Abstract
Methylophiopogonanone B (MO-B), which belongs to a group of homoisoflavonoids, present in Ophiopogon japonicus, has been identified as an active component with antioxidative and anti-tumor properties. The present study investigated whether MO-B may exert protective effects on human umbilical vein endothelial cells (HUVECs) against H2O2-induced injury in vitro, and whether the MO-B effects may be modulated by the NADPH pathway. HUVECs were treated with MO-B in the presence or absence of H2O2. Malondialdehyde (MDA), reactive oxygen species (ROS) levels, and superoxide dismutase (SOD) activity were analyzed to evaluate cell injury and the antioxidative potential of MO-B. The results revealed that MO-B inhibited the production of MDA and ROS, but enhanced SOD activity. Furthermore, MO-B could alleviate H2O2-induced apoptosis in HUVECs, which is consistent with the expression of apoptosis-associated genes and proteins in cells, including Bax/Bcl-2 and caspase-3. To explore the potential mechanism, the present study investigated the effects of MO-B on NADPH-related signaling via the analysis of neutrophil cytochrome b light chain (p22phox) expression, which is the membrane-associated subunit of NADPH oxidase. MO-B could improve the survival of endothelial cells and therefore may be a potential drug in the treatment of cardiovascular diseases.
Keywords: methylophiopogonanone B, homoisoflavonoids, apoptosis, nicotinamide adeninde dinucleotide phosphate
Introduction
Cardiovascular disease has a high incidence in numerous countries and is one of the most common threats to human health, accounting for 30% of all deaths (1). Atherosclerosis (AS) is the basis of various cardiovascular diseases. Numerous studies have demonstrated that vascular endothelial injury is the initial step of AS development (2,3). Oxidative stress can lead to the imbalance in intracellular antioxidant capacity, thus producing a large quantity of reactive oxygen species (ROS), inducing lipid peroxidation and biomacromolecular degeneration, which is considered to be the main factor leading to vascular endothelial cell injury (4). Therefore, the study of endothelial anti-oxidative stress injury and the inhibition of endothelial apoptosis is of great importance for the treatment of cardiovascular and cerebrovascular diseases.
The dried root-tuber of Ophiopogon japonicus has been historically used as a common agent in the clinical treatment of cardiovascular and cerebrovascular diseases (5). Radix Ophiopogonis is often used together with ginseng and Schisandra chinensis, and is an important raw material in traditional Chinese medicine in the form of shengmai powder, and application of shengmai injection and shenmai injection (6,7). The results of previous experiments and clinical studies indicate that Radix Ophiopogonis and its preparations have significant effects on the cardiovascular system, and can improve myocardial contractility, enhance cardiac blood output, and reduce cardiac load and myocardial oxygen consumption (8–10). In clinic, Radix Ophiopogonis has a notable effect on chronic cardiac insufficiency and coronary heart disease (11). Pharmacological experiments have demonstrated that Maidong injection protects against myocardial ischemia, and methylophiopogonanone B (MO-B) is one of the effective substances of Maidong injection (6,12). Using a male rabbit model of anterior descending coronary artery ligation to observe the influence of Maidong injection on microstructures in experimental myocardial infarction and myocardial ischemia, it was observed that the Maidong injection group exhibited an increased negative rate of myocardial damage than the control group; the incidence of myocardial infarction was lower compared with the control group (13). At present, the majority of studies on the main therapeutic substances of Radix Ophiopogonis are focused on saponins, while few are focused on homoisoflavonoids.
The MO-B of Radix Ophiopogonis is a major homoisoflavonoid monomer isolated from Ophiopogon japonicus. A recent study reported multiple activities of MO-B in various systems, with the highest antioxidant activity being exhibited in vitro (14). MO-B promotes Rho activation and tubulin depolymerisation (15), and inhibits hypoxia-inducible factor (HIF)-1 activity (16). Furthermore, Ito et al (17) reported that MO-B can inhibit melanosome transfer in normal human epidermal melanocytes. It has also been reported that MO-B exerts significant anti-tumor activities against HeLa cells (18); however, despite the various biological activities of MO-B, the cellular function of MO-B in the prevention of cardiovascular diseases in human umbilical vein endothelial cells (HUVECs), and its underlying molecular mechanism remain unknown. Thus, the present study investigated the effects of MO-B against injury on H2O2-exposed HUVECs in order to provide experimental evidence for its potential clinical use in the treatment of cardiovascular diseases.
Our study demonstrated that MO-B prevents HUVECs from H2O2-induced apoptosis by modulating nicotinamide adeninde dinucleotide phosphate (NADPH) signaling, caspase-3 and Bcl-2/(Bcl-2-associated X protein (Bax), indicating that MO-B could be a potential agent in promoting the viability of endothelial cells.
Materials and methods
Materials, reagents and antibodies
Ophiopogon japonicus was obtained from farms in Cixi (Zhejiang, China). MO-B was extracted from Ophiopogon japonicus using high-speed counter-current chromatography (19) and the yield was ~0.2–0.4 mg/g in tuber roots of Ophiopogon japonicus. High-performance liquid chromatography (HPLC) was conducted to measure the purity of MO-B, which was >97% (Fig. S1).HPLC was performed using a Shimadzu C18 column (5 µm 250×4.6 mm). The volume ratio of mobile solvents A (water) and B (acetonitrile) was maintained at 35:65, and the temperature was set at 30°C. The flow rate of the mobile phase was 1 ml/min. The detection wavelength was 285 nm. MO-B was then dissolved to 10, 20, 40 and 50 µM in dimethyl sulfoxide for cell treatment.
Antibodies targeting Bax (ab182733), Bcl-2 (ab182858), cleaved caspase-3 (ab32042), neutrophil cytochrome b light chain (p22phox; ab80896) and GAPDH (ab9482), goat anti-mouse horseradish peroxidase IgG (ab6789) and goat anti-rabbit IgG horseradish peroxidase (ab6721) secondary antibodies, were purchased from Abcam. Cell Counting Kit-8 (CCK-8), ROS and malondialdehyde (MDA) detection kits, radioimmunoprecipitation assay (RIPA) lysis buffer, a BCA Protein Assay kit and superoxide dismutase (SOD) assay kit with WST-8 were purchased from Beyotime Institute of Biotechnology.
