Abstract
Calcium phosphate-based bone substitutes have been widely used for bone repair, augmentation and reconstruction in bone implant surgery. While some of these substitutes have shown excellent biological efficacy, there remains a need to improve the performance of the current calcium phosphate-based bone substitutes. Strontium ions (Sr) can promote new osteogenesis, inhibit osteoclast formation and increase osteoconductivity. However, the therapeutic effect and mechanism of strontium-containing α-calcium sulfate hemihydrate (Sr-CaS) remains unclear. The present study created bone injuries in rats and treated the injuries with Sr-CaS. Then Cell Counting Kit-8, soft agar colony formation, flow cytometry, Transwell and Alizarin Red staining assays were performed to assess the bone cells for their proliferation, growth, apoptosis, invasion, and osteogenic differentiation abilities. The bone reconstructive states were measured by the microCT method, hematoxylin and eosin staining and Masson staining. Bone-related factors were analyzed by the reverse transcription-quantitative PCR assay; transforming growth factor (TGF)-β, mothers against decapentaplegic homolog (Smad)2/3 and β-catenin expression was measured by western blot analysis and osteocalcin (OCN) expression was assessed by immunohistochemistry. Sr-CaS did not significantly affect the proliferation and apoptosis of bone marrow stem cells (BMSCs), but did accelerate the migration and osteogenic differentiation of BMSCs in vitro. Sr-CaS promoted bone repair and significantly increased the values for bone mineral density, bone volume fraction, and trabecular thickness, but decreased trabecular spacing in vivo in a concentration-dependent manner. In addition, Sr-CaS dramatically upregulated the expression levels of genes associated with osteogenic differentiation (Runt-related transcription factor 2, Osterix, ALP, OCN and bone sialoprotein) both in vitro and in vivo. Sr-CaS also increased Smad2/3, TGF-β and phosphorylated-β-catenin protein expression in vitro and in vivo. These results indicated that materials that contain 5 or 10% Sr can improve bone defects by regulating the TGF-β/Smad signaling pathway.
Keywords: strontium-containing α-calcium sulfate hemihydrate, bone repair, transforming growth factor-β/Smad signaling pathway
Introduction
Bone defects caused by tumor surgery, infection or congenital bone malformation, are usually treated by bone grafting to increase bone regeneration (1). Bone grafting is one of the most common surgical procedures performed to increase bone regeneration via the surgical implantation of materials and ranks second only to blood transfusion and tissue transplantation (2). According to incomplete statistics, more than two million bone grafts are performed worldwide each year (3). At present, the materials used for bone reconstruction are mainly obtained from autograft and allograft materials (4,5). The use of autologous bone for bone grafts offers several advantages, such as superior bone conduction, inducibility and osteogenesis. As a result, autogenous bone grafts have become the ‘gold standard’ for use in promoting bone regeneration. However, the limited supply of autologous bone and the difficulties with obtaining appropriate donors limit the use of autogenous bone grafts in clinical practice (6).
Bone substitute materials are divided into synthetic and biological materials, which are characterized by their morphology, chemical composition, and crystallinity (7). These materials have to meet specific criteria, such as having the proper surface configuration and structure needed to simulate the desired effect in the body (8). As calcium sulfate (CaS) is biodegradable and biocompatible (9,10), it is frequently used as an insulating material for bone defects, where it serves to inhibit the growth of fibrous tissues and promote bone growth (11,12). CaS serves as an external fixator and can be used in distraction osteogenesis to cure osteomyelitis and bone tumors (13). CaS increases the time of distraction osteogenesis in the case of synchronous biodegradation. Studies have also suggested that CaS helps to maintain the connections between bone and periosteum, which promotes osteogenesis (14–17); in addition, CaS plays an essential role bone formation (18). However, a previous study showed that CaS does not significantly alter CaS levels in the bone matrix and CaS does not have good osteoconductive properties or stimulate osteogenesis (19). Therefore, it is beneficial to add stimulatory agents such as trace elements to orthopedic implant materials to promote bone growth.
A growing body of evidence indicates that strontium (Sr) promotes the differentiation of osteoblasts and inhibits the formation and absorption of osteoclasts (20,21). A previous study showed that Sr is beneficial for the synthesis of collagen in vitro (22). Therefore, the implantation of a bone substitute material followed by its continuous treatment with Sr might help to stimulate bone growth in a specific desired location. Bone implant materials containing SR have been extensively used in bone tissue engineering studies. For example, SR-containing mesoporous bioactive glass has the advantages of providing good bone formation bioactivity, enhanced mechanical strength and ion release regulation, and therefore can expected to be widely used for stimulating bone regeneration (23). Sr substituted bioactive glass is more effective at accelerating bone formation than is bioactive glass without Sr (24,25).
In the present study, co-precipitation and hydrothermal techniques were used to prepare a novel Sr-CaS material, and also established a tibia bone defect model in Sprague Dawley (SD) rats. The biocompatibility and safety of different concentrations of Sr-CaS in the model were investigated. the effects and mechanisms of different Sr-CaS concentrations on bone repair were also investigated in vitro and in vivo.
Materials and methods
Animals
Healthy male specific pathogen free SD rats (7-weeks-old, 180–230 g) were provided by the Experimental Animal Center of Southern Medical University (Guangzhou, China). After the SD rats were purchased, they were raised for 7–10 days according to the national standard (freely available food and water, 50–60% humidity, 21–25°C and a 12-h light and dark cycle) prior to being used in experiments. All protocols used for animal studies were approved by the Animal Ethics Committee of Southern Medical University.
Sr-CaS extraction preparation
The method of extraction preparation was referred to in a previous study by Li et al (26). The original extraction of Sr-CaS material was kept at concentration of 3 g/ml and 25% dilution extraction was generated by diluting with normal saline by 1:3.
Establishment of the animal model
The bone defect models were created as previously described (27,28). The rats received general anesthesia by an intravenous injection of 3% pentobarbital sodium (30 mg/kg body weight). Next, the skin over the proximal tibia was incised and the periosteum was cleared using a periosteal elevator. A micro-burr with a 0.8 mm tip was used to create a defect (3 mm wide and 5 mm long) in both tibias, starting at 10 mm below the articular surface in the anteromedial cortex. The defects and intramedullary canals were washed with physiological saline to remove any residual bone and bone marrow.
Experimental groups
SD rats were assigned to four different groups based on the materials that were used to fill their defects: i) A blank group in which no material was implanted into the bone defects; ii) a CaS group in which CaS was implanted into the bone defects; iii) a 5% Sr-CaS group in which 5% Sr-CaS was implanted into the bone defects and iv) a 10% Sr-CaS group in which 10% Sr-CaS was implanted into the bone defects. The defects were filled flush to the anterior cortex with the paste-form material prior to being allowed to set in situ. After surgery, the skin was carefully sutured and the rats were injected with an antibiotic (3% penicillin) for 2 days. All mice recovered well from surgery and were housed separately in plastic cages. Food and water were available ad libitum.
