Abstract
Osteosarcoma (OS) is one of the most malignant tumors in children and young adults. To better understand the underlying mechanism, five related datasets deposited in the Gene Expression Omnibus were included in the present study. The Bioconductor ‘limma’ package was used to identify differentially expressed genes (DEGs) and the ‘Weighted Gene Co-expression Network Analysis’ package was used to construct a weighted gene co-expression network to identify key modules and hub genes, associated with OS. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes overrepresentation analyses were used for functional annotation. The results indicated that 1,405 genes were dysregulated in OS, including 927 upregulated and 478 downregulated genes, when the cut off value was set at a ≥2 fold-change and an adjusted P-value of P<0.01 was used. Functional annotation of DEGs indicated that these genes were involved in the extracellular matrix (ECM) and that they function in several processes, including biological adhesion, ECM organization, cell migration and leukocyte migration. These findings suggested that dysregulation of the ECM shaped the tumor microenvironment and modulated the OS hallmark. Genes assigned to the yellow module were positively associated with OS and could contribute to the development of OS. In conclusion, the present study has identified several key genes that are potentially druggable genes or therapeutics targets in OS. Functional annotations revealed that the dysregulation of the ECM may contribute to OS development and, therefore, provided new insights to improve our understanding of the mechanisms underlying OS.
Keywords: osteosarcoma, differently expressed gene, gene expression microarray, meta-analysis
Introduction
Osteosarcoma (OS) is a primary malignant bone tumor arising from primitive transformed cells of a mesenchymal origin (1) and it is the 8th most common form of childhood cancer (2). Although OS is a rare malignancy overall, it is the most common malignant tumor found in the bone tissue of children and usually requires chemotherapy and surgical treatment (3,4). The management of OS has improved over the past few decades, with the 5-year survival rate increasing from 20–30 to 60–70% (5). However, relapse and pulmonary metastasis remain big challenges in the management of OS (6). A greater understanding of the underlying mechanisms of OS will improve its management.
It has been reported that most cases of OS harbor chromosomal abnormalities and gene mutations (7,8). In total, ~70% of patients with OS showed loss-of-function mutations in the gene encoding the retinoblastoma-associated protein (9,10). Somatic mutations that lead to the loss of tumor suppressor functions are a pivotal step in OS pathogenesis, and there are a variety of genetic events that lead to the development of OS (11). Systematic research from the genetic perspective may help to improve our understanding of the mechanism underlying OS.
Gene microarrays are a powerful tool to obtain gene expression profiles. Comparisons made between normal and tumor samples can lead to the identification of dysregulated genes; most diseases have specific gene expression profiles and abnormal regulation patterns (12). A common practice for the identification of differentially expressed genes (DEGs) is to filter results using fold change, P-values and false discovery rates (13,14). Weighted gene co-expression network analysis (WGCNA) can be used to identify groups of genes with similar functions, known as gene modules (15). Genes in the same module tend to have similar expression patterns and, therefore, may have similar functions. Genes with the most connectivity in a module are called hub genes, these genes are more relevant to the functionality of the module (14). WGCNA has been widely accepted as an investigation tool to identify hub genes in cancer studies.
In the present study, microarray gene expression data derived from the same platform were extracted from the Gene Expression Omnibus (GEO) database to identify DEGs in OS. WGCNA was used to identify gene modules that were closely associated with OS. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were used for functional annotations. PharmGKB (16), oncoKB (17), Clinical Interpretations of Variants in Cancer (CIViC) (18) were used to check if potentially druggable targets could be found in closely related modules in OS. The results of the present study may increase the understanding of the molecular mechanisms underlying OS and contribute to the clinical management of OS.
Materials and methods
Search strategy
The GEO database (www.ncbi.nlm.nih.gov/geo/) was used to retrieve relevant studies (19–23) that used the Affymetrix Human Genome U133 Plus 2.0 platform (GPL570; Affymetrix; Thermo Fisher Scientific, Inc.) to explore the mRNA expression profiles in tumor tissues from patients with OS or bone marrow mesenchymal stromal cells (BM-MSCs) from healthy controls. Search terms including ‘osteosarcoma’, ‘cancer’ or ‘tumor’ or ‘neoplasm’ or ‘carcinoma’ or ‘sarcoma’, ‘mesenchymal stromal cells’ and ‘GPL570’ were used. The species was limited to Homo sapiens.
Study selection
Inclusion criteria: i) Studies that used OS tissues from patients or BM-BMCs from healthy controls to explore the RNA expression profiles; and ii) for studies that used mixed tissue types from patients with OS or healthy controls, only the data from OS tissues and normal BM-BMCs were included. Exclusion criteria: i) Studies that used cell lines derived from OS or human mesenchymal stromal cells were excluded; ii) studies that used BM-MSCs extracted from patients with osteoarthritis or osteoporosis were excluded; and iii) studies with only ‘chp’ type original files, other than ‘cel’ type files, were also excluded.
Data extraction and pre-processing procedures
Raw data from eligible studies were retrieved from GEO. GEO accession number, author, country, submission year, platform and detailed patient information, as well as available information from healthy controls, were obtained from the metadata. Data were extracted by two researchers independently and conflicts were resolved by consulting a third senior researcher. Raw data were normalized using the R ‘affy’ package (version 1.62.0; http://bioconductor.org/packages/affy) with robust-multi array average methods, as described previously (24–26). Mean expression values were calculated for genes measured by multiple detection probes. DEGs between patients with OS and control tissues were compared using the Bioconductor ‘limma’ package (version 3.40.2; http://bioconductor.org/packages/limma) (27). Genes with a fold change ≥2 and an adjusted P<0.01 were considered as DEGs.
Functional characterization of DEGs
Microarray probe IDs were converted to Ensemble IDs and gene symbols using ‘hgu133plus2.db’ R package (version 3.2.3; http://bioconductor.org/packages/hgu133plus2.db) (28). To interpret the biological significance of DEGs, GO enrichment of cellular component, biological process and molecular function, as well as KEGG pathway enrichment analysis were conducted using Bioconductor ‘clusterProfiler’ R package (version 3.10.0; http://bioconductor.org/packages/clusterProfiler) (29). The ‘Disease Ontology semantic and enrichment analysis’ (DOSE) package (version 3.10.0; http://bioconductor.org/packages/DOSE) (30) was used to find genes closely associated with OS.
