Abstract
Advanced glycation end products (AGEs) have been reported to serve an important role in the stiffening of cardiac tissues and myocardial cell injury. Serious myocardial cell injury can result in various heart diseases with high mortality. Halofuginone (HF), which possesses marked anti-inflammatory and antifibrotic effects, has recently been applied to inhibit the effects of cardiac stress. The present study aimed to investigate the potential effects of HF and its underlying mechanism in the treatment of AGEs-induced H9C2 cardiomyocyte damage. The western blot results of the present study demonstrated that HF may reduce the expression levels of myocardial injury markers, including myoglobin, creatine kinase MB and cardiac troponin I. In addition, flow cytometric analysis indicated that the production of reactive oxygen species (ROS) was significantly decreased by HF. Additionally, endoplasmic reticulum (ER) stress was suppressed in response to treatment with HF, as observed by low expression levels of ER stress-associated proapoptotic proteins (CCAAT/enhancer-binding protein homologous protein and cleaved caspase-12); overexpression of prosurvival proteins (growth arrest and DNA damage-inducible protein GADD34 and binding immunoglobulin protein) was also reported. Furthermore, the expression levels of microtubule-associated proteins 1A/1B light chain 3B (LC3)II/LC3I and Beclin 1 were elevated, whereas P62 expression levels were reduced following treatment with HF. These findings, together with immunofluorescence staining of LC3, indicated that HF may induce autophagy. Finally, the protective effects of HF on AGEs-treated H9C2 cells were reversed following treatment with the inhibitor 3-methyladenine, as indicated by inhibition of autophagy, and increases in apoptosis, ROS production and the ER stress response. Collectively, the findings of the present study suggested that the protective effects of HF against AGEs-induced myocardial cell injury may be associated with the induction of autophagy and amelioration of ROS-mediated ER stress and apoptosis. These findings may contribute to the development of a novel therapeutic method to inhibit the progression of myocardial cell injury.
Keywords: halofuginone, endoplasmic reticulum stress, myocardial injury, apoptosis, autophagy
Introduction
Advanced glycation end products (AGEs) are produced during the irreversible Maillard reaction between carbohydrates and proteins (1). The accumulation of AGEs may lead to myocardial fibrosis, thereby facilitating myocardial injury (2,3). The progression of myocardial injury can lead to numerous events associated with cardiovascular disease, including myocardial ischemia, heart failure and myocardial infarction (4). Therefore, early and appropriate treatments are required to inhibit the progression of myocardial injury; however, the treatment of myocardial injury in the early stage remains a major challenge.
Halofuginone (HF, 7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl) is a halogenated derivative of febrifugine, which is the major active ingredient of Dichroa febrifuga. Several studies have suggested that HF exerts beneficial effects on various diseases models, including rheumatoid arthritis (5), radiation-induced lung injury (6), and burn-induced hepatic and renal damage in rats (7). A previous study indicated that the amino acid response pathway may be activated by HF in cardiac tissue (8). Additionally, HF may also alleviate the structural and functional effects of cardiac stress (8). The present study aimed to demonstrate the potential effects of HF in the treatment of myocardial injury.
Evidence has demonstrated that an increase in autophagy may protect against myocardial dysfunction in mice (9,10). In addition, endoplasmic reticulum (ER) stress-associated cardiomyocyte apoptosis is a major contributor to myocardial injury, according to published research (11). The ER is a vital organelle responsible for numerous cellular processes; for example, the ER can maintain calcium homeostasis, protein folding and post-translational modifications, and can detect oxidative stress (12,13). Furthermore, the ER has been reported to be involved in the regulation of apoptosis and the inflammatory response via various pathways (14). Therefore, myocardial injury may be alleviated by the regulation of autophagy and ER stress-associated apoptosis. Coincidentally, previous studies have indicated that HF serves an important role in regulating cell apoptosis and autophagy (15). Therefore, whether HF may affect ER stress to reduce cardiomyocyte injury requires further investigation.
In the present study, the possible protective effects and underlying molecular mechanisms of HF on cardiomyocyte injury were investigated. AGEs-induced H9C2 cell lines were incubated with HF at various concentrations. The results of the present study suggested that HF may protect AGEs-induced H9C2 cells against damage via the suppression of ER stress-associated apoptosis and the induction of autophagy.
Materials and methods
Cell culture and treatment
The rat cardiomyocyte cell line H9C2 was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 10 U/ml penicillin-streptomycin at 37°C in an atmosphere containing 5% CO2. The medium was replaced every 3 days. When cell confluence reached 70–80%, cells were passaged. Bovine serum albumin (BSA)-AGEs were prepared as previously described (1,2,16,17). Briefly, 0.3% BSA (cat. no. 9048-46-8; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was incubated with 50 mM D-glucose in 5% CO2/95% air at 37°C for 12 weeks. AGE-BSA specific fluorescence determinations were performed by measuring emission at 440 nm on excitation at 370 nm using a fluorescence spectrophotometer (Hitachi, Japan). AGE-BSA was stored at −70°C until use. The H9C2 cellular damage model was established via pretreatment with AGEs (400 µg/ml) for 12 h at 37°C.
