Abstract
The present study aimed to investigate the mechanism by which cyclooxygenase-2 (COX-2) promotes the metastasis of MG-63 osteosarcoma cells through the PI3K/AKT/NF-κB pathway. To achieve this, a recombinant lentivirus containing the COX-2 gene was constructed in order to overexpress COX-2; a recombinant lentivirus containing a control sequence was also constructed. A Transwell chamber migration assay was performed to quantify the migration of the COX-2-transduced cells, and of cells treated with a COX-2 inhibitor (NS398) or a PI3K inhibitor (LY294002). Immunofluorescence assays were performed to determine changes in E-cadherin, vimentin and NF-κB expression levels. ELISAs were performed to quantify the levels of matrix metallopeptidase (MMP)-2, MMP-9 and vascular endothelial growth factor (VEGF) in the culture medium. Western blot analysis was conducted to measure the protein expression levels of MMP-2, MMP-9, PI3K, phosphorylated (p-) PI3K, AKT, p-AKT, inhibitor of NF-κΒ kinase (IKK) and p-IKK. The results demonstrated that the migration ability of the COX-2-overexpressing MG-63 cells was significantly increased compared with the control cells. The migration ability of cells treated with NS398 or LY294002 was significantly decreased. Compared with the control cells, E-cadherin expression was significantly decreased in COX-2-overexpressing cells, while the expression levels of vimentin, MMP-2, MMP-9, VEGF, p-PI3K, p-AKT and p-IKK were significantly increased. Compared with the control cells, E-cadherin expression was significantly increased in cells treated with NS398 or LY294002, while the expression levels of vimentin, MMP-2, MMP-9, VEGF, p-PI3K, p-AKT, and p-IKK were significantly decreased. The total protein levels of PI3K, AKT and IKK were not changed among the treatment groups. In summary, COX-2 overexpression decreased the expression levels of the epithelial protein E-cadherin and increased the expression levels of the mesenchymal proteins vimentin, MMP-2 and MMP-9, as well as promoted cell migration, by activating the PI3K/AKT/NF-κB signaling pathway.
Keywords: cyclooxygenase-2, epithelial-mesenchymal transition, osteosarcoma, PI3K/AKT/NF-κB signaling
Introduction
Osteosarcoma is a common and highly malignant osteoblastic tumor that originates from mesenchymal cells. Osteosarcoma has high metastasis and recurrence rates (1), more than 85% of patients eventually succumbing to the disease due to lung metastases (2), before the advent of multimodality treatment methods. Therefore, metastasis is considered to be the primary cause of mortality in patients with osteosarcoma (2,3). The prevention of metastases is expected to effectively reduce the rate of mortality from osteosarcoma. The mechanisms of tumor metastasis are highly complex, as the pathogenesis of tumors varies due to differences in the genetic background and the microenvironment of the tumor. It has been reported that epithelial-mesenchymal transition (EMT) has a pivotal role in the metastatic process in many types of tumor cells (4) and promotes the metastasis of epithelial neoplasms (5). However, as osteosarcoma arises from cells of a mesenchymal origin, it is unclear whether EMT is necessary for the metastasis of osteosarcoma. In a previous study on EMT-related transcription factors, it was found that the knockout of twist family bHLH transcription factor 2 (Twist2) promoted EMT and metastasis of osteosarcoma cells; therefore, Twist2 functions as a tumor suppressor gene (6). Knockout and overexpression experiments revealed that snail family transcription repressor 2 (SNAI2) regulated the invasion and metastasis of osteosarcoma cells, and that knockout of SNAI2 resulted in significantly decreased motility, remodeling of the actin cytoskeleton and loss of cellular protrusions, which contributed to the negative regulation of tumor invasion and metastasis (7).
Cyclooxygenase-2 (COX-2), an inducible cyclooxygenase and a rate-limiting enzyme in prostaglandin synthesis, is associated with inflammatory diseases, and can promote angiogenesis and tissue invasion in cancer (8,9). In osteosarcomas, the rate of tumors with a positive expression of COX-2 is 67–92%, and the expression of COX-2 in osteosarcoma stem cells is 141-fold greater than its expression in osteosarcoma cells (10–12). Previous studies have also found that the overexpression of COX-2 in osteosarcomas increased the expression levels of matrix metallopeptidase (MMP)-2 and MMP-9 (13), and promoted cell motility and invasiveness (14). However, further work is required to determine whether COX-2 can promote EMT and migration in osteosarcoma cells.
Previous studies have reported that the PI3K/AKT/NF-κB signaling pathway is abnormally activated in many metastatic tumors (15,16). while other studies have found that mutations in AKT may lead to a higher metastatic risk in patients with osteosarcoma (17,18). However, to the best of our knowledge, the relationship between COX-2 and the PI3K/AKT/NF-κB pathway has not been characterized in the context of osteosarcoma. Therefore, in the present study, the effects of COX-2 on EMT and migration in MG-63 osteosarcoma cells were investigated with respect to PI3K/AKT/NF-κB signaling.
Materials and methods
Cells and reagents
The 293T cell line and the osteosarcoma MG-63 cell line were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences.
