Abstract
Recurrence rate ranges from 12% to 40% of all cases of Clostridium difficile infection (CDI) and proposes an exceptional clinical challenge. Conventionally, treatment options of CDI have been limited to regimes of established antibiotics (eg, pulsed/tapered vancomycin) or “improvised” alternative antibiotics (eg. teicoplanin, tigecycline, nitazoxanide or rifaximin) occasionally even in combination, but faecal microbiota transplantation is emerging as a useful and quite safe alternative. In recent years, promising new strategies have emerged for effective prevention of recurrent CDI (rCDI) including new antimicrobials (eg, fidaxomicin) and monoclonal antibodies (eg, bezlotoxumab). Despite promising progress in this area, difficulties remain for making the best use of these resources due to uncertainty over patient selection. This positioning review describes the current epidemiology of rCDI, its clinical impact and risk factors, some of the measures used for treating and preventing rCDI, and some of the emerging treatment options. It then describes some of the barriers that need to be overcome.
Keywords: Clostridium difficile infection, recurrences, fidaxomicin, bezlotoxumab, faecal transplantation
INTRODUCTION
Clostridium difficile infection (CDI) is the most common cause of nosocomial antibiotic-associated diarrhea worldwide, one of the most frequent healthcare-associated infections and the source of a growing number of cases of diarrhea in the community [1, 2]. The current picture of CDI is alarming with a mortality rate ranging between 3% and 15% and a CDI recurrence rate ranging from 12% to 40%, especially when it has been treated with metronidazole or vancomycin. The incidence of subsequent recurrent CDI (rCDI) increases with prior episodes of CDI, 15-35% risk after primary CDI to 35-65% risk after the first recurrent episode. Certain host or pathogen factors have been associated with an increased risk of rCDI or CDI-related adverse outcomes: age ≥ 65 years, compromised immunity, severe CDI, prior CDI episode (s), and infection with the BI/NAP1/027 strain [3]. rCDI is one of the most challenging and a very difficult to treat infections. Standard guidelines provide recommendations on treatment of primary CDI. However, treatment choices for rCDI are limited.
The key to preventing recurrent infection is identifying those patients at the greatest risk (table 1). Factors accepted to present a risk of initial CDI include older age and comorbidities. As with initial infection, the risk of recurrence increases with ageing. Poor baseline health status has also been identified as a risk factor. Past exposure to health care has also been found to be a significant risk factor. It has been found that chronic kidney disease with or without dialysis and chemotherapy increased the risk of recurrence at older ages. Usually, proton pump inhibitor and antibiotic use have also been implicated in risk of recurrence.
Table 1.
Proposed and potential risk factors for recurrent Clostridium difficile infection (rCDI)
| Risk factors group | Factors included in the group |
|---|---|
| Host risk factors | • Age ≥65 years • Prior CDI episode • Host genetics • Compromised immune system • Chronic renal failure • Low C. difficile-specific antitoxin antibody levels |
| Severity of CDI episode | • Severe primary CDI |
| Pathogen-specific factors | • C. difficilestrain type (ribotype 027, 078, or 244) |
| Exogenous factors (Exposures) | • Proton pump inhibitors/antacids • Previous fluoroquinolone use • Ongoing antibiotic use |
CDI: Clostridium difficile infection. Note: Data collated from multiple references included in the bibliography.
Antibiotics are the major risk factor for the promotion and development of an episode of CDI, as well as the prolongation or perpetuation of symptoms and a lesser response to specific treatment. In addition, they are one of the main factors favoring the appearance of recurrences. Antibiotic use causes an antibiotic-related loss of gut microbial communities that protect against gut infection, thereby facilitating the germination and vegetative growth of the organism when it enters the gut of vulnerable people [4]. Frequently, this factor is not easily modified and many patients need to continue receiving antibiotics for the mandatory treatment of their severe or complicated infectious syndromes.
RECURRENT CDI AND ANTIBIOTICS
Antibiotic therapy causes alterations of the intestinal microbial composition, enabling C. difficile colonization and consecutive toxin production leading to disruption of the colonic epithelial cells [5]. The risk of CDI is increased up to six-fold during antibiotic therapy and in the subsequent month afterwards. Although nearly all antibiotics have been associated with CDI, clindamycin, third-generation cephalosporins, penicillins, and fluoroquinolones have traditionally been considered to pose the greatest risk.
An association between CDI and antimicrobial treatment > 10 days has also been demonstrated. Antibiotics which have been less commonly associated with CDI include macrolides, sulfonamides, and tetracyclines (table 2). Even very limited exposure, such as single dose surgical antibiotic prophylaxis, can increase patients risk for both C. difficile colonization or infection.
Table 2.