Cell grouping
H9C2 cells were randomly divided into five groups: Control group, H9C2 cells without any treatment; AGEs group, H9C2 cells treated with 400 µg/ml AGEs; three experimental groups, AGEs-treated cells plus different concentrations of HF (0.5, 2 and 8 nM) for 24 h. For autophagy assay, the H9C2 cells were randomly divided into 4 groups: AGEs group, AGEs-induced cells; AGEs + 3-MA group, AGEs-induced cells treated with 3-MA (5 mM) for 24 h; AGEs + HF group, AGEs-induced cells treated with HF (8 nM) for 24 h and AGEs + 3-MA + HF group, AGEs-induced cells treated with 3-MA (5 mM) and HF (8 nM) for 24 h.
Cell viability
The viability of H9C2 cells was evaluated via an MTT assay (Sigma-Aldrich; Merck KGaA). Cells were seeded into 96-well plates at a concentration of 6×103 cells/well and were cultured for 12 h at 37°C under 5% CO2. Subsequently, the culture medium was replaced with fresh medium containing HF (CAS no. 64924-67-0; Sigma-Aldrich; Merck KGaA) at different concentrations (0, 0.5, 1, 2, 4, 8, 10, 20, 40, 80, 100 and 200 nM), and cells were cultured for 48 h at 37°C. The MTT solution was added to the cells and formazan crystals were dissolved using dimethyl sulfoxide. The optical density values were detected using a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 550 nm.
Western blot analysis
H9C2 cells were lysed in lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) and the protein concentration was determined using a Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg protein was separated via 10% SDS-PAGE, after which, they were transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). After blocking with 5% BSA for 1 h at room temperature, the samples were probed with primary antibodies against myoglobin (Mb; 1:1,000; cat. no. 25919; Cell Signaling Technology, Inc., Danvers, MA, USA), creatine kinase MB (CK-MB; 1:1,000; cat. no. ab31832; Abcam), cardiac troponin I (cTnI; 1:1,000; cat. no. ab47003; Abcam), CCAAT/enhancer-binding protein homologous protein (CHOP; 1:1,000; cat. no. 2895; Cell Signaling Technology, Inc.), cleaved caspase-12 (1;1,000; cat. no. 2202; Cell Signaling Technology, Inc.), growth arrest and DNA damage-inducible protein GADD34 (GADD34; 1:2,000; cat. no. ab9869; Abcam), binding immunoglobulin protein (BiP; 1:1,000; cat. no. 3177; Cell Signaling Technology, Inc.), cleaved caspase-3 (1:1,000; cat. no. 9661; Cell Signaling Technology, Inc.), cleaved caspase-9 (1:1,000; cat. no. 9509; Cell Signaling Technology, Inc.), microtubule-associated proteins 1A/1B light chain 3B (LC3; 1:1,000; cat. no. 4108; Cell Signaling Technology, Inc.), Beclin 1 (1:1,000; cat. no. 3495; Cell Signaling Technology, Inc.), P62 (1:1,000; cat. no. 88588; CST) and GAPDH (1:200; cat. no. ab9485; Abcam) overnight at 4°C. The next day, membranes were incubated with a horseradish peroxidase goat anti-mouse immunoglobulin G secondary antibody (1:2,000; cat. no. ab205719; Abcam) for 1.5 h at room temperature. Protein bands were visualized using an enhanced chemiluminescent (ECL) kit (Bio-Rad Laboratories, Inc.). Densitometric analysis was performed using ImageJ software version 1.48 (National Institutes of Health, Bethesda, MD, USA).
Measurement of reactive oxygen species (ROS)
The production of ROS in the H9C2 cell line was detected by measuring 2′,7′-dichlorodihydrofluorescein (DCFH)-derived fluorescence via flow cytometry. Cells were cultured with 10 µM DCFH-diacetate (CAS no. 4091-99-0; Sigma-Aldrich; Merck KGaA) in serum-free DMEM at 37°C under 5% CO2 for 1 h. Subsequently, the cells were routinely collected and suspended in PBS. Cells were analyzed via flow cytometry by measuring DCF fluorescence at an excitation wavelength of 488 nm and an emission wavelength of 519 nm. FACSDiva software version 5.0.2 (BD Biosciences, San Jose, CA, USA) was used to analyze fluorescence intensities and determine ROS production.
Detection of cell apoptosis by flow cytometry
Cells (1.5×105−1×106) were suspended and immobilized in 75% cold ethanol (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 12 h at 4°C. Subsequently, cells were stained with 5 µl Annexin V-fluorescein isothiocyanate (FITC) and 5 µl propidium iodide (PI) for 15 min at room temperature in the dark. Cell apoptotic index was determined by Annexin V-FITC/PI ratio, as detected by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). FACSDiva software version 5.0.2 (BD Biosciences) was used for data acquisition and analysis.
