Abstract
Osteoarthritis (OA) is one of the most prevalent types of chronic joint diseases. Chondrocytes survival is closely associated with the destruction of joints in patients with OA. Long noncoding RNAs (lncRNAs) serve a critical role in OA. However, to the best of our knowledge, the role of lncRNAs NR024118 in OA has not been examined. In the present study, the expression levels of NR024118 in lipopolysaccharide (LPS)-induced chondrocytes was measured using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and the apoptosis levels of cells was determined using flow cytometry. The levels of scavenged reactive oxygen species and expression levels of the antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and heme oxygenase-1 (HO-1) were measured using specialized detection kits. The expression of interleukin (IL)-1β, IL-6 and IL-18 were measured using ELISA. Expression of the cell apoptosis markers Bcl-2, Bax, Bcl-2-like protein 11, NF-κB, phosphorylated (p)-NF-κB inhibitor β (IκBβ), IκBβ, p-transcription factor p65 (p65) and p65, and nuclear factor erythroid-2 related factor 2 (Nrf2) signaling pathways-associated proteins, Nrf2, HO-1 and quinone oxidoreductase-1 were detected by western blot analysis and RT-qPCR. The results indicated that in ATDC5 cells, apoptosis, oxidative stress and inflammation were significantly increased and the expression level of NR024118 was significantly decreased by LPS-mediated induction. NR024118 overexpression significantly reversed the effects of LPS treatment in the ATDC5 cell line. In addition, the overexpression of NR024118 decreased NF-κB expression levels and activated the Nrf2 signaling pathways in LPS-induced ATDC5 cells. The present study demonstrated that NR024118 attenuated the effects of LPS-induction on ATDC5 cells. These results suggest that NR024118 may be a potential target for diagnosis and treatment of OA.
Keywords: lncRNA NR024118, osteoarthritis, nuclear factor κB, nuclear factor erythroid-2 related factor 2, inflammatory
Introduction
Osteoarthritis (OA) is identified as the degradation of articular cartilage, and it affects >43 million people globally. It is the most common cause of disability and disproportionately affects the elderly (1,2). OA is associated with a number of risk factors, including ageing, obesity and acetabular dysplasia, and has a hereditary aspect (3,4). Deterioration of the cartilage is generally accepted as the primary cause of OA occurrence. Chondrocytes are considered to be the target of biomechanical factors leading to abnormal functional changes in these cells, resulting in physiological changes that cause OA (5). OA is commonly treated with joint replacement surgery and anti-inflammatory drugs, although the inflammatory drugs have several side effects (6). Improving the understanding of the underlying molecular mechanisms of inflammatory injury of cartilage cells in OA may result in the development of improved therapeutic options.
Long noncoding RNAs (lncRNAs) are noncoding transcripts that are >200 nucleotides in length and lack protein encoding capacity (7). Although lncRNAs were initially thought to be transcriptional noise, they are now regarded as molecules with diverse functional roles in regulating a range of cellular functions. Different lncRNAs have exhibited differential expression patterns in different diseases and are involved in various developmental and pathological conditions of a number of diseases (8). A recent study suggested that lncRNAs serve a vital role in OA pathogenesis (9). lncRNA NR024118 is a recently identified lncRNA that is closely associated with the pathophysiology of a number of diseases, including cancer, cardiovascular diseases, neural diseases and rheumatoid arthritis (RA). However, the potential effects of lncRNA NR024118 in OA remain unknown.
NF-κB is a well-studied and key inflammatory molecule. Activation of the NF-κB pathway results in the production of pro-inflammatory cytokines, including tumor necrosis factor α, interleukin (IL)-1β and IL-6 (10). Nuclear factor erythroid-2 related factor 2 (Nrf2) is an important transcription factor and master regulator of the cellular response to oxidative stress and the inflammatory process, which increases the expression of antioxidant and cytoprotective enzymes and downstream proteins including catalase (CAT), superoxide dismutase (SOD), quinone oxidoreductase-1 (NQO1) and heme oxygenase-1 (HO-1) (11), and the Nrf2 pathway serves an important role in lipopolysaccharide (LPS)-induced inflammatory injury (12).