Principal component analysis (PCA) of DEGs in patients with OS and controls
PCA analyses were conducted using the ClustVis online tool (https://biit.cs.ut.ee/clustvis/) developed by Metsalu et al (31). Due to limitations on the file size that can be uploaded, only gene expression values of DEGs were included in the PCA analysis. Groups (OS or control) and gender were two of the clinical traits that were used in the PCA analysis.
WGCNA
To identify key gene modules in OS, WGCNA was conducted with the R ‘WGCNA’ package (version 1.46) (32). Normalized gene expression data were used in WGCNA. Soft-connectivity was calculated using the default parameters. Topology networks and gene modules were constructed using one-step network construction.
Hub genes are a group of genes that tend to have high connectivity with other genes and are expected to play pivotal biological roles. The connections between the top 30 hub genes were visualized using VisAnt software (version 5.51; http://visant.bu.edu). Functional annotations, including GO and KEGG enrichment analyses, were used to highlight the most overrepresented GO terms and KEGG pathways in modules that were closely associated with OS. To determine if any of the hub genes in the modules were abnormally expressed, log2 fold change (log2FC) was used to characterize the expression pattern and enrichment scores were used for characterizing the connectivity of genes in the yellow module.
Gene mutations may prevent the proper function of the corresponding protein by affecting protein structure or expression. PharmGKB (16), oncoKB (17), and CIViC (18) are three databases that provide information about the treatment implications of specific cancer gene alterations, and how these mutations affect response to treatment. In the present study, these databases were used to identify any potentially druggable targets in the modules that were found to be closely related with OS.
Tumor infiltrating immune cell profiling using CIBERSORT
Tumor microenvironments are critical to tumor cell survival and proliferation. Tumor infiltrating leukocytes are usually present in the microenvironment of solid tumors. The CIBERSORT webtool (version 1.06; http://cibersort.stanford.edu) (33) was used to estimate the abundancy of tumor-infiltrating immune cells in the OS microenvironment. LM22, which consisted of gene expression data from 22 distinct immune cell types, was used as reference in the present study (34).
Results
Characteristics of the included studies
In total, five eligible studies were included in the present study (19–23). RNA expression data were extracted from 48 patients with OS and 12 BM-MSCs from these previous studies (Table SI). Details of the included studies are shown in Table I. For GSE18043 and GSE36474, only data from three eligible individuals were included from each dataset (19,22). There were two biological replicates in GSE35331 (21), the results from the first set were included in the present study. More detailed information of the included individuals from each study can be found in Table SI. The original gene expression files from the included individuals were downloaded from the GEO website.
Table I.
Characteristics of the included studies.
| Author, year | Country | GEO accession | Platform | OS cases | Controls | Samples type | (Refs.) |
|---|---|---|---|---|---|---|---|
| Vella et al, 2016 | The Netherlands | GSE87437 | GPL570 | 21 | NA | High-grade osteosarcoma | (23) |
| Kobayashi et al, 2009 | Japan | GSE14827 | GPL570 | 27 | NA | Fresh frozen tumor specimens | (20) |
| Hamidouche et al, 2009 | Germany | GSE18043 | GPL570 | NA | 3 | BM-MSCs | (19) |
| André et al, 2013 | Belgium | GSE36474 | GPL570 | NA | 3 | BM-MSCs | (22) |
| Guilloton et al, 2012 | France | GSE35331 | GPL570 | NA | 6 | BM-MSCs | (21) |
OS, osteosarcoma; NA, not available; BM-MSCs, bone marrow mesenchymal stromal cells.
Identification and functional annotation of DEGs in OS
The Bioconductor ‘affy’ package was used to pre-process raw data for background correction and normalization. In total, expression values from 54,613 probes representing 20,188 known genes with symbols were analyzed in the present study. The mean expression values of multiple probes corresponding to each gene were calculated as the final expression value. The Bioconductor ‘limma’ package was used to identify DEGs. When the cutoff values were set as |log2FC|>1 (adjusted P<0.01), 1,405 genes were found to be dysregulated (including 927 up- and 478 downregulated genes) in OS compared with controls (Table SII). When cutoff values were set as |log2FC|>2 (adjusted P<0.01), there were 354 genes dysregulated (including 224 up- and 130 downregulated genes) in OS compared with controls. The top 10 most up- and downregulated genes are shown in Tables II and III. PCA analysis revealed that these DEGs could distinguish OS from normal controls and that there was no disparity between males and females (Fig. S1).
Table II.
Top 10 upregulated genes in osteosarcoma.
| Gene symbol | Log2FC | AveExp | t-score | P-value | Padj |
|---|---|---|---|---|---|
| CPE | 6.01 | 9.77 | 13.95 | <0.001 | <0.001 |
| MMP9 | 5.96 | 10.45 | 9.99 | <0.001 | <0.001 |
| SPARCL1 | 5.93 | 8.60 | 13.18 | <0.001 | <0.001 |
| S100A4 | 5.92 | 10.98 | 23.45 | <0.001 | <0.001 |
| CA2 | 5.60 | 8.08 | 9.62 | <0.001 | <0.001 |
| COL15A1 | 5.29 | 8.75 | 12.64 | <0.001 | <0.001 |
| ACP5 | 5.19 | 9.01 | 7.89 | <0.001 | <0.001 |
| C1QC | 5.18 | 8.93 | 16.32 | <0.001 | <0.001 |
| MMP13 | 5.17 | 8.91 | 6.80 | <0.001 | <0.001 |
| MRC1 | 5.00 | 7.55 | 15.24 | <0.001 | <0.001 |
AveExp, average expression across all samples; Log2FC, Log2(fold change); Padj, adjusted P-value; t-score, statistic value for t-test.
Table III.