Classification of antibiotics based on the risk of developing CDI or recurrence
| Low risk | Moderate risk | High risk |
|---|---|---|
| Aminoglycosides Vancomycin Metronidazole Rifampicin Antipseudomonal penicillins |
Ampicillin Amoxycillin Macrolides Tetracyclines Cotrimoxazole |
Clindamycin Quinolones Cephalosporins Amoxycillin/clavulanatePiperacillin/tazobactam Carbapenems Aztreonam |
Recurrent CDI can be defined as reappearance of symptoms following the completion of a course of therapy resulting in complete resolution of those symptoms [6]. European guidelines define recurrence as symptoms occurring within 8 weeks after the onset of a previous episode, provided the symptoms from the previous episode resolved after completion of initial treatment. However, studies offer different definitions.
Around a quarter of all patients with confirmed CDI will develop a recurrence. Those patients who have had a first recurrence are at increased risk of further recurrence (or multiply rCDI) – up to 60% of patients with a second recurrence will have further infections. Recurrence can occur either as a relapse with the same strain (as the consequence of germinating resident spores remaining in the colon after antibiotic treatment has stopped) or as a reinfection with a different strain (from an environmental source) [7]. Ultimately, distinction between recurrence and reinfection can only be achieved if the strain of C. difficile is “typed” using molecular epidemiology.
Studies comparing patients with recurrent infection, those with non-recurrent infection, and those without infection, have demonstrated both greater use of hospital resources and increased mortality. rCDI has been associated with a 2.5-fold higher hospital readmission rate, four-fold longer hospital stay, and 33% higher mortality rate compared to primary CDI [8].
Recurrences are associated with an impaired immune response to C. difficile toxins and/or alteration of the colonic microbiota, but nevertheless recurrent episodes are less severe compared to initial episodes and some studies reported a decline in the proportion of severe cases according to the number of recurrent episodes (47% for initial episodes, 31% for first recurrences, 25% for second, and 17% for third in a Canadian study) [9].
Even though consensus regarding factors associated with CDI recurrence is not universal, algorithms have been developed to predict CDI recurrence with good sensitivity. Scoring prediction models could be important tools for prioritizing more individualized, costly, or resource intensive treatment and prevention strategies. Tools to predict the risk of rCDI could be especially useful as advancements in therapies for prevention emerge. Risk stratification allowing identification of patients at risk for recurrence may translate into a more cost-effective approach to decrease rates of rCDI [3]. Unfortunately, existing models have used limited sample sizes, have not been validated externally, or have been found to perform poorly in predicting rCDI. Studies of models to predict rCDI are summarized in table 3.
Table 3.
List of risk prediction scales and scoring systems for recurrent CDI
| Study | Variables o parameters included in the score | Comments |
|---|---|---|
| Hu et al. [44] | Age > 65 years Severe disease based on Horn index Additional antibiotic use |
Small sample size; Score performed poorly in a prospective internal validation cohort (53.8% sensitive, 76.5% specific); Will require external validation to determine clinical utility. |
| Miller et al. [45] | Age Treatment with systemic antibiotics during CDI therapy Temperature Leukocyte count sCr Albumin |
Useful for predicting severity and response to treatment, but found to be a poor predictor of recurrence. |
| Zilberberg et al. [46] | Age Community onset CDI Prior hospitalization Fluoroquinolone use at onset of CDI Other high risk antibiotic use at onset of CDI Gastric acid suppression |
Derived from a large retrospective single center cohort study and cross-validated in the same population. Performed poorly in an external validation study (C statistic 0.59) |
| D’agostino et al. [19] | Age > 75 years > 10 unformed bowel movements in 24 hours sCr > 1.2 mg/dL Prior episode of CDI CDI treatment received (vancomycin or fidaxomicin) |
Derived and validated from a large prospective dataset. Poorly predictive of recurrent CDI (C statistic 0.54) |
| Escobar et al. [47] | Comparison of 4 models 1) Basic Model: age, prior GI surgery, Immunosuppression status, Locus of CDI onset, Admission from skilled nursing facility 2) Enhanced Model: Based on 14 variables extracted from EMR 3) Automated Model: Based on several variables generated in real time. |
Derived from a large multicenter retrospective cohort andinternally validated in a separate cohort. None of the models performed well (C statistics 0.59—0.61). |
| Viswesh et al. [48] | CDI present on admission Temperature > 37.8 °C at admission Leukocyte count > 15,000 cells/mm3 Nosocomial CDI Abdominal distention |
Derived from a large retrospective single center cohort study and cross-validated in the same population. Will require external validation to determine clinical utility. |
| Cobo et al. (GEIH-CDI Score) [20] | Age Prior CDI in last year Positive direct toxin test Persistence of diarrhea on day 5 of treatment |
Derived from a retrospective multicenter cohort and validated in a separate cohort (including several of the same centers from derivation cohort). Moderately predictive for recurrent CDI |
| Reveles et al. [49] | Prior 3rd or 4th generation cephalosporin use Prior proton pump inhibitor use Prior antidiarrheals Non-severe CDI Community onset CDI |
Large retrospective national cohort of veterans. Clinical prediction rule correlated strongly with recurrence (R2 = 0.94) in an internal validation cohort. Will require external validation to determine clinical utility. |
CDI: Clostridium difficile infection; sCr: serum creatinine; GI: gastrointestinal; EMR: electronic medical records.