In the present study, the potential effects of NR024118 on apoptosis and inflammatory damage in LPS-treated chondrocytes were measured. Furthermore, the effects of NR024118 on LPS-induced activation of the NF-κB signaling pathways and inhibition of the Nrf2 signaling pathway were examined. The results of the present study may provide insight into the identification of novel therapeutic and diagnostic targets for diagnosing and treating patients with OA.
Materials and methods
Cell culture and treatment
The murine chondrogenic ATDC5 cell line was obtained from the Cell Bank Cell Engineering Division, RIKEN BioResource Research Center and cultured in DMEM/Ham Nutrient Mixture F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5% CO2. Cells were treated with 5 µg/ml LPS (5 mg/ml; Sigma-Aldrich; Merck KGaA) for 5 h to stimulate inflammatory injury.
Transfection
The NR024118 sequence (TCAAAGACCACCACCATCTTCCTCAATGGCAACCGCGAGCGGCCCTTGGATGTGTTTTGTGACATGCAGACTGACGGAGGAGGTTGGCTGGTGTTCCAGCGCCGCATGGACGGACAGACAGACTTCTGGAGAGACTGGGAGGAGTACGCCCATGGTTTTGGGAACATCTCCAGGGAATTCTGGCTGGGCAATGAGGCCCTTCACAGCCTCACGCAGGCTGGAGACTACTCTATGCGTGTGGACCTGCGGGCCGGAAAGGAAGCCGTGTTCGCCCAGTATGACTTCTTCCGAGTAGACTCAGCGAAGGAGAACTATCGTCTACACCTAGGGGGCTACCATGGGACCGCGGGTGACTCTATGAGCTACCACAGCGGCAGTGCCTTTTCTGCCCGTGATCGAGACCCCAATAACTTGCTCATCTCCTGCGCTGTCTCCTATCGTGGGGCTTGGTGGTACAGGGACTGTCACTACGCCAATCTCAATGGGCTCTATGGGAGCACAGTGGATCACCAGGGAGTGAGCTGGTACCACTGGAAGGGCTTCGAGTTCTCGGTGCCCTTCACGGAAATGAAGCTGAGACCCAGAAACTTCCAGGCCCCCACCAGGGGCACCTGAGCCTGCTGCCCACCTCACTCACACCCTGGTATGACTGCCGAGCACTGAGGGGTTGTGCCCAGAGAAGAGCCAGTGTGTCTCTACTGTGCCTAGCTCACCGAGGAAGCCTTCTCTGCCACAGTCTCACAGCACCATGTTTACAGGGGGGAGGGGAGGGAAATGGAGCAATAAAGGAGAA) was synthesized and subcloned into a pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.). The pcDNA3.1-NR024118 (NR024118) or empty vector was transfected into chondrocytes using Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols, and the concentration is as followed: pcDNA 3.1: Lipofectamine=2 µg: 4 µl. After 48 h, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to evaluate the efficiency of the transfections.
Cell viability assay
Cell viability assay was assessed using a Cell Counting Kit-8 (CCK-8) assay Dojindo Molecular Technologies, Inc.). Briefly, cells (5×103 cells/well) were seeded in a 96-well cell culture plate following LPS treatment and/or transfection with pcDNA3.1-NR024118 and 20 µl CCK-8 solution was added into each well and incubated at 37°C for 2 h. The absorbance at 450 nm of each well was measured using a microplate reader (Bio-Rad Laboratories, Inc.).
Cell apoptosis assay
Cell apoptosis was detected using fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI) staining (BD Biosciences). Briefly, following LPS treatment and/or transfection with pcDNA3.1-NR024118, ATDC5 cells were collected and stained with 10 µl Annexin V-FITC and 5 µl PI in the dark at 37°C for 25 min. Subsequently, the percentage of apoptotic cells were determined using flow cytometry (Guava Technologies, Inc.; Luminex Corporation). FlowJo software version 10.0 (FlowJo LLC) was used to analyze the data.
