Abstract
It has been reported that loss and degradation of epidermal melanocytes is closely associated with the pathogenesis of vitiligo. In addition, CD8+ T and regulatory T (Treg) cells serve an important role during these two processes. MicroRNA-155 (miR-155) is known to contribute to the pathogenesis of vitiligo; however, the mechanism by which miR-155 regulates the development of vitiligo remains unclear. In the present study, naïve T and CD8+ T cells were isolated from a patient with non-segmental vitiligo by flow cytometry. The cells were differentiated into Treg cells by treatment with interleukin-2, transforming growth factor-β and retinoic acid. In addition, miR-155 agonists and antagonists were used to investigate the effect of miR-155 on the proliferation of CD8+ T cells, Treg cells and melanocytes. The results demonstrated that the miR-155 agonist significantly decreased the rate of CD8+ T cell growth, as well as promoted the proliferation of melanocytes by inducing an increase in the percentage of Treg cells. By contrast, the miR-155 antagonist inhibited the proliferation of melanocytes by decreasing the percentage of Treg cells. miR-155 protected melanocyte survival by increasing the number of Treg cells and by decreasing the number of CD8+ T cells. Therefore, these data may provide a new prospect for the treatment of vitiligo.
Keywords: vitiligo, microRNA-155, regulatory T cells, CD8+ T cells
Introduction
Vitiligo is a common acquired disease characterized by white spots on the skin, which affects 0.1–2% of the population worldwide (1). Accumulating evidence suggests that vitiligo is caused by the loss and degradation of epidermal melanocytes (2). Several hypotheses have been proposed for the development of this disease, including autoimmunity (3), cytotoxic metabolites, neural and genetic causes (4), and induction of oxidative stress (5,6). These factors have been suggested to explain the mechanisms underlying the melanocyte degradation, although the exact pathogenesis remains unknown.
Recent studies have demonstrated that vitiligo is an autoimmune response targeting melanocytes (7,8). Cytotoxic CD8+ T cells can specifically recognize melanocytes, which can in turn be isolated from the lesions of vitiligo subjects. In addition, the count of CD8+ T cells in the peripheral blood of patients with vitiligo is significantly increased, particularly in the advanced stages of vitiligo (9,10). Therefore, CD8+ T cells may serve a critical role during the processes of melanocyte loss and degradation.
CD4+CD25+ regulatory T (Treg) cells comprise a suppressive T cell subset that reduces the inflammatory activity of immune cells by direct contact, enabling the secretion of anti-inflammatory cytokines, such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) (11,12). Treg cells inhibit the activity of autoimmune T cells, namely CD4+ and CD8+ T cells (13). Dwivedi et al (14) have demonstrated that Treg cells were significantly decreased in active generalized vitiligo. In addition, Ben Ahmed et al (15) confirmed that the functional defect of Treg cells was involved in the pathogenesis of vitiligo. Therefore, the decrease in the number of natural Treg cells may cause the activation of CD8+ T cells, which can in turn damage the structure of melanocytes and lead to immune function disorders.
MicroRNAs (miRNAs) are small conserved non-coding RNA molecules, which have been found to serve key roles in normal cellular processes (16). Previous studies have proposed that miR-155 is a crucial regulator in the process of inflammation and immunity (17,18). In addition, miR-155 can increase the differentiation of Treg cells by activating the transcription of forkhead box P3 (Foxp3), a marker of Treg cells (19,20). A recent study has demonstrated that miR-155 was dysregulated in patients with vitiligo, and that the expression levels of the melanogenesis-associated genes in melanocytes and keratinocytes were inhibited by this miRNA (21). Furthermore, Yao et al (22) demonstrated that miR-155 regulated the differentiation of Treg cells by activating the JAK/STAT pathway. The present study further demonstrated that miR-155 upregulated the levels of Foxp3, a marker of Treg cell activity. However, this result was different from the findings of other studies. For instance, Karagiannidis et al (23) indicated that the upregulation of Foxp3 levels by glucocorticoids increased IL-10 expression. In addition, Ganesh et al (24) reported that IL-1β can increase the levels of Foxp3 and TGF-β. Despite these promising studies, the mechanisms by which miR-155 regulates the development of vitiligo remain unclear. Thus, the present study aimed to investigate the role of miR-155 in the development of vitiligo.
