Abstract
Mast cells serve a key role in the occurrence and development of allergy. As an important growth factor of mast cells, stem cell factor (SCF) has an effect on the apoptosis, chemotaxis, adhesion, degranulation and other biological characteristics of mast cells. However, there are few studies regarding the effect of SCF signal on the production of cytokines from mast cells, particularly Th2 type cytokines. In the present study, the expression and secretion of IL-13 in P815 cells stimulated by SCF were detected by fluorescence quantitative PCR and ELISA, and western blotting and EMSA were used to detect ERK phosphorylation and activation of CREB in stimulated P815 cells. The results demonstrated that the production of IL-13 was significantly increased in P815 cells stimulated by SCF (1–100 ng/ml; P<0.01). There was an obvious phosphorylation of ERK and CREB activation in P815 cells stimulated by SCF (50 ng/ml). Compared with the SCF single stimulation group, the production of IL-13 was significantly reduced in P815 cells stimulated with U0126 (ERK-MEK/pathway inhibitor) or H-89 (CREB inhibitor) combined with SCF stimulation group (P<0.01). However, JSI-124 (JAK/STAT3 pathway inhibitor), Wortmannin (PI3K/Akt pathway inhibitor) and PDTC (NF-κB inhibitor) had no effect on the role of SCF promoting the P815 cells producing IL-13. Therefore, SCF signaling promotes mast cell P815 to produce IL-13, and this effect is associated with the MEK-ERK-CREB signaling pathway.
Keywords: stem cell factor, mast cells, interleukin 13
Introduction
Mast cells (MCs) are important effector cells and immune regulatory cells in vivo (1). It originates from hematopoietic stem cells in bone marrow, matures in the peripheral tissues, distributes in the mucosa and connective tissue of whole body (2,3). MCs were mainly divided into mucous membranes and connective tissue MCs two subgroups according to the distribution site and particles containing tryptase or chymase (4). Many stimulus may activate the MCs through the FcεRIdependent and non-dependent (such as c-Kit, TLR) way. Activated MCs can produce three types of effector molecules: The first is material stored in particles, such as 5-HT, histamine, tryptase and chymase; the second category is the new synthetic substances such as lipid metabolites, prostaglandins; the third category is cytokines, such as IL-1, 3, TNF-α, VEGF and so on (5–7). It is precisely because of the generation of so many types of effector molecules, so MCs can participate in a variety of biological processes of body. Now studies show that MCs play a key role in the development of allergy (8,9). Stem cell factor (SCF) (i.e. c-Kit ligand), an important growth factor, has soluble and membrane-bound two forms. SCF can be produced from both fibroblasts and endothelial cells in vivo (10,11). MCs characteristically express SCF receptor c-Kit. SCF is a potential growth factor of MCs, in addition to affecting the development of MCs, but also on its apoptosis, chemotaxis, adhesion, degranulation and other biological characteristics (12,13). But there are few studies on the effect of SCF signal on the production of cytokines (especially Th2 type cytokines) in MCs. In the present study, we investigated the effects of SCF on the production of IL-13 and its mechanisms in mouse mast cell line P815 cells.
Materials and methods
Cell lines and experimental reagents
Mouse mast cell line P815 cells purchased from Shanghai Institute of life sciences, Chinese Academy of sciences. DMEM (high glucose type) culture was purchased from Thermo Fisher Company. Fetal bovine serum is product of Hangzhou Sijiqing biological company. Fluorescein (PE-Cy5)-labeled CD117 (c-Kit) antibody was purchased from eBioscience company. Recombinant mouse SCF and U0126 was respectively purchased from PeproTech and Gene Operation. JSI-124 and Curcumin are the product of Sigma company. Wortmannin, NP-40 lysate, β-actin, p42/44 antibody, Phospho-p42/44 antibody, horseradish peroxidase labeled goat anti-mouse IgG antibody, the BCA Protein Assay kit, nuclear proteins and cytoplasmic protein extraction kit, H-89, PDTC, chemiluminescent EMSA kit, biotin-labeled EMSA probes of CREB were purchased from Beyotime Institute of Biotechnology (Haimen, China). Mouse Interleukin 13 (IL-13) ELISA kit was purchased from Wuhan Huamei Biological Engineering Co., Ltd. Trizol Reagent was purchased from Invitrogen Corporation. TransScript First-Strand cDNA Synthesis SuperMix and TransStart Top Green qPCR SuperMix are products of Transgen Biotech company.