Cytokine analysis
The concentrations of IL-1β, IL-6 and IL-18 were determined using specific ELISA kits (Mouse IL-1β/ IL-1F2 Quantikine ELISA kit, cat. no. MLB00C; Mouse IL-6 Quantikine ELISA kit, cat. no. M6000B, and Mouse IL-18 ELISA, cat. no. 7625, respectively; R&D Systems, Inc.,) according to the manufacturer's protocols.
Measurement of intracellular reactive oxygen species (ROS)
The intracellular ROS levels were measured using the probe CM-H2DCFDA (Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, following LPS treatment and/or transfection with pcDNA3.1-NR024118, the cells were incubated with DCF-DA at 37°C for 30 min. Subsequently, the cells were trypsinized and suspended in PBS. Cells were added with PI (1 µg/ml) on ice in the dark, and the fluorescence intensities were measured at 485 and 535 nm using Cell Lab Quanta SC MPL flow cytometer (Beckman Coulter, Inc.). Acquisition and analysis of the data were performed the software of the flow cytometer.
Measurement of antioxidant enzyme activity
The activity of SOD was determined using a SOD assay kit (cat. no. A001-3-2; Nanjing Jiancheng Bioengineering Institute). MDA levels were determined using an MDA assay kit (cat. no. A003-1-2; Nanjing Jiancheng Bioengineering Institute) and CAT levels were examined using a CAT assay kit (cat. no. A007-1-1; Nanjing Jiancheng Bioengineering Institute) according to the manufacturers' protocols.
Western blot analysis
ATDC5 cells were lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology) and supplemented with protease inhibitors (Roche Diagnostics). The protein concentration was quantified using a bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 20 µg protein per lane was resolved using 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (EMD Millipore). The membrane was blocked in 5% skimmed milk at room temperature for 1 h and incubated with appropriate primary antibodies (dilution 1:1,000) at 4°C overnight. The primary antibodies used in the present study were: Anti-phosphorylated (p)-NF-κB inhibitor β (IκBβ; cat. no. ab59195; Abcam), anti-IκBβ (cat. no. ab32135; Abcam), anti-NF-κB transcription factor p65 (p65) antibody (cat. no. 8242; Cell Signaling Technology, Inc), anti-p-NF-κB p65 antibody (cat. no. 3033; Cell Signaling Technology, Inc), Bcl-2 (cat. no. ab196495; Abcam), Bax (cat. no. ab182733; Abcam), Bcl-2-like protein 11 (Bim; cat. no. ab7888; Abcam), Nrf2 (cat. no. 12721; Cell Signaling Technology, Inc), HO-1 (cat. no. 82206; Cell Signaling Technology, Inc), NQO1 (cat. no. 3187; Cell Signaling Technology, Inc) and GAPDH (cat. no. ab181603, Abcam). Subsequently, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (dilution 1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The signals were visualized by enhanced chemiluminescence kit (GE Healthcare) and Image Lab™ Software version 4.1 (Bio-Rad Laboratories, Inc.) and protein expression levels were quantified using ImageJ version 1.46 (National Institutes of Health).
RT-qPCR
Total RNA from cultured cells was extracted using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. A total of 2 µg RNA was reversed transcribed into cDNA using the PrimeScript™ RT reagent kit with DNA Eraser (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol using the following conditions: Initial incubation at 37°C for 15 min, followed by incubation at 85°C for 5 sec. NR024118 expression was quantified using SYBR1 Green RealTime PCR Master mix (Invitrogen; Thermo Fisher Scientific, Inc.) in an ABI PRISM® 7300 PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR reactions were performed using the following conditions: Initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 sec and anneal/extend at 63°C for 60 sec. The primers sequences were: NR024118 forward, 5′-GCTGCCCACCTCACTCAC-3′; NR024118 reverse, 5′-CTTTATTGCTCCATTTCCCTC-3′; Nrf2 forward, 5′-TTCCTCTGCTGCCATTAGTCAGTC-3′; Nrf2 reverse, 5′-GCTCTTCCATTTCCGAGTCACTG-3′; NQO-1 forward, 5′-AAGAGCCCTGATTGTACTGGC-3′; NQO-1 reverse, 5′-GCGTCCTTCCTTATATGCTAGAGA-3′; HO-1 forward, 5′-GTGACAGAAGAGGCTAAGACCG-3′; HO-1 reverse, 5′-ACAGGAAGCTGAGAGTGAGGAC-3′; GAPDH forward, 5′-GGGAAACTGTGGCGTGAT-3′; GAPDH reverse, 5′-GAGTGGGTGTCGCTGTTGA-3′. GAPDH was used as the endogenous control and the data was quantified using the 2−ΔΔCq method (13).
