown. (A) The effects of siRNA sequences targeting Sam68 on the mRNA expression of Sam68 in in vitro cultured podocytes. Three pairs of siRNA sequences that targeted Sam68 and one pair of control-siRNA were designed and synthesized. The third pair of siRNA sequence was selected for further experiments based on the interference effect. NG (5.3 mM) group. Values are expressed as the mean ± SEM. *P<0.05 NG+Sam68-siRNA1 or NG+Sam68-siRNA2 vs. NG; #P<0.01, NG+Sam68-siRNA1-3 vs. NG+Con-siRNA. (B) The effects of Sam68 siRNA on the mRNA expression of Sam68 in HG-cultured podocytes. The mRNA level of Sam68 was examined with real-time-PCR, and was calculated with the 2−ΔΔCq method. (C) Double immunofluorescence staining of Sam68 (red), synaptopodin-identified podocytes (green), DAPI-stained nuclei (blue) and merged images (purple) in cultured podocytes treated with HG for 48 h. (D) The protein expression of Sam68 was detected by immunoblotting in the nuclear extract of podocytes incubated with HG (30 mM) for 48 h. Histone H3 was used as the nuclear protein loading control. (E) Densitometric analysis of three independent experiments displayed in D. NG (5.3 mM) group; HG (30 mM) group; MA, NG (5.3 mM)+MA (24.7 mM) group, as an osmolality control; HG+Sam68-siRNA, HG (30 mM)+Sam68-siRNA (50 nM) group; HG+Con-siRNA, HG (30 mM)+non target-siRNA (50 nM) group. Values are expressed as the mean ± SEM. *P<0.05, HG or HG+Con-siRNA vs. NG; #P<0.05, HG+Sam68-siRNA vs. HG. Sam68, Src-associated substrate during mitosis of 68 kDa; HG, high glucose; PCR, polymerase chain reaction; NG, normal glucose; HG, high glucose; MA, mannitol.
Sam68 mediates HG-induced podocyte apoptosis
In agreement with previous results, the number of Annexin V-FITC+/PI− (apoptotic) cells in the HG group (16.18±1.09%) was significantly greater than that in the NG group (9.2±0.44%) and the mannitol group (10.20±0.29%) at 48 h (Fig. 3; all P<0.01). As anticipated, mannitol treatment did not have this effect, thus indicating that the apoptosis effect under HG did not result from high osmolarity.
Figure 3.
Sam68 mediates HG-induced podocyte apoptosis. (A) Podocytes cultured under different conditions were stained with Annexin V-FITC/PI for flow cytometric analysis. Cells that stained positive for Annexin V-FITC and negative for PI were considered apoptotic. (B) Histogram revealing changes in the percentage of apoptotic cells. NG (5.3 mM) group; HG group (30 mM); MA, NG (5.3 mM)+MA (24.7 mM) group, as an osmolality control; HG+Sam68-siRNA, HG (30 mM)+Sam68-siRNA (50 nM) group; HG+Con-siRNA, HG (30 mM)+non target-siRNA (50 nM) group. Data are expressed as the mean ± SEM. The experiments were performed in triplicate. *P<0.01, HG or HG+Con-siRNA vs. NG; #P<0.01, HG+Sam68-siRNA vs. HG. Src-associated substrate during mitosis of 68 kDa; HG, high glucose; NG, normal glucose; HG, high glucose; MA, mannitol.
To assess the effect of Sam68 on apoptosis regulation, Sam68-siRNA was further used to determine the role of Sam68 in podocyte apoptosis induced by HG treatment. As presented in Fig. 3, the apoptosis-inducing effect of HG was abolished by Sam68-siRNA knockdown (16.18±1.09% vs. 11.45±0.60%, P<0.01). These results demonstrated that Sam68 mediates HG-induced podocyte apoptosis and may play a proapoptotic role in HG-induced podocyte apoptosis.
Sam68 regulates Bax and Bcl-2 expression in podocytes under HG
To further investigate the probable mechanism of Sam68-mediated apoptosis in podocytes treated with HG, the effect of Sam68 on the protein expression of proapoptotic Bax and antiapoptotic Bcl-2 was investigated. As revealed in Fig. 4, HG increased the pro-apoptotic Bax protein and decreased the anti-apoptotic Bcl-2 protein in in vitro cultured podocytes, in contrast, in Sam68-siRNA-knockdown podocytes, the pro-apoptotic Bax protein was decreased while the anti-apoptotic Bcl-2 protein was increased (P<0.01). According to these results, Sam68 is implicated in the regulation of Bax and Bcl-2 expression in HG-treated podocytes.
Figure 4.