The lentiviral vector plvx-DsRed (containing DsRed, a red fluorescent protein) and recombinant lentivirus plvx-COX2-DsRed (containing DsRed and COX-2) were constructed by Sangon Biotech Co., Ltd. High glucose DMEM was purchased from Gibco (Thermo Fisher Scientific, Inc). FBS was purchased from Beijing Sijiqing Biotechnology Co., Ltd. (https://www.11467.com/beijing/co/739711.htm). Trypsin, RIPA lysis buffer, super-sensitive enhanced chemiluminescence (ECL) reagent, SDS-PAGE gel preparation kits, SDS-PAGE sample loading buffer, SDS-PAGE electrophoresis buffer, Matrigel matrix and western blot transfer buffer were purchased from Beyotime Institute of Biotechnology. Human MMP-2 (ml058669), MMP-9 (ml058617) and vascular endothelial growth factor (VEGF; ml064281) ELISA kits were purchased from Shanghai Meilian Biotechnology Co., Ltd. E-cadherin (Abcam, ab194982), vimentin (Abcam, ab193555), MMP-2 (Abcam, ab92536), MMP-9 (Abcam, ab38898), PI3K (Abcam, ab191606), phosphorylated (p)-PI3K (Bioss, bs4605), AKT (Abcam, ab179463), p-AKT (Abcam, ab81283), IKK (Abcam, ab178870), p-IKK (Abcam, ab38515) and NF-κB (Abcam, ab207297) rabbit monoclonal antibodies were purchased from Abcam and Bioss. DyLight 488-conjugated goat anti-rabbit immunoglobulin G (IgG; GTX213110-04) was purchased from GeneTex Inc., and horseradish peroxidase-conjugated goat anti-rabbit IgG (7074P2) was purchased from Cell Signaling Technology, Inc. The COX-2 inhibitor NS398 and the PI3K inhibitor LY294002 were purchased from MedChemExpress LLC.
Cell culture
MG-63 and 293T cells were cultured in high-glucose DMEM containing 10% FBS at 37°C in a 5% CO2 incubator. Subculture was performed when the cells reached a confluence of 80–90%.
Experimental grouping and lentiviral infection
The plvx-DsRed (1 µl) or plvx-COX2-DsRed (1 µl) plasmid stock solution (1.67 µg/ml) was transfected (Polybrene, Thermo Fisher Scientific, Inc.) to 293T cells in log phase for virus propagation, in order to obtain recombinant plvx-DsRed and plvx-COX2-DsRed lentiviruses with a titer of 5×1010 particle forming units/µl. The virus was collected 72 h after transfection.
MG-63 cells were subcultured at a confluence of 50–60% and the corresponding lentiviruses with an optimal multiplicity of infection (MOI; 25) were individually added; the expression of DsRed in the cells was observed and recorded after 24 h. The experimental groups were as follows: i) plvx-DsRed group, MG-63 cells infected with the empty lentiviral vector plvx-DsRed; ii) plvx-COX2-DsRed group, MG-63 cells infected with the recombinant lentivirus plvx-COX2-DsRed; iii) NS398 group, MG-63 cells infected with plvx-COX2-DsRed and then treated with the COX-2 inhibitor NS398 (3.8 µM) for 24 h; and iv) LY294002 group, MG-63 cells infected with plvx-COX2-DsRed and then treated with the PI3K inhibitor LY294002 (5 µM) for 24 h.
Transwell chamber migration and invasion assays
For the migration assay, cells in the plvx-DsRed, plvx-COX2-DsRed, NS398 and LY294002 groups were collected and digested with pancreatin. Single-cell suspensions of 1.5×105 cells/ml were prepared using serum-free medium. For each group, 400 µl/well of the suspension was loaded into the upper chamber of the Transwell insert and 600 µl of culture medium containing 20% FBS was added to the lower chamber; six parallel controls were established for each group. After culturing at 37°C in a 5% CO2 incubator for 24 h, the Transwell inserts were removed from the culture plate and fixed in anhydrous methanol at −20°C for 5 min. Cells in the upper chamber, which had not migrated, were gently removed using a cotton swab. The migrated cells were stained with 0.25% crystal violet for 5 min and subsequently washed with PBS to remove the excess crystal violet. The stained cells were observed and images captured using a microscope (magnification, ×200, routine light microscopy) and cell counting was performed in 6 randomly selected fields to determine the number of migrated cells.
The invasion assay was performed in the same manner as the migration assay, except that Matrigel-coated Transwell chambers were used.