Statistical analysis
Data are presented as the mean ± standard deviation from at least 3 experimental repeats. Statistical analysis was performed using GraphPad Prism v.5 (GraphPad Software, Inc.). A one-way analysis of variance followed by Tukey's post hoc test or an unpaired two-tailed t-test was used to analyze the data. P<0.05 was considered to indicate a statistically significant difference.
Results
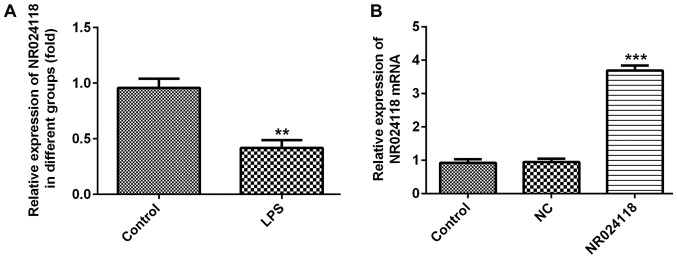
NR024118 expression is downregulated in LPS-induced in ATDC5 cells
Compared with the control group, NR024118 expression was significantly decreased in ATDC5 cells following treatment with LPS (Fig. 1A). To additionally investigate the biological role of NR024118 in OA, the expression of NR024118 was overexpressed by transfecting cells with pcDNA3.1-NR02411. Relative expression of NR024118 was significantly increased in cells transfected with pcDNA-NR02411 compared with the control.
Figure 1.
LPS induces the downregulation of NR024118 in ATDC5 cells. (A) NR024118 expression was detected by RT-qPCR. (B) Expression levels of NR024118 in ATDC5 cells transfected with pCDNA3.1-NR024118 (NR024118) or empty vectors were measured by RT-qPCR. Data are presented as the mean ± standard deviation. **P<0.01 and ***P<0.001 vs. control group. LPS, lipopolysaccharide; NR024118, pCDNA3.1-NR024118; NC, negative control; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
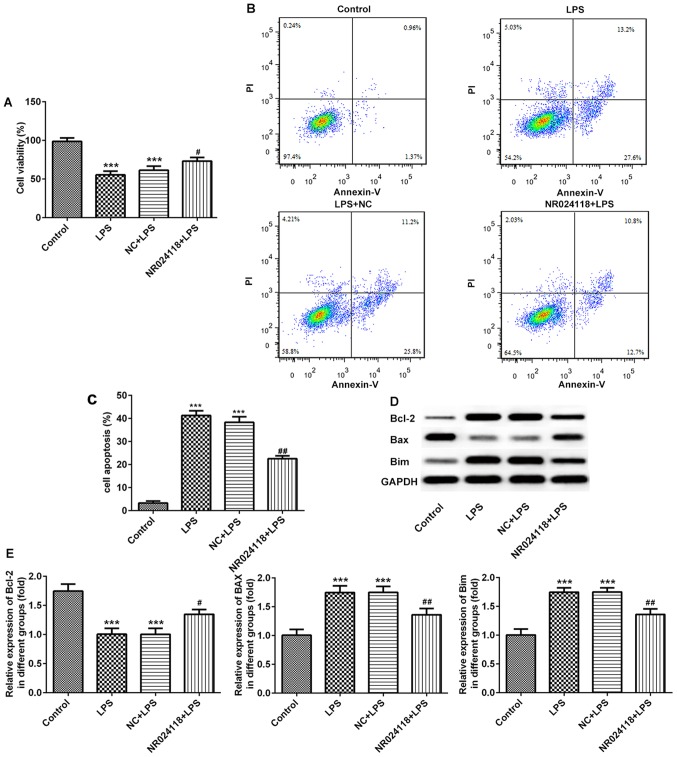
Overexpression of NR024118 attenuates LPS-induced cell apoptosis in ATDC5 cells
The effect of NR024118 on LPS-induced chondrocyte apoptosis was determined using a CCK-8 assay and flow cytometry. The results indicated that the levels of the LPS-induced decrease in cell viability and induction of apoptosis were decreased in cells transfected with NR024118 (Fig. 2A-C). In addition, in the LPS-induced cells, Bcl-2 and Bim expression were upregulated, and Bax was downregulated compared with the control; these results were reversed in cells transfected with NR024118.