Top 10 downregulated genes in osteosarcoma.
| Gene symbol | Log2FC | AveExp | t-score | P-value | Padj |
|---|---|---|---|---|---|
| KRTAP1-5 | −5.36 | 5.76 | −17.48 | <0.001 | <0.001 |
| DKK1 | −4.68 | 7.14 | −7.45 | <0.001 | <0.001 |
| STC2 | −4.25 | 6.78 | −16.57 | <0.001 | <0.001 |
| TFPI2 | −4.23 | 5.06 | −11.62 | <0.001 | <0.001 |
| PTX3 | −4.19 | 8.95 | −7.46 | <0.001 | <0.001 |
| RGS4 | −4.08 | 6.30 | −12.49 | <0.001 | <0.001 |
| NPR3 | −4.07 | 5.77 | −16.82 | <0.001 | <0.001 |
| DSP | −4.00 | 6.85 | −8.51 | <0.001 | <0.001 |
| LTBP2 | −3.99 | 7.91 | −12.64 | <0.001 | <0.001 |
| VGLL3 | −3.91 | 5.93 | −8.69 | <0.001 | <0.001 |
AveExp, average expression across all samples; Log2FC, Log2(fold change); Padj, adjusted P-value; t-score, statistic value for t-test.
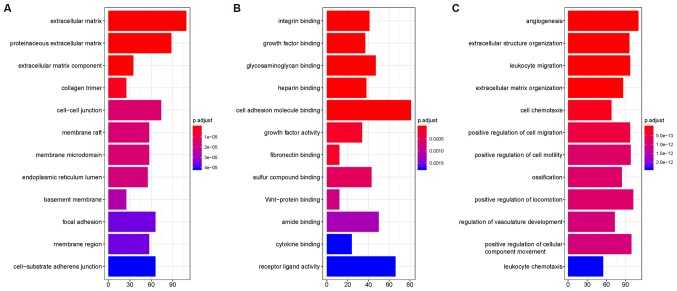
GO overrepresentation analysis showed that DEGs were enriched in terms including ‘extracellular matrix’, ‘proteinaceous extracellular matrix’, ‘extracellular matrix component’, ‘collagen trimer’, ‘cell-cell junction’, ‘membrane raft’, ‘membrane microdomain’, ‘endoplasmic reticulum lumen’ and ‘basement membrane’. Their functions included ‘integrin binding’, ‘growth factor binding’, ‘glycosaminoglycan binding’, ‘heparin binding’, ‘cell adhesion molecule binding’, ‘growth factor activity’, ‘fibronectin binding’, ‘sulfur compound binding’ and ‘Wnt-protein binding’. These DEGs participated in biological processes including ‘angiogenesis’, ‘extracellular structure organization’, ‘leukocyte migration’, ‘extracellular matrix organization’, ‘cell chemotaxis’, ‘positive regulation of cell migration’, ‘positive regulation of cell motility’, ‘ossification’ and ‘positive regulation of locomotion’. More detailed information can be found in Fig. 1. Dysregulation of the extracellular matrix (ECM) could shape the tumor microenvironment and further modulate cancer hallmarks (35). These results also suggest that dysregulation of the ECM may contribute to OS development and metastasis (Fig. 2).
Figure 1.
Top 12 terms of GO overrepresentation analysis (adjusted P<0.01). Overrepresented GO (A) cellular components, (B) molecular function and (C) biological process terms. The x-axis shows the number of differentially expressed genes in an overrepresented GO term. GO, Gene Ontology.
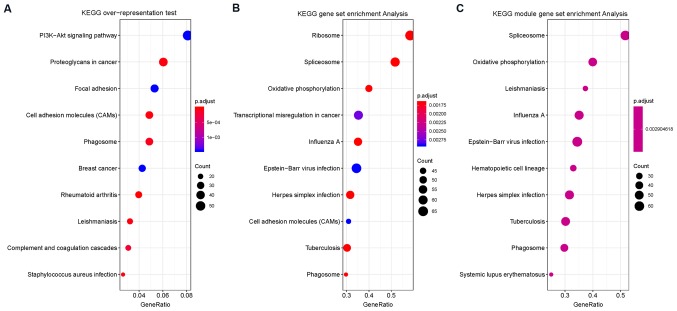
Figure 2.
Top 10 terms of KEGG enrichment analysis (adjusted P<0.01). (A) Overrepresented and (B) enriched KEGG pathways. (C) Enriched KEGG modules. The x-axis shows the ratio of genes enriched in a KEGG pathway. KEGG, Kyoto Encyclopedia of Genes and Genomes.
KEGG overrepresentation analysis demonstrated that DEGs were enriched in ‘PI3K-Akt signaling pathway’, ‘proteoglycans in cancer’, ‘focal adhesion’, ‘cell adhesion molecules (CAMs)’, ‘phagosome’, ‘breast cancer’, ‘rheumatoid arthritis’, ‘leishmaniasis’, ‘complement and coagulation cascades’ and ‘staphylococcus aureus infection’. KEGG enrichment analysis showed that DEGs were enriched in ‘ribosome’, ‘spliceosome’, ‘oxidative phosphorylation’, ‘transcriptional dysregulation in cancer’, ‘influenza A’, ‘Epstein-Barr virus infection’, ‘herpes simplex infection’, ‘cell adhesion molecules (CAMs)’, ‘tuberculosis’ and ‘phagosome’.