C-statistic [equivalent to the area under the Receiver Operating Characteristic (ROC) curve] is a standard measure of the predictive accuracy of a logistic regression model. C-statistic refer to the probability that predicting the outcome is better than chance. It is used to compare the goodness of fit of logistic regression models. Values for this measure range from 0.5 to 1.0. A value of 0.5 indicates that the model is no better than chance at making a prediction of membership in a group and a value of 1.0 indicates that the model perfectly identifies those within a group and those not. Models are typically considered reasonable when the C-statistic is higher than 0.7 and strong when C exceeds 0.8.
Recurrence of symptoms after initial therapy for C. difficile presents a clinical challenge. As the incidence of rCDI is rising, there is an unmet need for therapies and strategies to prevent rCDI. Despite the great efforts made over the past 10 years to face the rCDI burden, there are still gray areas in our knowledge on rCDI management. Promising treatments include fidaxomicin (FDX), faecal microbiota transplantation (FMT) and monoclonal antibodies [10].
THERAPIES AND STRATEGIES TO PREVENT RCDI
In general, treatment goals of CDI are to resolve the infection, reduce gut dysbiosis, prevent recurrence, prevent transmission among individuals, improve quality of life, and reduce healthcare costs. Along with medical management, some patients may require surgical intervention (which nowadays is required less commonly). In patients diagnosed with CDI, consideration should be given to discontinuation of the offending antibiotic if clinically appropriate. The use of antibiotics along with treatment for CDI is associated with lower cure rates and higher rates of recurrence.
Then we discuss in this section three current strategies for the prevention and treatment of rCDI.
Fidaxomicin use. FDX is a new class of narrow-spectrum macrocyclic antibiotics that inhibits bacterial RNA polymerase. FDX is a bactericidal drug that seems to be more specific over C. difficile than metronidazole and vancomycin, with less disruption of the fecal microbiota [11]. Moreover, FDX also decreases both spore production and relapse rates [12], and its low absorption can prevent systemic side effects, reaching high fecal concentrations and remaining in the gastrointestinal tract with reduced impact on the intestinal microbiota [13]. Compared to vancomycin treatment, FDX was associated with a lower rate (~50%) of second-occurrence relapses 4 weeks after the infection in patients with no prior episode of CDI [14]. A post hoc exploratory intent to treat (ITT) time-toevent analysis showed a 40% reduction in persistent diarrhea, recurrence or death at the 40-day follow-up (95% CI, 26-51%; p<0.0001) [13]. This evidence argues in favor that specific treatment developed against C. difficile can greatly improve clinical outcomes, although several patient groups were excluded from the trials. Nevertheless, there is still a margin for further improvement since FDX fails in 12% of treatments. In addition, FDX is effective and safe for the treatment of CDI in critical patients, immunosuppressed patients, or patients with chronic renal failure [15].
Vancomycin and FDX are now recommended as a first line treatment options for CDI. Both are considered to have similar therapeutic efficacy (87.7-88.2% with FDX and 85.8-86.8% with vancomycin) though FDX has a significantly lower recurrence rate (15.4% vs. 25.3%, p < 0.005), respectively [14, 16]. Hence, FDX is recommended from the first episode of infection to ensure maximum efficacy in patients with well-contrasted recurrence risk factors (elderly people, concomitant antibiotic use and severe underlying disease) [17, 18]. Due to its higher cost, real-world use of FDX is likely to be reserved for patients with first or later recurrences. Several studies have developed scoring systems that allow the more high-risk patients to be treated earlier [19, 20] and show cost-effectiveness [21].
Furthermore, in 60 years-old patients and older, extended-pulsed FDX (EPFDX) (200 mg oral tablets, twice daily on days 1–5, then once daily on alternate days on days 7–25) was superior to standard-dose vancomycin for sustained clinical cure of CDI and significant reduction in recurrence rates [22]. A recent Spanish economic model showed that EPFDX is cost-effective compared with vancomycin for the first-line treatment of CDI in patients aged 60 years and older [23].
Faecal microbiota transplantation (FMT). Some patients with CDI (primary episodes and recurrences) that do not respond to conventional antibiotic treatments of first choice may be cured by FMT [24, 25], an intervention first described in treating pseudomembranous colitis in 1958.