Figure 2.
Overexpression of NR024118 alleviates LPS-induced cell apoptosis in ATDC5 cells. (A) Cell viability was detected by CCK-8 assay. (B) Cell apoptosis was detected by flow cytometry. (C) Quantitative analysis of the flow cytometry data. (D) The expression levels of apoptosis-associated factors Bax, Bcl-2 and Bim was detected by western blot analysis. (E) Quantitative analysis the protein expression. Data are presented as the mean ± standard deviation. ***P<0.001 vs. control group. #P<0.05 and ##P<0.01 vs. LPS group. LPS, lipopolysaccharide; NR024118, pCDNA3.1-NR024118; NC, negative control; Bim, Bcl-2-like protein 11; FITC, fluorescein isothiocyanate.
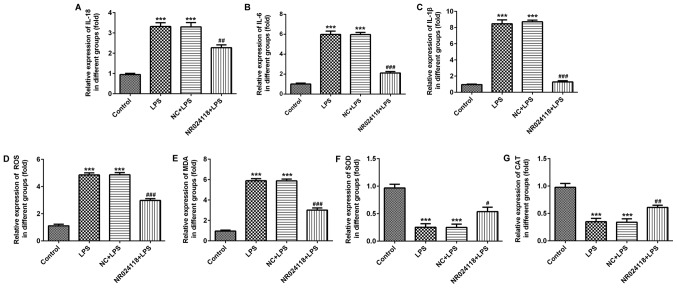
Overexpression of NR024118 attenuates LPS-induced inflammatory injury in ATDC5 cells
Following stimulation of cells with 5 µg/ml LPS, the levels of IL-18, IL-1β and IL-6 were significantly increased compared with the control group. Overexpression of NR024118 significantly decreased the expression of IL-1β, IL-6 and IL-18 in LPS-induced cells (Fig. 3A-C). In addition, ROS production was decreased by NR024118 overexpression (Fig. 3D). LPS stimulation significantly increased the levels of MDA (Fig. 3E) and decreased the levels of SOD (Fig. 3F) and CAT (Fig. 3G) compared with the control group. These alterations in production and activity were significantly reversed by the overexpression of NR024118.
Figure 3.
Overexpression of NR024118 alleviates LPS-induced inflammatory injury in ATDC5 cells. The concentration of inflammatory cytokines (A) IL-18, (B) IL-6 and (C) IL-1β in the culture supernatant of ATDC5 cells was detected by ELISA. (D) ROS, (E) MDA, (F) SOD and (G) CAT expression were detected by specific commercial kits. Data are presented as the mean ± standard deviation. ***P<0.001 vs. control group. #P<0.05, ##P<0.01 and ###P<0.001 vs. LPS group. LPS, lipopolysaccharide; IL, interleukin; ROS, reactive oxygen species; SOD, superoxide dismutase; MDA, malondialdehyde; CAT, catalase; NR024118, pCDNA3.1-NR024118; NC, negative control.