Sample collection
The mice were closely observed and recorded data for the following parameters: Postoperative animal spirit, food intake, activity, wound drainage and swelling. At 8 h post-surgery, specimens of tibia were obtained and analyzed by micro-computed tomography (CT) scans. Consecutive micro-CT cross section images of regions of interest were obtained and the values for various parameters were calculated three-dimensional model visualization software (CTVol ver. 2.0, Bruker). At 8 weeks after surgery, the rats were sacrificed, the amount of osteotylus in the bone defects was evaluated and decalcified specimens were prepared for routine sectioning.
Cell culture and treatment
Bone marrow mesenchymal stem cells (BMSCs) were isolated from cavities of the femurs and tibias of SD rats as previously described (28). The isolated BMSCs were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 2 mM l-glutamine and penicillin (100 U/ml)/streptomycin (100 g/ml) at 37°C in a humidified atmosphere of 5% CO2. The Sr-CaS stock material was diluted 1:3 with DMEM. The BMSCs were divided into the following four groups based on the different materials used for treatment: Blank group (treated with an equal amount of PBS), CaS group, 5% Sr-CaS group and 10% Sr-CaS group, respectively.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR) assay
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract the total RNA from the rat tissues and treated BMSCs. A 50 ng sample of total RNA was used to synthesize cDNA with incubation at 37°C for 15 min and then at 85°C for 5 sec by using the reagents in a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan). The RT-qPCR assay was carried out for 40 cycles with pre-denaturation at 95°C for 30 sec, and then denaturation at 95°C for 5 sec, and finally annealing/extension at 60°C for 30 sec by using Bestar™ qPCR MasterMix (cat. no. 2043; DBI Bioscience, Shanghai, China) on an ABI 7500 detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers used in the present study are shown in Table I. GAPDH was used as internal reference gene, and relative expression of candidate genes were calculated using 2−ΔΔCt methods (29). All the primers were synthesized by Invitrogen (Thermo Fisher Scientific, Guangzhou, China).
Table I.
The sequence of primers used in the present study.
| ID | Sequence (5′-3′) | Product length (bp) |
|---|---|---|
| GAPDH F | CCTCGTCTCATAGACAAGATGGT | 169 |
| GAPDH R | GGGTAGAGTCATACTGGAACATG | |
| ALP F | TGTAGGTGCTGTGGTCAAGG | 177 |
| ALP R | AGAGTGACGGTGTCGTAGCC | |
| RUNX2 F | GAATGATGAGAACTACTCTGCCG | 144 |
| RUNX2 R | GGATTTGTGAAGACCGTTATGG | |
| OCN F | CAACAATGGACTTGGAGCCC | 133 |
| OCN R | ATAGATGCGCTTGTAGGCGT | |
| OPG F | GCTCCTGGCACCTACCTAAA | 157 |
| OPG R | ACTCCTGTTTCACGGTCTGC | |
| BSP F | CTGACCAGTTATGGCACCAC | 278 |
| BSP R | TAATCCTGACCCTCGTAGCC |
ALP, alkaline phosphatase; OCN, osteocalcin; R, reverse; F, forward; OPG, osteoprotein; BSP, bone sialoprotein.
Western blotting assay
Total proteins were extracted by using RIPA buffer (P0013B; Beyotime Institute of Biotechnology) containing phenylmethylsulfonyl fluoride and the protein concentration in each extract was measured using a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.). Aliquots of total protein (~20 µg) were separated by 10% SDS-PAGE. The separated protein bands were transferred onto nitrocellulose membranes (EMD Millipore, Billerica, MA, USA), which were subsequently blocked by incubation in 5% low fat dried milk for 2 h at room temperature. After being washed, the membranes were incubated overnight with primary antibodies at 4°C; after which, they were incubated with horseradish peroxidase conjugated donkey-anti-rabbit secondary antibodies at room temperature for 2 h. Immunostaining was detected with the use of enhanced chemiluminescent reagents (P0018FS, Beyotime Institute of Biotechnology). The primary antibodies used in the study were Smad2/3 (Cell Signaling Technology, Inc., Danvers MA, USA; cat. no. 3102, dilution 1:1,000), phosphorylated (p)-Smad2/3 (Cell Signaling Technology, Inc.; cat. no. 8828, dilution 1:1,000), TGF-β (Abcam, Cambridge, MA, USA; cat. no. ab31013, dilution 1:1,000), β-catenin (Cell Signaling Technology, Inc.; cat. no. 9562, dilution 1:1,000), p-β-catenin (Cell Signaling Technology, Inc.; cat. no. 4270, dilution 1:1,000) and GAPDH (Abcam; cat. no. ab9385, dilution 1:5,000). Then secondary anti-rabbit antibodies (Abcam; cat. no. ab7097, dilution 1:10,000) were used. Bands were quantified using Image-Pro Plus (ver. 6.0; Media Cybernetics, Inc., USA).
Cell proliferation assay
Cell proliferation ability was evaluated by using a Cell Counting Kit-8 assay (CCK-8; Sigma-Aldrich; Merck KGaA). In brief, the treated BMSCs were seeded into 96-well plates. Next, 15 µl of CCK-8 solution was added to each well and incubated for 3 h. The optical density of each well was measured with a microtiter plate reader (SpectraMax; Molecular Devices, LLC, Sunnyvale, CA, USA).
Colony formation assay
The treated BMSCs were seeded into 7 cm culture dish at a density of 200 cell/well and maintained in complete medium for 14 days at 37°C in a humidified atmosphere with 5% CO2. The cell colonies were fixed with 4% paraformaldehyde for 15 min at room temperature and then stained with 0.5% crystal violet for 15 min at room temperature. The numbers of colonies were counted under a light microscope.
ELISA
Cells were plated into a 96-well plate at a density of 1×104 cells/well. Next, cell lysis buffer was added to each well; after which, the plate was centrifuged and the supernatant fractions were collected. Alkaline phosphatase (ALP) concentrations were then measured using an ALP assay kit (cat. no. A059-2; Nanjing Jiancheng Bioengineering Institute).
Cell apoptosis analysis
The treated BMSCs were harvested and washed with PBS. The BMSCs were then fixed in 70% ethanol for 2 h at room temperature and treated with Annexin V-fluorescein isothiocyanate and PI (KeyGen Biotech Co., Ltd.) for 10 min in the dark. The results were analyzed by flow cytometer (BD Biosciences; Becton-Dickinson and Company, Franklin Lakes, NJ, USA) with FlowJo software (ver. 7.6.1, FlowJo LLC).