Determination of changes in E-cadherin, vimentin and NF-κB protein expression levels using immunofluorescence assays
Cells from each group were collected and digested with pancreatin. Single-cell suspensions of 1×104 cells/ml were prepared using high-glucose DMEM containing 10% FBS. For each group, a 6-well plate was inoculated with 1 ml/well of the suspension and cultured at 37°C in a 5% CO2 incubator for 24 h. Subsequently, the cells were washed with PBS, fixed with 4% paraformaldehyde at room temperature for 15 min, washed again with PBS, permeabilized in 0.25% Triton for 15 min and blocked with blocking buffer (5% BSA in 0.25% Triton) for 30 min at 37°C. After the removal of excess blocking buffer, primary antibodies in blocking buffer were added at the following dilutions: E-cadherin (1:500), vimentin (1:500) and NF-κB (1:200). The cells were incubated at 4°C overnight, washed with PBS and the DyLight 488-conjugated secondary antibody (1:2,000) was added. After incubation at room temperature for 1 h, the cells were washed with PBS. Cells were imaged using a fluorescent microscope. The optical density (OD) values of the cells were analyzed as the integrated optical density (IOD)/total area, and IOD values were calculated using Image-Pro Plus (version 6.0, Media Cybernetics, Inc.). The concentration of DAPI used 15 µg/ml, for 5 min at 37°C.
Determination of changes in the levels of MMP-2, MMP-9 and VEGF in the supernatant of MG-63 cells using ELISA
The culture supernatant was obtained from the cells of each group following growth for 48 h in the media and centrifugation (1,200 × g for 5 min at 37°C). Changes in the levels of MMP-2, MMP-9 and VEGF were measured using ELISA kits, according to the manufacturer's instructions.
Determination of changes in the levels of PI3K, p-PI3K, AKT, p-AKT, IKK and p-IKK protein expression using western blotting
Cells from each group were collected, lysed on ice with RIPA buffer, collected in a centrifuge tube and further lysed for 30 min. After centrifugation at 13,000 × g for 10 min at 4°C, the supernatant was collected and protein concentration measured with a BCA assay. After the addition of 4X sample loading buffer, the cells were boiled for 5 min to allow sufficient denaturation and stored at −20°C before use. SDS-PAGE was performed using a 10% separating gel and 5% stacking gel. The loading volumes were determined based on protein concentration (20 µg per lane). After electrophoresis, the samples were transferred onto PVDF membranes and blocked with 5% skimmed milk/TBST (0.1% Tween-20) at 37°C for 90 min. Subsequently, the cells were incubated overnight with the following primary antibodies: PI3K (1:1,000), p-PI3K (1:2,000), AKT (1:750), p-AKT (1:1,000), IKK (1:1,000) or p-IKK (1:1,500). Membranes were washed with PBS, incubated for 1.5 h at room temperature with the secondary antibody (1:6,000), washed with PBS again, and then visualized using ECL reagent. The OD values of the samples were analyzed using Image-Pro Plus.
Statistical analysis
The experimental data (n=3) were analyzed using SPSS 21.0 (IBM Corp.). Quantitative values were expressed as the mean ± standard error of the mean. Comparisons between multiple groups were performed using one-way ANOVA, and pairwise comparisons were performed using the least significance difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Generation of recombinant lentivirus-infected MG-63 cells overexpressing COX-2
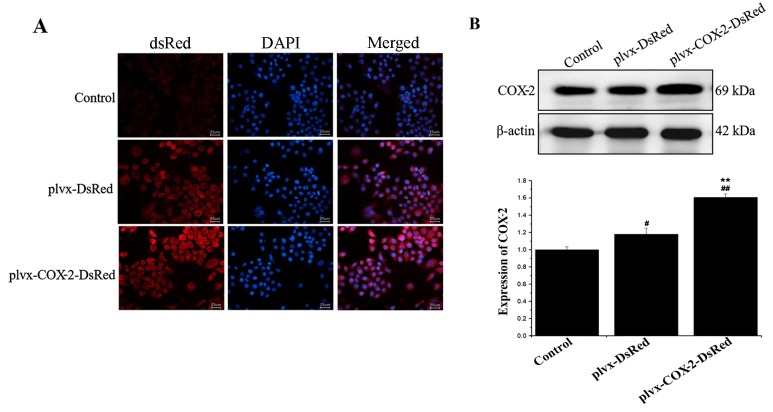
As shown in Fig. 1A, strong red fluorescence was observed in the plvx-DsRed and plvx-COX2-DsRed infected groups. Western blotting results further demonstrated a significant increase in COX-2 protein expression levels in cells of the plvx-COX2-DsRed group compared with that in the plvx-DsRed group (Fig. 1B). These results indicated that the lentiviral infections and COX-2 overexpression were successful.
Figure 1.
Detection of COX-2 protein expression following lentiviral transduction. (A) Based on the intensity of red fluorescence (encoded by the dsRed protein), the lentiviral infection of each group was observed by fluorescence microscopy. (B) The protein expression levels of COX-2 in each group were determined using western blotting. #P<0.05, ##P<0.01 vs. control; **P<0.01 vs. plvx-DsRed. COX-2, cyclooxygenase-2; plvx-DsRed, empty vector; plvx-COX2-DsRed, vector overexpressing COX-2.