Materials and methods
Patient samples
All samples were obtained from the Wenzhou Medical University, between April 2017 and May 2018. Peripheral blood and skin tissues were obtained from one patient with non-segmental vitiligo (male, 49-year-old). The disease status of the patient was stable. In addition, the normal T cells were obtained from a healthy donor (male, 53-years-old). The exclusion criteria were: patients with severe liver, kidney disease, or cardiovascular diseases; participants subjected with other associated dermatoses during the last 6 months, such as psoriasis. The research was approved by the Ethics Committee of Wenzhou Medical University (Wenzhou, China; approval no. YS2019050). The patient and the healthy donor provided informed consent for their participation in the study.
Purification of naive T and CD8+ T cells
Peripheral blood mononuclear cells were obtained from the patient with vitiligo and healthy donor by Ficoll-Hypaque density gradient centrifugation. For purification of naïve T cells and CD8+ T cells, single cell suspensions of peripheral blood mononuclear cells were enriched by immunomagnetic bead selection using MACS Miltenyi system (Miltenyi Biotech, Inc.) as previously described (25). In addition, flow cytometry was used for sorting naïve T cells (CD3+CD4+CD45RA+ T cells) and CD3+CD8+ T cells. The purity of CD3+CD4+CD45RA+ T and CD3+CD8+ T cells was also evaluated using flow cytometry. Naïve T cells were enriched by depletion of magnetically labeled contaminating CD3+, CD4+, and CD45RA+ cells. CD8+ T cells were enriched by depletion of magnetically labeled contaminating CD3+, CD8+ cells. The highly enriched (90%) naïve T cells or CD8+ T cells were subsequently stained with anti-CD3 (cat. no. 64-0037-41, 1:100 dilution), anti-CD4 (cat. no. 15-0049-42, 1:100 dilution), anti-CD8 (cat. no. MHCD0800-4, 1:100 dilution) and anti-CD45RA (cat. no. 11-0458-41, 1:100 dilution) antibodies were provided by Thermo Fisher Scientific, Inc. Cells at a concentration of 4×107 cells/ml in staining buffer were incubated with indicated antibodies for 30 min on ice, followed by three washes with staining buffer.
Differentiation of naïve T cells to Treg cells
The isolated naive T cells (4×105 cells/per well) were seeded into 6-well plates coated with anti-CD3 and anti-CD28 and cultured in RPMI 1640 medium (Thermo Fisher Scientific) overnight. The cells were treated with all these reagents, including IL-2 (100 U/ml, R&D Systems, Inc.), TGF-β (10 ng/ml, R&D), and retinoic acid (10 nM, R&D Systems, Inc.), and incubated in RPMI 1640 medium (Thermo Fisher Scientific, Inc.) at 4°C for 30 min. After 4 days of stimulation, flow cytometry was used to determine the purity of CD4+CD25+FoxP3+ Treg cells (26).
Nucleofection
A human T Cell Nucleofector® kit (Lonza Inc.) and a nucleofector device (Lonza) were used for nucleofection. Initially, 1×107 Treg cells were resuspended in 100 µl Nucleofector® solution. Subsequently, 100 pM oligonucleotides (Thermo Fisher Scientific, Inc.) were added to the solution and mixed gently. The oligonucleotides included an miR-155 agonist (pre-miR-155) and its control (pre-miR-ctrl), as well as an miR-155 antagonist (anti-miR-155) and the corresponding control (anti-miR-ctrl). The miRNA sequences were as follows: pre-miR-155, 5′-CCCCUAUCACGAUUAGCAUUAAUU-3′; pre-miR-ctrl, 5′-AACCCCUAUCACGAUUAGCAUUAA-3′; anti-miR-155, 5′-UUAAUGCUAAUCGUGAUAGGGGUU-3′; and anti-miR-ctrl, 5′-AACCCCUAUCACGAUUAGCAUUAA-3′.
Thus, the oligonucleotides mixtures were carefully transferred to the electroporation cuvettes and placed in the nucleofector device, and the Treg cells were nucleofected in the X-01 program. Finally, the cells (5×105 cells/well) were transferred to a 12-well plate, prepared with 1.5 ml human T cell nucleofector medium and incubated at 37°C in a 5% CO2 incubator until analysis (22).
Cell culture
Primary melanocytes were isolated from the vitiligo patient by suction blistering and cultured in Hu 16 medium [consisted of Ham's F12 nutrient mixture (Thermo Fisher Scientific, Inc.) supplemented with 50 µg/ml gentamicin, 20 ng/ml fibroblast growth factor (Sigma Aldrich; Merck KGaA), 20 µg/ml isobutylmethylxanthine (Sigma Aldrich; Merck KGaA), 10 ng/ml cholera toxin (Sigma Aldrich; Merck KGaA)] at 37°C in a 5% CO2 incubator. The cell density in the culture flasks was 5×105/ml, and the base factor (100 µg/ml of geneticin) was added to the medium to remove keratinocytes and fibroblasts on the third day. Subsequently, the cells were seeded in 6-well plates at a density of 1×105 cells/ml, placed in an incubator for 4 h and inoculated with CD8+ T cells, Treg cells, pre-miR-155, or CD8+ T cells, Treg cells, anti-miR-155 respectively, and incubated for 72 h at 37°C.