Immunofluorescence staining of LC3
H9C2 cells from each group were washed with PBS three times and fixed with 4% paraformaldehyde for 15 min at 4°C. Cells were permeabilized with Tris-buffered saline (TBS) containing 0.25% Triton X-100 (TBSX) three times for 10 min each at 4°C, and then blocked with 10% horse serum (cat. no. H8890; Sigma-Aldrich; Merck KGaA) in TBSX for 1 h at 4°C. Subsequently, cells were incubated with a primary antibody against LC3 (1: 500; cat. no. NB100-2220; Novus Biologicals Canada ULC, Oakville, ON, Canada) overnight at 4°C, followed by incubation with a swine anti-rabbit FITC-conjugated secondary antibody (1:50; cat. no. F0205; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for 30 min at 4°C. Cells were then stained with 1 µg/ml DAPI at 37°C for 5 min. Cells were mounted with anti-fading medium. Three randomly chosen microscopic fields were analyzed under a confocal laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany) and quanitified using ImageJ software version 1.48 (National Institutes of Health).
Statistical analysis
Experiments were repeated at least three times and the data are presented as the means ± standard deviation. Statistical comparisons between different groups were conducted using one-way analysis of variance followed by Bonferroni post hoc test using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
HF alleviates AGEs-induced H9C2 cellular damage
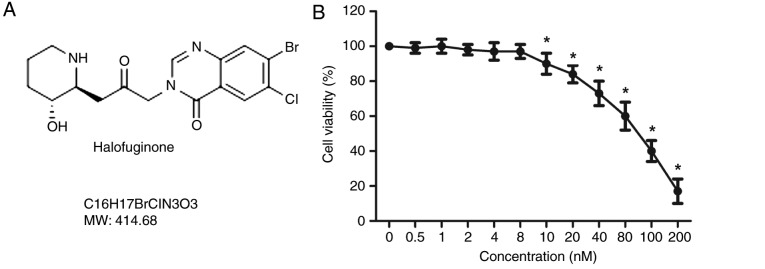
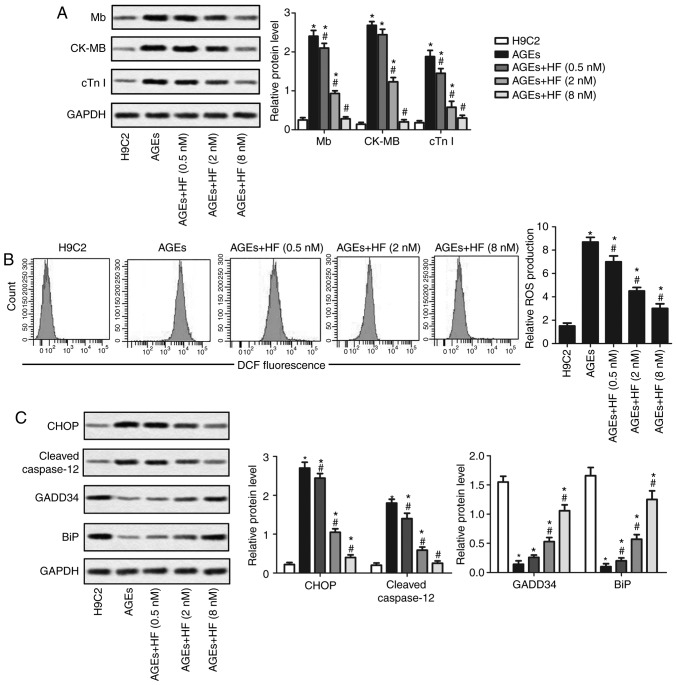
The structure of HF is presented in Fig. 1A. An MTT assay was used to detect the viability of H9C2 cells treated with HF at various concentrations (0, 0.5, 1, 2, 4, 8, 10, 20, 40, 80, 100 and 200 nM), for 48 h. As presented in Fig. 1B, the cell viability was significantly decreased in H9C2 cells treated with HF at concentrations >8 nM. To exclude cell toxicity, concentrations of 0.5, 2 and 8 nM were selected for subsequent analysis. The expression levels of the markers of myocardial injury (Mb, CK-MB and cTnI) were analyzed by western blotting. The results suggested that the expression levels of the three markers were significantly increased in the AGEs group compared with in the control group. Conversely, Mb, CK-MB and cTnI expression levels were significantly reduced in the experimental groups treated with HF compared with in the AGEs-only group (Fig. 2A). These results indicated that HF may mitigate AGEs-induced myocardial injury.
Figure 1.
Effects of HF on H9C2 cell viability. (A) Structure of HF. (B) H9C2 cells were treated with HF at various concentrations (0, 0.5, 1, 2, 4, 8, 10, 20, 40, 80, 100 and 200 nM) for 48 h; cell viability was measured using an MTT assay. HF, halofuginone; MW, molecular weight. *P<0.05 vs. the 0 nM group.