Cell culture
HUVECs were obtained from Procell Life Science & Technology Co., Ltd. and the STR validation of the cell line was performed by the company, which revealed no cross contamination of human cells. HUVECs were grown in Ham's F-12 K medium with 0.1 mg/ml Heparin, 0.03–0.05 mg/ml Endothelial Cell Growth Supplement, 10% fetal bovine serum and 1% penicillin/streptomycin (Procell Life Science & Technology Co., Ltd.), and maintained at 37°C in a humidified incubator with 5% CO2. The cells were sub-cultured every 2–3 days with 0.25% trypsin digestion. Cells between passages 5–12 were used for the subsequent the experiments.
Cell viability assay
To evaluate cell viability, cells were enzymatically harvested as aforementioned, counted in a hemocytometer and sub-cultured in 96-well plates at a density of 5×103 cell/well. HUVECs cultivated for 24 h in medium at 37°C without (control) or with MO-B (10, 20, 40 and 50 µM) were incubated with H2O2 (1,000 µM) for 60 min. Finally, the medium was discarded and 100 µl fresh medium containing 10% CCK-8 agent was added to each well for incubation for 1 h at 37°C. The absorbance at 450 nm was measured using a Varioskan Flash reader (Thermo Fisher Scientific, Inc.).
MDA and SOD assays
HUVECs were cultured at a density of 2×105 cells/well in 6-well plates and cultured overnight at 37°C before being treated for 24 h without (control) or with MO-B (10, 20, 40 and 50 µM), and then stimulated with H2O2 (1,000 µM) for 6 h. The aforementioned assay kits (Beyotime Institute of Biotechnology) were then used to measure the MDA levels and SOD activity, respectively, according to the manufacturer's protocols.
Intracellular ROS quantification
The level of intracellular ROS was determined by the change in fluorescence emission of the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Briefly, 2×105 HUVECs were cultured into 6-well plates at 37°C and treated with the indicated concentrations of MO-B (10, 40 and 50 µM) for 24 h, followed by treatment with H2O2 (1,000 µM) for 1 h. Cells were trypsinized and washed with PBS, and then incubated with 10 mmol/l DCFH-DA for 30 min at 37°C. Subsequently, cells were washed with PBS twice and analyzed using a BD ACCURIC6 PLUS flow cytometer and BD Accuri C6 software (BD Biosciences).
Analysis of apoptosis
After treatment with or without MO-B (10, 20 and 40 µM) for 24 h, cells were exposed to 1,000 µM H2O2 for 6 h and then washed with ice-cold PBS. Apoptosis was analyzed using an Annexin V-fluorescein isothiocyanate/propidium iodide Apoptosis Detection Kit (Beyotime Institute of Biotechnology). Cells were stained according to the manufacturer's instructions. The proportion of apoptotic cells in 1×105 labeled cells was quantified using a BD Accuri C6 Plus flow cytometry and BD Accuri C6 software (BD Biosciences).
Reverse transcription-quantitative PCR (RT-qPCR)
Cells were pretreated at 37°C without (control) or with MO-B (10, 20 and 50 µM) for 24 h and then exposed to 1,000 µM H2O2 for 6 h. The cells were then used for RNA extraction.
Total RNA extraction was performed using the RNAiso Plus reagent (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocols. The concentration of RNA was determined by measuring the absorbance at 260 and 320 nm using a Nanodrop 2000 (Thermo Fisher Scientific, Inc.). Complementary DNA was generated from 500 ng total RNA using Super Script II Reverse Transcriptase (Takara Biotechnology Co., Ltd.), according to the manufacturer's protocol. The reverse transcription reaction was as follows: 37°C for 30 min and 85°C for 5 min. qPCR analysis was performed using SYBR® GREEN PCR master mix in a reaction volume of 20 µl using a 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The annealing temperature used was 60°C for 30 sec. Relative gene expression was calculated by the 2−ΔΔCq method (20), and the values were normalized to the endogenous reference β-actin. Primer sequences were as follows (forward, 5′-3′ and reverse, 5′-3′): p22phox, CAGTGGTACTTTGGTGCCTACTCCandGGTGGAGCCCTTCTTCCTCT; Bcl-2, CGACGACTTCTCCCGCCGCTACCGC and CCGCATGCTGGGGCCGTACAGTTCC; Bax: TCCACCAAGAAGCTGAGCGAG and GTCCAGCCCATGATGGTTCT; caspase-3, AATTGTGGAATTGATGCGTGATGT and ATAATAACCAGGTGCTGTGGAGTA; β-actin, GTGGGGCGCCCCAGGCACC and CTCCTTAATGTCACGCACGATTTC.
Western blot analysis
Cells were pretreated with MO-B (10, 20, 40 and 50 µM) for 24 h and then exposed to 1,000 µM H2O2 for 6 h. Subsequently, the cells were collected and lysed in RIPA lysis buffer containing 2 mM PMSF, and the protein concentration in cell lysates was determined by BCA assay. Cell lysates containing 60 µg total protein were subjected to 12% SDS-PAGE (Bis-Tris Midi-Gels; Thermo Fisher Scientific, Inc.) and then transferred to PVDF membranes. The membranes were blocked in TBS with 0.1% Tween-20 (TBST) containing 1% BSA at 4°C overnight, and then incubated with primary antibodies (1:5,000) in TBST at room temperature for 2 h. The membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000) in TBST at room temperature for 2 h. Proteins were visualized using an ECL kit (Beyotime Institute of Biotechnology) and detected using a Chemi Doc XRS imaging system (Bio-Rad Laboratories). GAPDH was used as a loading control.