COX-2 overexpression promotes the migration and invasion of MG-63 cells
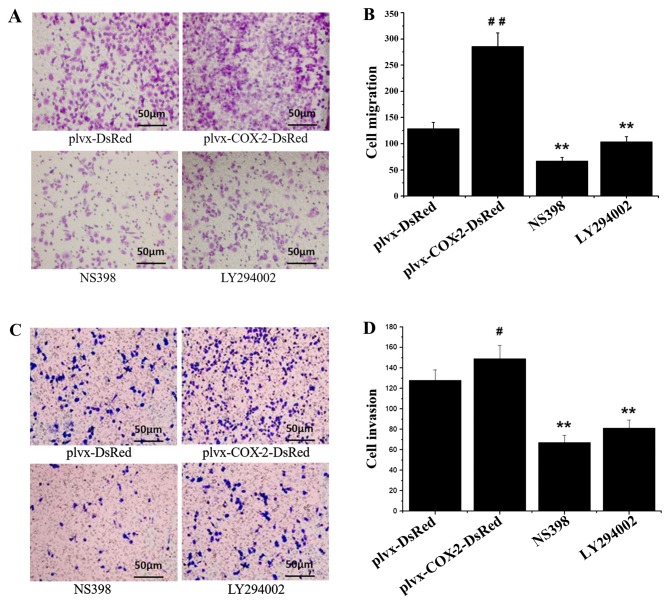
Results of the Transwell chamber assay indicated that the number of migrated and invaded MG-63 cells was significantly higher in the plvx-COX2-DsRed group compared with the plvx-DsRed group (Fig. 2). The number of migrated and invaded cells were significantly decreased in the NS398- and LY294002-treated groups compared with the cells infected with plvx-COX2-DsRed alone (Fig. 2). The number of migrated and invaded cells in the NS398 and LY294002 groups were not significantly different from each other (Fig. 2). These results indicated that COX-2 overexpression promoted the migration and invasion of MG-63 cells.
Figure 2.
COX-2 overexpression increases migration and invasion in MG-63 cells. MG-63 cells were infected with control or COX-2-overexpressing lentivirus, and treated with or without the COX-2 inhibitor NS398 or the PI3K inhibitor LY294002. (A) Transwell chambers were used to detect changes in the migration ability of each group. (B) Quantification of cell migration. (C) Matrigel-coated Transwell chambers were used to detect changes in the invasion ability of each group. (D) Quantification of cell invasion. Magnification, ×200. #P<0.05, ##P<0.01 vs. plvx-DsRed; **P<0.01 vs. plvx-COX2-DsRed. COX-2, cyclooxygenase-2; plvx-DsRed, empty vector; plvx-COX2-DsRed, vector overexpressing COX-2.
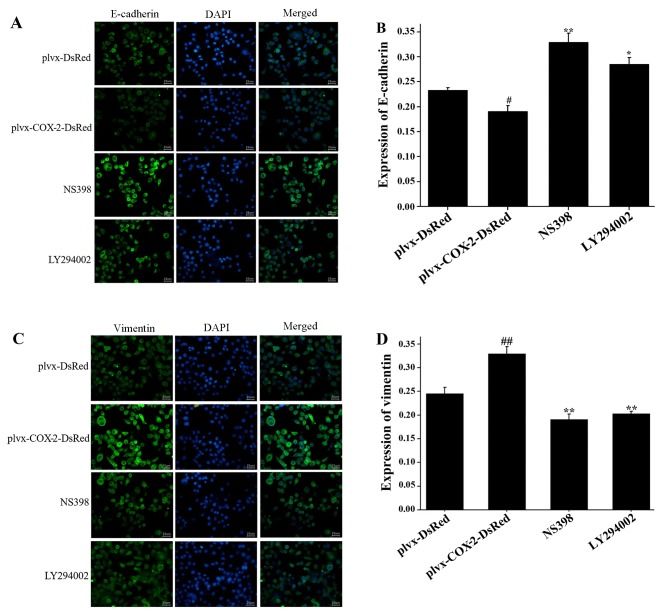
COX-2 overexpression inhibits the expression of E-cadherin and promotes the expression of vimentin in MG-63 cells
As shown in Fig. 3A and B, the results of the immunofluorescence assay indicated that E-cadherin expression was significantly lower in MG-63 cells infected with plvx-COX2-DsRed compared with cells infected with plvx-DsRed control. Expression of E-cadherin was significantly higher in cells treated with NS398 or LY294002 compared with cells infected with plvx-COX2-DsRed alone (Fig. 3A and B). The expression of vimentin was significantly higher in MG-63 cells infected with plvx-COX2-DsRed than in cells infected with plvx-DsRed (Fig. 3C and D). Vimentin expression was significantly lower in cells treated with NS398 or LY294002 than in cells infected with plvx-COX2-DsRed alone (Fig. 3C and D).
Figure 3.
Expression of E-cadherin and vimentin is regulated by COX-2. MG-63 cells were infected with control or COX-2-overexpressing lentivirus, and treated with or without the COX-2 inhibitor NS398 or the PI3K inhibitor LY294002. (A) The expression of E-cadherin (green) in each group was determined using immunofluorescence. (B) Quantification of E-cadherin expression. (C) The expression of vimentin in each group. (D) Quantification of vimentin expression. Magnification, ×400. #P<0.05, ##P<0.01 vs. plvx-DsRed; *P<0.05, **P<0.01 vs. plvx-COX2-DsRed. COX-2, cyclooxygenase-2; plvx-DsRed, empty vector; plvx-COX2-DsRed, vector overexpressing COX-2.