FMT procedure is based on transplanting stool from healthy donors (people without diseases as malignancy, metabolic or autoimmune disease or infections like HIV or active hepatitis) in order to restore gut microbiome which is disrupted in CDI, suppressing C. difficile overgrowth [26]. Donor feces (≥50 g obtained preferably within <6 hours after evacuation) are diluted with water or normal saline, homogenized and filtrated and are administrated through enema, colonoscopy (100–700 mL of stool suspension delivered to the caecum or terminal ileum, as it seems to obtain a better result), nasogastric or nasojejunal tube, or in capsules. For patients with systemic illnesses, capsules may be the best option, followed by nasoenteric tube, but in patients at risk of aspiration, enema or colonoscopy should be a better choice [27]. However, combination of several of those methods is recommended in complex cases. Common adverse events after FMT, that are usually self-limitated, include gastrointestinal discomfort (abdominal pain, bloating, flatulence, diarrhoea, constipation, vomiting or belching) and endoscopy-related complications (like aspiration during sedation). Before FMT, patients are given antimicrobial therapy directed at CDI for at least 4 days and 1 day before FMT, bowel lavage is performed in most of them.
FMT has proven to be safe and effective, showing a rate of cure of recurrent CDI >90% when associated to antibiotic cessation. Nowadays, following the recommendations of British Society of Gastroenterology and Healthcare Infection Society guidelines from 2018 [28], FMT may be offered to patients with recurrent CDI who have had at least two recurrences, or those who have had one recurrence and have risk factors for further episodes, including severe and complicated CDI. However, in Spain it is still not a routine procedure and the potential benefit of FMT in primary CDI remains uncertain.
In a recent systematic review with meta-analysis of donor features, procedures and outcomes in 168 clinical studies of FMT (including ulcerative colitis and Crohn’s disease), a final cure rate for CDI of 95.6% was observed [29]. Cure rates in CDI and final remission rates for inflammatory bowel disease were comparable across all routes of FMT administration. Overall adverse event incidence was <1%, mostly gastrointestinal-related. Adverse event rates did not differ significantly between routes of FMT administration or indication. Reports of its safety in certain immunocompromised populations, such as those with inflammatory bowel disease, those who have received a solid organ transplant [30] or suffering from a oncohaematological disease, appear reassuring and their outcomes are becoming better known.
Monoclonal antibodies (Bezlotoxumab). A new approach to the prevention of recurrent C. difficile infection (CDI) is the administration of monoclonal antibodies against C. difficile toxins in addition to antibiotic therapy as a form of passive immunity. Bezlotoxumab is the first of its kind, fully humanized monoclonal antibody directed against C. difficile toxin B. Binding to toxin B neutralizes the toxin and prevents damage to colonic cells.
Bezlotoxumab is currently approved for the prevention of rCDI in patients on treatment for CDI and who are at high risk for recurrence. Approved dose is a single 10 mg/kg administered intravenously during active C. difficile therapy, up to treatment day 14. Bezlotoxumab does not require dosage adjustment in either renal or hepatic impairment and no drug– drug interactions are anticipated or published [31].
Two phase III clinical double-blind trials (MODIFY I and MODIFY II) studied the ability of this antibody to reduce the recurrence of CDI in 2,655 patients. In these trials, it was shown that the addition of bezlotoxumab to the standard of care antibiotics for primary or recurrence C. difficile infections resulted in a lower rate of recurrence compared with placebo (17% vs 28% in MODIFY I and 16% vs 26% in MODIFY II; p<0.001). These results representing a 40% relative reduction rate (p < 0.0001) and a number needed to treat of 10 patients. Bezlotoxumab had no effect on clinical cure (clinical cure of 80% bezlotoxumab vs 80% placebo). Moreover, the absolute difference in rCDI rate was greater in subpopulations at high risk of CDI recurrence than in the overall population [32].
A post hoc analysis evaluated the efficacy of bezlotoxumab in patients with previously identified “high-risk” rCDI (risk factors including age ≥ 65 years, compromised immunity, severe CDI, prior CDI episode, and infection with ribotypes 027/078/244). All of the categories demonstrated a statistically significant reduction in CDI recurrence, with the exception of infection with ribotypes 027/078/244. When further stratified by number of underlying risk factors, there was a greater impact on prevention of recurrence as the number of risk factors increased. While participants with ≥3 risk factors had the greatest reduction of rCDI with bezlotoxumab, those with 1 or 2 risk factors may also benefit [3]. In addition to the rCDI risk factors evaluated in the above study, data presented at the 2016 IDWeek conference evaluated the efficacy of bezlotoxumab in prevention of CDI recurrence in patients receiving concomitant antibiotics. rCDI was observed in 18% of bezlotoxumab treated patients who received concomitant antibiotics compared with 28% of placebo subjects together with concomitant antibiotics. These preliminary data suggest that the efficacy of bezlotoxumab was maintained in patients with concomitant antibiotics [33].