Flow cytometry analysis
The activation of CD8+ T cells, and the ratio of Treg, CD4−CD8+ was evaluated with FACSCalibur flow cytometry (BD Biosciences, Franklin Lake, NJ, USA). The induction of cell apoptosis was detected using the Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (BD Biosciences, Franklin Lake, NJ, USA) following the manufacturer's protocol. Briefly, cells were harvested and washed with PBS twice. Next, the cells were resuspended and stained with 2 µl Annexin V and 2 µl PI for 15 min at 25°C in the dark. The number of apoptotic cells was quantified by flow cytometry.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific, Inc.) following the manufacturer's procedure. cDNA synthesis was synthesized by using a SuperScript IV Reverse Transcriptase kit (Thermo Fisher Scientific, Inc.). For miR-155 analysis, cDNA was synthesized using the PrimeScript® RT reagent kit (Takara Bio, Inc., Otsu, Japan), miR-155 RT primers (Thermo Fisher Scientific, Inc.) and 1 µg of total RNA. qPCR was then performed using the SYBR Premix Ex Taq II kit (Takara Bio, Inc.). The PCR conditions were as follows: 95°C for 5 min; then 45 cycles consisting of 94°C for 30 sec and 59 °C for 45 sec. The primer sequences used in qPCR are listed in Table I. The relative levels of the genes were normalized to those of the human β-actin gene and evaluated by the comparative quantification cycle (2−∆∆Cq) method (27).
Table I.
Primers used in polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β-actin | 5′-TGACGTGGACATCCGCAAAG-3′ | 5′-CTGGAAGGTGGACAGCGAGG-3′ |
| Foxp3 | 5′-GATCACCTCTTGGATGAGAAGG-3′ | 5′-TGTGGAAGAACTCTGGAAAGGT-3′ |
| IL-10 | 5′-GCCAGAGCCACATGCTCCTA-3′ | 5′-GATAAGGCTTGGCAACCCAAGTAA-3′ |
| TGF-β1 | 5′-GTGTGGAGCAACATGTGGAACTCTA-3′ | 5′-CGCTGAATCGAAAGCCCTGTA-3′ |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | |
| miR-155 | 5′-GCGCCGTTAATGCTAATCGTGAT-3′ |
Foxp3, forkhead box P3; IL-10, interleukin-10; TGF-β1, transforming growth factor-β; miR-155, microRNA-155.
ELISA analysis
The levels of IL-10 and TGF-β1 in the cell culture supernatant were detected using the corresponding ELISA kits (Neobioscience) according to the manufacturer's procedures.
Western blot analysis
Cells were lysed in RIPA buffer (Thermo Fisher Scientific, Inc.), and then the supernatants of cell lysates were collected. After that, BCA™ Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used to detect the concentration of proteins in the supernatants. Total protein was separated on 12% sodium dodecyl sulfate gels using polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (EMD Millipore). The blotted membranes were blocked in 5% BSA (Sigma Aldrich; Merck KGaA, Darmstadt, Germany) and incubated with primary antibodies overnight at 4°C. On the following morning, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The membranes were washed again, and the proteins were detected using a chemiluminescence detection kit (Thermo Fisher Scientific, Inc.), and Image Lab™ Software (Bio-Rad Laboratories, Inc.) was used to quantify the intensity of the bands. The primary antibodies against Foxp3 (cat. no. ab215206, 1:1,000 dilution) and GAPDH (cat. no. ab181602, 1:1,000 dilution), and the secondary antibody (cat. no. ab150077, 1:5,000 dilution) used in this experiment were provided by Abcam. GAPDH was used as a loading control.
Statistical analysis
The data are expressed as the mean ± standard deviation. All statistical analyses were performed with GraphPad Prism software (version 6.01; GraphPad Software, Inc.). Student's t-test (two-sided) was applied for comparison of continuous variables between two groups, while statistical differences among multiple groups were analyzed by one-way analysis of variance followed by Tukey's test. For all tests, a P<0.05 was considered to indicate a statistically significant difference, and a P<0.01 was considered to indicate a highly significant difference.