Cell culture
P815 cells were suspended in DMEM complete solution (containing 4.0 mM L-glutamine, 4500 mg/l glucose, 10% fetal bovine serum, 50,000 U/l gentamicin, 1 mmol/l sodium pyruvate), and cultured in 37°C, 5% CO2 incubator. P815 cells were seeded in 24-well plates and cells density is 5×105 cells/ml. After cultured in DMEM without serum for starvation 12 h, the cells were treated with different concentrations of SCF for different time.
Detection of c-kit receptor on P815 cell surface by flow cytometry
The cultured cells were washed 2 times with the staining buffer solution. P815 cells were incubated with PE-Cy5-c-Kit antibody (concentration based on the specification instruction) in 100 µl reaction system at dark 4°C. After washed two times with staining buffer, stained cells were fixed with 1% paraformaldehyde (PFA), then were detected by flow cytometry.
Reverse transcription-quantitaive polymerase chain reaction (RT-qPCR)
After P815 cells were washed two times with PBS, their total RNA was extracted using Trizol reagent. cDNA was reversed to synthesized in accordance with manufacturer's instructions. PCR amplification was conducted in 25 µl reaction system (including 2×TS Top Green qPCR SuperMix 12.5 µl, Passive Reference Dye 0.5 µl, cDNA 1 µl, Forward Primer 0.5 µl, Reverse Primer 0.5 µl, RNase-free water 10 µl). PCR reaction conditions (two step method): 94°C 30s, 94°C 5s, 60°C 30s, a total of 40 cycles. IL-13 primer sequences are listed below: Upstream primer: 5′-GCAGCAGCTTGAGCACATT-3′, downstream primer: 5′-GGCATAGGCAGCAAACCA-3′. Gene expression was analyzed using 2−ΔΔCq method (14).
ELISA
To collect the culture supernatant of P815 cells under various conditions, the concentration of IL-13 in supernatant was detected by ELISA according to manufacturer's instructions.
Western blot analysis
Cytoplasmic protein was extracted from P815 cells of control group and SCF stimulation group according to manufacturer's instructions. After quantified by BCA method, the protein was electrophoresed by SDS-PAGE (10% separating gel and 5% stacking gel). After electrophoresis, NC film was used to transfer the protein. The membrane was blocked 2 h with 5% BSA, and washed 30 min using TBST, then the membrane was incubated with primary antibody or loading control antibody overnight at 4°C. After the membrane was washed 30 min with TBST, then was incubated with HRP-labeled second antibody 2 h. The positive signal was detected by chemiluminescence method, and then the light density analysis was carried out.
Electrophoretic mobility shift assay (EMSA)
Cell nuclear proteins were collected from P815 cells in the control group and the SCF stimulation group and biotin-labeled CREB probe and nuclear protein binding reaction was detected according to Beyotime kit instructions. Probe and protein mixture was electrophoresis 45 min at 120V using 12% non-denaturing PAGE gel. After electrophoresis, the protein was transferred to nylon membrane using 380 mA constant current for 1 h and was cross-linked 10 min using ultraviolet rays. Enhanced chemiluminescence method was used for the detection of biotin labeled CREB probes. CREB consensus oligo sequences are as follows:
5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′,
3′-TCTCTAACGGACTGCAGTCTCTCGATC-5′.