Bezlotoxumab was generally well tolerated and had a safety profile similar to that of placebo. The most commonly reported adverse drug responses are infusion related reactions (10%). Side effects within 4 weeks of administration reported in ≥ 4% of patients in the MODIFY I and II trials included nausea (7%), pyrexia (5%), and headache (4%). These did not differ significantly from placebo. Heart failure was not seen in preclinical trials but reported in 17 (2.2%) bezlotoxumab and 7 (0.9%) placebo treated patients in Phase III trials. Heart failure was more frequently observed in patients with a history of congestive heart failure [31, 32, 34]. Additionaly, recent studies showed that bezlotoxumab added to standard of care antibiotic therapy compared to standard of care alone is a cost-effective treatment to prevent the recurrence of CDI in high-risk patients especially in patients ≥ 65 years old, with severe CDI and immunocompromised [35, 36].
Its novel mechanism of action, apparent lack of impact on the fecal microbiome, and safety profile make it an attractive adjunctive therapy for prevention of rCDI. One of the weaknesses is that much of the published data come from the drug pharmaceutical sponsored MODIFY trials. Real world clinical experience and independent investigations will be helpful to verify the clinical efficacy in high-risk populations [37].
CONCLUSIONS AND FUTURE DIRECTIONS
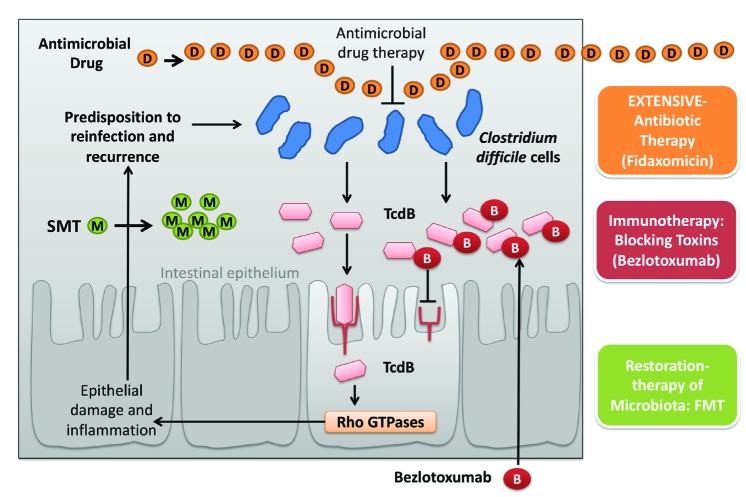
Treatment and prevention of rCDI remain difficult. Patients who are able to restore their natural gut microbiota or mount an effective immune response to the toxins and/or the bacterium recover from the infection (figure 1), whereas patients who fail to do so are susceptible to recurrent CDI. Although newer strategies are available or in the pipeline, further studies are required to identify those patients in whom these treatments are likely to be both clinically and cost-effective [38].
Figure 1.
Evaluating and combining strategies against recurrences of CDI
Treatment of CDI has become complicated due to the emergence of strains with increased toxigenicity and sporulation rate, together with rampant antibiotics use that disrupts colonization resistance of the colonic microbiota. As a result, there is a critical need for non-antibiotic treatments. Therapies based on inhibiting the toxins, bacterial structures responsible for colonization, virulence and restoration of the gut microbiota are the most important non-antibiotic targets to combat CDI. New discovered targets in C. difficile could become the focus of future therapeutic agents. Inhibiting colonization and virulence factors during CDI will disrupt pathogen persistence and decrease exposure to the inflammatory toxins, allowing the immune system to clear the infection [39].
The high risk of recurrence has led to multiple emerging therapies that target toxin activity, recovery of the intestinal microbial community, and elimination of latent C. difficile in the intestine. The high incidence of rCDI has driven new research on improved prevention such as the emerging use of probiotics, intestinal microbiome manipulation during antibiotic therapies, vaccinations, and newer antibiotics that reduce the disruption of the intestinal microbiome [40]. A novel approach in the manipulation of the microbiome would include the administration of non-toxigenic C. difficile strains [4]. In a clinical trial, non-toxigenic C. difficile was administered to those with CDI, with the aim of outcompeting toxigenic C. difficile from its reservoir within the gut. CDI recurrence rates were 30% in those receiving placebo in comparison with 11% in those receiving non-toxigenic C. difficile [41]. While anti-toxin vaccines could be another viable preventative measure, they are currently not as effective and more clinical trials will be needed to identify an efficacious and safe vaccine. Early data suggest reduced seroconversion in older people subjected to this active immunization, those most at risk of CDI, and also very likely in immunosuppressed patients.