Overexpression of NR024118 inhibits LPS-induced NF-κB pathway activation and promotes Nrf2 pathway activation in ATDC5 cells
Western blot analysis was used to detect the activation of the NF-κB and Nrf2 signaling pathways in ATDC5 cells following LPS treatment and/or transfection with pcDNA3.1-NR024118 transfection. As indicated in Fig. 4A-C, LPS treatment resulted in the activation of the NF-κB pathway by increasing the expression levels of total and p-IκBβ and total and p-p65. Overexpression of NR024118 significantly inhibited the LPS-induced NF-κB pathway activation as the expression levels of total and p-IκBβ and total and p-p65 in ATDC5 cells were decreased. In addition, LPS treatment significantly activated the Nrf2 pathway by increasing the protein (Fig. 4D-G) and mRNA (Fig. 4H-J) expression levels of the Nrf2 signaling pathway associated molecules, Nrf2, HO-1 and NQO1. Overexpression of NR024118 significantly increased the protein and mRNA expression levels of Nrf2, HO-1 and NQO1.
Figure 4.
Overexpression of NR024118 inhibits LPS-induced NF-κB pathway activation and promotes Nrf2 pathway activation in ATDC5 cells. (A) Western blot analysis was performed to evaluate the protein expression levels of p-IκBβ, t-IκBβ, p-p65 and t-p65 following treatment with 5 µg/ml LPS and/or pCDNA3.1-NR024118 transfection in ATDC5 cells. (B) Quantitative analyses of the ratios of p/t-IκBβ and (C) p/t-p65 were performed. (D) Western blot analysis was performed to evaluate the protein expression of NQO-1, NRF2 and HO-1 following treatment with 5 µg/ml LPS treatment and/or pCDNA3.1-NR024118 transfection in ATDC5 cells. Quantitative analyses of the protein expression of (E) NQO-1, (F) NRF2 and (G) HO-1 were performed. RT-qPCR was conducted to analyze the mRNA expressions of (H) NQO-1, (I) NRF2 and (J) HO-1 following treatment with 5 µg/ml LPS and/or pCDNA3.1-NR024118 transfection in ATDC5 cells. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. #P<0.05 and ##P<0.01 vs. LPS group. LPS, lipopolysaccharide; NR024118, pCDNA3.1-NR024118; NC, negative control; IκBβ, NF-κB inhibitor β; p65, transcription factor p65; p-, phosphorylated; t-, total; NQO-1, quinone oxidoreductase-1; NRF2, nuclear factor erythroid-2 related factor 2; HO-1, heme oxygenase-1.
Discussion
OA is a widespread joint disorder that plagues millions of people globally and is a major cause of disability in the elderly (14). In the present study, NR024118 expression was significantly increased in LPS-induced ATDC5 cells. Overexpression of NR024118 reversed the LPS-induced decrease in the viability of ATDC5 cells, and also inhibited the LPS-induced increase in apoptosis, oxidative stress injury and secretion of proinflammatory cytokines, and activation of the NF-κB pathways. In addition, the overexpression of NR024118 effectively augmented the LPS-induced changes in the protein expression levels of Nrf2, HO-1 and NQO1, and activation of the Nrf2 pathway.
Inflammatory damage of the articular cartilage serves a vital role in articular cartilage degeneration and OA progression (15). LPS has been used to mimic OA in vitro and to examine potential drugs for OA (16). Therefore, in the present study, LPS-induced cells were used as a model of OA in vitro. Consistent with data from a previous study (16), LPS treatment significantly decreased ATDC5 cell viability, increased cell apoptosis, oxidative stress and secretion of inflammatory cytokines.
Recently, lncRNAs have been demonstrated to be involved in a variety of biological processes, and a number of different types of diseases, including OA. Numerous lncRNAs, including HOTAIR (17), MALAT1 (18) and MEG3 (19) function to degrade cartilage in inflammatory injury, and are therefore considered as promising therapeutic targets of OA. A previous study indicated that shikonin inhibited the secretion and expression of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via lncRNA-NR024118 (20). The results of the present study demonstrated that NR024118 was significantly upregulated in LPS-treated ATDC5 cells and that the overexpression of NR024118 reversed LPS-induced cell damages in ATDC5 cells.
