Abstract
Resistance rates in ESKAPE microorganisms included in the EARS-net surveillance database from Spain have increased in most of the cases. In 2017, multi-drug resistant isolates rose to 5.5% in Escherichia coli and 13.0% in Klebsiella pneumoniae. Carbapenemase producing Enterobacterales (CPE) have also increased in Spain over the last years with a current spread of throughout the country. EuSCAPE project revealed dominance of OXA-48 carbapenemase with lower prevalence of KPC, VIM or NDM enzymes. Increase of faecal carriers and presence of carbapenemases in the socalled high-risk clones have boosted the persistence and dissemination of CPE. One of these clones, the ST307 K. pneumoniae, has been associated with the spread of KPC carbapenemases and emergence of KPC variants conferring resistance to ceftazidime-avibactam combination.
Keywords: ESKAPE microorganisms, carbapenemase producing Enterobacterales, ceftazidime-avibactam
INTRODUCTION
Traditionally in developed countries, the problem of nosocomial antimicrobial resistance has been mainly associated with a particular group of microorganisms, “the ESKAPE bugs”[1]. While many bacteria remain susceptible to antimicrobial agents, this group (composed of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) presents a potential series of mechanisms to evade the lethal or inhibitory action of antimicrobial agents. The high antibiotic exposure due to excessive antimicrobial prescription or its inappropriate use, acquisition of resistance mechanisms either by mutational events or gene transfer and clonal spread have been the causes of their increase. Nowadays this group has been extended to other clinically relevant microorganisms and includes the overall Enterobacterales, Clostridioides difficile and all Enterococcus species.
In order to fight against the ESKAPE organisms, strategies such as “10 × ’20” proposed by The Infectious Diseases Society of America (IDSA) were developed. The aim of this initiative was the creation of sustainable global antibacterial drug research and development enterprise with the power in the short term to develop 10 new, safe, and efficacious systemically administered antibiotics by 2020 [2] especially the ESKAPE pathogens, continue to increase in frequency and cause significant morbidity and mortality. New antimicrobial agents are greatly needed to treat infections caused by Gramnegative bacilli (GNB). This was necessary due to the decrease in the number of new systemic antibacterial agents approved by the Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in the EU, despite the need for new antibiotic compounds. Moreover, the high rates of resistance among these microorganisms have led the World Health Organization (WHO) to recommend prioritization in the development of new antibiotics against them [3].