Results
Purification of naive T and CD8+ T cells
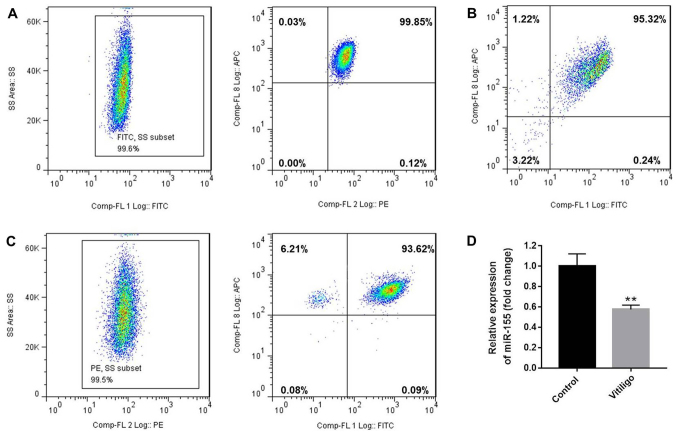
Initially, flow cytometry was used to assess the number of naïve T cells (CD3+CD4+CD45RA+ T cells) and CD3+CD8+ T cells. The purity of CD3+CD4+CD45RA+ (99.45%, Fig. 1A) and CD3+CD8+T (Fig. 1B) cells was also evaluated by flow cytometry, and was found to be >95%. Next, the isolated naive T cells were differentiated to Treg cells following treatment with IL-2, TGF-β and retinoic acid (10 nM). After 4 days of stimulation, the purity of CD4+CD25+FoxP3+ Treg cells was detected to be 93.15% (Fig. 1C). In addition, it was observed that the level of miR-155 in T cells of the patient with vitiligo was downregulated compared with that in the healthy donor (Fig. 1D).
Figure 1.
Purity of CD3+CD4+CD45RA+ T cells, CD3+CD8+ T cells and CD4+CD25+FoxP3+ Treg cells. CD3+CD4+CD45RA+ T cells and CD3+CD8+ T cells were purified by magnetic cell sorting, and their purity was determined by flow cytometry. (A) The purity of CD3+CD4+CD45RA+ T cells was 99.45% (CD3+ T cells, 99.6%; CD4+CD45RA+ T cells, 99.85%). (B) The purity of CD3+CD8+ T cells was 95.32%. (C) The purity of CD4+CD25+FoxP3+ Treg cells was 93.15% (CD4+ T cells, 99.5%; CD25+FoxP3+ T cells, 93.62%). (D) miR-155 expression in T cells of the patients with vitiligo and healthy donor was detected by reverse transcription quantitative polymerase chain reaction. **P<0.01 vs. control (healthy donor). Treg, regulatory T; miR-155, microRNA-155; FoxP3, forkhead box P3.
miR-155 increases the percentage of Treg cells, and the secretion of IL-10 and TGF-β1 in the cell culture medium
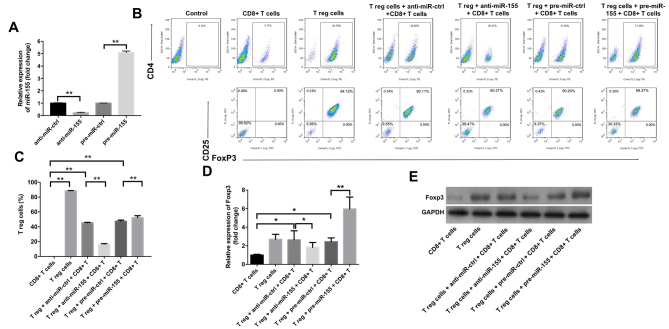
The study subsequently examined the effects of miR-155 on the differentiation of Treg cells using flow cytometry. As indicated in Fig. 2A, treatment with anti-miR-155 significantly downregulated the level of miR-155 in Treg cells, while pre-miR-155 exhibited the opposite effect, as compared with the corresponding control groups. In addition, the results revealed that anti-miR-155 caused a significant decrease in the percentage of Treg cells in the cell culture medium, while pre-miR-155 markedly increased this percentage (Fig. 2B and C). Furthermore, it was observed that anti-miR-155 significantly inhibited the gene and protein levels of Foxp3, while pre-miR-155 exhibited the opposite effects (Fig. 2D and E).