Transwell assay
Treated BMSCs (1×105 cells/ml) were re-suspended in 0.2 ml of culture medium and added to the upper chambers of a Transwell plate, while 0.8 ml of culture medium containing 20% FBS was added to the lower chambers. After incubation for 48 h in a cell incubator at 37°C with 5% CO2, the non-invading cells on the membrane surface were removed and the migrated cells were fixed with 4% paraformaldehyde for 15 min at room temperature and subsequently stained with 0.5% crystal violet (Beyotime Institute of Biotechnology, Beijing, China) for 10 min at room temperature. The number of migrated cells was then counted under a light microscope (OLYMPUS CX41; Olympus Corporation).
Alizarin Red staining
Treated BMSCs (5×105 cells/ml) were seeded into 6-well plates and maintained in complete medium for 24 h at 37°C in a 5% CO2 atmosphere. Next, the cells were washed with PBS, fixed with 95% ethanol for 15 min at room temperature and then stained with 1% Alizarin Red for 5 min at room temperature. After washing, the staining results were obtained by using a light microscope (OLYMPUS CX41; Olympus Corporation).
Hematoxylin and eosin (H&E) staining and Masson staining
The tissues from each group were fixed in 4% paraformaldehyde for 24 h at room temperature and then decalcified using Quick Decalcifying Solution (cat. no. G1107; Servicebio, Wuhan, China). Next, the tissues were embedded with paraffin and 5-µm sections were obtained for dehydration with different concentrations of alcohol. For H&E staining, 5-µm sections were stained with H&E solution (hematoxylin: 5 min; eosin: 2 min) (cat. no. H8070; Beijing Solarbio Science & Technology Co., Ltd.). For Masson staining, 5-µm sections were first treated with Ponceau solution for 5 min, then with phosphomolybdic acid solution for 4 min and finally with aniline blue solution for 10 min. All the procedures were performed at room temperature. The stained sections were analyzed by light microscopy (OLYMPUS CX41; Olympus Corporation).
Immunohistochemistry
Slides with 4-µm thick tissue sections were treated with 3% H2O2 for 25 min at room temperature in the dark and then washed 3 times with PBS. The slides were then incubated in 3% bovine serum albumin (AR1006, Wuhan Boster Biological Technology, Ltd.) at room temperature for 30 min, followed by an overnight incubation with the primary antibodies (ab13418, dilution: 1:100; Abcam) at 4°C. Next, the slides were incubated with the secondary antibodies (Abcam; cat. no. ab150113, dilution 1:1,000), followed by treated with chromogenic agent, DAB (K5007, Dako; Agilent Technologies, Inc.) at room temperature for 50 min and then incubated with 3,3′-diaminobenzidine. Finally, the slides were stained with hematoxylin for 3 min at room temperature and dehydrated with different concentrations of alcohol. The stained tissues were observed under a light microscope (Nikon Eclipse TI-SR; Nikon Corporation, Tokyo, Japan).
Statistical analysis
All data in this study was presented as mean ± standard deviation. One-way analysis of variance followed by Tukey's test was used to analyze the data. P<0.05 was considered to indicate a statistically significant difference. All the experiments in this study were repeated in triplicate.
Results
Sr-CaS does not alter the proliferation and apoptosis of BMSCs
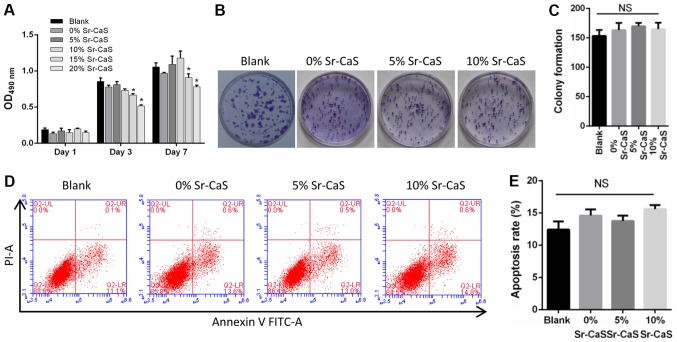
To explore the effect of Sr-CaS on BMSCs, compounds containing different levels of calcium and strontium ions were prepared [0% Sr (pure CaS), 5% Sr-CaS, and 10% Sr-CaS, respectively]. These compounds were then used to treat BMSCs for periods for 1, 3 and 7 days, respectively. The results from the CCK-8 and soft agar colony formation assays revealed that none of the compounds significantly affected the proliferative ability of the BMSCs (Fig. 1A-C). These results were further confirmed by flow cytometry assays (Fig. 1D and E). Therefore, 5 and 10% Sr-CaS (25% dilution ratio) had no significant cytotoxicity when administered to BMSCs.
Figure 1.
Effects of Sr-CaS on BMSC proliferation and apoptosis at 7 days after treatment. (A) The proliferative ability of BMSCs treated with 0, 5 or 10% Sr-CaS at 1, 3, and 7 days after treatment, respectively. *P<0.05 vs. Blank. (B) The soft agar colony formation assay was used to analyze the effect of 0, 5 and 10% Sr-CaS on the proliferative ability of BMSCs. (C) The number of cell colonies was counted. (D) BMSCs were treated with 0, 5 or 10% Sr-CaS for 7 days, and their apoptosis rates were determined by flow cytometry performed with Annexin V/PI staining. (E) The percentage of apoptotic cells was calculated. Sr, strontium; BMSCs, bone marrow stem cells; CaS, calcium sulphate; PI, propidium iodide; OD, optical density; NS, not significant.