Identification of key modules and genes closely associated with OS
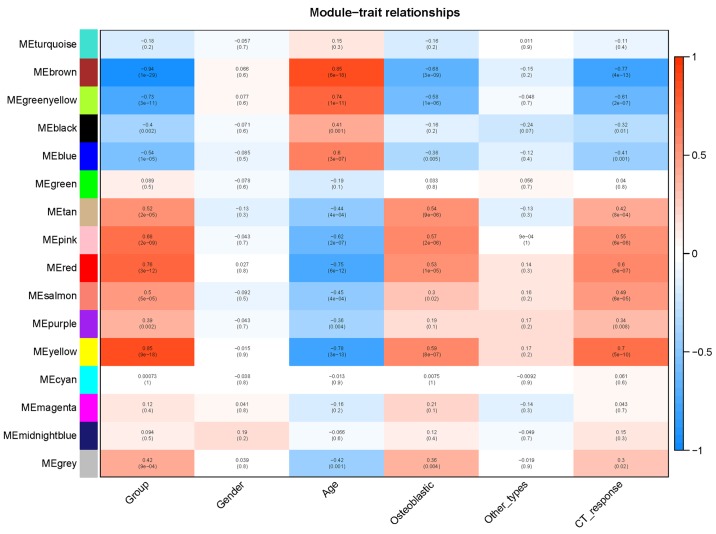
Gene expression values from all genes and samples were included in WGCNA. Soft-thresholding was selected with a power of 12, a minimum module size of 30 and a medium sensitivity to cluster splitting (Fig. S2). A module-trait association heatmap was plotted to identify modules that were significantly associated with clinical traits (Fig. 3). As shown in Fig. 3, the yellow, red and pink modules are positively related with OS status, osteoblastic tumor type and chemotherapy response; these three modules are negatively associated with age. The blue module is negatively associated with OS and positively associated with age. Modules with a height of <0.25 were merged. In total, 15 gene modules were identified and the dendrogram displayed together with the color assignment is shown in Fig. 4.
Figure 3.
Module-trait relationship heatmap for different traits and gene modules. The yellow, red and pink gene modules are positively related to OS, osteoblastic status and CT response; these modules are negatively associated with age. The brown and greenyellow modules are positively related to age and negatively related to OS, osteoblastic status and CT response. Values in the figure indicate the correlation coefficient between modules and clinical traits. Values in brackets are the P-values for the association test. OS, osteosarcoma; CT, chemotherapy; ME, module.
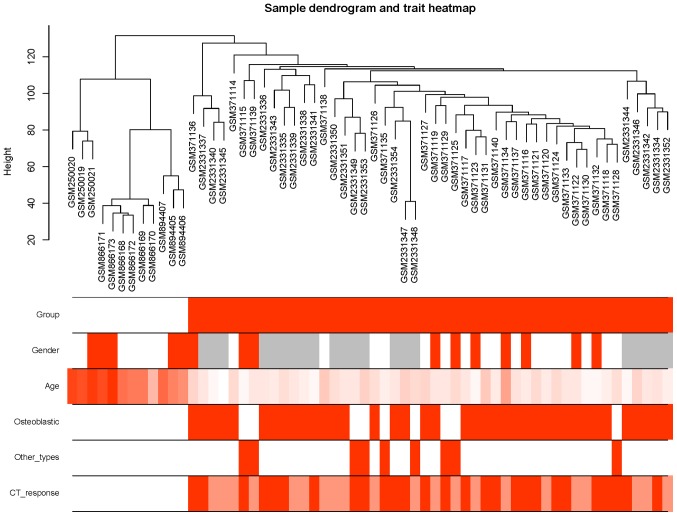
Figure 4.
Sample dendrogram and trait heatmap for the different traits. Control and OS samples can be classified. The average age in the control group was higher than in the OS group. For gender, histology type (osteoblastic and other types) and CT response, no significant patterns were found. Group: White, BM-MSCs; orange: OS. Gender: White, male; orange, female; grey, not reported. Age: Color scale from white (young) to orange (older). Osteoblastic type: White, no; orange, yes. Other types: White, osteoblastic type; orange, other types (except osteoblastic type). CT response: White, not applicable; dark orange, poor; light orange, good. OS, osteosarcoma; CT, chemotherapy.
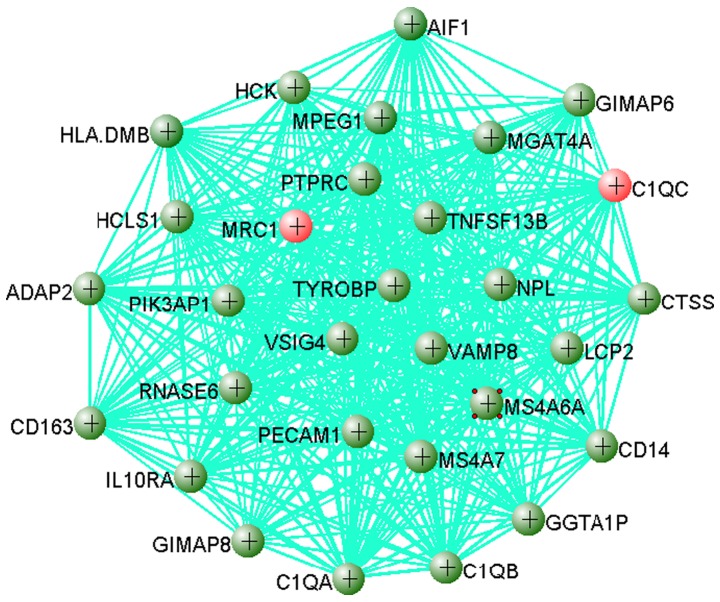
Genes in these modules may play a pivotal role in OS. The results showed that 383 out of 749 genes in the yellow module were dysregulated, including 358 upregulated genes and 25 downregulated genes when the cutoff value was set as |log2FC|>1 and adjusted P<0.01. The top 30 hub genes from the yellow module were extracted to visualize their connections using VisAnt software (Fig. 5). As shown in Fig. 5, C1QC and MRC1 are two hub genes that were upregulated in OS, which indicated that these two genes may play important roles in OS.
Figure 5.
Gene-gene interaction network of the top 30 genes in the yellow module visualized using VisAnt software. Red circle indicate that the gene is overexpressed in OS while green circles indicate that the expression of this gene is unchanged. The small red circles around MS4A6A indicate that there are more connections to this gene. OS, osteosarcoma.