Figure 2.
miR-155 upregulated the percentage of Treg cells in primary melanocytes. Anti-miR-ctrl, anti-miR-155, pre-miR-ctrl and pre-miR-155 were transfected into Treg cells. (A) The level of miR-155 in Treg cells was detected by RT-qPCR. (B) Flow cytometry graphs and (C) percentage of Treg cells, detected after 3 days of stimulation. Representative fluorescence-activated cell sorting images from a single case are shown. (D) mRNA and (E) protein expression levels of Foxp3 in T cells, detected by RT-qPCR and western blot analysis, respectively, at 3 days after transfection. Collective results from three independent experiments are shown. *P<0.05 and **P<0.01. miR-155, microRNA-155; Treg, regulatory T; FoxP3, forkhead box P3; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; ctrl, control.
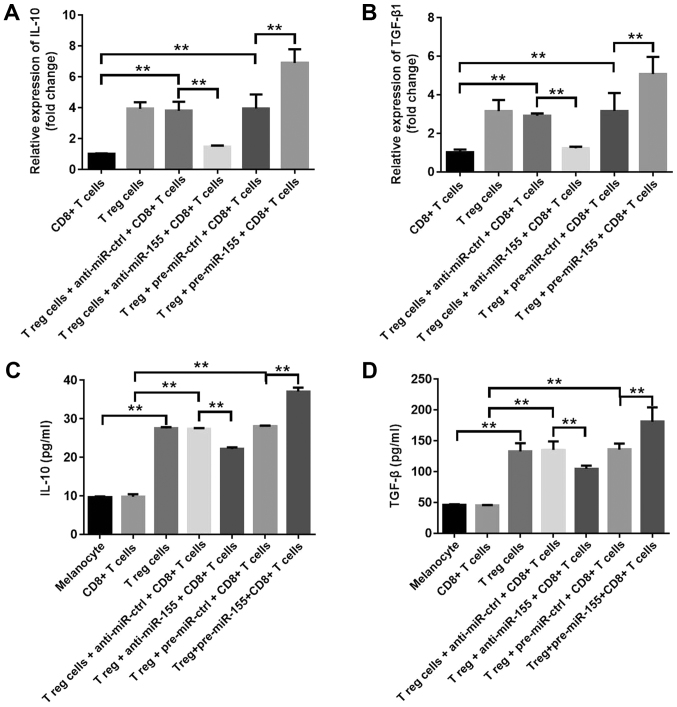
In order to investigate the effects of miR-155 on the function of Treg cells, the mRNA levels of IL-10 and TGF-β1 were assessed in T cells, and the extracellular secretions of these cytokines in the culture medium were also examined. The results indicated that pre-miR-155 significantly increased IL-10 and TGF-β1 mRNA expression levels, while anti-miR-155 markedly downregulated the levels of these cytokines (Fig. 3A and B). Furthermore, the extracellular secretions of IL-10 and TGF-β1 were significantly increased in the pre-miR-155 group, which was consistent with the previous findings, while they were downregulated in the anti-miR-155 group (Fig. 3C and D). These data suggested that miR-155 increased the percentage of Treg cells, and promoted the secretion of IL-10 and TGF-β1 in the cell culture medium.
Figure 3.
miR-155 increased the secretion of IL-10 and TGF-β1 in the cell culture supernatant. (A) IL-10 and (B) TGF-β1 mRNA levels were detected using reverse transcription-quantitative polymerase chain reaction at 3 days after transfection. (C) IL-10 and (D) TGF-β1 levels in the cell culture medium were detected by ELISA at 3 days after transfection. Data are representative of three independent experiments. **P<0.01. miR-155, microRNA-155; IL-10, interleukin-10; TGF-β1, transforming growth factor-β1; Treg, regulatory T; ctrl, control.
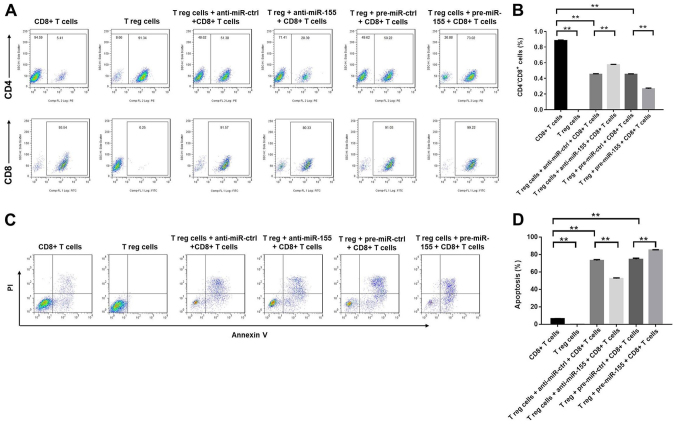
miR-155 decreases the percentage of CD8+ T cells by inducing apoptosis in the cell culture medium
The effects of miR-155 on CD8+ T cells were subsequently evaluated using flow cytometry. The application of anti-miR-155 significantly increased the percentage of CD8+ cells, while pre-miR-155 exhibited the opposite effect (Fig. 4A and B). In addition, Treg cells induced the apoptosis of CD8+ T cells, which was further enhanced by treatment with pre-miR-155, and reduced by treatment with anti-miR-155 (Fig. 4C and D). In conclusion, the results demonstrated that miR-155 decreased the percentage of CD8+ T cells by promoting the induction of apoptosis.