Figure 2.
HF alleviates AGEs-induced H9C2 cell injury and ER stress. H9C2 cells were randomly divided into five groups: Control group, normal H9C2 cells; AGEs group, AGEs-induced cells; and three experimental groups, AGEs-induced cells treated with HF at different concentrations (0.5, 2 and 8 nM) for 24 h. (A) Expression levels of myocardial injury markers (Mb, CK-MB and cTnI) were detected using western blotting. (B) Relative ROS production was measured by flow cytometry. (C) Expression levels of ER stress-associated proapoptotic proteins (CHOP and cleaved caspase-12) and prosurvival proteins (GADD34 and BiP) were investigated by western blotting. GAPDH was used as an endogenous reference. Experiments were repeated at least three times, and data are presented as the means ± standard deviation. *P<0.05 vs. the H9C2 group; #P<0.05 vs. the AGEs group. AGEs, advanced glycation end products; BiP, binding immunoglobulin protein; CK-MB, creatine kinase-muscle/brain; CHOP, CCAAT/enhancer-binding protein homologous protein; cTnI, cardiac troponin I; DCF, 2.7-dihydrochlorofluorescein; GADD34, growth arrest and DNA damage-inducible protein GADD34; HF, halofuginone; Mb, myoglobin; ROS, reactive oxygen species.
HF mitigates ER stress
To determine whether HF may affect ROS-mediated ER stress, the production of ROS was examined using flow cytometry in H9C2 cells. As presented in Fig. 2B, ROS production was significantly increased in the AGEs group compared with in the control group. In addition, the production of ROS was significantly decreased in the experimental groups compared with in the AGEs group. Western blotting was applied to investigate the expression of ER stress-associated proapoptotic (CHOP and cleaved caspase-12) and prosurvival proteins (GADD34 and BiP). The findings indicated that HF may inhibit the upregulation of proapoptotic proteins and increase the expression of prosurvival proteins in AGEs-treated cells (Fig. 2C). These results suggested that HF may alleviate ER-associated stress.
HF inhibits AGEs-induced ER-associated H9C2 apoptosis
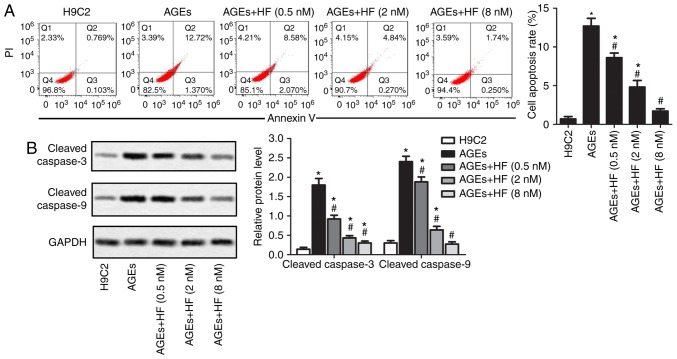
To detect the effects of HF on ER-associated H9C2 cell apoptosis induced by AGEs, the present study investigated cell apoptosis and the expression of apoptosis-associated proteins within H9C2 cells. As presented in Fig. 3A, AGEs-induced cell apoptosis may be suppressed by HF. Apoptotic markers (cleaved caspase-3 and cleaved caspase-9) were detected via western blotting. The results demonstrated that HF inhibited the overexpression of apoptotic markers induced by AGEs (Fig. 3B). These results indicated that HF may inhibit AGEs-induced ER-associated H9C2 apoptosis.
Figure 3.
HF inhibits AGEs-induced ER-associated H9C2 cell apoptosis. H9C2 cells were randomly divided into five groups: Control group, normal H9C2 cells; AGEs group, AGEs-induced cells; and three experimental groups, AGEs-induced cells treated with HF at different concentrations (0.5, 2 and 8 nM) for 24 h. (A) Flow cytometry was used to determine cell apoptosis rate. (B) Expression levels of apoptosis-associated proteins (cleaved caspase-3 and cleaved caspase-9) were analyzed by western blotting. Experiments were repeated at least three times, and data are presented as the means ± standard deviation. *P<0.05 vs. the H9C2 group; #P<0.05 vs. the AGEs group. AGEs, advanced glycation end products; HF, halofuginone; PI, propidium iodide.