DEGs from the yellow module were investigated using the PharmGKB, oncoKB and CIViC databases to identify potentially druggable targets (16–18). The results showed that MERTK and SYK were druggable according to the CIViC database (18,36,37). Gene variations in CXCR4, FCGR2A, MGAT4A, NCOA1, PIK3R1, RGS5, RRAS2 and SOD2 may be predictive markers or have targetable variations according to the PharmGKB database (16,38–47). A total of 10 oncogenes (CXCR4, ERG, FLT1, IGF1, KDR, LYN, MITF, PIK3CG, REL and SYK), six tumor suppressor genes (MITF, MAP3K1, MOB3B, NFKBIA, PRDM1 and SAMHD1) and one gene (CSF1R), belonging to neither oncogene or tumor suppressor gene categories, were identified, according to the oncoKB database. Some mutations in MITF are oncogenic while others can repress the development of cancer (17). Therefore, MITF was classified as both a tumor suppressor and an oncogenic gene. However, no further evidence could support these findings in OS as the present study only included gene expression results from microarray analysis where no gene variation data were available. These findings were predominantly reported in breast cancer, colorectal cancer and prostatic neoplasms, with only a few studies in OS.
Tumor infiltrating immune cell profiles in OS
The results of the present study showed that M0 and M2 macrophages were two major types of immune cells found in OS tissues. Some memory resting CD4+ T cells were present in OS tissues. In BM-MSCs, memory resting CD4+ T cells and naïve B cells were the two major types of immune cells identified (Fig. S3).
Discussion
Bioinformatic approaches are widely used for the clinical prediction of cancer diagnosis and gene research. By using DEG analysis in combination with WGCNA, biologically meaningful genes and gene modules can be identified as candidate biomarkers (32,48). The present study identified five previous studies related to OS from GEO; 1,405 genes were found dysregulated in OS compared with BM-MSCs. These genes were found to be involved in the ECM, according to the results from functional annotations. WGCNA analysis showed that the yellow module was positively associated with OS. A total of 30 hub genes were selected to visualize their connections. Several DEGs in the yellow module were found to be potentially druggable genes, according to the CIViC, PharmGKB and oncoKB databases. CXCR4 belongs to the chemokine receptor family and is an oncogene that can mediate metastasis in cancers (49). It is overexpressed in breast cancer (50), ovarian cancer (51), melanoma (52), and prostate cancer (53,54). A previous study investigating gastric cancer showed that CXCR4 mRNA expression was positively correlated with docetaxel sensitivity (55), indicating that docetaxel may be effective in patients with OS who have a high level of CXCR4 mRNA expression. These findings may be helpful for guiding the clinical management of OS.
Yang et al (56) conducted a meta-analysis of OS microarray data in 2014 to better understand the underlying mechanism of OS. In this previous study, data was included from different microarray platforms, and results from OS tissue samples and cell lines were also included. The study revealed that ‘ECM-receptor interaction’ and the ‘cell cycle’ were highly enriched KEGG pathways, and several hub genes were identified, including PTBP2, RGS4 and FXYD6 (56). The present study provided some improvements in the inclusion criteria and the analytic methods used. Evidence suggests that cell lines from different laboratories are heterogeneous in various ways (57), therefore, gene expression profiles generated from OS cell lines were excluded from the present study. BM-MSCs from individuals with no signs of malignancies were selected as controls as OS has been reported to originate from BM-MSCs (1). Furthermore, as different microarray platforms may have different probes to represent the same gene, results from different platforms are not usually directly comparable. In the present study, only RNA expression data generated from Human Genome U133 Plus 2.0 array (GPL570) using tissue samples were included to reduce bias. Public databases were used to investigate key genes in order to identify potentially druggable genes or therapeutic targets.
Results from GO and KEGG enrichment analysis suggested that the DEGs identified participated in the ECM; this may contribute to OS development, which was consistent with a previous study by Yang et al (56). Many ECM proteins are significantly dysregulated during the progression of cancer, causing both biochemical and biomechanical changes (58). It has been reported that cancer cells can degrade the ECM, and promote metastasis by facilitating tumor associated angiogenesis and inflammation (59,60). Accumulation of ECM proteins can provide a suitable microenvironment to promote cancer cell proliferation and metastasis (61–63). Miyata et al (64) reported that MMP2 and MMP9 could promote the mobility of vascular epithelial cells by remodeling the ECM. In the present study, both MMP9 and MMP13 were found to be overexpressed in OS.
The yellow gene module was positively related to OS and thus, may play an important role in the development of OS. The top 30 hub genes were selected to visualize the gene-gene interactions. Each of these genes were upregulated >2-fold. In total, 17 out of the 30 hub genes identified were from the innate immune system, including C1QA, C1QB, C1QC, CD14, CTSS, HCK, HLA-DMB, IL10RA, LCP2, MRC1, PECAM1, PIK3AP1, PTPRC, RNASE6, TNFSF13B, TYROBP and VAMP8. C1QA, C1QB and C1QC encode the A-, B- and C-chain of the serum complement subcomponent C1q, respectively. The protein encoded by CD14 is a component of the innate immune system and CD163 functions as an innate immune sensor in bacteria (65,66). CTSS acts as a pivotal role in antigen presentation and can promote cell growth during tumorigenesis (67). GGTA1P can produce an immunogenic carbohydrate structure in Homo sapiens and the aberrant expression of this gene is associated with autoimmune disorders. GIMAP6 and GIMAP8 are members of the GTPase of immunity-associated protein family, regulating lymphocyte survival and homeostasis (68). The protein encoded by HCK may play a role in neutrophil migration and neutrophil degranulation (66). Expression of HCLS1 is not restricted to hematopoietic cell lineages (69). HLA-DMA and HLA-DMB are both required for the normal assembly of peptides onto major histocompatibility complex class II molecules (70,71). HLA-DMB is upregulated in the tumor tissues of patients of a Caucasian decent, but not of African-American descent, and is positively correlated with an increase in T cell infiltration and an improved prognosis (72). In mouse models, PECAM1 is associated with dysregulated osteoclastogenesis and hematopoiesis (73). PTPRC is also known as the CD45 antigen, which belongs to the protein tyrosine phosphatase (PTP) family (74). PTPs can regulate a variety of cellular processes, including cell growth, differentiation, the mitotic cell cycle and oncogenic transformation (74). CENTA2, encoded by ADAP2, can bind β-tubulin and increase its stability (75). AIF1 expression is induced by cytokines and interferon, and may promote the activation of macrophages and the growth of vascular smooth muscle cells (76). It has been reported that tumor-associated macrophages can suppress the T cell-mediated anti-tumor immune response (77). OS is a type of cold tumor, largely due to the anti-inflammatory M2 macrophages enriched in the tumor microenvironment, which can repress tumor-infiltrating T cells (78,79). Results from CIBERSORT revealed that M0 and M2 macrophages were two major types of immune cell found in OS compared with BM-BMCs. This is consistent with the results of previous studies that have been reviewed by Kelleher et al (78), indicating that AIF1 may play an important role in the OS microenvironment. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 can downregulate the immune system by suppressing T cell-mediated inflammatory activity in order to prevent the immune system from killing cancer cell (80). However, a previous study showed that a PD-1 inhibitor is only effective in metastatic OS, as only metastatic OS expressed PD-1 (81). In the present study, PD-1 and PD-L1 were downregulated in OS compared with BM-MSCs.