Figure 4.
miR-155 decreased the percentage of CD8+ T cells via promoting apoptosis in the cell culture medium. Anti-miR-ctrl, anti-miR-155, pre-miR-ctrl and pre-miR-155 were transfected into Treg cells. (A) Flow cytometry images and (B) the percentage of CD8+ cells, as determined at 3 days after transfection. Representative fluorescence-activated cell sorting images from a single case are shown. (C) The apoptosis of CD8+ T cells was detected using the Annexin V-FITC/PI Apoptosis Detection kit. (D) Cell apoptosis rate was quantified by flow cytometry. Data are representative of three independent experiments. **P<0.01. miR-155, microRNA-155; Treg, regulatory T; ctrl, control.
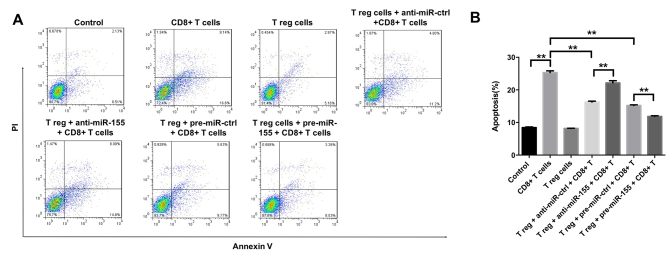
Treg cells and/or miR-155 inhibit the induction of melanocyte apoptosis by CD8+ T cells
To further investigate the effects of miR-155 on melanocytes, the induction of melanocyte apoptosis was detected by flow cytometry, following successful transfection with miR-155 mimics and subsequent 3 days of cell incubation. It was observed that CD8+ T cells were able to induce apoptosis in melanocytes, which was partly reversed by the function of Treg cells (Fig. 5A and B). In addition, the CD8+ T cell-induced apoptosis was markedly inhibited by the application of pre-miR-155 and significantly enhanced by anti-miR-155 (Fig. 5A and B). Taken together, the data suggested that Treg cells and/or miR-155 were able to inhibit the induction of melanocyte apoptosis by CD8+ T cells.
Figure 5.
Treg cells and/or miR-155 inhibited the apoptosis of melanocytes induced by CD8+ T cells. (A) Apoptosis of melanocytes was detected with the Annexin V-FITC/PI Apoptosis Detection kit and quantified by flow cytometry at 3 days after transfection. The percentages of positive cells are shown in each panel. (B) Percentage of apoptotic cells, as quantified by flow cytometry. The collective results of three independent experiments are shown in the histogram. **P<0.01. miR-155, microRNA-155; Treg, regulatory T; ctrl, control.
Discussion
It has recently been reported that miR-155 can modulate the expression of melanogenesis-associated genes both in keratinocytes and melanocytes, suggesting its important role during the pathogenesis of vitiligo (21). The present study revealed that miR-155 was able to protect melanocytes from CD8+ T lymphocytes by regulating the activity of Treg cells. The overexpression of miR-155 promoted the differentiation and function of Treg cells. It was further demonstrated that miR-155 was able to inhibit the differentiation of CD8+ T cells and decrease the apoptotic rate of the melanocytes.
Cell-mediated autoimmunity is associated with the degradation of melanocytes in vitiligo (28). Previous studies have reported an apparent increase in the number of CD8+ T cells and a significant reduction in the number of Treg cells in patients with generalized vitiligo, which indicates that the infiltration of CD8+ T cells and the deregulation of natural Treg cells may be closely associated with the pathogenesis of this disease (29,30). In the present study, it was demonstrated that the anti-miR-155 group exhibited a decrease in the percentage of Treg cells and an increase in the percentage of CD8+ T cells. In addition, transfection of the cells with anti-miR-155 significantly increased the apoptotic rate of the melanocytes. A study by Le Poole and Mehrotra (31) indicated that a considerably low number of Treg cells was able to effectively interfere with depigmentation when transferred into depigmenting mice. The present study is in accordance with these previous findings, demonstrating that the pre-miR-155 group can decrease the apoptotic rate of melanocytes by increasing the number of Treg cells. The current study further revealed that miR-155 exerted a positive regulation on the differentiation of Treg cells.