HF promotes autophagy of H9C2 cells under AGEs-induced ER stress
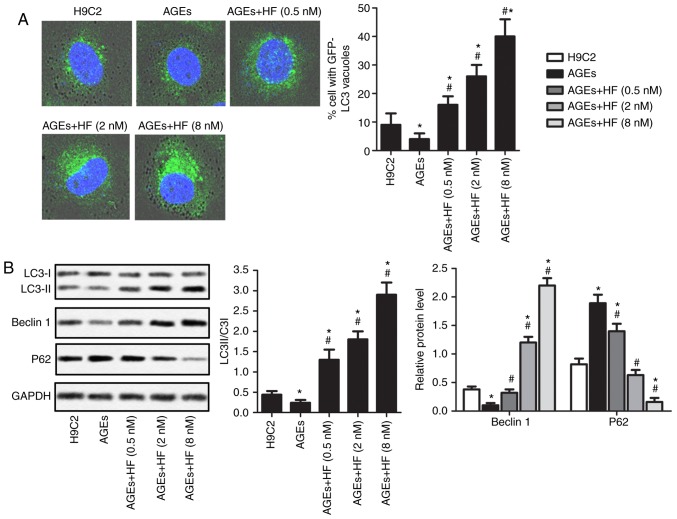
To determine the survival of H9C2 cells under AGEs-induced ER stress, the expression levels of autophagy-associated proteins were investigated in the present study. As presented in Fig. 4A, AGEs-induced cells exhibited reduced cytoplasmic staining of LC3 compared with in non-AGEs-induced cells. In addition, AGEs-induced H9C2 cells demonstrated increased LC3 staining in the cytoplasm in response to increasing concentrations of HF. To further verify these observations, the expression levels of the autophagy-associated proteins (LC3II/LC3I, Beclin 1 and P62) were analyzed using western blotting (Fig. 4B). The results revealed that AGEs significantly upregulated the expression levels of P62, but reduced the expression levels of LC3II/LC3I and Beclin 1 compared with in normal cells. Conversely, the expression levels of P62 were significantly decreased, whereas the expression levels of LC3II/LC3I and Beclin 1 were significantly increased in AGEs-induced cells treated with HF compared with in cells induced only with AGEs. These results suggested that HF may promote AGEs-induced ER-associated H9C2 cell autophagy.
Figure 4.
HF promotes the autophagy of H9C2 cells under AGEs-induced ER stress. H9C2 cells were randomly divided into five groups: Control group, normal H9C2 cells; AGEs group, AGEs-induced cells; and three experimental groups, AGEs-induced cells treated with HF at different concentrations (0.5, 2 and 8 nM) for 24 h. (A) Expression levels of LC3 were detected via immunofluorescence (magnification, ×400). (B) Expression levels of autophagy-associated proteins (LC3, Beclin 1 and P62) were measured by western blotting. Experiments were repeated at least three times, and data are presented as the means ± standard deviation. *P<0.05 vs. the H9C2 group; #P<0.05 vs. the AGEs group. AGEs, advanced glycation end products; GFP, green fluorescent protein; HF, halofuginone; LC3, microtubule-associated proteins 1A/1B light chain 3B.
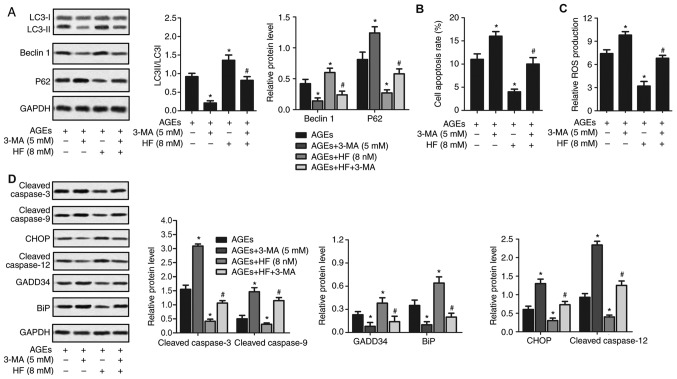
HF protects H9C2 cells from AGEs-induced damage via inducing autophagy
To investigate the protective effects of HF on H9C2 cells, the expression of autophagy-associated (LC3, Beclin 1 and P62), apoptosis-associated (cleaved caspase-3 and cleaved caspase-9) and ER stress-associated (CHOP, cleaved caspased-12, GADD34 and BiP) proteins were detected in AGEs-induced cells treated with 3-MA. As presented in Fig. 5, the LC3II/LC3I ratio, and the expression levels of Beclin 1, BiP and GADD34 were significantly reduced, whereas ROS production, and P62, caspase-3, caspase-9, CHOP and caspase-12 expression levels were upregulated in the AGEs + 3-MA group compared with in the AGEs-only group. Conversely, the LC3II/LC3I ratio, and the expression levels of Beclin 1, BiP and GADD34 were significantly increased, whereas ROS production, and P62, caspase-3, caspase-9, CHOP and caspase-12 expression levels were significantly suppressed in the AGEs + HF group compared with in the AGEs-only group. Furthermore, the LC3II/LC3I ratio, and the expression levels of Beclin 1, BiP and GADD34 were significantly decreased, whereas ROS production, and P62, caspase-3, caspase-9, CHOP and caspase-12 expression levels were increased in the AGEs + 3-MA + HF group compared with in the AGEs + HF group. These results indicated that 3-MA reversed the protective effects of HF in H9C2 cells.
Figure 5.