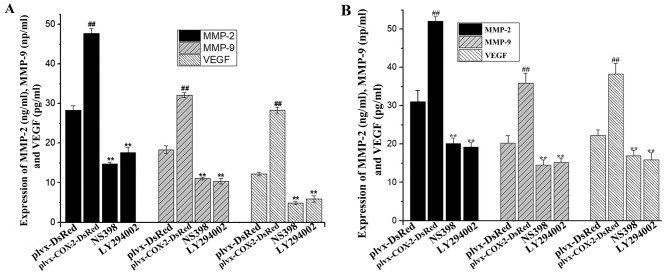
COX-2 overexpression increases the secreted levels of MMP-2, MMP-9 and VEGF in MG-63 cells
ELISA results indicated that the levels of MMP-2, MMP-9 and VEGF were significantly higher in the culture supernatant of cells infected with plvx-COX2-DsRed than of cells infected with plvx-DsRed (Fig. 4A). The expression levels of MMP-2, MMP-9 and VEGF in MG-63 cells were also significantly higher in cells infected with plvx-COX2-DsRed than in cells infected with plvx-DsRed (Fig. 4B). By contrast, the levels of MMP-2, MMP-9 and VEGF in the culture supernatant were significantly lower in MG-63 cells treated with NS398 or LY294002 compared with cells infected with plvx-COX2-DsRed alone (Fig. 4A and B).
Figure 4.
Expression of MMP-2, MMP-9 and VEGF is regulated by COX-2. MG-63 cells were infected with control or COX-2-overexpressing lentivirus, and treated with or without the COX-2 inhibitor NS398 or the PI3K inhibitor LY294002. The change in MMP-2, MMP-9 and VEGF levels (A) in the supernatant and (B) inside the cell was determined using ELISAs. ##P<0.01 vs. plvx-DsRed; **P<0.01 vs. plvx-COX2-DsRed. COX-2, cyclooxygenase-2; MMP, matrix metallopeptidase; VEGF, vascular endothelial growth factor; plvx-DsRed, empty vector; plvx-COX2-DsRed, vector overexpressing COX-2.
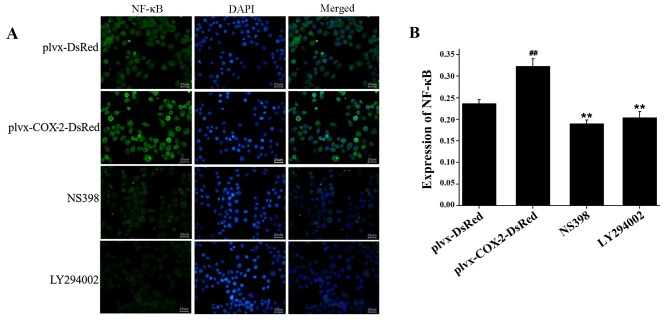
COX-2 overexpression promotes the expression of NF-κB
Immunofluorescence assay results indicated that the total protein expression levels of NF-κB were significantly higher in MG-63 cells infected with plvx-COX2-DsRed than in cells infected with plvx-DsRed (Fig. 5). The total protein expression levels of NF-κB were significantly lower in cells treated with NS398 or LY294002 than in cells infected with plvx-COX2-DsRed alone (Fig. 5).
Figure 5.
Expression of NF-κB p65 protein is regulated by COX-2. MG-63 cells were infected with control or COX-2-overexpressing lentivirus, and treated with or without the COX-2 inhibitor NS398 or the PI3K inhibitor LY294002. (A) The expression of NF-κB p65 (green) in each group was determined by immunofluorescence. (B) Quantification of NF-κB p65 expression. ##P<0.01 vs. plvx-DsRed; **P<0.01 vs. plvx-COX2-DsRed. COX-2, cyclooxygenase-2; plvx-DsRed, empty vector; plvx-COX2-DsRed, vector overexpressing COX-2.
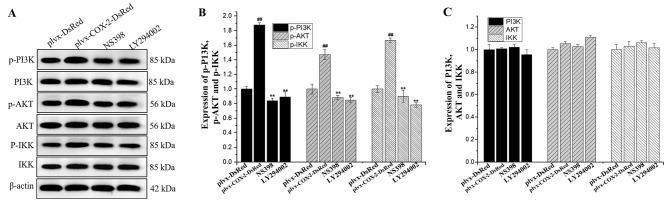
COX-2 overexpression promotes the phosphorylation of PI3K, AKT and IKK proteins
Western blotting results indicated that the expression levels of p-PI3K, p-AKT and p-IKK were significantly higher in MG-63 cells infected with plvx-COX2-DsRed than in cells infected with plvx-DsRed (Fig. 6). The expression levels of p-PI3K, p-AKT and p-IKK were significantly lower in cells treated with NS398 or LY294002 than in cells infected with plvx-COX2-DsRed alone (Fig. 6). However, no significant differences were observed in the total protein levels of PI3K, AKT, and IKK among the groups.
Figure 6.