The protein encoded by MGAT4A can regulate the availability of serum glycoproteins, and may participate in oncogenesis and differentiation (82). As OS is frequently infiltrated by immune cells, including M2 macrophages and T cells (83,84), these findings may be instrumental in developing a better understanding of the mechanisms underlying OS. Further studies are warranted to explore whether and how personalized chemotherapy along with targeted therapy, including PD1 inhibitors, can benefit patients with primary OS.
Several advantages of the present study should be mentioned. Firstly, the inclusion criteria for relevant studies has been improved, only RNA expression data from the same platform and from tissues were included in the present study. Additionally, DEG analysis was combined with WGCNA analysis, which reduced the number of genes closely related to OS. Key genes from the yellow module were further compared using the CIViC, PharmGKB and oncoKB databases, and several promising druggable targets were identified. However, there were also several limitations to the present study. The five studies included were from The Netherlands, Japan, Germany, Belgium and France; stratified analysis was not performed on these data due to the relatively small samples in each study, and the heterogeneity of the tissues used in the different studies were unmodifiable, which should be improved in further studies.
In conclusion, the present study identified a group of DEGs in OS using meta-analysis and bioinformatics analysis, and several key genes that may contribute to OS were identified. Functional annotations of these hub genes indicated that the ECM is involved in the development of OS. The present study improved our understanding of the mechanisms underlying the development of OS.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- OS
osteosarcoma
- GEO
Gene Expression Omnibus
- DEGs
differently expressed genes
- WGCNA
weighted gene co-expression network analysis
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- ECM
extracellular matrix
- CAMs
cell adhesion molecules
- GIMAP
GTPase of immunity-associated protein
- PTP
protein tyrosine phosphatase
- BM-MSCs
bone marrow mesenchymal stromal cells
Funding
The present study was supported by the Key Research Program for Higher Educational Institutes of Henan Province (grant no. 19B310007) and the Key R&D and Transfer Program for the Institute of Science and Technology of Henan Province (grant nos. 192102310402 and 192102310034). The funding body had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript or the decision to submit the paper for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JSh and CZ conceived the idea. JSu, HX and MQ retrieved and screened all relevant studies, and conducted the bioinformatic analyses. JSh prepared the manuscript with all other authors contributing to its completion.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment-where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Pediatric and adolescent osteosarcoma, Springer. 2009:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6:85–92. doi: 10.1016/S1470-2045(05)01734-1. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity and apoptosis pathway. Mol Cancer Ther. 2008;7:3461–3469. doi: 10.1158/1535-7163.MCT-08-0530. [DOI] [PubMed] [Google Scholar]
- 5.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempf-Bielack B, Bielack SS, Jürgens H, Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G, Kabisch H, et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 7.Kruzelock RP, Murphy EC, Strong LC, Naylor SL, Hansen MF. Localization of a novel tumor suppressor locus on human chromosome 3q important in osteosarcoma tumorigenesis. Cancer Res. 1997;57:106–159. [PubMed] [Google Scholar]
- 8.Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: Emerging biology and candidate therapies. Crit Rev Oncog. 2015;20:173–197. doi: 10.1615/CritRevOncog.2015013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belchis DA, Meece CA, Benko FA, Rogan PK, Williams RA, Gocke CD. Loss of heterozygosity and microsatellite instability at the retinoblastoma locus in osteosarcomas. Diagn Mol Pathol. 1996;5:214–219. doi: 10.1097/00019606-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Benassi MS, Molendini L, Gamberi G, Ragazzini P, Sollazzo MR, Merli M, Asp J, Magagnoli G, Balladelli A, Bertoni F, Picci P. Alteration of pRb/p16/cdk4 regulation in human osteosarcoma. Int J Cancer. 1999;84:489–493. doi: 10.1002/(SICI)1097-0215(19991022)84:5<489::AID-IJC7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3:221–243. doi: 10.1007/s40744-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lage K, Hansen NT, Karlberg EO, Eklund AC, Roque FS, Donahoe PK, Szallasi Z, Jensen TS, Brunak S. A large-scale analysis of tissue-specific pathology and gene expression of human disease genes and complexes. Proc Natl Acad Sci USA. 2008;105:20870–20875. doi: 10.1073/pnas.0810772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Cao J. A close examination of double filtering with fold change and t test in microarray analysis. BMC Bioinformatics. 2009;10:402. doi: 10.1186/1471-2105-10-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 16.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, Altman RB, Klein TE. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92:414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, Rudolph JE, Yaeger R, Soumerai T, Nissan MH, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol. 2017 May 16; doi: 10.1200/PO.17.00011. (Epub ahead of print). doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith M, Spies NC, Krysiak K, McMichael JF, Coffman AC, Danos AM, Ainscough BJ, Ramirez CA, Rieke DT, Kujan L, et al. CIViC is a community knowledgebase for expert crowdsourcing the clinical interpretation of variants in cancer. Nat Genet. 2017;49:170–174. doi: 10.1038/ng.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamidouche Z, Fromigué O, Ringe J, Häupl T, Vaudin P, Pagès JC, Srouji S, Livne E, Marie PJ. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci USA. 2009;106:18587–18591. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi E, Masuda M, Nakayama R, Ichikawa H, Satow R, Shitashige M, Honda K, Yamaguchi U, Shoji A, Tochigi N, et al. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther. 2010;9:535–544. doi: 10.1158/1535-7163.MCT-09-0774. [DOI] [PubMed] [Google Scholar]