In Spain, the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) made a study with the objective of determining the clinical impact on mortality of multi-drug resistant (MDR) infections in our country. During a one-week period (March 2018), all MDR infections were investigated in 82 hospitals. This involved a total of 903 patients infected by MDR microorganisms of whom 177 died during the first month. If this data were extrapolated to the total of hospitals in the country, this would imply a total of 35,400 annual deaths in patients presenting infections due to MDR microorganisms [4].
ANTIMICROBIAL RESISTANCE IN ESKAPE ORGANISMS IN SPAIN
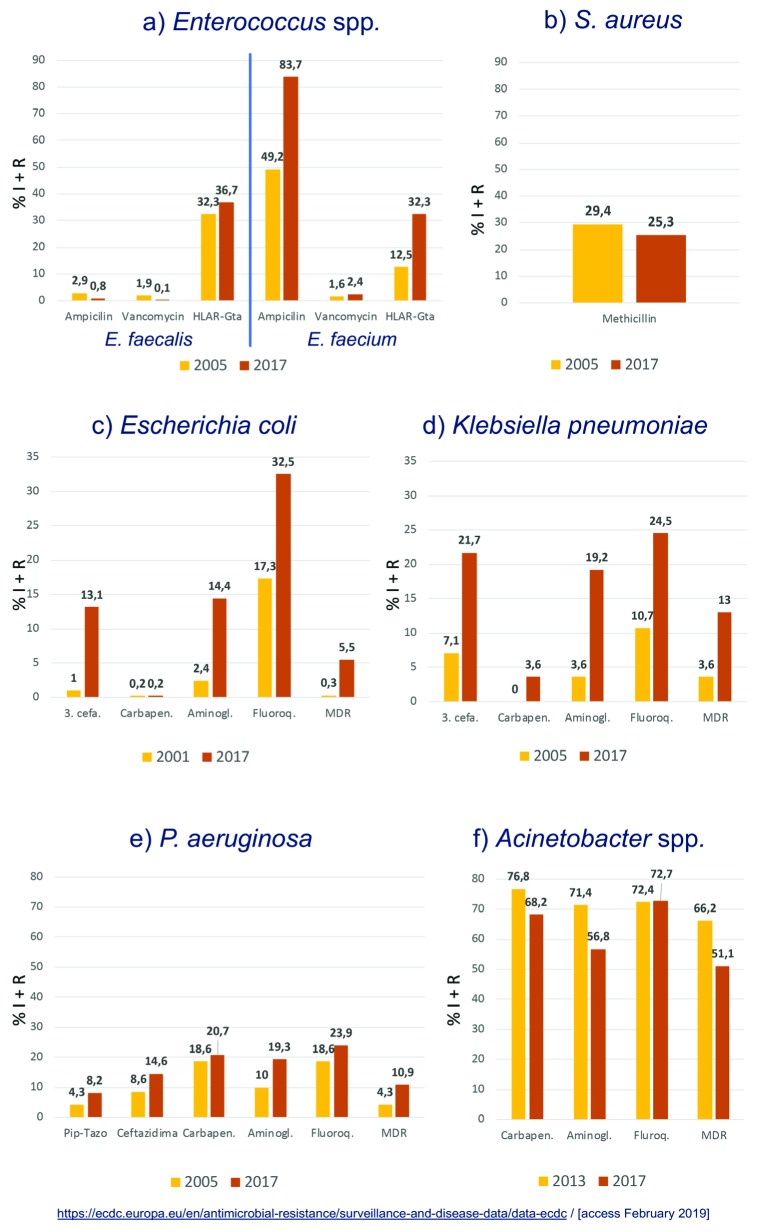
Currently, in Spain, resistance among the ESKAPE organisms has mainly increased over the years, a fact documented in the EARS-net surveillance study [5] (figure 1). In Enterococcus faecalis, unlike E. faecium, a low percentage of resistance (considering the intermediate and resistant categories) to ampicillin (0.8%) and vancomycin (0.1%) whereas a 36.6% of high-level aminoglycoside resistance was observed in 2017. This is very similar to that observed in 2005. On the contrary, in E. faecium, ampicillin resistance has increased with respect to 2005 from 49.2% to 83.7%, the high-level aminoglycoside resistance has also experimented an increment (12.5 to 32.3%) but vancomycin resistance rates have remained low (2.4%). In S. aureus, methicillin resistance has slightly decreased from 29.4% to 25.3%.
Figure 1.
Percentage of non-susceptible ESKAPE isolates. Data obtained from the EARS-net data base (https://ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc, access February 2019)
In Acinetobacter spp ., resistance to carbapenems and aminoglycosides has decreased (68.2% and 56.8%, respectively) and also, the rate of MDR isolates (51.1%) remain minor. In P. aeruginosa, we have more resistance than in 2005 to piperacillin-tazobactam (8.2%), ceftazidime (14.6%), carbapenems (20.7%), aminoglycosides (19.3%), fluoroquinolones (23.9%) and overall a higher percentage of MDR microorganisms (10.9%). Focusing on Enterobacterales, and specifically in E. coli and K. pneumoniae, the percentages of resistant isolates have increased in both species: third generation cephalosporin resistance has increased in E. coli (1.0% to 13.1%) and K. pneumoniae (7.1% to 21.7%). This trend was also observed for aminoglycosides, fluoroquinolones and carbapenems. As a consequence, the percentage of MDR E. coli has increased up to 5.5% and the MDR K. pneumoniae up to 13.0% in 2017 (figure 1), the latter might also include carbapenemase producers.