Phosphorylation levels of PI3K, AKT and IKK are regulated by COX-2. (A) The expression levels of PI3K, p-PI3K, AKT, p-AKT, IKK and p-IKK proteins in each group were determined using western blotting. (B) Quantification of the expression levels of p-PI3K, p-AKT and p-IKK. (C) Quantification of the expression levels of total PI3K, AKT and IKK proteins. ##P<0.01 vs. plvx-DsRed; **P<0.01 vs. plvx-COX2-DsRed. COX-2, cyclooxygenase-2; IKK, inhibitor of NF-κΒ; p-, phosphorylated; plvx-DsRed, empty vector; plvx-COX2-DsRed, vector overexpressing COX-2.
Discussion
The results of the present study demonstrated that COX-2 overexpression decreased the expression of E-cadherin and increased the levels of vimentin, MMP-2, MMP-9 and VEGF through the activation of the PI3K/AKT/NF-κB signaling pathway, enhancing the invasive ability of MG-63 osteosarcoma cells.
Metastatic invasion and migration are important hallmarks of malignant tumors, and the acquisition of these characteristics leads to a significant increase in the mortality rate of cancer (19,20). Currently, the mechanisms by which tumor metastasis occurs remain poorly understood; however, many studies have indicated that tumor metastasis is not an isolated event, but the influence of a combination of internal and external factors leads to changes in the signaling networks within tumor cells following an accumulation-mutation model, When the accumulation of such changes reaches a critical threshold, tumor cells acquire metastatic characteristics resulting from the changes in these gene-signaling networks (21,22).
Osteosarcoma is a highly malignant tumor that is extremely prone to metastasis during the early stages and has a high incidence of hematogenous metastasis, which often occurs during the early stages, and has high recurrence and metastasis rates (3). The lungs are the most common site of osteosarcoma metastasis, and lung metastases are associated with poor clinical efficacy and prognosis (3). As there are relatively few studies investigating the mechanism of osteosarcoma metastasis, the present study was conducted to explore the mechanism by which the metastasis of osteosarcoma occurs using the human osteosarcoma cell line MG-63. In osteosarcoma tissue and SaOS-2 cells, the positive expression rate of COX-2 can be as high as 67–92% (10,11). Furthermore, in osteosarcoma, the expression of COX-2 in stem cells spheres is 141-fold greater than in daughter adherent cells, thus endowing these tumors with the characteristics of drug resistance, metastasis and recurrence (12). COX-2 is also the predominant rate-limiting enzyme in prostaglandin synthesis; its overexpression in tissues of advanced osteosarcoma results in the production of large amounts of prostaglandin 2, which aggravates inflammation and promotes metastasis.
In the present study, COX-2 overexpression increased the migratory and invasive ability of MG-63 cells, while the inhibition of COX-2 activity decreased MG-63 cell migration and invasion. Furthermore, it was found that COX-2 promoted the production of MMP-2, MMP-9, VEGF and vimentin, and inhibited E-cadherin production, in MG-63 cells. The use of the COX-2 inhibitor NS398 reversed the aforementioned changes in protein expression caused by the overexpression of COX-2. MMP-2 and MMP-9 can promote cell metastasis through the degradation of the extracellular matrix (23,24). The increased expression of vimentin and decreased expression of E-cadherin also promotes metastasis (25,26). These changes indicated that the cells were undergoing EMT, leading to the dedifferentiation of epithelial cells into mesenchymal cells, and changes to cell morphology and polarity, providing cells with the conditions for the acquisition of metastatic characteristics. Therefore, it can be deduced that COX-2 affects metastasis by influencing the EMT process in osteosarcoma MG-63 cells.
Multiple signaling pathways are involved in the acquisition of tumor characteristics by cells, including the ERK pathway, the Wnt/β-catenin pathway, the PI3K/AKT pathway, the NF-κB pathway and the transforming growth factor (TGF)-β1/Smad pathway. These pathways can be activated through continuous stimulation by a large number of external signals or by mutation-induced changes in pathway components, thus promoting tumor incidence and growth. Previous studies have found that activation of the PI3K/AKT pathway in osteosarcoma promotes cell proliferation and metastasis, provides cells with drug resistance, participates in angiogenesis and regulates changes in the cell cycle (17,27). Other previous studies have revealed that the PI3K/AKT pathway is abnormally activated during lung metastasis of osteosarcoma cells. He et al (18) analyzed the relationship between AKT single nucleotide polymorphisms and osteosarcoma, and demonstrated that Chinese patients with osteosarcoma who possessed the genotype AA of AKT rs6973569 had a higher risk of metastasis. Furthermore, a study by Guo et al (28) showed that TGF-β1 could induce the metastasis of Saos-2 cells through the activation of the PI3K/AKT signaling pathway. Hou et al (29) found that the knockdown of TGF-α inhibited the activation of the PI3K/AKT/NF-κB signaling pathway, which downregulated the expression of intercellular adhesion molecule-1 and resulted in decreased distant metastases of osteosarcoma cells. Another previous study showed that the inhibition of the AKT pathway decreased MMP-2 secretion, thereby inhibiting the development of pulmonary metastasis in nude mice implanted with LM8 cells (30). In addition, a previous study reported that the blockade of the Ras/PI3K/AKT signaling pathway in a xenograft mouse model of osteosarcoma decreased the expression and activity of MMP-1, MMP-2 and MMP-9, leading to a decreased level of LM8 cell metastasis (31).