NF-κB activation exhibits a crucial role in the LPS-induced inflammatory response (21). LPS stimulation increases the phosphorylation of IκBβ and NF-κB p65, increasing the transcription of multiple inflammatory cytokines, including IL-6 and IL-1β (22). The NF-κB signaling pathway is generally considered to be a central regulator of the chondrocyte inflammatory response (23). A previous study has suggested that lncRNA MALAT1 mitigated the LPS-induced inflammatory damage in ATDC5 cells by inactivating NF-κB pathways (18). The Nrf2 signaling pathway is an important endogenous antioxidant pathway. Increasing evidence has suggested the presence of crosstalk between the NF-κB and Nrf2 signaling pathways. For example, Nrf2 has been indicated to inhibit NF-κB pathway activation by increasing antioxidant activity and effectively neutralizing excessive NF-κB activation when exposed to LPS (24). Nrf2 suppressed lupus nephritis through neutralizing ROS and by negatively regulating the NF-κB signaling pathway (25). Sauchinone inhibits IL-1β-induced catabolism and hypertrophy in mouse chondrocytes, attenuating OA via the Nrf2/HO-1 and NF-κB pathways (26). Nrf2 is hypothesized to suppress the pro-inflammatory pathways mediated by NF-κB signaling (27,28). The results of the present study exhibited an activation of the NF-κB and Nrf-2 pathway in ATDC5 cells following treatment with LPS. In the present study, p-IκBβ and p-p65 expression was increased following LPS stimulation, which was additionally increased by the overexpression of NR024118. In addition, NR024118 overexpression effectively augmented the LPS-induced the protein and mRNA expression levels of Nrf2, HO-1 and NQO1, and activation of the Nrf2 pathway.
In conclusion, the present study demonstrated that NR024118 levels were downregulated in LPS-induced chondrocytes. NR024118 overexpression alleviated the LPS-induced cell apoptosis, oxidative stress and inflammatory injury through the NF-κB and Nrf-2 signaling pathways. These results suggested that NR024118 may be a potential target for diagnosis and treatment of patients with OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by Nanjing Medical Science and Technique Development Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XM wrote the paper. XM, JT, and WZ performed the experiments. XM and JT analyzed the data. YZ designed the experiments and improved the manuscript. All authors read and approved the manuscript, and agreed to be accountable for all aspects of the study in ensuring that the integrity and accuracy of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tong W, Geng Y, Huang Y, Shi Y, Xiang S, Zhang N, Qin L, Shi Q, Chen Q, Dai K, Zhang X. In vivo identification and induction of articular cartilage stem cells by inhibiting NF-κB signaling in osteoarthritis. Stem Cells. 2015;33:3125–3137. doi: 10.1002/stem.2124. [DOI] [PubMed] [Google Scholar]
- 2.Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of posttraumatic osteoarthritis. J Athl Train. 2017;52:491–496. doi: 10.4085/1062-6050-51.5.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mithoefer K. Complex articular cartilage restoration. Sports Med Arthrosc Rev. 2013;21:31–37. doi: 10.1097/JSA.0b013e318266f0c3. [DOI] [PubMed] [Google Scholar]
- 4.Yucesoy B, Charles LE, Baker B, Burchfiel CM. Occupational and genetic risk factors for osteoarthritis: A review. Work. 2015;50:261–273. doi: 10.3233/WOR-131739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Bay-Jensen AC, Slagboom E, Chen-An P, Alexandersen P, Qvist P, Christiansen C, Meulenbelt I, Karsdal MA. Role of hormones in cartilage and joint metabolism: Understanding an unhealthy metabolic phenotype in osteoarthritis. Menopause. 2013;20:578–586. doi: 10.1097/GME.0b013e3182745993. [DOI] [PubMed] [Google Scholar]