Sam68 regulates Bax and Bcl-2 expression in HG-cultured podocytes. (A) The protein expression of Bax and Bcl-2 were determined by western blotting analysis (n=3). (B and C) Densitometric analysis of three independent experiments presented in A. NG (5.3 mM) group; HG, (30 mM); MA, NG (5.3 mM) + MA (24.7 mM) group, as an osmolality control; HG+Sam68-siRNA, HG (30 mM)+Sam68-siRNA (50 nM) group; HG+Con-siRNA, HG (30 mM)+non target-siRNA (50 nM) group. All data are expressed as the mean ± SEM. *P<0.05 HG or HG+Con-siRNA vs. NG; #P<0.05 HG+Sam68-siRNA vs. HG. Src-associated substrate during mitosis of 68 kDa; HG, high glucose; NG, normal glucose; HG, high glucose; MA, mannitol.
Discussion
Podocytes are highly differentiated cells that play a crucial role in the function of the glomerular filtration barrier (4,5). Podocyte depletion due to apoptosis plays a pivotal role in the initiation and development of DN (9,21). Previous results have demonstrated that a decrease in podocyte number due to apoptosis or detachment contributes to the development of proteinuria and glomerulosclerosis in DN (8,9,22,23). Therefore, preventing or inhibiting podocyte apoptosis may be a promising therapeutic target for treatment of DN.
In the present study, it was first revealed that the mRNA and protein expression of Sam68 were significantly increased in HG-cultured podocytes in vitro. Moreover, the effect of apoptosis induced by HG was abolished by Sam68 knockdown. The first evidence that HG mediates podocyte apoptosis was provided through activation of Sam68, a specific target of the Src tyrosine kinase in mitosis. It was also further determined that Sam68 may mediate HG-induced apoptosis by significantly increased Bax expression and decreased Bcl-2 expression (P<0.01). These data indicated that the Sam68/Bax/Bcl-2 signaling pathway may play a proapoptotic role in HG-induced podocyte apoptosis.
Sam68, which was originally identified as a substrate for Src kinase phosphorylation, is a member of the STAR family of KH domain-containing RNA-binding proteins, which link signaling pathways to RNA processing (11,12). Sam68 is broadly expressed in various tissues and cell types, and it regulates a variety of cellular processes, such as alternative splicing, gene transcription and signal transduction (14,15,18). Sam68 has also been identified as an important signaling molecule that regulates NF-κB activation and apoptosis mediated by the tumor necrosis factor receptor (17). Moreover, several tumor-associated proteins, including Bcl-x, cyclin D1b and CD44 are targets of Sam68 (18,24,25). Previous studies have demonstrated the importance of Sam68 in carcinogenesis and have provided evidence that Sam68 promotes tumor development (26,27), whereas other studies have reported a tumor-suppressive function of Sam68 (28). The present study demonstrated that the expression of Sam68 was increased in podocytes treated with HG in vitro. Knockdown of Sam68 with siRNA in HG-treated podocytes attenuated apoptosis, as assessed by flow cytometry, thus indicating that Sam68 may exert a proapoptotic function in podocytes. Podocyte apoptosis has been reported to play a vital role in the pathophysiology of DN. Bax and Bcl-2 are two key markers of cell apoptosis that play critical roles in a variety of cell systems, including podocytes (29,30). The present study investigated whether Bax and Bcl-2 are also involved in Sam68-induced apoptosis in podocytes treated with HG. The present study demonstrated that the expression of the pro-apoptotic protein Bax increased, whereas that of the anti-apoptotic protein Bcl-2 decreased in in vitro HG-cultured podocytes, and this effect was attenuated by Sam68 knockdown, whereas mannitol had no such effect.
Several limitations of this study must be taken into account. Firstly, the concrete mechanism on Sam68 expression-triggered apoptosis is still lacking. Does NF-κB or other signaling pathways contribute to the changes, through transcriptional induction or inhibition of Bax or Bcl-2? Secondly, apoptosis was evaluated with only one method (flow cytometry based on Annexin V staining). Thirdly, only apoptosis was studied, cell viability was not investigated. These issues aforementioned should be further studied in future research.