In view of the aforementioned studies, it can be deduced that activation or inhibition of the PI3K/AKT signaling pathway in osteosarcoma can affect tumor cell metastasis. Further experiments were conducted to verify whether this pathway is a signal pathway dependent on COX2 to promote MG63 cell metastasis. The findings of the present study indicated that when COX-2-overexpressing MG-63 cells were treated with the PI3K inhibitor LY294002, the invasive ability of the cells decreased significantly. In addition, the expression of MMP-2, MMP-9, VEGF and vimentin decreased significantly, while the expression of E-cadherin significantly increased, indicating that inhibition of PI3K activity reversed the increased invasive ability of MG-63 cells caused by COX-2 overexpression. Furthermore, quantification of the protein expression levels of PI3K, p-PI3K, AKT, p-AKT, IKK and p-IKK revealed that COX-2 overexpression in MG-63 cells was accompanied by an increase in the phosphorylation levels of PI3K, AKT and IKK. Conversely, the inhibition of COX-2 or PI3K activity resulted in a reversal of the increased phosphorylation of PI3K, AKT and IKK, while the total protein levels did not change. Activated IKK can activate NF-κB through the degradation of IκB (inhibitor of NF-κB), allowing the import of NF-κB into nuclei (32). A previous study found that the nuclear import of NF-κB can initiate MMP expression and promote EMT (33). In the present study, it was found that the protein expression of NF-κB was upregulated by COX-2 overexpression and reduced by the inhibition of COX-2 and PI3K.
In conclusion, the present study reported for the first time, to the best of our knowledge, that COX-2 overexpression promoted EMT and invasion in osteosarcoma MG-63 cells by activating the PI3K/AKT/NF-κB signaling pathway. This provides a theoretical basis for the development of drugs targeting COX-2. However, owing to the complexity of intracellular signaling networks and the limitations of in vitro experiments, further studies are required to verify whether COX-2 can be used as a therapeutic target for the development of drugs to treat osteosarcoma.
Acknowledgements
The authors would like to thank Dr Lu (College of Chemical Engineering, Lanzhou University) for providing excellent technical assistance and helpful discussion.
Funding
The present study was supported by a grant from The Natural Science Foundation of Gansu province (grant no. 1606RJZA126).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ and NC conceptualized and designed the study. HZ, PQ and TZ made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data and figures. XZ and TZ wrote the manuscript. XZ, HZ, PQ and TZ agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. NC revised the manuscript critically and advised revisions. All authors have read and approved the manuscript, and take public responsibility for appropriate portions of the content.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.He X, Gao Z, Xu H, Zhang Z, Fu P. A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J Orthop Surg Res. 2017;12:5. doi: 10.1186/s13018-016-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morel AP, Hinkal GW, Thomas C, Fauvet F, Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux I, Tissier A, Gras B, et al. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS Genet. 2012;8:e1002723. doi: 10.1371/journal.pgen.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amatangelo MD, Goodyear S, Varma D. c-Myc expression and MEK1-induced Erk2 nuclear localization are required for TGF-beta induced epithelial-mesenchymal transition and invasion in prostate cancer. Carcinogenesis. 2012;33:1965–1975. doi: 10.1093/carcin/bgs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharili AS, Allen S, Smith K, Price J, McGonnell IM. Snail2 promotes osteosarcoma cell motility through remodelling of the actin cytoskeleton and regulates tumor development. Cancer Lett. 2013;333:170–179. doi: 10.1016/j.canlet.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujii M, Kawano S, Dubois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/S0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 10.Masi L, Recenti R, Silvestri S, Pinzani P, Pepi M, Paglierani M, Brandi ML, Franchi A. Expression of cyclooxygenase-2 in osteosarcoma of bone. Appl Immunohistochem Mol Morphol. 2007;15:70–76. doi: 10.1097/01.pai.0000213131.63417.fa. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez NI, Hoots WK, Koshkina NV, Morales-Arias JA, Arndt CA, Inwards CY, Hawkins DS, Munsell MF, Kleinerman ES. COX-2 expression correlates with survival in patients with osteosarcoma lung metastases. J Pediatr Hematol Oncol. 2008;30:507–512. doi: 10.1097/MPH.0b013e31816e238c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang LY, Gatenby EL, Kamida A, Whitelaw BA, Hupp TR, Argyle DJ. Global gene expression analysis of canine osteosarcoma stem cells reveals a novel role for COX-2 in tumour initiation. PLoS One. 2014;9:e83144. doi: 10.1371/journal.pone.0083144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EJ, Choi EM, Kim SR, Park JH, Kim H, Ha KS, Kim YM, Kim SS, Choe M, Kim JI, Han JA. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp Mol Med. 2007;39:469–476. doi: 10.1038/emm.2007.51. [DOI] [PubMed] [Google Scholar]