Sr-CaS promotes the migration and osteogenic differentiation of BMSCs after 7 days after treatment
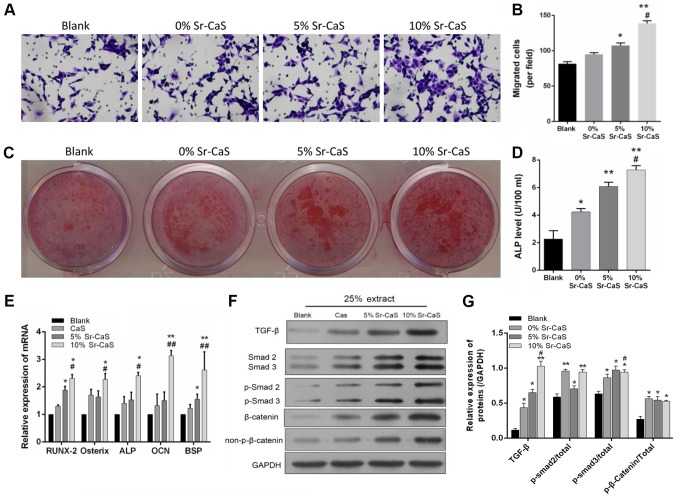
To further investigate the effect of Sr-CaS on BMSC migration and osteogenic differentiation, BMSCs that had been treated with 0, 5, or 10% Sr-CaS for 7 days were used in Transwell and Alizarin Red staining assays. The results showed that the migratory ability of BMSCs in the 5 Sr-CaS and 10% Sr-CaS groups were significantly increased when compared with BMSCs in the blank group (P<0.05). Furthermore, the migratory ability of BMSCs in the 10% Sr-CaS group was significantly increased compared with BMSCs in the 5% Sr-CaS group (P<0.01; Fig. 2A and B). The results of Alizarin Red staining indicated that Sr-CaS could promote the osteogenic differentiation of BMSCs and this effect increased in conjunction with the increase in Sr concentration. ELISA was then used to assess the ALP levels in the treated BMSCs. The results showed that Sr-CaS significantly increased ALP levels and those levels gradually increased in conjunction with the increase in Sr concentration (P<0.05; Fig. 2D).
Figure 2.
Effects of Sr-CaS on BMSC migration and osteogenic differentiation at 7 days after treatment. (A) The effects of 0, 5, and 10% Sr-CaS on the invasive ability of BMSCs were measured by a Transwell assay at 7 days after treatment. (B) The numbers of migrated cells are shown. *P<0.05 and **P<0.01 vs. blank group; #P<0.05 vs. 5% Sr-CaS group. (C) The osteoblastic differentiation ability of BMSCs treated with 0, 5 or 10% Sr-CaS for 7 days was assessed by Alizarin Red staining. (D) ALP levels were detected by ELISA. *P<0.05 and **P<0.01 vs. blank group; #P<0.05 vs. 5% Sr-CaS group. (E) BMSCs were treated with 0, 5 or 10% Sr-CaS for 7 days, and their levels of RUNX2, Osterix, ALP, OCN, and BSP mRNA expression were analyzed by the reverse transcription-quantitative PCR assay. TGF-β, Smad2/3, and β-catenin protein expression was measured by (F) western blotting and (G) densitometry analysis. *P<0.05 and **P<0.01 vs. blank group; #P<0.05, ##P<0.01 vs. CaS group. ALP, alkaline phosphatase; Sr, strontium; BMSCs, bone marrow stem cells; CaS, calcium sulphate; OCN, osteocalcin; TGF, transforming growth factor; BSP, bone sialoprotein; p-Smad, phosphorylated mothers against decapentaplegic homolog; RUNX2, runt-related transcription factor 2.
In addition, the influence of Sr-CaS on the expression of various genes associated with the osteogenic differentiation of BMSCs was analyzed. The results of the present study demonstrated that the levels of Runt-Related Transcription Factor 2 (RUNX2), Osterix, ALP, OCN and bone sialoprotein (BSP) expression were significantly elevated in the 10% Sr-CaS group when compared with those in the blank group (P<0.05). Furthermore, those levels of gene expression were directly associated with the Sr concentration (Fig. 2E). It was also found that Sr-CaS upregulated TGF-β, Smad2/3 and β-catenin expression related to the Sr content (P<0.05; Fig. 2F and G).
Sr-CaS, as bone-rebuilding material, promotes bone repair
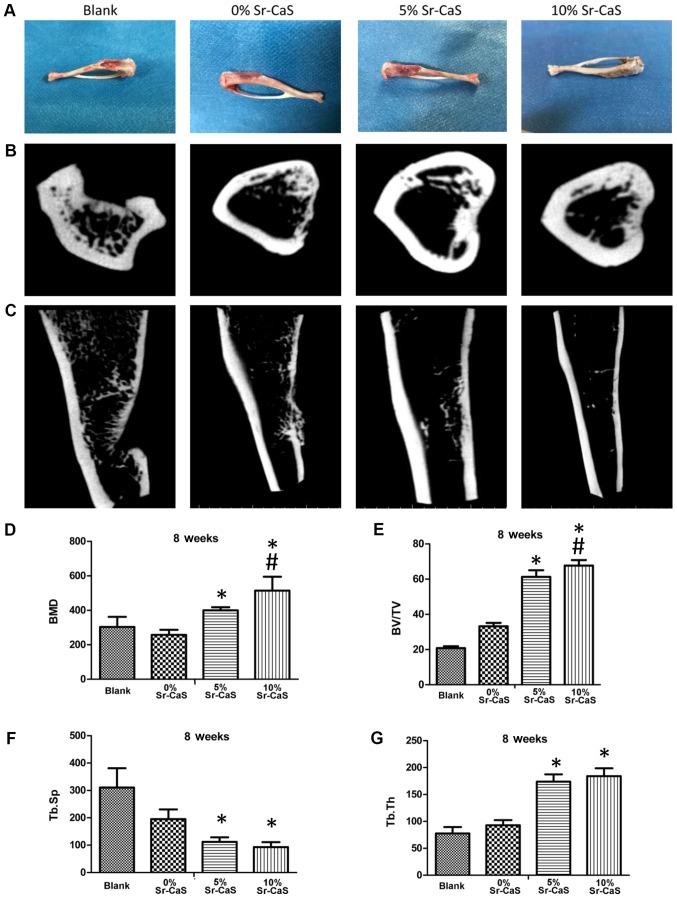
SD rats with shin defects were treated with 0, 5 or 10% Sr-CaS for 2 months and their bone reconstructive states were recorded. As shown in Fig. 3A, the tibia bone defects in the blank treatment group continued to be severe, while the tibia bone defects in the 0, 5 and 10% Sr-CaS treatment groups showed improvement when compared with those in the blank group. Moreover, the tibia bone defects showed their greatest improvement in 10% Sr-CaS group.
Figure 3.
Sr-CaS, as a bone-rebuilding material, promotes bone repair. (A) After treatment of the rat shin defects with 0, 5 or 10% Sr-CaS, the bone reconstructive states were observed. (B and C) The micro-computed tomography method was used to detect the effect of 0, 5, and 10% Sr-CaS on bone rebuilding in rats with a shin defect. (B) aerial view, (C) parallel perspective. The values for BMD (D) BV/TV (E) Tb.Sp (F) and Tb.Th (G) were calculated. *P<0.05 vs. blank group; #P<0.05 vs. 5% Sr-CaS group. BMD, bone mineral density; BV/TV, relative bone volume; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; Sr, strontium; CaS, calcium sulphate; FITC, fluorescein isothiocyanate; OD, optical density.