Statistical analysis
Data are expressed as the mean ± standard error from three independent experiments. Statistical analysis was performed with statistical software SPSS 18.0 (SPSS, Inc.). Data were analyzed by one-way analysis of variance followed by the Least Significant Difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
MO-B protects HUVECs against H2O2-induced cell death
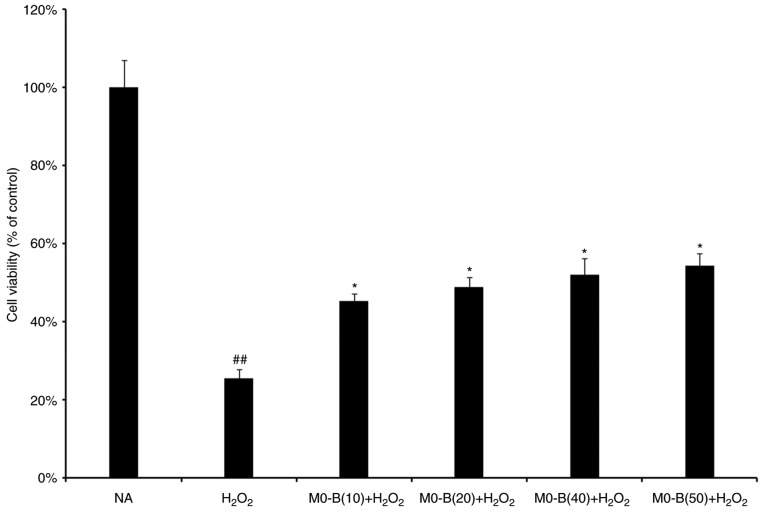
To determine the protective effects of MO-B on HUVECs under H2O2 stress, a CCK-8 assay was performed. As presented in Fig. 1, H2O2 treatment for 24 h significantly inhibited HUVEC viability compared with in untreated cells. However, pretreatment of cells with MO-B for 24 h at concentrations of 10, 20, 40 and 50 µm significantly ameliorated the effects of H2O2 on cytotoxicity compared with H2O2 treatment alone; cell activity increased by 30% under 50 µM MO-B treatment (Fig. 1). This suggested that MO-B could protect endothelial cells against H2O2-induced cell death in a concentration-dependent manner.
Figure 1.
Viability of human umbilical vein endothelial cells as detected by a Cell Counting Kit-8 assay. Data are expressed as the mean ± standard error from five independent experiments. ##P<0.01 vs. NA group. *P<0.05 vs. H2O2 group. MO-B, methylophiopogonanone B; NA, control.
MO-B attenuates H2O2-induced oxidative stress in HUVECs
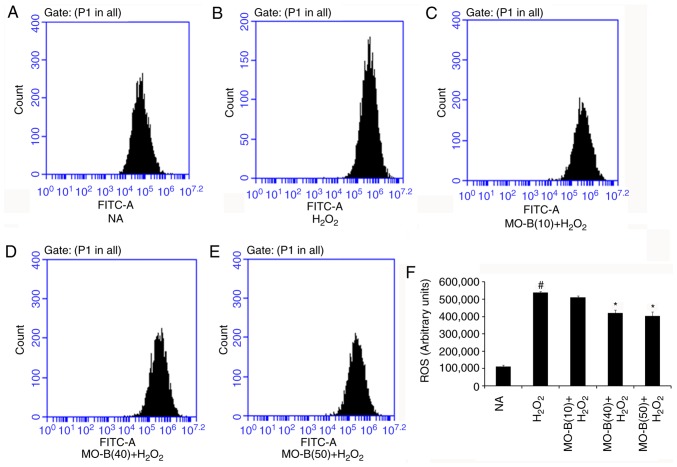
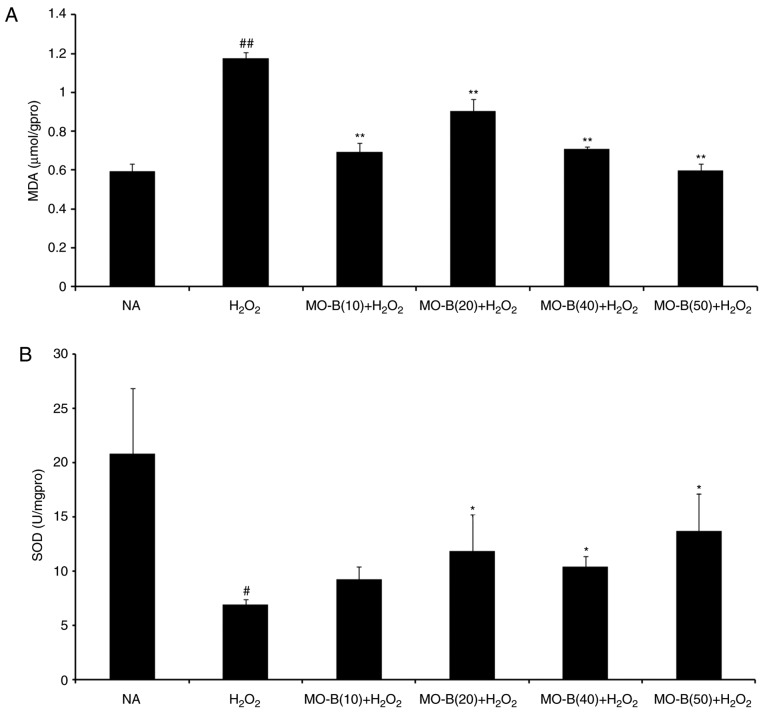
To further examine whether MO-B could protect HUVECs via an antioxidant mechanism, the intracellular production of ROS and MDA, and SOD activity were investigated. As presented in Fig. 2, intracellular ROS levels were significantly increased in the H2O2-treated group compared with the control group, while 40 and 50 µM MO-B significantly reduced ROS levels compared with H2O2 treatment alone. This indicated that H2O2 exerts its cytotoxicity via oxidative injury and MO-B can mitigate such injury. Similarly, the levels of MDA, which is an indicator of lipid peroxidation (21), were significantly increased under H2O2 treatment compared with the control, but decreased with MO-B pretreatment compared with H2O2 treatment alone (Fig. 3A). This suggested that the increased ROS and MDA produced are scavenged by MO-B, indicating its antioxidative properties. Subsequently, the antioxidant enzyme SOD was examined. SOD is a type of superoxide free radical scavenger that naturally occurs in living organisms, and is an active substance capable of eliminating harmful substances produced during metabolic processes (22). In our study, SOD activity was significantly decreased in HUVECs treated with H2O2 for 6 h compared with untreated cells, while significant increases with MO-B pretreatment were observed compared with H2O2 treatment alone (Fig. 3B).