HF protects H9C2 cells from AGEs-induced damage by inducing autophagy. H9C2 cells were randomly divided into four groups: AGEs group, AGEs-induced cells; AGEs + 3-MA group, AGEs-induced cells treated with 3-MA (5 mM) for 24 h; AGEs + HF group, AGEs-induced cells treated with HF (8 nM) for 24 h and AGEs + 3-MA + HF group, AGEs-induced cells treated with 3-MA (5 mM) and HF (8 nM) for 24 h. (A) Western blot analysis of the autophagy-associated proteins (LC3, Beclin 1 and P62). GAPDH was used as an endogenous reference. Flow cytometry was used to determine (B) cell apoptosis rate and (C) relative ROS production. (D) Expression levels of apoptosis-associated proteins (cleaved caspase-3 and cleaved caspase-9), ER stress-associated proapoptotic proteins (CHOP and cleaved caspase-12) and prosurvival proteins (GADD34 and BiP) were detected via western blot analysis. GAPDH was used as an endogenous reference. Experiments were repeated at least three times, and data are presented as the means ± standard deviation. *P<0.05 vs. the AGEs group; #P<0.05 vs. the AGEs + HF group. 3-MA, 3-methyladenine; AGEs, advanced glycation end products; BiP, binding immunoglobulin protein; CHOP, CCAAT/enhancer-binding protein homologous protein; ER, endoplasmic reticulum; GADD34, growth arrest and DNA damage-inducible protein GADD34; HF, halofuginone; LC3, microtubule-associated proteins 1A/1B light chain 3B; ROS, reactive oxygen species.
Discussion
Uncontrolled myocardial cell injury has been reported to be the major cause of various cardiovascular diseases, including myocardial infarction, myocarditis and heart failure (18). Patients suffering from heart disease are administered various medical treatments; however, the rates of morbidity and mortality remain high and continue to rise (19). Therefore, an advanced therapeutic method is urgently required for the treatment of myocardial cell injury prior to the onset of severe effects.
Previous studies have demonstrated that numerous Chinese herbs exert a positive effect on myocardial cell injury (20–22). For example, polydatin defends cardiomyocytes against myocardial infarction injury via upregulation of sirtuin-3 expression (20). In addition, the apoptosis of cultured neonatal cardiomyocytes induced by daunorubicin is prevented following treatment with Astragalus membranaceus (21). Andrographolide has been reported to increase the levels of cellular-reduced glutathione and defend cardiomyocytes against hypoxia/reoxygenation damage (22). These reports indicate the potential of Chinese herbs in the treatment of myocardial cell injury.
HF, which has been used as an antiprotozoal for 20 years, has been identified to possess notable anti-inflammatory and antifibrotic effects (23). At present, HF has been applied in certain models of cardiovascular disease. According to Qin et al (8), HF not only activates the amino acid response pathway in the heart, but also attenuates the structural and functional effects of cardiac stress. Mb, CK-MB and cTnI are the most widely established and useful biomarkers of myocardial injury. In the present study, HF protected H9C2 cardiomyocytes against AGEs-induced damage. The expression of myocardial injury markers (Mb, CK-MB and cTnI) was significantly decreased in response to by HF; therefore, the present study aimed to understand the mechanisms underlying HF-regulated cardiomyocyte survival.
ER fulfills various cellular functions, including Ca2+ homeostasis, protein synthesis and maturation, and stress responses. The dysregulation of any of these functions may induce ER stress. Studies have suggested that ER stress is often induced by increases in ROS generation within the myocardium (24,25); the overexpression of ROS may further result in the dysregulation of the ER, complex unfolded protein response signaling pathway and cell apoptosis. Increasing evidence has indicated that ER stress-induced apoptosis may exert a vital effect on myocardial injury (26). Therefore, reducing ROS-mediated ER stress and apoptosis may be considered a therapeutic target for the treatment of myocardial injury. HF has been reported to suppress the generation of ROS, lipid peroxidation and myeloperoxidase activity to reduce oxidative ischemia/reperfusion injury (27). This was supported by the findings of the present study, in which ROS production was significantly suppressed by HF in AGEs-induced H9C2 cells.
Cell apoptosis may be induced by chronic or unresolved ER stress by activating the CHOP and caspase-12 signaling pathway (28). The results obtained from the present study demonstrated the anti-apoptotic effects of HF. According to Bodanovsky et al (29), the abundance of apoptotic nuclei decreases in response to HF in the diaphragm of mdx mice. Furthermore, it has been reported that the expression levels of the proapoptotic marker B-cell lymphoma 2 (Bcl-2)-associated X are reduced, whereas those of the anti-apoptotic marker Bcl-2 are increased in myofibers following treatment with HF (15). In the present study, ER stress was inhibited via the suppression of proapoptotic proteins (CHOP and cleaved caspase-12) and the upregulation of prosurvival proteins (GADD34 and BiP) in AGEs-treated H9C2 cells induced by HF. Furthermore, cell apoptosis was also reduced in response to HF; decreased expression levels of caspase-3 and caspase-9 were detected in the present study. These results suggested that HF may alleviate AGEs-induced myocardial injury via inhibiting ER-associated apoptosis.