Tregs can mediate their suppressive activity by a cellular contact dependent mechanism or by suppressor cytokines, including TGF-β1 and IL-10 (23,24). In the current study, it was observed that miR-155 increased the percentage of Treg cells, and promoted the secretion of IL-10 and TGF-β1 in the cell culture medium. Nevertheless, a previous study by Gracias et al (32) reported that miR-155 overexpression augmented anti-viral CD8+ T cell responses in C57Bl/6 mice. The contrary results were observed in the present study, which may be due to the different species investigated. In addition, TGF-β1 and IL-10 have been reported to exhibit an inhibitory effect on autoimmune responses, and a decrease in TGF-β1 and IL-10 levels in the skin compromised the local immune suppressing function, leading to an autoimmune reaction against the melanocytes in the patients with vitiligo (23,24).
A previous study has demonstrated that CD8+ T cells induce the apoptosis of autologous melanocytes at the perilesional margins of vitiligo patients (33). Several previous studies have also reported that cytotoxic CD8+ T cells can specifically recognize melanocytes in patients with vitiligo (10,11). Therefore, the cytotoxic effect of CD8+ T cells on melanocytes has been suggested as a key factor during the pathogenesis of vitiligo (34,35). In addition, CD69 and CD137 serve an important role in CD8+ T cell activation (36). CD69 was reported to be an early surface marker of activated T cells, while CD137 was only expressed on the surface of activated T cells (37). These data suggested that miR-155 was able to inhibit the activation of CD8+ T cells by regulating the differentiation of Treg cells.
However, there are certain limitations in the present study. The study included solely cellular assays, and the experiments and conclusions were based on samples obtained from only one patient. Therefore, further experiments are required to clarify the function of miR-155 during the pathogenesis of vitiligo.
In conclusion, the present study indicated that miR-155 positively regulated the number of Treg cells, which prevented the degradation of melanocytes from CD8+ T cells. Therefore, it is proposed that miR-155 may serve as a potential therapeutic target for the treatment of patients with vitiligo.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The National Natural Science Foundation of China (grant nos. 81703105, 81571395 and 81771531) and Wenzhou Science & Technology Bureau of China (grant no. Y20190576).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ML, ZJL, JL and FL analyzed and interpreted the patient data, and were major contributors in the development of the first draft of the present manuscript. QZ, ZML and YW participated in experiment design, tissue collection and experiment execution. KW and YX participated in experiment design, tissue collection, and reviewed and approved the final draft of the manuscript prior to submission.
Ethics approval and consent to participate
Ethics approval for the present study was provided by the Wenzhou Medical University Ethics Committee. Informed consent was obtained from the patient and healthy donor.
Patient consent for publication
Informed consent was obtained from the patient and healthy donor.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206–1212. doi: 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, Cole JB, Gowan K, Holland PJ, Bennett DC, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676–680. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 4.Gey A, Diallo A, Seneschal J, Leaute-Labreze C, Boralevi F, Jouary T, Taieb A, Ezzedine K. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756–761. doi: 10.1111/bjd.12166. [DOI] [PubMed] [Google Scholar]
- 5.Shi Q, Zhang W, Guo S, Jian Z, Li S, Li K, Ge R, Dai W, Wang G, Gao T, Li C. Oxidative stress-induced overexpression of miR-25: The mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016;23:496–508. doi: 10.1038/cdd.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W, Zhao Y, Kong Y, Zhang W, Ma W, Li W, Wang K. Geniposide prevents H2 O2 -induced oxidative damage in melanocytes by activating the PI3K-Akt signalling pathway. Clin Exp Dermatol. 2018;43:667–674. doi: 10.1111/ced.13409. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Rauf A, Khan H, Meher BR, Hassan SSU. A holistic review on the autoimmune disease vitiligo with emphasis on the causal factors. Biomed Pharmacother. 2017;92:501–508. doi: 10.1016/j.biopha.2017.05.095. [DOI] [PubMed] [Google Scholar]
- 8.Strassner JP, Harris JE. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr Opin Immunol. 2016;43:81–88. doi: 10.1016/j.coi.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, Vyth-Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 10.Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003;83:683–695. doi: 10.1097/01.LAB.0000069521.42488.1B. [DOI] [PubMed] [Google Scholar]