In conclusion, the expression of Sam68 was investigated in in vitro cultured mouse podocytes with HG stimulation and it was determined whether this expression led to podocyte apoptosis. The mechanism of Sam68-mediated apoptosis in podocytes treated with HG was also further explored. The results revealed that the apoptosis-promoting effect of HG was abolished by Sam68 knockdown. HG also significantly increased Sam68 expression in a time and dose-dependent manner, and this effect was completely blocked by Sam68 knockdown. Moreover, it was revealed that HG increased Bax, and decreased Bcl-2, protein expression. These results indicated that preventing or inhibiting the Sam68/Bax/Bcl-2 pathway may be a promising therapeutic target for treating podocyte apoptosis under hyperglycemic conditions.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81470930).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
LZ and WS conceived the present study. YC performed the experiments and drafted the manuscript. SLiu, BY, HZ, SLia, JM and XL interpreted and analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, et al. US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, Hall YN, Hirsch IB, de Boer IH. Diabetes and CKD in the United States Population, 2009–2014. Clin J Am Soc Nephrol. 2017;12:1984–1990. doi: 10.2215/CJN.03700417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal MA, Patrakka J. Understanding podocyte biology to develop novel kidney therapeutics. Front Endocrinol (Lausanne) 2018;9:409. doi: 10.3389/fendo.2018.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogo AB. The targeted podocyte. J Clin Invest. 2011;121:2142–2145. doi: 10.1172/JCI57935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankland SJ. The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 7.Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Díaz AG. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018;2018:1875870. doi: 10.1155/2018/1875870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y, Chen S, Xu J, Zhu Q, Ye X, Ding D, Yao W, Lu Y. Dysregulation of lncRNAs GM5524 and GM15645 involved in high-glucose-induced podocyte apoptosis and autophagy in diabetic nephropathy. Mol Med Rep. 2018;18:3657–3664. doi: 10.3892/mmr.2018.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. doi: 10.2337/diabetes.55.01.06.db05-0894. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Lim S, Park M, Choi J, Kim J, Han H, Yoon K, Kim K, Lim J, Park S. Ubiquitination-dependent CARM1 degradation facilitates Notch1-mediated podocyte apoptosis in diabetic nephropathy. Cell Signal. 2014;26:1774–1782. doi: 10.1016/j.cellsig.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 12.Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta. 2003;1653:73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Najib S, Martin-Romero C, Gonzalez-Yanes C, Sánchez-Margalet V. Role of Sam68 as an adaptor protein in signal transduction. Cell Mol Life Sci. 2005;62:36–43. doi: 10.1007/s00018-004-4309-3. [DOI] [PubMed] [Google Scholar]
- 14.Frisone P, Pradella D, Di Matteo A, Belloni E, Ghigna C, Paronetto MP. SAM68: Signal transduction and RNA metabolism in human cancer. Biomed Res Int. 2015;2015:528954. doi: 10.1155/2015/528954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez-Jiménez F, Sánchez-Margalet V. Role of Sam68 in post-transcriptional gene regulation. Int J Mol Sci. 2013;14:23402–23419. doi: 10.3390/ijms141223402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun W, Qin R, Wang R, Ding D, Yu Z, Liu Y, Hong R, Cheng Z, Wang Y. Sam68 promotes invasion, migration, and proliferation of fibroblast-like synoviocytes by enhancing the NF-κB/P65 pathway in rheumatoid arthritis. Inflammation. 2018;41:1661–1670. doi: 10.1007/s10753-018-0809-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishnan P, Baltimore D. Sam68 is required for both NF-κB activation and apoptosis signaling by the TNF receptor. Mol Cell. 2011;43:167–179. doi: 10.1016/j.molcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paronetto MP, Achsel T, Massiello A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176:929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 2014;5:151. doi: 10.3389/fendo.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Jiang Y, Han J, Hu J, He T, Yan T, Huang N, Zhang Q, Mei H, Liao Y, Huang Y, Chen B, et al. Atgl deficiency induces podocyte apoptosis and leads to glomerular filtration barrier damage. FEBS J. 2017;284:1070–1081. doi: 10.1111/febs.14038. [DOI] [PubMed] [Google Scholar]
- 24.Paronetto MP, Cappellari M, Busa R, Pedrotti S, Vitali R, Comstock C, Hyslop T, Knudsen KE, Sette C. Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68. Cancer Res. 2010;70:229–239. doi: 10.1158/0008-5472.CAN-09-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Mol Cell Biol. 2006;26:362–370. doi: 10.1128/MCB.26.1.362-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielli P, Busà R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr Relat Cancer. 2011;18:R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 27.Song L, Wang L, Li Y, Xiong H, Wu J, Li J, Li M. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J Pathol. 2010;222:227–237. doi: 10.1002/path.2751. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SJ, Resnick RJ, Shalloway D. Sam68 exerts separable effects on cell cycle progression and apoptosis. BMC Cell Biol. 2004;5:5. doi: 10.1186/1471-2121-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin T, Zhang L, Liu S, Chen Y, Zhang H, Zhao X, Li R, Zhang Q, Liao R, Huang Z, et al. WWC1 promotes podocyte survival via stabilizing slit diaphragm protein dendrin. Mol Med Rep. 2017;16:8685–8690. doi: 10.3892/mmr.2017.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian X, Tan J, Liu L, Chen S, You N, Yong H, Pan M, You Q, Ding D, Lu Y. MicroRNA-134-5p promotes high glucose-induced podocyte apoptosis by targeting bcl-2. Am J Transl Res. 2018;10:989–997. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.