In the heart, autophagy contributes to cell homeostasis via degrading excessive proteins and aged organelles, as observed in numerous heart disease models (30,31). Inhibiting autophagy can cause adverse effects in cardiomyocytes (32,33). According to Chen et al (15), HF serves a dual regulatory role in the initiation stage of autophagy via the liver kinase B1-5′AMP-activated protein kinase-unc-5 like autophagy activating kinase 1 (ULK1) or protein kinase B-mammalian target of rapamycin complex 1-ULK1 signaling pathways. The effect of this regulation always depends on the nutritional conditions of cells (15). In addition, Qin et al (8) observed that HF treatment alone, or in combination with endothelin-1, suppresses the expression of P62, but increases the number of LC3B-positive vesicles in cardiomyocytes, thus suggesting that HF may induce autophagy in cardiomyocytes. Additionally, the present study demonstrated that HF may protect H9C2 cells against AGEs-induced injury via the induction of autophagy, as observed by the increased LC3II/LC3I ratio and Beclin 1 expression, and decreased P62 expression in HF-treated cells.
3-MA is an autophagy antagonist, which is widely used for its anti-autophagic activity in various cell types. In the present study, 3-MA reversed the protective effects of HF in H9C2 cells; 3-MA suppressed autophagy, and increased apoptosis, production and the ER stress response. These findings indicated that autophagy may serve a vital role in myocardial cell injury.
In conclusion, the present study revealed that HF may protect H9C2 cells from AGEs-induced damage at concentrations of 0.5, 2 and 8 nM. In addition, ROS- mediated ER stress was suppressed by HF in AGEs-induced H9C2 cells. Therefore, ER-stress-associated cell apoptosis was inhibited, leading to the survival of H9C2 cardiomyocytes. Furthermore, HF induced autophagy, which is a beneficial process for cardiomyocyte homeostasis; conversely, 3-MA inhibited the effects of HF on AGEs-induced H9C2 cell damage. The present study revealed the protective effects of HF against AGEs-induced myocardial cell injury; however further investigation is required to understand the mechanism underlying the effects of HF on myocardial injury. The results of the present study may contribute towards the further analysis of HF in clinical trials.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- HF
halofuginone
- AGEs
advanced glycation end products
- ROS
reactive oxygen species
- ER stress
endoplasmic reticulum stress
- Mb
myoglobin
- CK-MB
creatine kinase-MB
- 3-MA
3-methyladenine
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YHL performed cell culture and treatments. WLZ was responsible for cell viability and ROS measurements. HYZ was involved in western blot and flow cytometry analysis. DWY conducted the immunofluorescence staining and autophagy assay. XNS collected the data and performed statistical analysis. QH was involved in designing the study and drafting the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Corman B, Duriez M, Poitevin P, Heudes D, Bruneval P, Tedgui A, Levy BI. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci USA. 1998;95:1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rani N, Bharti S, Bhatia J, Nag TC, Ray R, Arya DS. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem Biol Interact. 2016;250:59–67. doi: 10.1016/j.cbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng S, Wang K, Huang M, Qiu Q, Xiao Y, Shi M, Zou Y, Yang X, Xu H, Liang L. Halofuginone inhibits TNF-α-induced the migration and proliferation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Int Immunopharmacol. 2017;43:187–194. doi: 10.1016/j.intimp.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Calik M, Yavas G, Calik SG, Yavas C, Celik ZE, Sargon MF, Esme H. Amelioration of radiation-induced lung injury by halofuginone: An experimental study in Wistar-Albino rats. Hum Exp Toxicol. 2017;36:638–647. doi: 10.1177/0960327116660753. [DOI] [PubMed] [Google Scholar]
- 7.Cerit KK, Karakoyun B, Yüksel M, Ercan F, Tuğtepe H, Dagli TE, Yeğen BÇ. Halofuginone alleviates burn-induced hepatic and renal damage in rats. J Burn Care Res. 2017;38:e384–e394. doi: 10.1097/BCR.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 8.Qin P, Arabacilar P, Bernard RE, Bao W, Olzinski AR, Guo Y, Lal H, Eisennagel SH, Platchek MC, Xie W, et al. Activation of the amino acid response pathway blunts the effects of cardiac stress. J Am Heart Assoc. 2017;6(pii):e004453. doi: 10.1161/JAHA.116.004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao P, Kuai J, Gao J, Sun L, Wang Y, Yao L. Delta opioid receptor agonist attenuates lipopolysaccharide-induced myocardial injury by regulating autophagy. Biochem Biophys Res Commun. 2017;492:140–146. doi: 10.1016/j.bbrc.