Figure 2.
Effects of MO-B on ROS generation induced by H2O2. (A) NA group. (B) H2O2 group. (C) 10 µM MO-B. (D) 40 µM MO-B. (E) 50 µM MO-B. (F) Histogram analysis of ROS generation of HUVECs. Data are expressed as means ± standard error of the mean from three independent experiments. #P<0.05 vs. NA group, *P<0.05 vs. H2O2 group. FITC, fluorescein isothiocyanate; MO-B, methylophiopogonanone B; NA, control.
Figure 3.
Effects of MO-B on MDA levels and intracellular SOD activities induced by H2O2 in human umbilical vein endothelial cells. Cells were treated with or without MO-B for 24 h followed by H2O2 for 4 h. (A) The release of MDA. (B) The level of SOD. Data are expressed as means ± standard error from three independent experiments. ##P<0.01 and #P<0.05 vs. NA group, **P<0.01 and *P<0.05 vs. H2O2 group. MDA, malondialdehyde; MO-B, methylophiopogonanone B; NA, control; SOD, superoxide dismutase.
MO-B ameliorates H2O2-induced apoptosis in HUVECs
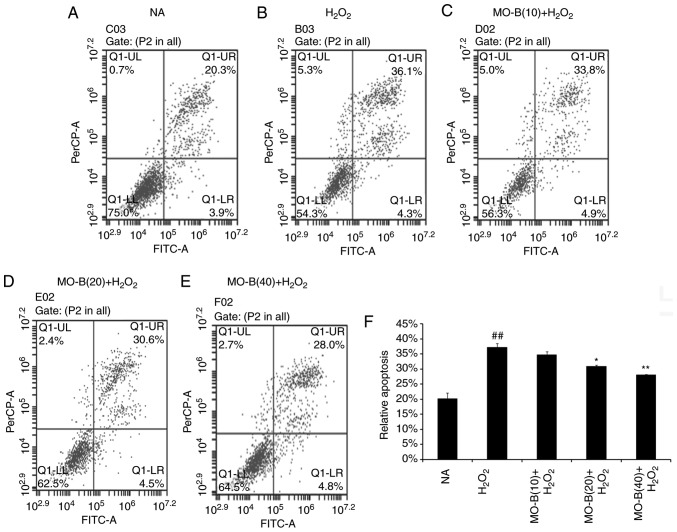
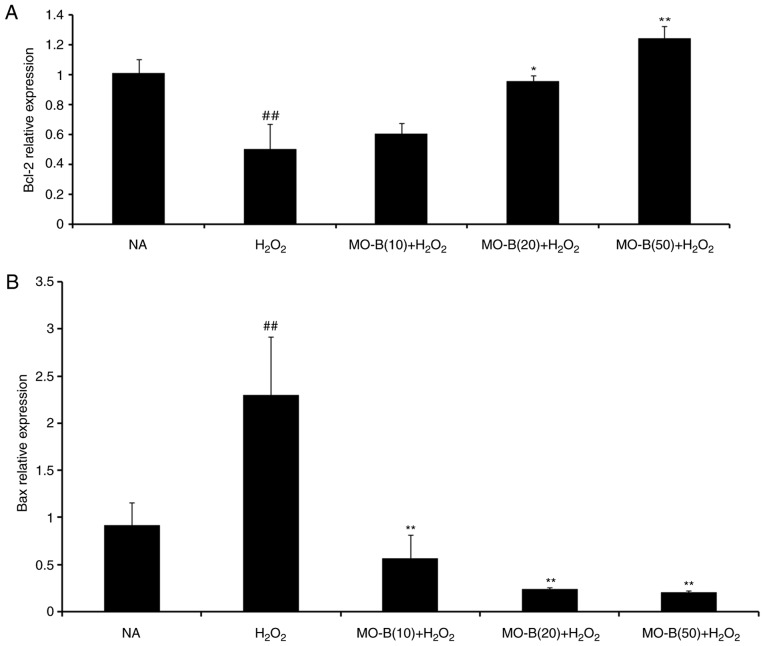
To further illustrate the effects of MO-B on H2O2-induced vascular endothelial cell injury, cell apoptosis was measured by the Annexin V-FITC and PI double-staining method, and the number of apoptotic cells of HUVECs was determined by flow cytometry. As shown in Fig. 4B, the percentage of double-positive cells (upper right quadrant; 36.1%) was significantly increased compared with the normal group (20.3%) (Fig. 4A and F). This level of apoptosis was significantly ameliorated by the three concentrations of MO-B tested, ranging from 33.8 to 20.8%, compared with H2O2 treatment alone (Fig. 4C-F). These results indicate that MO-B could protect HUVECs from H2O2-induced apoptosis. Apoptosis is an orderly cell death process regulated by apoptosis-associated genes, including the Bcl-2 family and caspases family and cytochrome c. The Bcl-2 family is composed of proteins that regulate apoptosis by inducing (pro-apoptotic) or inhibiting (anti-apoptotic) this process (23). The Bcl-2 family plays a dual role in the regulation of apoptosis (24). Thus, the present study detected the mRNA expression of Bcl-2 in HUVECs, which prevents apoptosis, and that of Bax, which promotes apoptosis. As presented in Fig. 5A and B, H2O2 significantly promoted Bax mRNA expression, but suppressed that of Bcl-2 in HUVECs compared with the control group. Pretreatment of MO-B led to significantly downregulated Bax and increased Bcl-2 expression compared with H2O2 treatment alone. Similar results were observed for protein expression in which H2O2 alone markedly increased the level of Bax but decreased the Bcl-2 protein expression, while MO-B could dose-dependently ameliorate the effects of H2O2 (Fig. 6). The results indicated that H2O2 induced cell apoptosis, which was reversed with MO-B pretreatment.