- 14.Urakawa H, Nishida Y, Naruse T, Nakashima H, Ishiguro N. Cyclooxygenase-2 overexpression predicts poor survival in patients with high-grade extremity osteosarcoma: A pilot study. Clin Orthop Relat Res. 2009;467:2932–2938. doi: 10.1007/s11999-009-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji C, Guo H, Zhang P, Kuang W, Fan Y, Wu L. AnnexinA5 promote glioma cell invasion and migration via the PI3K/Akt/NF-κB signaling pathway. J Neurooncol. 2018;138:469–478. doi: 10.1007/s11060-018-2818-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhu LB, Jiang J, Zhu XP, Wang TF, Chen XY, Luo QF, Shu Y, Liu ZL, Huang SH. Knockdown of Aurora-B inhibits osteosarcoma cell invasion and migration via modulating PI3K/Akt/NF-κB signaling pathway. Int J Clin Exp Pathol. 2014;7:3984–3991. [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y, Liang G, Yuan B, Yang C, Gao R, Zhou X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol. 2015;36:1477–1486. doi: 10.1007/s13277-014-2631-4. [DOI] [PubMed] [Google Scholar]
- 18.He ML, Wu Y, Zhao JM, Wang Z, Chen YB. PIK3CA and AKT gene polymorphisms in susceptibility to osteosarcoma in a Chinese population. Asian Pac J Cancer Prev. 2013;14:5117–5122. doi: 10.7314/APJCP.2013.14.9.5117. [DOI] [PubMed] [Google Scholar]
- 19.Orgaz JL, Ladhani O, Hoek KS, Fernández-Barral A, Mihic D, Aguilera O, Seftor EA, Bernad A, Rodríguez-Peralto JL, Hendrix MJ, et al. ‘Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma’. Oncogene. 2009;28:4147–4161. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luan W, Yao Q, Xin N, Bu X, Xia Y, Wang J, Ruan H, Ma S, Xu B. miR-204-5p acts as a tumor suppressor by targeting matrix metalloproteinases-9 and B-cell lymphoma-2 in malignant melanoma. Onco Targets Ther. 2017;10:1237–1246. doi: 10.2147/OTT.S128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent CT, Fuxe J. EMT, inflammation and metastasis. Semin Cancer Biol. 2017;47:168–169. doi: 10.1016/j.semcancer.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2008;40:643–650. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amano S, Akutsu N, Matsunaga Y, Nishiyama T, Champliaud MF, Burgeson RE, Adachi E. Importance of balance between extracellular matrix synthesis and degradation in basement membrane formation. Exp Cell Res. 2001;271:249–262. doi: 10.1006/excr.2001.5387. [DOI] [PubMed] [Google Scholar]
- 24.Liao CL, Chu YL, Lin HY, Chen CY, Hsu MJ, Liu KC, Lai KC, Huang AC, Chung JG. Bisdemethoxycurcumin suppresses migration and invasion of human cervical cancer HeLa cells via inhibition of NF-ĸB, MMP-2 and −9 pathways. Anticancer Res. 2018;38:3989–3997. doi: 10.21873/anticanres.12686. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Yang D, Beckford J, Alachkar H. Upregulation of the EMT marker vimentin is associated with poor clinical outcome in acute myeloid leukemia. J Transl Med. 2018;16:170. doi: 10.1186/s12967-018-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gou Y, Zhai F, Zhang L, Cui L. RUNX3 regulates hepatocellular carcinoma cell metastasis via targeting miR-186/E-cadherin/EMT pathway. Oncotarget. 2017;8:61475–61486. doi: 10.18632/oncotarget.18424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao G, Cai C, Yang T, Qiu X, Liao B, Li W, Ji Z, Zhao J, Zhao H, Guo M, et al. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS One. 2013;8:e53906. doi: 10.1371/journal.pone.0053906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo YS, Zhao R, Ma J, Cui W, Sun Z, Gao B, He S, Han YH, Fan J, Yang L, et al. βig-h3 promotes human osteosarcoma cells metastasis by interacting with integrin α2β1 and activating PI3K signaling pathway. PLoS One. 2014;9:e90220. doi: 10.1371/journal.pone.0090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou CH, Lin FL, Tong KB, Hou SM, Liu JF. Transforming growth factor alpha promotes osteosarcoma metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem Pharmacol. 2014;89:453–463. doi: 10.1016/j.bcp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Aizawa J, Sakayama K, Kamei S, Kidani T, Yamamoto H, Norimatsu Y, Masuno H. Effect of troglitazone on tumor growth and pulmonary metastasis development of the mouse osteosarcoma cell line LM8. BMC Cancer. 2010;10:51. doi: 10.1186/1471-2407-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsubaki M, Satou T, Itoh T, Imano M, Ogaki M, Yanae M, Nishida S. Reduction of metastasis, cell invasion, and adhesion in mouse osteosarcoma by YM529/ONO-5920-induced blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathway. Toxicol Appl Pharmacol. 2012;259:402–410. doi: 10.1016/j.taap.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.