The results of microCT examinations also showed obvious tibia bone defects in the blank group. Moreover, those defects were severely depressed, indicating their poor degree of restoration. In the Sr-CaS groups, the bone-marrow cavity at the defect site was closed, but the periosteum was still thin and the connection between the two ends remained incomplete. In the 5 and 10% Sr-CaS groups, the periosteum was markedly thickened when compared with periostea in the 0% Sr-CaS group (Fig. 3B and C).
Values were also obtained for BMD, BV/TV, Tb.Sp and Tb.Th. Those data revealed that the values for BMD, BV/TV, and Tb.Th were significantly increased, while the values for Tb.Sp were significantly decreased in the 5 Sr-CaS and 10% Sr-CaS groups when compared with those values in the blank group. Furthermore, the values for BMD and BV/TV in the 10% Sr-CaS group were significantly increased compared with those in the 5% Sr-CaS group (P<0.05; Fig. 3D-G).
Sr-CaS improves the tibia bone defects
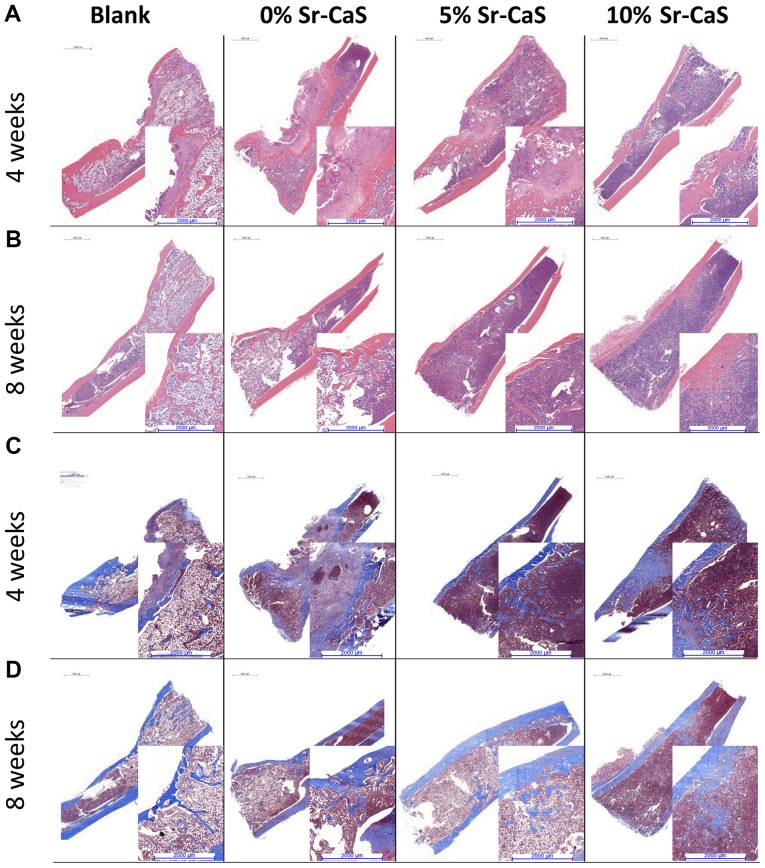
To further explore the effect of Sr-CaS on tibia bone defects, specimens of defective bone tissue were obtained from the SD rats that had been implanted with 0, 5 or 10% Sr-CaS for 4 and 8 weeks, respectively. The H&E and Masson staining results showed poor recovery of the bone defects in the blank group, where there were few new collagen fibers, and the bone calcium content was low. After the implantation of Sr-CaS, the bone remodeling process significantly improved, and the bone defects showed signs of recovery. At the same time, the rate of collagen fiber synthesis and the bone calcium content increased in conjunction with the increase in Sr content (Fig. 4).
Figure 4.
Effect of Sr-CaS on the tibia bone defects. The tibia bone defects as detected by hematoxylin and eosin staining after treatment with 0, 5 or 10% Sr-CaS for (A) 4 weeks and (B) 8 weeks. Masson staining was used to help determine the severity of the bone defects after they had been treated with 0, 5 or 10% Sr-CaS for (C) 4 weeks and (D) 8 weeks. Scale bar, 2,000 µm. Sr, strontium; CaS, calcium sulphate.
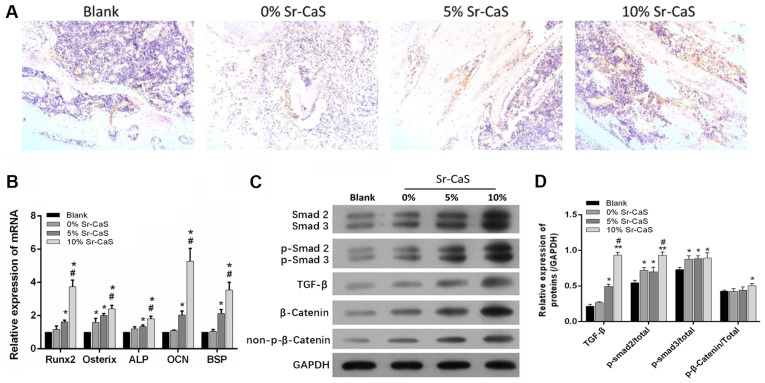
Sr-CaS accelerates bone repair via the TGF-β/Smad signaling pathway
To further explore the effects of Sr-CaS on the expression levels of bone-related factors after creation of a tibia defect, the defective tissues from the bone defect animal models at 8 weeks post-implantation surgery were collected. IHC assays were performed to detect the levels of OCN expression in those tissues. The results showed that OCN expression was increased in both the 5 Sr-CaS group and 10% Sr-CaS group relative to its expression in the blank group, and OCN expression was highest in 10% Sr-CaS group (Fig. 5A). Data from RT-qPCR assays showed that RUNX2, Osterix, ALP, OCN and BSP expression were significantly upregulated in the 5 Sr-CaS group and 10% Sr-CaS group relative to their expression levels in the blank group (P<0.05). OCN expression was highest in the 10% Sr-CaS group, where it was significantly increased compared with the 5% Sr-CaS group (P<0.05; Fig. 5B).
Figure 5.