CARBAPENEMASES-PRODUCING ENTEROBACTERALES
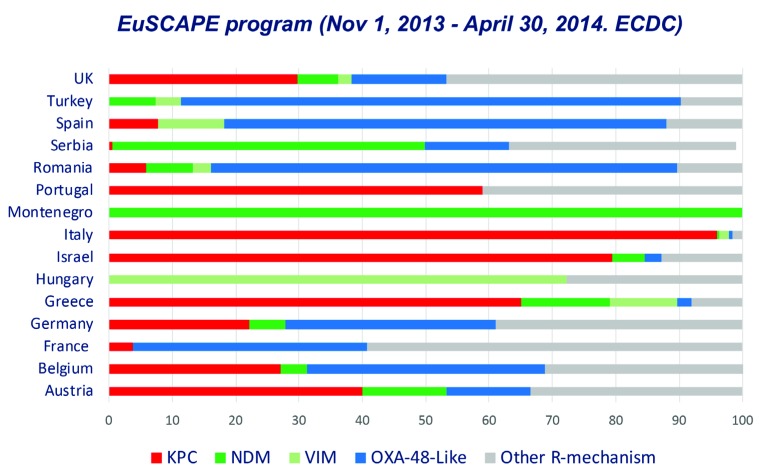
The problem of carbapenems resistance in Enterobacterales lies in a series of factors that are promoting their emergence, persistence and rapid dispersion. The increased prevalence of faecal carriage and co-colonization with carbapenemaseproducing Enterobacterales (CPE), the dispersion of MDR highrisk clones, the presence of co-resistance to other antimicrobials, including colistin resistance and now, the appearance of resistance determinants to new ß-lactam-ß-lactamase inhibitor combinations are the main factor driving this trend. In Europe, the EuSCAPE program performed a survey not only to determine the occurrence of carbapenemase-producing K. pneumoniae and E. coli in European hospitals but also to depict differences in the spread of different carbapenemases in different countries (figure 2) [6].
Figure 2.
Occurrence of different carbapenemases in carbapenemase-producing Klebsiella pneumoniae in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE) (Data obtained from reference [6])
For the same reasons, the complexity in the carbapenemase distribution in Enterobacterales in Spain has increased along the last years. The first detection of CPE in our country was associated with sporadic cases of metallo-ß-lactamases (MBLs) in Barcelona in 2003 [7]. Later in 2007, local outbreaks in different hospitals in the Madrid area due to VIM and KPC carbapenemases were described in patients with no history of travel abroad [8, 9]. A national multicenter study performed in 2009 only demonstrated a very low prevalence [10]. The detection of the first imported cases of NDM occurred in 2010 and the appearance of extra-hospitalary cases with no previous sanitary contact [11] has continued until today with local outbreaks and dispersion in different areas of OXA-48-carrying Enterobacterales [12]. Higher prevalence of OXA-48 was also highlighted in the EuSCAPE project with lower prevalence of KPC, VIM and NDM carbapenemases.
As mentioned before, one of the reasons of CPE dispersion is the increasing number of patients colonized with CPE. In the article of Hernández-García et al. [13], incidence of colonization by CPE in our hospital, during a follow-up period between March 2014 and March 2016, was 2% (161/8,209) of patients, and of these 0.9% were colonized at admission and 1.1% acquired colonization during admission. The principal colonizer was K. pneumoniae (54%) followed by E. coli (19%) mainly as OXA-48 (64.1%) and VIM-1 (26.8%) producers. Also, 20% of patients were colonized with two or three different CPE (co-colonization).
Other factors fueling emergence and dispersion of CPE is the presence of carbapenemases in the so-called MDR highrisk clones. A recent study performed in Spain analyzing the population structure of CPE revealed that carbapenemases concentrate in a few clones when compared with the susceptible population. These MDR high-risk clones are the cause of multiple outbreaks throughout our country as the one described in Cordoba [14]. This originated in a patient transferred from an Italian hospital, and there was a range of 67 infected and 14 colonized patients and a mortality of 30% due to a K. pneumoniae-ST512-KPC-3 resistant to third generation cephalosporins, carbapenems, tobramycin, amikacin, fluoroquinolones and colistin. Also, there is a significant percentage of CPE isolates from clinical samples in ICU admitted patients. This was demonstrated in a recent multicenter study performed in Spain in 8 hospitals, with 23.1% of ESBL-producing Klebsiella spp. and 20% of carbapenemase-producing Klebsiella spp. [15]. These isolates also showed high co-resistances to non-β-lactam antimicrobials.