- 21.Guilloton F, Caron G, Ménard C, Pangault C, Amé-Thomas P, Dulong J, De Vos J, Rossille D, Henry C, Lamy T, et al. Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood. 2012;119:2556–2567. doi: 10.1182/blood-2011-08-370908. [DOI] [PubMed] [Google Scholar]
- 22.André T, Meuleman N, Stamatopoulos B, De Bruyn C, Pieters K, Bron D, Lagneaux L. Evidences of early senescence in multiple myeloma bone marrow mesenchymal stromal cells. PLoS One. 2013;8:e59756. doi: 10.1371/journal.pone.0059756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vella S, Tavanti E, Hattinger CM, Fanelli M, Versteeg R, Koster J, Picci P, Serra M. Targeting CDKs with roscovitine increases sensitivity to DNA damaging drugs of human osteosarcoma cells. PLoS One. 2016;11:e0166233. doi: 10.1371/journal.pone.0166233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 25.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson M, Falcon S, Pages H, Li N. hgu133plus2. db: Affymetrix human genome U133 Plus 2.0 Array annotation data (chip hgu133plus2) R package version 2. 2012 [Google Scholar]
- 29.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G, Wang LG, Yan GR, He QY. DOSE: An R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics. 2015;31:608–609. doi: 10.1093/bioinformatics/btu684. [DOI] [PubMed] [Google Scholar]
- 31.Metsalu T, Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlegel J, Sambade MJ, Sather S, Moschos SJ, Tan AC, Winges A, DeRyckere D, Carson CC, Trembath DG, Tentler JJ, et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J Clin Invest. 2013;123:2257–2267. doi: 10.1172/JCI67816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Gaillard S, Phillip JM, Huang TC, Pinto SM, Tessarollo NG, Zhang Z, Pandey A, Wirtz D, Ayhan A, et al. Inhibition of spleen tyrosine kinase potentiates paclitaxel-induced cytotoxicity in ovarian cancer cells by stabilizing microtubules. Cancer Cell. 2015;28:82–96. doi: 10.1016/j.ccell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsusaka S, Cao S, Hanna DL, Sunakawa Y, Ueno M, Mizunuma N, Zhang W, Yang D, Ning Y, Stintzing S, et al. CXCR4 polymorphism predicts progression-free survival in metastatic colorectal cancer patients treated with first-line bevacizumab-based chemotherapy. Pharmacogenomics J. 2017;17:543–550. doi: 10.1038/tpj.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 40.Tamura K, Shimizu C, Hojo T, Akashi-Tanaka S, Kinoshita T, Yonemori K, Kouno T, Katsumata N, Ando M, Aogi K, et al. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22:1302–1307. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- 41.Pander J, van Huis-Tanja L, Böhringer S, van der Straaten T, Gelderblom H, Punt C, Guchelaar HJ. Genome wide association study for predictors of progression free survival in patients on capecitabine, oxaliplatin, bevacizumab and cetuximab in first-line therapy of metastatic colorectal cancer. PLoS One. 2015;10:e0131091. doi: 10.1371/journal.pone.0131091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartmaier RJ, Richter AS, Gillihan RM, Sallit JZ, McGuire SE, Wang J, Lee AV, Osborne CK, O'Malley BW, Brown PH, et al. A SNP in steroid receptor coactivator-1 disrupts a GSK3β phosphorylation site and is associated with altered tamoxifen response in bone. Mol Endocrinol. 2012;26:220–177. doi: 10.1210/me.2011-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascual T, Apellániz-Ruiz M, Pernaut C, Cueto-Felgueroso C, Villalba P, Álvarez C, Manso L, Inglada-Pérez L, Robledo M, Rodríguez-Antona C, Ciruelos E. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS One. 2017;12:e0180192. doi: 10.1371/journal.pone.0180192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volz N, Stintzing S, Zhang W, Yang D, Ning Y, Wakatsuki T, El-Khoueiry RE, Li JE, Kardosh A, Loupakis F, et al. Genes involved in pericyte-driven tumor maturation predict treatment benefit of first-line FOLFIRI plus bevacizumab in patients with metastatic colorectal cancer. Pharmacogenomics J. 2015;15:69–76. doi: 10.1038/tpj.2014.40. [DOI] [PubMed] [Google Scholar]
- 45.Rokavec M, Schroth W, Amaral SM, Fritz P, Antoniadou L, Glavac D, Simon W, Schwab M, Eichelbaum M, Brauch H. A polymorphism in the TC21 promoter associates with an unfavorable tamoxifen treatment outcome in breast cancer. Cancer Res. 2008;68:9799–9808. doi: 10.1158/0008-5472.CAN-08-0247. [DOI] [PubMed] [Google Scholar]
- 46.Glynn SA, Boersma BJ, Howe TM, Edvardsen H, Geisler SB, Goodman JE, Ridnour LA, Lønning PE, Børresen-Dale AL, Naume B, et al. A mitochondrial target sequence polymorphism in manganese superoxide dismutase predicts inferior survival in breast cancer patients treated with cyclophosphamide. Clin Cancer Res. 2009;15:4165–4173. doi: 10.1158/1078-0432.CCR-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosó V, Herrero MJ, Santaballa A, Palomar L, Megias JE, de la Cueva H, Rojas L, Marqués MR, Poveda JL, Montalar J, Aliño SF. SNPs and taxane toxicity in breast cancer patients. Pharmacogenomics. 2014;15:1845–1858. doi: 10.2217/pgs.14.127. [DOI] [PubMed] [Google Scholar]
- 48.DiLeo MV, Strahan GD, den Bakker M, Hoekenga OA. Weighted correlation network analysis (WGCNA) applied to the tomato fruit metabolome. PLoS One. 2011;6:e26683. doi: 10.1371/journal.pone.0026683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 50.Holm NT, Byrnes K, Li BD, Turnage RH, Abreo F, Mathis JM, Chu QD. Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J Surg Res. 2007;141:53–59. doi: 10.1016/j.jss.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: An independent prognostic factor for tumor progression. Gynecol Oncol. 2006;103:226–233. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 52.Longo-Imedio MI, Longo N, Treviño I, Lázaro P, Sánchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. 2005;117:861–865. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: A system review and meta-analysis. Oncotarget. 2015;6:5022–5040. doi: 10.18632/oncotarget.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–542. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie L, Wei J, Qian X, Chen G, Yu L, Ding Y, Liu B. CXCR4, a potential predictive marker for docetaxel sensitivity in gastric cancer. Anticancer Res. 2010;30:2209–2216. [PubMed] [Google Scholar]