Sr-CaS accelerates bone repair via the TGF-β/Smad signaling pathway. (A) The levels of OCN expression in bone defects at 8 weeks after implantation surgery were assessed by immunohistochemistry. Magnification, ×100. (B) The levels of RUNX2, Osterix, ALP, OCN, and BSP mRNA expression in the defective bone tissues implanted with 0, 5 or 10% Sr-CaS for 8 weeks were analyzed by the reverse transcription-quantitative PCR. *P<0.05 vs. blank group; #P<0.05 vs. 5% Sr-CaS group. Smad2/3, p-Smad2/3, TGF-β, β-catenin, and p-β-catenin expression in bone defects treated with 0, 5 or 10% Sr-CaS for 8 weeks was examined using (C) western blotting and (D) densitometric analysis. *P<0.05 and **P<0.01 vs. blank group; #P<0.05 vs. CaS group. Sr, strontium; CaS, calcium sulphate; TGF, transforming growth factor; ALP, alkaline phosphatase; p-Smad, phosphorylated-mothers against decapentaplegic homolog; OCN, osteocalcin; BSP, bone sialoprotein; RUNX2, runt-related transcription factor 2.
A previous study has suggested involvement of the TGF-β/Smad signaling pathway in the bone reconstruction process (30). Therefore, whether Sr-CaS might affect the TGF-β/Smad signaling pathway to promote bone repair was investigated. As shown in Fig. 5C and D, the levels of Smad2/3, p-Smad2/3, TGF-β, β-catenin, and p-β-catenin protein expression were all upregulated in the tissues implanted with CaS compared with tissues in the blank group. Furthermore, the expression levels of proteins involved in the TGF-β/Smad signaling pathway gradually increased in conjunction with the increase in Sr content (Fig. 5C and D).
Discussion
In recent years, a number of advances in cytobiology have been incorporated into the new discipline of bioengineering. At the same time, tissue engineering performed with diverse scaffolds has been widely applied in medical science (31–33). However, more efficient techniques are needed to accelerate osteogenic differentiation and bone formation in clinical practice. BMSCs are a class of osteoprogenitor cells (34). Research has suggested that BMSCs can differentiate into osteoblasts and be used to treat diseases caused by insufficient bone formation (35).
A previous study has shown that Sr activates the calcium-sensing receptor of osteoblasts, stimulates the production of osteoprotein (36,37) and then induces the production of osteoclasts by inhibiting the expression of receptor activator of nuclear factor-κB (38). In addition, Sr inhibits BMSC proliferation and promotes bone differentiation in a dose-dependent manner (39).
At the same time, when rat osteoclasts were co-cultured with primary mature rabbit osteoclasts, Sr was shown to reduce bone resorption and osteoclast formation (40). Numerous clinical and animal studies have demonstrated that strontium ranelate suppresses bone absorption and promotes bone formation (41–43). At present, strontium ranelate has been used in Europe as a prescribed treatment for senile menopausal osteoporosis (44).
A number of studies have suggested that Sr can improve the osteoconductive properties of CaS (44,45). Sr-CaS is a novel bone substitute material with high levels of biocompatibility and biological activity (26,45). Sr-enriched biomaterials have shown good efficacy and safety when used to promote bone formation and remodeling in animal models (46). In the present study, BMSCs were treated with 0, 5 or 10% Sr-CaS. The results showed that 5 and 10% Sr-CaS did not adversely affect the proliferation and apoptosis rates of BMSCs, suggesting the low toxicity of those formulations. However, 5 and 10% Sr-CaS did promote BMSC migration and osteogenic differentiation. In addition, bone defect animal models were created using a previously described method (27,28) and filled the defects with different materials. When used as a bone-rebuilding material, Sr-CaS improved the bone defects in tibias by promoting bone repair, which is consistent with results in previous studies (27,28). The present study further confirmed that 10% Sr-CaS could effectively promote bone repair.
RUNX2 is a transcription factor that functions during osteoblast differentiation. Previous studies showed that silencing of RUNX2 blocked the differentiation of osteoblasts in mice (47,48). Patients with cleidocranial dysplasia caused by heterozygous mutations in the RUNX2 gene are characterized by having an underdeveloped collarbone, short stature, excess teeth, a patent fontanelle and other bone growth-related defects (49). Osterix is a zinc finger transcription factor present in osteoblasts and plays a leading role in the osteoblast differentiation process (49). A previous study proved that RUNX2 can regulate the levels of Osx (osterix) (50). ALP is widely distributed in human skeletal, kidney, liver, intestinal and placental tissues. OCN is a vitamin k-dependent calcium binding protein. ALP and OCN are two typical biomarkers of osteoblasts, and are involved in osteogenic differentiation and the mineralization of extracellular matrix during bone formation and repair (51,52). Previous studies have shown that RUNX2 stimulates the differentiation of mesenchymal stem cells into osteoblasts by regulating OCN and ALP activity (47,53). A previous study has also indicated that BSP significantly affects bone matrix and bone tumor growth (54). In the present study, it was verified that Sr-CaS dramatically upregulates RUNX2, Osterix, ALP, OCN and BSP expression, suggesting that it accelerates bone repair.
TGF-β plays an important role in keeping bone formation and resorption in balance, and thereby promotes the differentiation of BMSCs into bone tissue and inhibits osteoclast formation (55). Smads are directly involved in the signal transduction processes mediated by members of the TGF-β superfamily (56). A previous study indicated that TGF-β receptor conducted by TGF-β can recruit Smads, like Smad2/3 and finally enhance the factor related to bone mineralization, including RUNXs and osterix (57). However, TGF-β also has been reported as a double-edged sword in maintenance of articular cartilage metabolic homeostasis and the pathogenesis of arthritis (58). Research suggests that TGF-β participated functions with Smad2/3 (59). Li et al (60) indicate that Smad2/3 can be activated by TGF-β and then osteogenesis has demonstrated to be strengthen. Furthermore, β-catenin is one of the most important factors in osteogenesis (61). Also, it demonstrated to be modulated by TGF-β (62). In the present study, it was proved that Sr-CaS upregulated the levels of Smad2/3, p-Smad2/3, TGF-β, β-catenin and p-β-catenin expression, suggesting that it promotes bone repair via the TGF-β/Smad signaling pathway.
The present study suggest that Sr-CaS can promote osteogenic differentiation via the TGF-β/Smad2/3 molecular signaling pathway both in vitro and in vivo. The implantation material used in the present study, which contained 10% Sr-CaS as a new component significantly promoted the improvement and healing of bone defects.
Acknowledgements
Not applicable.
Funding
This research was supported by the Key Projects of Science and Technology plan of Guangdong province (grant no. 2016B90913004).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
ZL and BY designed the experiments, ZL, ZY and HC conducted the experiments; ZL, YW, HX and XZ analyzed the data; BY and XZ validated the data analysis. ZL drafted the manuscript, and BY and XZ revised the manuscript. All authors read and approved the final version.