EMERGENCE OF CEFTAZIDIME-AVIBACTAM RESISTANCE
Nowadays, we have new compounds available designed to fight against CPE as new ß-lactam-ß-lactamase inhibitors combinations such as ceftazidime-avibactam and meropenemvarbobactamin and in the future imipenem-relebactam. The first one, already available in the US and EU, is a combination of ceftazidime, a classical third generation cephalosporin, and avibactam, a non-ß-lactam (diazabicyclooctane) ß-lactamase inhibitor, that is active against Ambler class A and C ß-lactamases and possesses activity against some Ambler class D enzymes, including OXA-48 producers.
Despite its short life in the clinical setting, isolates with acquired resistance to this combination has already been occasionally described. The mechanisms involved in this resistance include: a) Overexpression of extended-spectrum AmpC in E. cloacae and mutations in AmpC from P. aeruginosa; b) Increased hydrolytic activity of blaCTX-M-14 variants; c) KPC-K. pneumoniae with multiple resistance mechanisms such as KPC-3 overexpression plus porin deficiency (ompK35/ompK36) and SHV-12 with enhanced efflux activity (AcrAB); and d) double or triple blaKPC-3 mutations and blaKPC-2 mutations that confer resistance to ceftazidime-avibactam. Interestingly these last resistance mechanisms might produce a reversion of carbapenem susceptibility, a phenomenon that has been named as “collateral sensitivity”.
Many of these resistance mutations appear after ceftazidime-avibactam treatment, such as blaKPC mutations and lead to treatment failure and resistance development [16]. This had been reproduced with in vitro studies subjecting the strains of KPC producing K. pneumoniae to various concentrations of antibiotic and counting the number of colonies that grew [17]. Furthermore, in these strains with blaKPC gene mutation, at the same time as the development of ceftazidime-avibactam resistance, there was restoration of meropenem susceptibility occurred during or after ceftazidime-avibactam treatment [18].
In our hospital, we have detected a rapid dissemination of a KPC-3-producing K. pneumoniae ST307 clone. In a year period, we detected 353 patients with carbapenemase-producing K. pneumoniae isolates of whom 19.2% (68/353) were KpST307-KPC-3 producers. In two patients, ceftazidime-avibactam resistance developed associated with ceftazidime-avibactam treatment due to the emergence of a KPC-3 variant [19].
CONCLUSIONS
In summary, there has been an increase of ESKAPE organisms in Spain during the last years. In addition, CPE have been dispersed throughout all the country with a dominance of OXA-48 producing K. pneumoniae isolates but with an increasing prevalence of KPC producers and maintenance of VIM producers. This complexity in the carbapenemase-distribution among Enterobacterales is also due to high faecal carriers co-colonized with different CPE, the dispersion of MDR highrisk clones, and the co-resistance with non-ß-lactam antimicrobials, including colistin. Moreover, the emergence of resistance to new β-lactamβ-lactamase inhibitor combinations with restoration of carbapenem susceptibility due to new KPC mutations has also been detected in Spain.
FUNDING
The content and scientific background of this work was supported by the European Commission (grants R-GNOSIS-FP7-HEALTH-F3-2011-293 282512), the Plan Nacional de I+D+i 2013-2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0011) cofinanced by European Development Regional Fund “A way to achieve Europe” (ERDF), Operative program Intelligent Growth 2014-2020.
CONFLICT OF INTEREST
Authors have no conflicts of interest to declare with respect to the contents of this manuscript.
References
- 1.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis 2008;197:1079–81. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin DK, Gilbert D, Bonomo RA, Jones RN, Talbot GH, Brad-ley J, et al. 10 x ’20 Progress--Development of new drugs active against Gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1685–94. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline, including tuberculosis. 2017. (WHO/EMP/IAU/2017.12). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 4.Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) Registro hospitalario de pacientes afectados por las resistencias bacterianas. 2018. Available at: https://seimc.org/contenidos/noticias/2018/seimc-Registro_de_Pacientes_BMR.pdf.