Figure 4.
Effects of MO-B on the cell apoptosis of H2O2 induced HUVECs. (A-E) Cell apoptosis of H2O2-induced HUVECs was detected using flow cytometry. (F) Histogram analysis of the number of apoptotic cells of HUVECs. ##P<0.01 vs. NA group. **P<0.01 and *P<0.05 vs. H2O2 group. FITC, fluorescein isothiocyanate; HUVECs, human umbilical vein endothelial cells; MO-B, methylophiopogonanone B; NA, control.
Figure 5.
Effects of MO-B on Bcl-2, Bax, caspase-3 and p22phox mRNA expressions induced by H2O2. Cells were treated with increasing doses of MO-B for 24 h prior to stimulation with H2O2 (1 mmol/l) for 4 h. (A) Bcl-2 mRNA expression. (B) Bax mRNA expression. (C) Caspase-3 mRNA expression. (D) p22phox mRNA expression. Data are expressed as means ± standard error from three independent experiments. ##P<0.01 and #P<0.05 vs. NA group vs. H2O2 group, **P<0.01 and *P<0.05 vs. H2O2 group. Bax, Bcl-2-associated X protein; MO-B, methylophiopogonanone B; NA, control; p22phox, neutrophil cytochrome b light chain.
Figure 6.
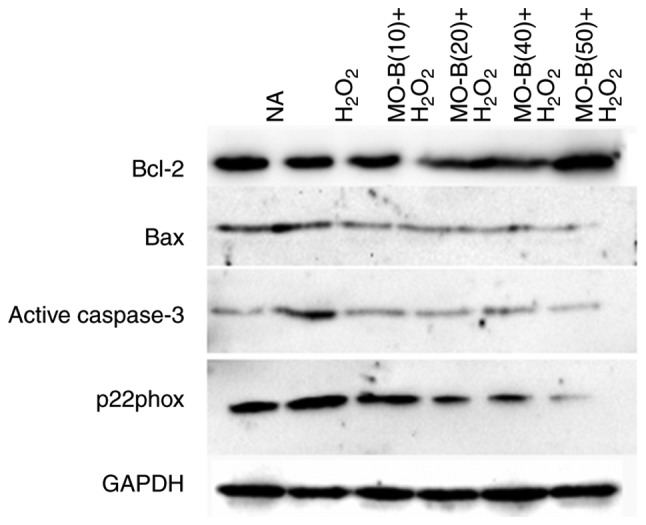
Effects of MO-B on Bcl-2, Bax, cleaved caspase-3 and p22phox protein expression induced by H2O2. Cells were treated with increasing doses of MO-B for 24 h prior to stimulation with H2O2 (1 mmol/l) for 6 h. Data are expressed as the mean ± standard error from three independent experiments. MO-B, methylophiopogonanone B; NA, control; p22phox, neutrophil cytochrome b light chain.
Furthermore, caspases belong to a family of cysteine proteases that are key mediators of programmed cell death or apoptosis (25). Caspase-3 is a marker of apoptosis, and the initiator and executor of this process (26). I addition, caspase-3 is the hub of various apoptotic signal transduction pathways outside the cell, and is the most important apoptotic protease, which is activated in the early stage of apoptosis (27). Activated caspase-3 causes a series of cascade reactions, which eventually lead to apoptosis. Hence, the activity of caspase-3 in HUVECs was examined. In Fig. 5C, H2O2 significantly increased caspase-3 mRNA expression in HUVECs compared with the control group. Pretreatment with MO-B (10, 20 or 50 µM) significantly inhibited caspase-3 mRNA expression compared with H2O2 treatment alone. Western blotting also revealed the same trend in active caspase-3 protein expression. There was an increase in active caspase-3 expression caused by H2O2 treatment, which could be inhibited by administration of MO-B (Fig. 6). Collectively, these results further suggested the pivotal role of caspase-3 activation in H2O2-induced apoptosis, as well as the anti-apoptotic effect of MO-B.
MO-B activates NADPH signaling via inhibition of p22phox overexpression
NADPH oxidase serves a key role in ROS generation, and p22phox is a modulatory subunit of NADPH oxidase (28). Thus, the effects of MO-B on H2O2-induced p22phox mRNA and protein expression in HUVECs were examined. A notable increase in p22phox mRNA levels was observed in the H2O2 group. Pretreatment of HUVECs with MO-B (>10 µM) significantly reversed H2O2-induced p22phox expression (Fig. 5D). A similar result was also observed for protein expression. H2O2 alone markedly increased the level of p22phox protein expression by 2-fold compared with the control group, while MO-B could dose-dependently attenuate its protein expression (Fig. 6). These results suggested that MO-B may exert its effects on p22phox expression by modulating the NADPH pathway.
Discussion
In the present study, 1 mM H2O2 effectively induced the apoptosis of HUVECs, while MO-B protected HUVECs from H2O2-induced apoptosis possibly via the stimulation of the NADPH oxidase pathway, acting as an antioxidant.