Ethics approval and consent to participate
All experimental protocols were approved by the Animal Ethics Committee of Southern Medical University. All experiments on animals were performed according the Animal Care and Use guidelines established by the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ishack S, Mediero A, Wilder T, Ricci JL, Cronstein BN. Bone regeneration in critical bone defects using three-dimensionally printed beta-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J Biomed Mater Res B Appl Biomater. 2017;105:366–375. doi: 10.1002/jbm.b.33561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–121. doi: 10.1016/j.bone.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 3.van Houdt CI, Tim CR, Crovace MC, Zanotto ED, Peitl O, Ulrich DJ, Jansen JA, Parizotto NA, Renno AC, van den Beucken JJ. Bone regeneration and gene expression in bone defects under healthy and osteoporotic bone conditions using two commercially available bone graft substitutes. Biomed Mater. 2015;10:035003. doi: 10.1088/1748-6041/10/3/035003. [DOI] [PubMed] [Google Scholar]
- 4.Lazar MA, Rotaru H, Baldea I, Bosca AB, Berce CP, Prejmerean C, Prodan D, Campian RS. Evaluation of the biocompatibility of new fiber-reinforced composite materials for craniofacial bone reconstruction. J Craniofac Surg. 2016;27:1694–1699. doi: 10.1097/SCS.0000000000002925. [DOI] [PubMed] [Google Scholar]
- 5.Sahoo N, Roy ID, Desai AP, Gupta V. Comparative evaluation of autogenous calvarial bone graft and alloplastic materials for secondary reconstruction of cranial defects. J Craniofac Surg. 2010;21:79–82. doi: 10.1097/SCS.0b013e3181c3ba58. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Yeung KW. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive Mater. 2017;2:224–247. doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenley RA, Yim K, Abrams J, Ron E, Turek T, Marden LJ, Hollinger JO. Biotechnology and bone graft substitutes. Pharm Res. 1993;10:1393–1401. doi: 10.1023/A:1018902720816. [DOI] [PubMed] [Google Scholar]
- 8.Barrere F, van Blitterswijk CA, de Groot K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomedicine. 2006;1:317–332. [PMC free article] [PubMed] [Google Scholar]
- 9.Scarano A, Orsini G, Pecora G, Iezzi G, Perrotti V, Piattelli A. Peri-implant bone regeneration with calcium sulfate: A light and transmission electron microscopy case report. Implant Dent. 2007;16:195–203. doi: 10.1097/ID.0b013e3180587ad8. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer CD, App GR. The use of plaster of paris in treating infrabony periodontal defects in humans. J Periodontol. 1971;42:685–690. doi: 10.1902/jop.1971.42.11.685. [DOI] [PubMed] [Google Scholar]
- 11.Hing KA, Wilson LF, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475–490. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel SR, Simon J, Alexander H, Dennis M, Ricci JL. Osseointegration on metallic implant surfaces: Effects of microgeometry and growth factor treatment. J Biomed Mater Res. 2002;63:706–713. doi: 10.1002/jbm.10408. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Zhang X, Li Z, Peng D. Management of combined bone defect and limb-length discrepancy after tibial chronic osteomyelitis. Orthopedics. 2011;34:e363–e367. doi: 10.3928/01477447-20110627-12. [DOI] [PubMed] [Google Scholar]
- 14.Villar CC, Cochran DL. Regeneration of periodontal tissues: Guided tissue regeneration. Dent Clin North Am. 2010;54:73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Li B, Li Q, Huang Z, Yin B, Ma P, Xu D, Wu Z, Qiu G. Effect of injectable composites of calcium sulfate and hyaluronate in enhancing osteogenesis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31:730–737. doi: 10.7507/1002-1892.201612145. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao L, Weng W, Chen X, Zhang J, Zhou Q, Cui J, Zhao Y, Shin JW, Su J. Promotion of in vivo degradability, vascularization and osteogenesis of calcium sulfate-based bone cements containing nanoporous lithium doping magnesium silicate. Int J Nanomedicine. 2017;12:1341–1352. doi: 10.2147/IJN.S124965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Liu HY, Lian X, Shi XL, Wang W, Cui FZ, Zhang Y. Osteogenesis of mineralized collagen bone graft modified by PLA and calcium sulfate hemihydrate: In vivo study. J Biomater Appl. 2013;28:12–19. doi: 10.1177/0885328211433618. [DOI] [PubMed] [Google Scholar]
- 18.Yin W, Sun Q, Ma L. Study on the effects of curculigoside on proliferation, differentiation, and calcification of mouse osteoblastic MC3T3-E1 cells. World Sci Technol. 2011;13:852–855. doi: 10.1016/S1876-3553(12)60026-X. [DOI] [Google Scholar]
- 19.Beuerlein MJ, Mckee MD. Calcium sulfates: What is the evidence? J Orthop Trauma. 2010;24(Suppl 1):S46–S51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]
- 20.Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 21.Marie PJ, Hott M, Modrowski D, De Pollak C, Guillemain J, Deloffre P, Tsouderos Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res. 1993;8:607–615. doi: 10.1002/jbmr.5650080512. [DOI] [PubMed] [Google Scholar]
- 22.Canalis E, Hott M, Deloffre P, Tsouderos Y, Marie PJ. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone. 1996;18:517–523. doi: 10.1016/8756-3282(96)00080-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Zhao S, Zhu Y, Huang Y, Zhu M, Tao C, Zhang C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10:2269–2281. doi: 10.1016/j.actbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Poh PS, Hutmacher DW, Stevens MM, Woodruff MA. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication. 2013;5:045005. doi: 10.1088/1758-5082/5/4/045005. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Blackwood KA, Doustgani A, Poh PP, Steck R, Stevens MM, Woodruff MA. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J Biomed Mater Res A. 2016;104:2109. doi: 10.1002/jbm.a.35801. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Xu CP, Hou YL, Song JQ, Cui Z, Wang SN, Huang L, Zhou CR, Yu B. A novel resorbable strontium-containing α-calcium sulfate hemihydrate bone substitute: A preparation and preliminary study. Biomed Mater. 2014;9:045010. doi: 10.1088/1748-6041/9/4/045010. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translat. 2015;3:95–104. doi: 10.1016/j.jot.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Rosario C, Rodriguez-Evora M, Reyes R, Delgado A, Evora C. BMP-2, PDGF-BB, and bone marrow mesenchymal cells in a macroporous beta-TCP scaffold for critical-size bone defect repair in rats. Biomed Mater. 2015;10:045008. doi: 10.1088/1748-6041/10/4/045008. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Xu L, Li K, Xie N, Xi Y, Wang Y, Zheng X, Chen X, Wang M, Ye X. Zinc-modified calcium silicate coatings promote osteogenic differentiation through TGF-β/smad pathway and osseointegration in osteopenic rabbits. Sci Rep. 2017;7:3440. doi: 10.1038/s41598-017-03661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004;13:343–355. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]