- 5.European Centre for Disease Prevention and Control Surveillance of antimicrobial resistance in Europe 2016, ECDC surveillance report. 2016. doi: 10.2900/296939. [DOI]
- 6.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasevi AT, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European sur-vey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 2017;17:153–63. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 7.Tórtola MT, Lavilla S, Miró E, González JJ, Larrosa N, Sabaté M, et al. First detection of a carbapenem-hydrolyzing metalloenzyme in two Enterobacteriaceae isolates in Spain. Antimicrob Agents Chemoth-er 2005;49:3492–4. doi: 10.1128/AAC.49.8.3492-3494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tato M, Coque TM, Rucz-Garbajosa P, Pintado V, Cobo J, Sader HS, et al. Complex clonal and plasmid epidemiology in the first outbreak of Enterobacteriaceae infection involving VIM-1 metallo-ß-lactamase in Spain: toward endemicity? Clin Infect Dis 2007;45:1171–8. doi: 10.1086/522288. [DOI] [PubMed] [Google Scholar]
- 9.Curiao T, Morosini MI, Ruiz-Garbajosa P, Robustillo A, Baquero F, Coque TM, et al. Emergence of blaKPC-3-Tn4401a associated with a pKPN3/4-like plasmid within ST384 and ST388 Klebsiella pneu-moniae clones in Spain. J Antimicrob Chemother 2010;65:1608–14. doi: 10.1093/jac/dkq174. [DOI] [PubMed] [Google Scholar]
- 10.Miró E, Agüero J, Larrosa MN, Fernández A, Conejo MC, Bou G, et al. Prevalence and molecular epidemiology of acquired AmpC β-lactamases and carbapenemases in Enterobacteriaceae iso-lates from 35 hospitals in Spain. Eur J Clin Microbiol Infect Dis 2013;32:253–9. doi: 10.1007/s10096-012-1737-0. [DOI] [PubMed] [Google Scholar]
- 11.Gijón D, Curiao T, Baquero F, Coque TM, Cantón R. Fecal carriage of carbapenemase-producing Enterobacteriaceae: A hidden reser-voir in hospitalized and nonhospitalized patients. J Clin Microbiol 2012;50:1558–63. doi: 10.1128/JCM.00020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paño-Pardo JR, Ruiz-Carrascoso G, Navarro-San Francisco C, Gómez-Gil R, Mora-Rillo M, Romero-Gómez MP, et al. Infections caused by oxa-48-producing Klebsiella pneumoniae in a tertiary hospital in Spain in the setting of a prolonged, hospital-wide out-break. J Antimicrob Chemother 2013;68:89–96. doi: 10.1093/jac/dks364. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-García M, Pérez-Viso B, Carmen Turrientes M, Díaz-Agero C, López-Fresneña N, Bonten M, et al. Characteriza-tion of carbapenemase-producing Enterobacteriaceae from col-onized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J Antimicrob Chemother 2018;73:3039–43. doi: 10.1093/jac/dky284. [DOI] [PubMed] [Google Scholar]
- 14.López-Cerero L, Egea P, Gracia-Ahufinger I, González-Padilla M, Rodríguez-López F, Rodríguez-Baño J, et al. Characterisation of the first ongoing outbreak due to KPC-3-producing Klebsiella pneu-moniae (ST512) in Spain. Int J Antimicrob Agents 2014;44:538–40. doi: 10.1016/j.ijantimicag.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.García-Fernández S, García-Castillo M, Bou G, Calvo J, Cercenado E, Delgado M, et al. Activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and Enterobacterales isolates recovered from intensive care unit patients in Spain: The SUPERIOR multi-centre study. Int J Antimicrob Agents 2019;53. doi: 10.1016/j.ijanti-micag.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibac-tam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016;63:1615–8. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, et al. In Vitro selection of ceftazidime-avibactam re-sistance in Enterobacteriaceae with KPC-3 carbapenemase . Antimicrob Agents Chemother 2015;59:5324–30. doi: 10.1128/aac.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapen-emase-producing K pneumoniae: A case report and review of liter-ature. Open Forum Infect Dis 2017;4:1–4. doi: 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-López J, Massone CA, Moreno Nuñez P, López Fresneña N, Morosini Reilly MI, Cantón R, et al. Dissemination of KPC-3-producing Klebsiella pneumoniae ST307 in a tertiary hospital in Madrid ( Spain ) associated with the emergence of ceftazidime-avibactam resistance Abstract: O0914; European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, 2019. Available at: https://www.eccmidlive.org/#!resources/dissemination-of-kpc-2-producing-klebsiel-la-pneumoniae-st307-in-a-tertiary-hospital-in-madrid-spain-associated-with-the-emergence-of-ceftazidime-avibactam-resistance-6deed3f6-3793-495f-99d1-8da85511da79.