There are >110 homoisoflavonoid compounds, which are a particular type of flavonoid, isolated from natural materials (29). Pharmacological activity studies have shown that homoisoflavonoids have anti-inflammatory (30,31), antioxidative (5,32,33), anti-tumor (34,35), antimicrobial properties (36). Other type of homoisoflavonoids, such as sappanin-type homoisoflavonoids from the fibrous roots of Polygonatum odoratum (Mill.) Druce, were reported to be potent glucose transporter 2 inhibitors with glucose-lowering properties (37). These compounds cause notable reductions in cardiovascular events and have been reported as potential cardiovascular drugs, which have notable antiangiogenic effects (38,39). MO-B is a homoisoflavonoid that, besides antioxidant (40) and anti-tumor activity (17), appears to have protective effects on the cardiovascular system (16). MO-A, a structural analogue of MO-B, can suppress ischemia/reperfusion-induced myocardial apoptosis in mice (41), and protects against cerebral ischemia/reperfusion injury and attenuates blood-brain barrier disruption in vitro (42). Similarly, MO-B caused the inhibition of HIF-1α activity (16). As HIF-1α was demonstrated to induce the production of the pleiotropic proinflammatory cytokine migration inhibitory factor (MIF) in macrophages (43), and MIF is implicated in several immunoinflammatory and autoimmune diseases and cancer (44–48), MO-B may negatively regulate MIF in several immunoinflammatory and autoimmune diseases. MO- A and MO-B are the major contributors to the total homoisoflavonoid content in Ophiopogon japonicas (40). Previous pharmacological studies have focused their attention on MO-A as a natural drug able to inhibit the progression of cardiovascular diseases (40,41). At present, there few studies have investigated MO-B compounds as of the low abundance in plants (16).
Apoptosis is a complex process that occurs via a series of physiological activities in cells, including lipid peroxidation, apoptotic gene and protein expression and apoptotic body formation. MDA can be applied as a biomarker to evaluate the degree of lipid peroxidation on the cell membrane (49), which is generated by oxidative stress in the organism (50). In the HUVEC oxidative damage model, increased levels of MDA were linked to greater toxicity and could affect normal cell functions by interacting with phospholipid proteins, and accumulating inside the cells (51), while the activity of SOD was significantly decreased; the antioxidant function of SOD is involved in preventing injuries to the cell membrane due to ROS (52). The percentage of apoptotic cells decreased in a dose-dependent manner in the presence of MO-B, indicating that MO-B can prevent the apoptosis of HUVECs and help to maintain cell integrity and activity. These findings suggested that MO-B protected cells from apoptosis via intracellular antioxidant enzymes. Furthermore, apoptotic gene expression was induced, including that of Bcl-2/Bax and caspase-3. The primary role of Bcl-2 family members is the regulation of apoptosis, and the ratio of Bcl-2/Bax determines the fate of cells upon exposure to various stimuli (53). In the present study, the mRNA expression levels of Bax were downregulated, while those of Bcl-2 were upregulated when cells were exposed to MO-B and H2O2. These findings were confirmed by measuring protein expression via western blot analysis. Caspase-3 is an initiator and executioner of apoptosis (54). Our study reported that MO-B suppress the apoptosis of endothelial cells caused by H2O2 via downregulation of cleaved-caspase-3.
The primary catalytic function of NADPH oxidase is to generate ROS (55). The enzyme is stimulated by hypertension, hypercholesterolemia, diabetes and ageing, and once activated, it causes oxidative stress, endothelial dysfunction and vascular inflammation, which are the early steps of arterial remodeling and atherogenesis (56). p22phox is an important component of NADPH oxidase, and can activate this enzyme (57), serving a critical role under oxidative stress in cardiovascular disease (58). In previous studies, the expression of p22phox both at the protein and mRNA level was closely followed by the release of ROS (51), and p22phox was upregulated under H2O2 stimulation, while antioxidant treatment with vitamin C or diphenyleneiodonium abrogated thrombin-induced ROS production and p22phox expression (59). In the present study, using a cell culture model, it was observed that MO-B could significantly reduce ROS levels in HUVECs, suggesting that MO-B may play an antioxidative role by inhibiting NADPH oxidase activity. Compared with the H2O2-induced group, the mRNA and protein expression levels of p22phox in the MO-B group exhibited a dose-dependent decrease, which supported our hypothesis. This suggested that MO-B may exert a protective effect via NADPH oxidase on HUVECs induced by H2O2; however, further investigation is required.
In conclusion, homoisoflavonoids from Radix Ophiopogonis may be potential therapeutic agents for treating cardiovascular disease. Homoisoflavonoids are a particular type of flavone compound, whose parental structure has an additional carbon atom than isoflavones. This type of compound is notably rare in plants, and is mainly distributed among Ophiopogon, Scilla, Eucomis and Muscari (29). Although the protective effects of MO-B on damaged endothelial cells have been confirmed in the present study, the associated pathological mechanisms remain unknown. In addition, a recent study investigated this compound in vivo, using a diet with low-dose and long-term concentrations of MO-B daily for 2 weeks (40). The clinical application of Radix Ophiopogonis has been reported; however, previous pharmacological research on Radix Ophiopogonis has mainly focused on its effects, while the activity of its individual chemical components was rarely studied (11). The association between the monomer components and the target is not clear; thus, further studies on the efficacy of the monomers are of great importance for the safety, effectiveness, controllability and stability of clinical application of this drug.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Provincial Natural Science Foundation of China (grant no. LY16C020002) and Zhejiang Provincial Science and Technology Project (grant no. 2018C02042).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YZ and LW made substantial contributions to the conception and design of the study. LW and YQ performed the experiments. YW, BL and LW made substantial contributions to the acquisition, analysis and interpretation of the data and wrote the paper. MB and RF designed the experiment, analyzed the data, and contributed reagents, materials and analysis tools. MB, LW and YZ reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: Origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–550. doi: 10.1038/35080097. [DOI] [PubMed] [Google Scholar]