- 32.Bajada S, Harrison PE, Ashton BA, Cassar-Pullicino VN, Ashammakhi N, Richardson JB. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg Br. 2007;89:1382–1386. doi: 10.1302/0301-620X.89B10.19103. [DOI] [PubMed] [Google Scholar]
- 33.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 34.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86:1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Guanghua Chen, Guizhi Huang, Hao Lin, Haojun Wu, Chen H. Bone marrow mesenchymal stem cell transplatation increases bone mineral density of an ovariectomized rat model of osteoporosis. Chin J Tissue Engineering Res. 2017;21:49–53. [Google Scholar]
- 36.Cesareo R, Napolitano C, Iozzino M. Strontium ranelate in postmenopausal osteoporosis treatment: A critical appraisal. Int J Womens Health. 2010;2:1–6. [PMC free article] [PubMed] [Google Scholar]
- 37.Kostenuik PJ, Shalhoub V. Osteoprotegerin: A physiological and pharmacological inhibitor of bone resorption. Curr Pharm Des. 2001;7:613–635. doi: 10.2174/1381612013397807. [DOI] [PubMed] [Google Scholar]
- 38.Chen QY, Liang GQ, Lin Y, Liu BL, Zhao-Hui LI. Effects of strontium ranelate on titanium particles stimulating mononuclear macrophage to secrete osteolysis factor and its RANK expression. Rheum Arthritis. 2015 [Google Scholar]
- 39.Li Y, Li J, Zhu S, Luo E, Feng G, Chen Q, Hu J. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;418:725–730. doi: 10.1016/j.bbrc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 40.Gentleman E, Fredholm YC, Jell G, Lotfibakhshaiesh N, O'Donnell MD, Hill RG, Stevens MM. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials. 2010;31:3949–3956. doi: 10.1016/j.biomaterials.2010.01.121. [DOI] [PubMed] [Google Scholar]
- 41.Nahass HE, Din NNE, Nasry SA. The effect of strontium ranelate gel on bone formation in calvarial critical size defects. Open Access Maced J Med Sci. 2017;5:994–999. doi: 10.3889/oamjms.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao S, Wang X, Li N, Chen Y, Su Y, Zhang J. Effects of strontium ranelate on bone formation in the mid-palatal suture after rapid maxillary expansion. Drug Des Devel Ther. 2015;9:2725–2734. doi: 10.2147/DDDT.S82892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karatas OH, Toy E, Demir A, Toy H, Kozacioglu S. Effects of strontium ranelate on sutural bone formation: A histological and immunohistochemical study. Aust Orthod J. 2016;32:139–147. [PubMed] [Google Scholar]
- 44.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Zhou Y, Yang S, Li JJ, Li X, Ma Y, Hou Y, Jiang N, Xu C, Zhang S, et al. Novel bone substitute composed of chitosan and strontium-doped α-calcium sulfate hemihydrate: Fabrication, characterisation and evaluation of biocompatibility. Mater Sci Eng C Mater Biol Appl. 2016;66:84–91. doi: 10.1016/j.msea.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 46.Neves N, Linhares D, Costa G, Ribeiro CC, Barbosa MA. In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: A systematic review. Bone Joint Res. 2017;6:366–375. doi: 10.1302/2046-3758.66.BJR-2016-0311.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang D, Okamura H, Qiu L. Upregulated osterix expression elicited by Runx2 and Dlx5 is required for the accelerated osteoblast differentiation in PP2A Cα-knockdown cells. Cell Biol Int. 2018;42:403–410. doi: 10.1002/cbin.10902. [DOI] [PubMed] [Google Scholar]
- 48.Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149:313–323. doi: 10.1007/s00418-018-1640-6. [DOI] [PubMed] [Google Scholar]
- 49.Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/S0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 50.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H, Choong P, McCarthy R, Chou ST, Martin TJ, Ng KW. In situ hybridization to show sequential expression of osteoblast gene markers during bone formation in vivo. J Bone Miner Res. 1994;9:1489–1499. doi: 10.1002/jbmr.5650090922. [DOI] [PubMed] [Google Scholar]
- 52.van Leeuwen JP, van Driel M, van den Bemd GJ, Pols HA. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr. 2001;11:199–226. [PubMed] [Google Scholar]
- 53.Takahashi T, Kato S, Suzuki N, Kawabata N, Takagi M. Autoregulatory mechanism of Runx2 through the expression of transcription factors and bone matrix proteins in multipotential mesenchymal cell line, ROB-C26. J Oral Sci. 2005;47:199–207. doi: 10.2334/josnusd.47.199. [DOI] [PubMed] [Google Scholar]
- 54.Riminucci M, Corsi A, Peris K, Fisher LW, Chimenti S, Bianco P. Coexpression of bone sialoprotein (BSP) and the pivotal transcriptional regulator of osteogenesis, Cbfa1/Runx2, in malignant melanoma. Calcif Tissue Int. 2003;73:281–289. doi: 10.1007/s00223-002-2134-y. [DOI] [PubMed] [Google Scholar]
- 55.Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leah E. Osteoarthritis: TGF-β overload at bones of cartilage degeneration. Nat Rev Rheumatol. 2013;9:382. doi: 10.1038/nrrheum.2013.81. [DOI] [PubMed] [Google Scholar]
- 59.Saito M, Ichikawa J, Ando T, Schoenecker JG, Ohba T, Koyama K, Suzuki-Inoue K, Haro H. Platelet-derived TGF-β induces tissue factor expression via the smad3 pathway in osteosarcoma cells. J Bone Miner Res. 2018;33:2048–2058. doi: 10.1002/jbmr.3537. [DOI] [PubMed] [Google Scholar]
- 60.Li H, Fan J, Fan L, Li T, Yang Y, Xu H, Deng L, Li J, Li T, Weng X, et al. MiRNA-10b reciprocally stimulates osteogenesis and inhibits adipogenesis partly through the TGF-β/SMAD2 signaling pathway. Aging Dis. 2018;9:1058–1073. doi: 10.14336/AD.2018.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Hu C, Wang G, Li L, Kong X, Ding Y, Jin Y. Nuclear factor-κB modulates osteogenesis of periodontal ligament stem cells through competition with β-catenin signaling in inflammatory microenvironments. Cell Death Dis. 2013;4:e510. doi: 10.1038/cddis.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amini Nik S, Ebrahim RP, Van Dam K, Cassiman JJ, Tejpar S. TGF-beta modulates beta-Catenin stability and signaling in mesenchymal proliferations. Exp Cell Res. 2007;313:2887–2895. doi: 10.1016/j.yexcr.2007.05.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.