CDI: Clostridium difficile infection; TcdB: C. difficile toxin B; B: Bezlotoxumab; D: Antimicrobial Drug; M: Microbiota (restored) from selected donors; SMT: Stool microbiota transfer; FMT: fecal microbiota transplant. Arrow: stimulation of the action or effect provocation ; Locked arrow: inhibition effect or blocking action. (modified and adapted from reference number 40)
At this moment there are more than fifteen antimicrobial molecules under study for CDI treatment in different phases of clinical trials: cadazolid, ridinilazole, surotomycin, rifaximin, rifampin, fusidic acid, tigecycline, LFF571, nitazoxanide, ramoplanin, auranofin, CRS3123, thuricin CD, lacticin 3147, NVB302, and acyldepsipeptide antimicrobials. In comparison with the traditional anti-CDI antimicrobial treatment, some of the novel antimicrobials offer several advantages, such as the favorable pharmacokinetic and pharmacodynamic profile, the narrow-spectrum activity against C. difficile that implicates a low impact on the gut microbiota composition, the inhibitory activity on C. difficile sporulation and toxins production [42]. Among these novel antimicrobials, the most active compounds in reducing spore production are cadazolid, ridinilazole, ramoplanin, CRS3123 and, potentially, the acyldepsipeptide antimicrobials. These antimicrobials may potentially reduce C. difficile environment spread and persistence, thus reducing CDI healthcare-associated acquisition and rCDI. However, some of them, as for example surotomycin, fusidic acid, etc., will not be available due to lack of superiority versus standard of treatment. The most C. difficile narrow-spectrum novel antimicrobials that allow preserve microbiota integrity are ridinilazole, cadazolid, auranofin, and thuricin CD.
Another strategy in the prevention of CDI would be a direct action on β-lactam antibiotics. Ribaxamase (SYN-004) is an orally administered β-lactamase that was designed to be given with systemic broad-spectrum antibiotics (intravenous β-lactam antibiotics) to degrade excess antibiotics in the upper gastrointestinal tract before they disrupt the gut microbiome and create a propensity to CDI [4]. In a recent study with patients treated with intravenous ceftriaxone for lower respiratory tract infections, oral ribaxamase reduced the incidence of CDI compared with placebo [43]. The findings of this study support continued clinical development of ribaxamase to prevent CDI.
In conclusion, the novel antimicrobial molecules under development for CDI have promising key features and advancements in comparison to the traditional anti-CDI antimicrobials. In the near future, some of these new molecules might be effective alternatives to fight against CDI and prevent more effectively rCDI.
References
- 1.Alcalá Hernández L, Reigadas Ramírez E, Bouza Santiago E. Clostrid-ium difficile infection. Med Clin (Barc) 2017; 148 (10): 456-63. doi: 10.1016/j.medcli.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Salavert Lletí M. Choice of treatment in Clostridium difficile-asso-ciated diarrhoea: Clinical practice guidelines or risk classifications. Enferm Infecc Microbiol Clin 2017; 35(10):613-6. doi: 10.1016/j.eimc.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Gerding DN, Kelly CP, Rahav G, Lee C, Dubberke ER, Kumar PN, et al. . Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018; 67(5):649-656. doi: 10.1093/cid/ciy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullish BH, Williams HR. Clostridium difficile infection and anti-biotic-associated diarrhoea. Clin Med (Lond) 2018; 18(3): 237-41. doi: 10.7861/clinmedicine.18-3-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schäffler H, Breitrück A. Clostridium difficile-From Coloniza-tion to Infection. Front Microbiol 2018; 9:646. doi: 10.3389/fmicb.2018.00646. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartelli M, Di Bella S, McFarland LV, Khanna S, Furuya-Kanamori L, Abuzeid N, et al. . 2019 update of the WSES guidelines for manage-ment of Clostridioides (Clostridium) difficile infection in surgical patients. World J Emerg Surg 2019; 14:8. doi: 10.1186/s13017-19-0228-3. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JH, Kim YS. Recurrent Clostridium difficile Infection: Risk Fac-tors, Treatment, and Prevention. Gut Liver 2019; 13(1):16-24. doi: 10.5009/gnl18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels LM, Kufel WD. Clinical review of Clostridium difficile infection: an update on treatment and prevention. Expert Opin Pharmacoth-er. 2018; 19 (16): 1759-69. doi: 10.1080/14656566.2018.1524872. [DOI] [PubMed] [Google Scholar]
- 9.Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, Marcil-Héguy A, Nault V, Valiquette L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis 2016; 62(5):574-580. doi: 10.1093/cid/civ95 [DOI] [PubMed] [Google Scholar]