- 3.Dimmeler S, Hermann C, Zeither AM. Apoptosis of endothelial cells: Contribution to the pathophysiology of atherosclerosis. Eur Cytokine Netw. 1998;9:697–698. [PubMed] [Google Scholar]
- 4.Sharifpanah F, Sauer H. Reactive oxygen species, oxidative stress, and cardiovascular diseases. In: Armstrong D, Stratton RD, editors. Oxidative Stress and Antioxidant Protection: The Science of Free Radical Biology and Disease. John Wiley & Sons Inc.; Hoboken, NJ: 2016. pp. 281–306. [DOI] [Google Scholar]
- 5.Zhu YZ, Huang SH, Tan BK, Sun J, Whiteman M, Zhu YC. Antioxidants in Chinese herbal medicines: A biochemical perspective. Nat Prod Rep. 2004;21:478–489. doi: 10.1039/b304821g. [DOI] [PubMed] [Google Scholar]
- 6.Lu LY, Zheng GQ, Wang Y. An overview of systematic reviews of shenmai injection for healthcare. Evid Based Complement Alternat Med. 2014;2014:840650. doi: 10.1155/2014/840650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HD, Xie YM, Wang LX, Wu JB. Systematic review of efficacy and safety of shenmai injection for chronic heart failure. Zhongguo Zhong Yao Za Zhi. 2014;39:3650–3661. (In Chinese) [PubMed] [Google Scholar]
- 8.Jun F, Xu Z. Advancement in research of pharmacological functions of Radix Ophiopogonis on cardiovascular system. J Nanjing Univ Tradit Chin Med. 2006;22:270–272. [Google Scholar]
- 9.Zhao M, Xu W, Shen HY, Shen PQ, Zhang J, Wang DD, Xu H, Wang H, Yan TT, Wang L, et al. Comparison of bioactive components and pharmacological activities of Ophiopogon japonicas extracts from different geographical origins. J Pharm Biomed Anal. 2017;138:134–141. doi: 10.1016/j.jpba.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Yu BY. Exploration on the modern research methodology of traditional chinese medicine, basing on the systemic research of Radix Ophiopogonis. Chin J Nat Med Jan. 2007;5:10–14. [Google Scholar]
- 11.Chen MH, Chen XJ, Wang M, Lin LG, Wang YT. Ophiopogon japonicas-A phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol. 2016;181:193–213. doi: 10.1016/j.jep.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Fan XH, Wang Y, Cheng YY. LC/MS fingerprinting of Shenmai injection: A novel approach to quality control of herbal medicines. J Pharm Biomed Anal. 2006;40:591–597. doi: 10.1016/j.jpba.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Gu SL, Xu SS, Ji K, Yang QH, Lu WW, Jia YS, Wang N. Effects of maidong on experimental myocardial infarction and submicrostructure in myocardial hypoxia. Shanghai J Tradit Chin Med. 1983;3:44–45. [Google Scholar]
- 14.Wang Y, Liu F, Liang Z, Peng L, Wang B, Yu J, Su Y, Ma C. Homoisoflavonoids and the antioxidant activity of Ophiopogon japonicus root. Iran J Pharm Res. 2017;16:357–365. [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y, Kanamaru AA, Akihiro T. A novel agent, methylophiopogonanone B, promotes Rho activation and tubulin depolymerization. Mol Cell Biochem. 2007;297:121–129. doi: 10.1007/s11010-006-9336-y. [DOI] [PubMed] [Google Scholar]
- 16.Fujii M, Egawa K, Hirai Y, Kondo M, Fujii K, Uekusa H, Akita H, Nose K, Toriizuka K, Ida Y. Dihydrochalcone designed from methylophiopogonanone B strongly inhibits hypoxia-inducible factor (HIF)-1α activity. Heterocycles. 2009;78:2061–2065. doi: 10.3987/COM-09-11684. [DOI] [Google Scholar]
- 17.Ito Y, Kanamaru A, Tada A. Effects of methylophiopogonanone B on melanosome transfer and dendrite retraction. J Dermatol Sci. 2006;42:68–70. doi: 10.1016/j.jdermsci.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Wang KW, Zhang H, Shen LQ, Wang W. Novel steroidal saponins from liriope graminifolia (Linn.) baker with anti-tumor activities. Carbohydr Res. 2011;346:253–258. doi: 10.1016/j.carres.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Wang L, Liu T, Mao Z, Ge Q, Mao J. Isolation of homoisoflavonoids from the fibrous roots of Ophiopogon japonicus by recycling high-speed counter-currentchromatography and online antioxidant activity assay. Acta Chromatogr. 2018 Oct 14; doi: 10.1556/1326.2018.00509. (Epub ahead of print). doi.org/10.1556/1326.2018.00509. [DOI] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004;57:453–455. (In Polish) [PubMed] [Google Scholar]
- 22.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- 23.Cleary ML, Smith SD, Sklar AJ. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 24.Gross A, Mcdonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 25.Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 27.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 28.Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K, Sun H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J Atheroscler Thromb. 2014;21:768–783. doi: 10.5551/jat.23697. [DOI] [PubMed] [Google Scholar]
- 29.Jiang HB, Huang J, Guo MJ, Zou P, Tian XQ. Recent advances in the study of natural homoisoflavonoids. Yao Xue Xue Bao. 2007;42:118–126. (In Chinese) [PubMed] [Google Scholar]