- 7.Jiang SD, Lu J, Deng ZH, Li YS, Lei GH. Long noncoding RNAs in osteoarthritis. Joint Bone Spine. 2017;84:553–556. doi: 10.1016/j.jbspin.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Sun J, Huang S, Su G, Pi G. lncRNA GAS5 overexpression reverses LPS-induced inflammatory injury and apoptosis through Up-regulating KLF2 expression in ATDC5 chondrocytes. Cell Physiol Biochem. 2018;45:1241–1251. doi: 10.1159/000487455. [DOI] [PubMed] [Google Scholar]
- 10.Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo C, Urgard E, Vooder T, Metspalu A. The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: Aging and anti-aging. Med Hypotheses. 2011;77:174–178. doi: 10.1016/j.mehy.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Niu J, Li H, Ke Y, Li R, Zhang Y, Lin J. Knee symptomatic osteoarthritis, walking disability, NSAIDs use and All-cause mortality: Population-based wuchuan osteoarthritis study. Sci Rep. 2017;7:3309. doi: 10.1038/s41598-017-03110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8:305–313. doi: 10.2174/138945007779940115. [DOI] [PubMed] [Google Scholar]
- 16.Chang CH, Hsu YM, Chen YC, Lin FH, Sadhasivam S, Loo ST, Savitha S. Anti-inflammatory effects of hydrophilic and lipophilic statins with hyaluronic acid against LPS-induced inflammation in porcine articular chondrocytes. J Orthop Res. 2014;32:557–565. doi: 10.1002/jor.22536. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Wang P, Jiang P, Lv Y, Dong C, Dai X, Tan L, Wang Z. Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene. 2016;586:248–253. doi: 10.1016/j.gene.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Pan L, Liu D, Zhao L, Wang L, Xin M, Li X. Long noncoding RNA MALAT1 alleviates lipopolysaccharide-induced inflammatory injury by upregulating microRNA-19b in murine chondrogenic ATDC5 cells. J Cell Biochem. 2018;119:10165–10175. doi: 10.1002/jcb.27357. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Chi X, Liu L, Wang Y, Mei X, Yang Y, Jia T. Long noncoding RNA maternally expressed gene 3 knockdown alleviates lipopolysaccharide-induced inflammatory injury by up-regulation of miR-203 in ATDC5 cells. Biomed Pharmacother. 2018;100:240–249. doi: 10.1016/j.biopha.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Yang KY, Chen DL. Shikonin inhibits inflammatory response in rheumatoid arthritis synovial fibroblasts via lncRNA-NR024118. Evid Based Complement Alternat Med. 2015;2015:631737. doi: 10.1155/2015/631737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci USA. 2006;103:13777–13782. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Liu H, Shi W, Liu H, Yang J, Xu D, Huang H, Wu L. Insights into the action mechanisms of traditional Chinese medicine in osteoarthritis. Evid Based Complement Alternat Med. 2017;2017:5190986. doi: 10.1155/2017/5190986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji B, Guo W, Ma H, Xu B, Mu W, Zhang Z, Amat A, Cao L. Isoliquiritigenin suppresses IL-1β induced apoptosis and inflammation in chondrocyte-like ATDC5 cells by inhibiting NF-κB and exerts chondroprotective effects on a mouse model of anterior cruciate ligament transection. Int J Mol Med. 2017;40:1709–1718. doi: 10.3892/ijmm.2017.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen XY, Dou YX, Luo DD, Zhang ZB, Li CL, Zeng HF, Su ZR, Xie JH, Lai XP, Li YC. β-Patchoulene from patchouli oil protects against LPS-induced acute lung injury via suppressing NF-κB and activating Nrf2 pathways. Int Immunopharmacol. 2017;50:270–278. doi: 10.1016/j.intimp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Jiang T, Tian F, Zheng H, Whitman SA, Lin Y, Zhang Z, Zhang N, Zhang DD. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014;85:333–343. doi: 10.1038/ki.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D, Jin S, Lin Z, Chen R, Pan T, Kang X, Huang H, Lin C, Pan J. Sauchinone inhibits IL-1β induced catabolism and hypertrophy in mouse chondrocytes to attenuate osteoarthritis via Nrf2/HO-1 and NF-κB pathways. Int Immunopharmacol. 2018;62:181–190. doi: 10.1016/j.intimp.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.