- 10.Ramsay I, Brown NM, Enoch DA. Recent Progress for the Effec-tive Prevention and Treatment of Recurrent Clostridium diffi-cile Infection. Infect Dis (Auckl). 2018; 11: 1178633718758023. doi: 10.1177/1178633718758023. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, Krulicki W. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis 2012; 55 Suppl 2:S132-42. doi: 10.1093/cid/cis338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen L, Sonenshein AL. Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis. 2012; 55 Suppl 2:S162-9. doi: 10.1093/cid/cis45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crook DW, Walker AS, Kean Y, Weiss K, Cornely OA, Miller MA, Es-posito R, Louie TJ, Stoesser NE, Young BC, Angus BJ, Gorbach SL, Peto TE; Study 003/004 Teams . Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis 2012; 55 Suppl2:S93-103. doi: 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gor-bach S, Sears P, Shue YK; OPT-80-003 Clinical Study Group . Fidax-omicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364(5): 422-31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 15.Penziner S, Dubrovskaya Y, Press R, Safdar A. Fidaxomicin thera-py in critically ill patients with Clostridium difficile infection. An-timicrob Agents Chemother. 2015; 59(3): 1776-81. doi: 10.1128/AAC.04268-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S; OPT-80-004 Clinical Study Group . Fidaxomicin versus vancomycin for infection with Clostridium difficile in Eu-rope, Canada, and the USA: a double-blind, non-inferiority, ran-domised controlled trial. Lancet Infect Dis.2012; 12(4):281-9. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 17.Bouza E, Cobo J, Almirante B; Grupo de Trabajo CLODIEXPAN . [Recommendations from a panel of experts on the usefulness of fidaxomicin for the treatment ofinfections caused by Clostridium difficile]. Rev Esp Quimioter 2019; 32(1): 50-59. PMid: [PMC free article] [PubMed] [Google Scholar]
- 18.Gerding DN, File TM Jr; McDonald LC. Diagnosis and treatment of Clostridium difficile infection. Infect Dis Clin Pract.2016; 24: 3–10. doi: 10.1097/IPC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Agostino RB Sr, Collins SH, Pencina KM, Kean Y, Gorbach S. Risk estimation for recurrent Clostridium difficile infection based on clinical factors. Clin Infect Dis 2014; 58(10): 1386-93. doi: 10.1093/cid/ciu107. [DOI] [PubMed] [Google Scholar]
- 20.Cobo J, Merino E, Martínez C, Cózar-Llistó A, Shaw E, Marrodán T, et al. ; Nosocomial Infection Study Group . Prediction of recurrent Clostridium difficile infection at the bedside: the GEIH-CDI score. Int J Antimicrob Agents 2018; 51(3):393-8. doi: 10.1016/j.ijantimi-cag.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Terrés C, Cobo Reinoso J, Grau Cerrato S, Mensa Pueyo J, Sa-lavert Lletí M, Toledo A, et al. . Economic assessment of fidaxomicin for the treatment of Clostridium difficile infection (CDI) in special populations (patients with cancer, concomitant antibiotic treat-ment or renal impairment) in Spain. Eur J Clin Microbiol Infect Dis 2015; 34(11):2213-23. doi: 10.1007/s10096-015-2472-0 [DOI] [PubMed] [Google Scholar]
- 22.Guery B, Menichetti F, Anttila VJ, Adomakoh N, Aguado JM, Bis-nauthsing K, et al. ; EXTEND Clinical Study Group . Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis 2018; 18(3):296-307. doi: 10.1016/S1473-3099(17)30751-X. [DOI] [PubMed] [Google Scholar]
- 23.Rubio-Terrés C, Aguado JM, Almirante B, Cobo J, Grau S, Salavert M, et al. . Extended-pulsed fidaxomicin versus vancomycin in patients 60 years and older with Clostridium difficile infection: cost-effectiveness analysis in Spain. Eur J Clin Microbiol Infect Dis 2019. April 13. doi: 10.1007/s10096-019-03503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal H, Perisetti A, Rehman MR, Singla U. New and emerg-ing therapies in treatment of Clostridium difficile infection. Eur J Gastroenterol Hepatol 2018; 30(6): 589-97. doi: 10.1097/MEG.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 25.Ling L, Stratton CW, Li C, Polage CR, Wu B, Tang Y. Advances in the diagnosis and treatment of Clostridium difficile infections. Emerg Microbes Infect 2018; 7(1): 15. doi: 10.1038/s41426-017-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooijevaar RE, Beurden YH Van, Terveer EM, Goorhuis A, Bauer MP, Keller JJ, et al. . Update of treatment algorithms for Clostridium difficile infection. Clin Microbiol Infect. 2018; 24(5):452–62. doi: 10.1016/j.cmi.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Bhutiani N, Schucht JE, Miller KR, Mcclave SA. Technical Aspects of Fecal Microbial Transplantation (FMT). Curr Gastroenterol Rep. 2018;20(7):30. doi: 10.1007/s11894-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 28.Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. . The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other po-tential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018; 67: 1920–41. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 29.Lai CY, Sung J, Cheng F, Tang W, Wong SH, Chan PKS, et al. . Sys-tematic review with meta-analysis: review of donor features, pro-cedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol Ther 2019; 49(4): 354-63. doi: 10.1111/apt.15116 [DOI] [PubMed] [Google Scholar]
- 30.Lin SC, Alonso CD, Moss AC. Fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with solid organ transplants: an institutional experience and review of the literature. Transpl Infect Dis 2018; 20(6): e12967. doi: 10.1111/tid.12967. [DOI] [PubMed] [Google Scholar]
- 31.Federal Drug Administration briefing document bezlotoxumab injection meeting of the Antimicrobial Drugs Advisory Committee; 2016. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiInfectiveDrugsAdvisoryCommittee/UCM505290.pdf.