- 56.Yang Z, Chen Y, Fu Y, Yang Y, Zhang Y, Chen Y, Li D. Meta-analysis of differentially expressed genes in osteosarcoma based on gene expression data. BMC Med Genet. 2014;15:80. doi: 10.1186/1471-2350-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Mi Y, Mueller T, Kreibich S, Williams EG, Van Drogen A, Borel C, Frank M, Germain PL, Bludau I, et al. Multi-omic measurements of heterogeneity in HeLa cells across laboratories. Nat Biotechnol. 2019;37:314–322. doi: 10.1038/s41587-019-0037-y. [DOI] [PubMed] [Google Scholar]
- 58.Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivanov V, Ivanova S, Roomi M, Kalinovsky T, Niedzwiecki A, Rath M. Naturally produced extracellular matrix inhibits growth rate and invasiveness of human osteosarcoma cancer cells. Med Oncol. 2007;24:209–217. doi: 10.1007/BF02698042. [DOI] [PubMed] [Google Scholar]
- 60.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim BG, An HJ, Kang S, Choi YP, Gao MQ, Park H, Cho NH. Laminin-332-rich tumor microenvironment for tumor invasion in the interface zone of breast cancer. Am J Pathol. 2011;178:373–381. doi: 10.1016/j.ajpath.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee S, Stewart S, Nagtegaal I, Luo J, Wu Y, Colditz G, Medina D, Allred DC. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res. 2012;72:4574–4586. doi: 10.1158/0008-5472.CAN-12-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyata Y, Kanda S, Nomata K, Hayashida Y, Kanetake H. Expression of metalloproteinase-2, metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in transitional cell carcinoma of upper urinary tract: Correlation with tumor stage and survival. Urology. 2004;63:602–608. doi: 10.1016/j.urology.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 65.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 66.Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 67.Héninger E, Krueger TE, Lang JM. Augmenting antitumor immune responses with epigenetic modifying agents. Front Immunol. 2015;6:29. doi: 10.3389/fimmu.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwefel D, Arasu BS, Marino SF, Lamprecht B, Köchert K, Rosenbaum E, Eichhorst J, Wiesner B, Behlke J, Rocks O, et al. Structural insights into the mechanism of GTPase activation in the GIMAP family. Structure. 2013;21:550–559. doi: 10.1016/j.str.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Fischer U, Michel A, Meese EU. Expression of the gene for hematopoietic cell specific protein is not restricted to cells of hematopoietic origin. Int J Mol Med. 2005;15:611–615. [PubMed] [Google Scholar]
- 70.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, Bergman C, Monaco JJ, Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 71.Fling SP, Arp B, Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–548. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- 72.Kinseth MA, Jia Z, Rahmatpanah F, Sawyers A, Sutton M, Wang-Rodriguez J, Mercola D, McGuire KL. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int J Cancer. 2014;134:81–91. doi: 10.1002/ijc.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Tworkoski K, Michaud M, Madri JA. Bone marrow monocyte PECAM-1 deficiency elicits increased osteoclastogenesis resulting in trabecular bone loss. J Immunol. 2009;182:2672–2679. doi: 10.4049/jimmunol.0802398. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan R, Morse B, Huebner K, Croce C, Howk R, Ravera M, Jaye M, Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci USA. 1990;87:7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuccotti P, Cartelli D, Stroppi M, Pandini V, Venturin M, Aliverti A, Battaglioli E, Cappelletti G, Riva P. Centaurin-α2 interacts with β-tubulin and stabilizes microtubules. PLoS One. 2012;7:e52867. doi: 10.1371/journal.pone.0052867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Autieri MV, Carbone C, Mu A. Expression of allograft inflammatory factor-1 is a marker of activated human vascular smooth muscle cells and arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1737–1744. doi: 10.1161/01.ATV.20.7.1737. [DOI] [PubMed] [Google Scholar]
- 77.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelleher FC, O'Sullivan H. Monocytes, macrophages and osteoclasts in osteosarcoma. J Adolesc Young Adult Oncol. 2017;6:396–405. doi: 10.1089/jayao.2016.0078. [DOI] [PubMed] [Google Scholar]
- 79.Han Q, Shi H, Liu F. CD163(+) M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int Immunopharmacol. 2016;34:101–106. doi: 10.1016/j.intimp.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 80.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 81.Lussier DM, O'Neill L, Nieves LM, McAfee MS, Holechek SA, Collins AW, Dickman P, Jacobsen J, Hingorani P, Blattman JN. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother. 2015;38:96–106. doi: 10.1097/CJI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida A, Minowa MT, Takamatsu S, Hara T, Oguri S, Ikenaga H, Takeuchi M. Tissue specific expression and chromosomal mapping of a human UDP-N-acetylglucosamine: Alpha1,3-d-mannoside beta1, 4-N-acetylglucosaminyltransferase. Glycobiology. 1999;9:303–310. doi: 10.1093/glycob/9.3.303. [DOI] [PubMed] [Google Scholar]
- 83.Buddingh EP, Kuijjer ML, Duim RA, Bürger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PC, Lankester AC, Cleton-Jansen AM. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin Cancer Res. 2011;17:2110–2119. doi: 10.1158/1078-0432.CCR-10-2047. [DOI] [PubMed] [Google Scholar]
- 84.van Ravenswaay Claasen HH, Kluin PM, Fleuren GJ. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab Invest. 1992;67:166–174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.