- 11.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of tregulatory cell function. Autoimmun Rev. 2008;7:370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, Brand D, Ramalingam R, Kiela PR, Horwitz DA, et al. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4:409–419. doi: 10.1093/jmcb/mjs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R. Decreased regulatory T-cells and CD4(+) /CD8(+) ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res. 2013;26:586–591. doi: 10.1111/pcmr.12105. [DOI] [PubMed] [Google Scholar]
- 15.Ben Ahmed M, Zaraa I, Rekik R, Elbeldi-Ferchiou A, Kourda N, Belhadj Hmida N, Abdeladhim M, Karoui O, Ben Osman A, Mokni M, Louzir H. Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res. 2012;25:99–109. doi: 10.1111/j.1755-148X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 16.Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, Rådinger M. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014;133:1429–1438. doi: 10.1016/j.jaci.2013.11.008. 1438.e1421-1427. [DOI] [PubMed] [Google Scholar]
- 18.Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R, et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity. 2013;38:742–753. doi: 10.1016/j.immuni.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liston A, Lu LF, O'Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempinska-Podhorodecka A, Milkiewicz M, Wasik U, Ligocka J, Zawadzki M, Krawczyk M, Milkiewicz P. Decreased expression of vitamin D receptor affects an immune response in primary biliary cholangitis via the VDR-miRNA155-SOCS1 pathway. Int J Mol Sci. 2017;18:E289. doi: 10.3390/ijms18020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Šahmatova L, Tankov S, Prans E, Aab A, Hermann H, Reemann P, Pihlap M, Karelson M, Abram K, Kisand K, et al. MicroRNA-155 is dysregulated in the skin of patients with vitiligo and inhibits melanogenesis-associated genes in melanocytes and keratinocytes. Acta Derm Venereol. 2016;96:742–747. doi: 10.2340/00015555-2394. [DOI] [PubMed] [Google Scholar]
- 22.Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, Liao YH. MicroRNA-155 modulates treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7:e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel PY, Menz G, Akdis CA, Blaser K, Schmidt-Weber CB. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Ganesh BB, Bhattacharya P, Gopisetty A, Sheng J, Vasu C, Prabhakar BS. IL-1β promotes TGF-β1 and IL-2 dependent Foxp3 expression in regulatory T cells. PLoS One. 2011;6:e21949. doi: 10.1371/journal.pone.0021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polanczyk MJ, Walker E, Haley D, Guerrouahen BS, Akporiaye ET. Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4 + CD25 + Foxp3 + and CD4 + CD25 - Foxp3 + T cells. J Transl Med. 2019;17:219. doi: 10.1186/s12967-019-1967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Klarquist J, Eby JM, Henning SW, Li M, Wainwright DA, Westerhof W, Luiten RM, Nishimura MI, Le Poole IC. Functional cloning of a gp100-reactive T-cell receptor from vitiligo patient skin. Pigment Cell Melanoma Res. 2016;29:379–384. doi: 10.1111/pcmr.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nigam PK, Patra PK, Khodiar PK, Gual J. A study of blood CD3+, CD4+, and CD8+ T cell levels and CD4+:CD8+ ratio in vitiligo patients. Indian J Dermatol Venereol Leprol. 2011;77:111. doi: 10.4103/0378-6323.74993. [DOI] [PubMed] [Google Scholar]
- 30.Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One. 2012;7:e37513. doi: 10.1371/journal.pone.0037513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Poole IC, Mehrotra S. Replenishing regulatory T cells to halt depigmentation in vitiligo. J Investig Dermatol Symp Proc. 2017;18:S38–S45. doi: 10.1016/j.jisp.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Zhou M, Wan Y, Xu A. CD8+ T cells from vitiligo perilesional margins induce autologous melanocyte apoptosis. Mol Med Rep. 2013;7:237–241. doi: 10.3892/mmr.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci (Lond) 2011;120:99–120. doi: 10.1042/CS20090603. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Yang S, Lei J, Hu W, Chen R, Lin F, Xu AE. Role of chemokines and the corresponding receptors in vitiligo: A pilot study. J Dermatol. 2018;45:31–38. doi: 10.1111/1346-8138.14004. [DOI] [PubMed] [Google Scholar]
- 36.Guan C, Li Q, Song X, Xu W, Li L, Xu A. Antroquinonol exerts immunosuppressive effect on CD8(+) T Cell proliferation and activation to resist depigmentation induced by H2O2. Oxid Med Cell Longev. 2017;2017:9303054. doi: 10.1155/2017/9303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arneth BM. Activation of CD4 and CD8 T cell receptors and regulatory T cells in response to human proteins. PeerJ. 2018;6:e4462. doi: 10.7717/peerj.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.