- 32.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305–317. doi: 10.1056/NEJ-Moa1602615. [DOI] [PubMed] [Google Scholar]
- 33.Mullane K, Wilcox M, Golan Y, Murata Y, Shoemaker K, Kelly M, et al. . Efficacy of Bezlotoxumab in Prevention of Clostridium difficile Infection Recurrence in Patients Receiving Concomitant Antibiotics. Poster # 2115. ID Week 2016.
- 34.Alonso CD, Mahoney MV. Bezlotoxumab for the prevention of Clostridium difficile infection: a review of current evidence and safety profile. Infect Drug Resist 2018; 12:1-9. doi: 10.2147/IDR.S159957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salavert M, Cobo J, Pascual A, Aragón B, Maratia S, Jiang Y, et al. . Cost-Effectiveness Analysis of Bezlotoxumab Added to Standard of Care Versus Standard of Care Alone for the Prevention of Recurrent Clostridium difficile Infection in High-Risk Patients in Spain. Adv Ther 2018; 35(11):1920-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhu VS, Dubberke ER, Dorr MB, Elbasha E, Cossrow N, Jiang Y, et al. . 2018. Cost-effectiveness of bezlotoxumab compared with pla-cebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis 2018; 66(3):355-362. doi: 10.1093/cid/cix809. [DOI] [PubMed] [Google Scholar]
- 37.Navalkele BD, Chopra T. Bezlotoxumab: an emerging monoclonal antibody therapy for prevention of recurrent Clostridium difficile infection. Biologics. 2018; 12: 11-21. doi: 10.2147/BTT.S127099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng Z, Ling L, Stratton CW, Li C, Polage CR, Wu B, et al. . Advances in the diagnosis and treatment of Clostridium difficile infections. Emerg Microbes Infect 2018; 7(1): 15. doi: 10.1038/s41426-017-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darkoh C, Deaton M, DuPont HL. Nonantimicrobial drug targets for Clostridium difficile infections. Future Microbiol 2017; 12: 975-85. doi: 10.2217/fmb-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dieterle MG, Rao K, Young VB. Novel therapies and preventative strategies for primary and recurrent Clostridium difficile infections. Ann N Y Acad Sci 2019; 1435(1): 110-38. doi: 10.1111/nyas.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerding DN, Meyer T, Lee C, Cohen SH, Murthy UK, Poirier A, et al. . Administration of spores of Non-toxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a rand-omized clinical trial. JAMA 2015; 313 (17): 1719-27. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 42.Petrosillo N, Granata G, Cataldo MA. Novel antimicrobials for the treatment of Clostridium difficile Infection. Front Med (Lausanne) 2018; 5:96. doi: 10.3389/fmed.2018.00096. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokai-Kun JF, Roberts T, Coughlin O, Le C, Whalen H, Stevenson R, et al. . Use of ribaxamase (SYN-004), a β-lactamase, to pre-vent Clostridium difficile infection in β-lactam-treated patients: a double-blind, phase 2b, randomised placebo-controlled trial. Lancet Infect Dis. 2019. May;19(5):487-96. doi: 10.1016/S1473-3099(18)30731-X. [DOI] [PubMed] [Google Scholar]
- 44.Hu MY, Katchar K, Kyne L, Maroo S, Tummala S, Dreisbach V, et al. . Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology 2009;136(4):1206-14. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 45.Miller MA, Louie T, Mullane K, Weiss K, Lentnek A, Golan Y, et al. . Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to thera-py. BMC Infect Dis 2013;13: 148. doi: 10.1186/1471-2334-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med 2014; 9(7): 418-23. doi: 10.1002/jhm.2189. [DOI] [PubMed] [Google Scholar]
- 47.Escobar GJ, Baker JM, Kipnis P, Greene JD, Mast TC, Gupta SB, et al. . Prediction of Recurrent Clostridium Difficile Infection Using Com-prehensive Electronic Medical Records in an Integrated Healthcare Delivery System. Infect Control Hosp Epidemiol 2017; 38(10):1196-1203. doi: 10.1017/ice.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viswesh V, Hincapie AL, Yu M, Khatchatourian L, Nowak MA. De-velopment of a bedside scoring system for predicting a first recur-rence of Clostridium difficile-associated diarrhea. Am J Health Syst Pharm 2017; 74(7):474-82. doi: 10.2146/ajhp160186. [DOI] [PubMed] [Google Scholar]
- 49.Reveles KR, Mortensen EM, Koeller JM, Lawson KA, Pugh MJV, Rum-bellow SA,et al. . Derivation and Validation of a Clostridium difficile Infection Recurrence Prediction Rule in a National Cohort of Veter-ans. Pharmacotherapy 2018; 38(3):349-56. doi: 10.1002/phar.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]