- 30.Hung TM, Thu CV, Dat NT, Ryoo SW, Lee JH, Kim JC, Na M, Jung HJ, Bae K, Min BS. Homoisoflavonoid derivatives from the roots of Ophiopogon japonicus and their in vitro anti-inflammation activity. Bioorg Med Chem Lett. 2010;20:2412–2416. doi: 10.1016/j.bmcl.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 31.Damodar K, Lee J, Kim JK, Jun JG. Synthesis and in vitro evaluation of homoisoflavonoids as potent inhibitors of nitric oxide production in RAW-264.7 cells. Bioorg Med Chem Lett. 2018;28:2098–2102. doi: 10.1016/j.bmcl.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 32.Siddaiah V, Maheswara M, Venkata Rao C, Venkateswarlu S, Subbaraju GV. Synthesis, structural revision, and antioxidant activities of antimutagenic homoisoflavonoids from Hoffmanosseggia intricata. Bioorg Med Chem Lett. 2007;17:1288–1290. doi: 10.1016/j.bmcl.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Zhou YF, Qi J, Zhu DN, Yu BY. Homoisoflavonoids from Ophiopogon japonicus and its oxygen free radicals (OFRs) scavenging effects. Chin J Nat Med. 2008;6:201–204. doi: 10.3724/SP.J.1009.2008.00201. [DOI] [Google Scholar]
- 34.El-Elimat T, Rivera-Chávez J, Burdette JE, Czarnecki A, Alhawarri MB, Al-Gharaibeh M, Alali F, Oberlies NH. Cytotoxic homoisoflavonoids from the bulbs of Bellevalia flexuosa. Fitoterapia. 2018;127:201–206. doi: 10.1016/j.fitote.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan CL, Kang ZY, Lin CR, Jiang Y, Liu JX, Tu PF. Two new homoisoflavonoids from the fibrous roots of Ophiopogon japonicus (Thunb.) Ker-Gawl. J Asian Nat Prod Res. 2009;11:876–879. doi: 10.1080/10286020903093161. [DOI] [PubMed] [Google Scholar]
- 36.Alali F, El-Elimat T, Albataineh H, Al-Balas Q, Al-Gharaibeh M, Falkinham JO, III, Chen WL, Swanson SM, Oberlies NH. Cytotoxic homoisoflavones from the bulbs of Bellevalia eigii. J Nat Prod. 2015;78:1708–1715. doi: 10.1021/acs.jnatprod.5b00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Fowler MI, Messenge DJ, Terry LA, Gu X, Zhou L, Liu R, Su J, Shi S, Ordaz-Ortiz JJ, et al. Homoisoflavonoids are potent glucose transporter 2(GLUT2) inhibitors: A potential mechanism for the glucose-lowering properties of Polygonatum odoratum. J Agric Food Chem. 2018;66:3137–3145. doi: 10.1021/acs.jafc.8b00107. [DOI] [PubMed] [Google Scholar]
- 38.Lee B, Sun W, Lee H, Basavarajappa H, Sulaiman RS, Sishtla K, Fei X, Corson TW, Seo SY. Design, synthesis and biological evaluation of photoaffinity probes of antiangiogenic homoisoflavonoids. Bioorg Med Chem Lett. 2016;26:4277–4281. doi: 10.1016/j.bmcl.2016.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin SA, Adhikari N, Gayen S, Jha T. Homoisoflavonoids as potential antiangiogenic agents for retinal neovascularization. Biomed Pharmacother. 2017;95:818–827. doi: 10.1016/j.biopha.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 40.He F, Xu BL, Chen C, Jia HJ, Wu JX, Wang XC, Sheng JL, Huang L, Cheng J. Methylophiopogonanone A suppresses ischemia/reperfusion-induced myocardial apoptosis in mice via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol Sin. 2016;37:763–771. doi: 10.1038/aps.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin M, Sun W, Gong W, Zhou Z, Ding Y, Hou Q. Methylophiopogonanone a protects against cerebral ischemia/reperfusion injury and attenuates blood-brain barrier disruption in vitro. PLoS One. 2015;10:e0124558. doi: 10.1371/journal.pone.0124558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richard V, Kindt N, Saussez S. Macrophage migration inhibitory factor involvement in breast cancer (Review) Int J Oncol. 2015;47:1627–1633. doi: 10.3892/ijo.2015.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Günther S, Fagone P, Jalce G, Atanasov AG, Guignabert C, Nicoletti F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov Today. 2019;24:428–439. doi: 10.1016/j.drudis.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Fagone P, Mazzon E, Cavalli E, Bramanti A, Petralia MC, Mangano K, Al-Abed Y, Bramati P, Nicoletti F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J Neuroimmunol. 2018;322:46–56. doi: 10.1016/j.jneuroim.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Presti M, Mazzon E, Basile MS, Petralia MC, Bramant A, Colletti G, Bramanti P, Nicoletti F, Fagone P. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol Lett. 2018;16:2881–2886. doi: 10.3892/ol.2018.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangano K, Mazzon E, Basile MS, Marco R, Bramanti P, Mammana S, Petralia MC, Fagone P, Nicoletti F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget. 2018;9:17951–17970. doi: 10.18632/oncotarget.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kindt N, Journe F, Laurent G, Saussez S. Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets. Oncol Lett. 2016;12:2247–2253. doi: 10.3892/ol.2016.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y, Zhu D, Qi J, Qin M, Yu B. Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity. J Pharm Biomed Anal. 2010;52:757–762. doi: 10.1016/j.jpba.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Yapislar H, Taskin E. L-carnosine alters some hemorheologic and lipid peroxidation parameters in nephrectomized rats. Med Sci Monit. 2014;20:399–405. doi: 10.12659/MSM.890528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 51.Jin Y, Liu K, Peng J, Wang C, Kang L, Chang N, Sun H. Rhizoma Dioscoreae Nipponicae polysaccharides protect HUVECs from H2O2-induced injury by regulating PPARγ factor and the NADPH oxidase/ROS-NF-κB signal pathway. Toxicol Lett. 2015;232:149–158. doi: 10.1016/j.toxlet.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Atig F, Raffa M, Ali HB, Abdelhamid K, Saad A, Ajina M. Altered antioxidant status and increased lipid per-oxidation in seminal plasma of tunisian infertile men. Int J Biol Sci. 2012;8:139–149. doi: 10.7150/ijbs.8.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 54.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.HYP.32.3.488. [DOI] [PubMed] [Google Scholar]
- 56.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol. 2012;56:216–231. doi: 10.1016/j.vph.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.San José G, Fortuño A, Beloqui O, Díez J, Zalba G. NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci (Lond) 2008;114:173–182. doi: 10.1042/CS20070130. [DOI] [PubMed] [Google Scholar]
- 59.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Görlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med. 2005;38:616–630. doi: 10.1016/j.freeradbiomed.2004.09.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.