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Giricz Z, Mentzer RM, Jr, Gottlieb RA. Autophagy, myocardial protection, and the metabolic syndrome. J Cardiovasc Pharmacol. 2012;60:125–132. doi: 10.1097/FJC.0b013e318256ce10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashem SI, Perry CN, Bauer M, Han S, Clegg SD, Ouyang K, Deacon DC, Spinharney M, Panopoulos AD, Izpisua Belmonte JC, et al. Brief report: Oxidative stress mediates cardiomyocyte apoptosis in a human model of danon disease and heart failure. Stem Cells. 2015;33:2343–2350. doi: 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Lu Q, Hu Z, Yu Y, Chen Q, Wang QK. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat Commun. 2017;8:133. doi: 10.1038/s41467-017-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: One touch, multiple functions. Biochim Biophys Acta. 2014;1837:461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Chen GQ, Gong RH, Yang DJ, Zhang G, Lu AP, Yan SC, Lin SH, Bian ZX. Halofuginone dually regulates autophagic flux through nutrient-sensing pathways in colorectal cancer. Cell Death Dis. 2017;8:e2789. doi: 10.1038/cddis.2017.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge J, Jia Q, Liang C, Luo Y, Huang D, Sun A, Wang K, Zou Y, Chen H. Advanced glycosylation end products might promote atherosclerosis through inducing the immune maturation of dendritic cells. Arterioscler Thromb Vasc Biol. 2005;25:2157–2163. doi: 10.1161/01.ATV.0000181744.58265.63. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Feng L, Wang S, Zhu Q, Lin J, Lou C, Xiang P, He B, Zheng Z, Tang D, Zuo G. Phytoestrogen calycosin-7-O-β-D-glucopyranoside ameliorates advanced glycation end products-induced HUVEC damage. J Cell Biochem. 2011;112:2953–2965. doi: 10.1002/jcb.23212. [DOI] [PubMed] [Google Scholar]
- 18.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: Cohort study in primary care. BMJ. 2016;354:i3477. doi: 10.1136/bmj.i3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Zhao Z, Shen M, Zhang Y, Duan J, Guo Y, Zhang D, Hu J, Lin J, Man W, et al. Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3. Biochim Biophys Acta. 2017;1863:1962–1972. doi: 10.1016/j.bbadis.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Luo Z, Zhong L, Han X, Wang H, Zhong J, Xuan Z. Astragalus membranaceus prevents daunorubicin-induced apoptosis of cultured neonatal cardiomyocytes: Role of free radical effect of Astragalus membranaceus on daunorubicin cardiotoxicity. Phytother Res. 2009;23:761–767. doi: 10.1002/ptr.2575. [DOI] [PubMed] [Google Scholar]
- 22.Woo AY, Waye MM, Tsui SK, Yeung ST, Cheng CH. Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J Pharmacol Exp Ther. 2008;325:226–235. doi: 10.1124/jpet.107.133918. [DOI] [PubMed] [Google Scholar]
- 23.Yavas G, Calik M, Calik G, Yavas C, Ata O, Esme H. The effect of Halofuginone in the amelioration of radiation induced-lung fibrosis. Med Hypotheses. 2013;80:357–359. doi: 10.1016/j.mehy.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZW, Zhu HT, Chen KL, Dong X, Wei J, Qiu C, Xue JH. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol. 2013;12:158. doi: 10.1186/1475-2840-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan X, Xun M, Dou X, Wu L, Han Y, Zheng J. Regulation of Na+-K+-ATPase effected high glucose-induced myocardial cell injury through c-Src dependent NADPH oxidase/ROS pathway. Exp Cell Res. 2017;357:243–251. doi: 10.1016/j.yexcr.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 27.Karadeniz Cerit K, Karakoyun B, Yüksel M, Özkan N, Cetinel Ş, Tolga Dağli E, Yeğen BÇ, Tuğtepe H. The antifibrotic drug halofuginone reduces ischemia/reperfusion-induced oxidative renal damage in rats. J Pediatr Urol. 2013;9:174–183. doi: 10.1016/j.jpurol.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Zhang M, Yin H. Signaling pathways involved in endoplasmic reticulum stress-induced neuronal apoptosis. Int J Neurosci. 2013;123:155–162. doi: 10.3109/00207454.2012.746974. [DOI] [PubMed] [Google Scholar]
- 29.Bodanovsky A, Guttman N, Barzilai-Tutsch H, Genin O, Levy O, Pines M, Halevy O. Halofuginone improves muscle-cell survival in muscular dystrophies. Biochim Biophys Acta. 2014;1843:1339–1347. doi: 10.1016/j.bbamcr.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Nishida K, Yamaguchi O, Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ Res. 2008;103:343–351. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- 31.Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–160. doi: 10.1161/CIRCRESAHA.110.237339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maron BJ, Roberts WC, Arad M, Haas TS, Spirito P, Wright GB, Almquist AK, Baffa JM, Saul JP, Ho CY, et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301:1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: Joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.