Statistical analysis
The experimental data is indicated as mean ± standard deviation, and one-way analysis of variance was used with the Least Significant Difference post hoc test for the comparison between groups. All analyses were carried out using SPSS 16. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of c-Kit receptor on the surface of mast cell P815
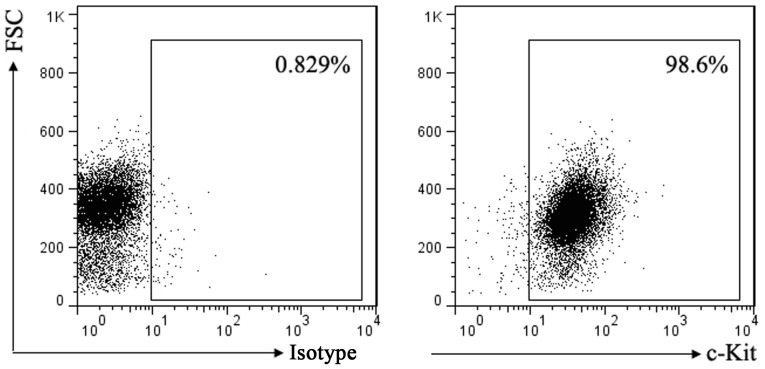
SCF corresponding receptor is c-Kit. To investigate the effect of SCF signal on mast cell, we first used flow cytometry to detect the expression of c-Kit in P815 cells. Result as shown in Fig. 1, almost all of the P815 cells membrane surface express c-Kit.
Figure 1.
Expression of c-Kit receptor on the surface of P815 cells was detected by flow cytometry. FSC, forward scatter.
Effect of SCF on IL-13 production in P815 cells
P815 cells were stimulated 6 h with different concentrations of SCF
The supernatant in culture well was detected by ELISA, and the results were shown in Fig. 2. SCF (1–100 ng/ml) can promote P815 cells to produce IL-13, among which the 10–50 ng/ml effect is the most obvious. After P815 cells were stimulated with SCF (50 ng/ml) 6 h, IL-13 gene expression in P815 cells is increased by about 3-fold (Fig. 3). P815 cells were stimulated with SCF (50 ng/ml) at different times, the content of IL-13 in supernatant are shown in Fig. 4. Among them, the production of IL-13 reachs a higher level when P815 cells were stimulated 6–24 h.
Figure 2.

Content of IL-13 in supernatant of P815 cells stimulated by SCF. P815 cells were seeded in 24-well plates at a density of 5×105/ml. After P815 cells were stimulated for 6 h by different concentrations of SCF (1–100 ng/ml), the content of IL-13 in the supernatant of P815 cells was detected by ELISA. *P<0.01 vs. 0 ng/ml. IL-13, interleukin 13; SCF, stem cell factor.
Figure 3.
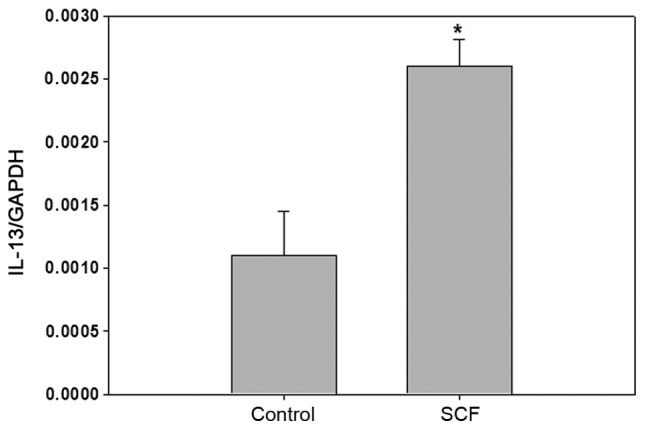
IL-13 gene expression of P815 cells stimulated by SCF. P815 cells (cell density, 5×105/ml) were seeded in 24-well plates. After P815 cells were stimulated for 6 h with SCF (50 ng/ml), the IL-13 gene expression of P815 cells was detected by reverse transcription-quantitative PCR. *P<0.01. IL-13, interleukin 13; SCF, stem cell factor.
Figure 4.

Secretion of IL-13 in supernatant of P815 cells stimulated different time by SCF (50 ng/ml). P815 cells (cell density, 5×105/ml) were seeded in 24-well plates. After P815 cells were stimulated 6, 24, 48 or 72 h with SCF (50 ng/ml), the content of IL-13 in the supernatant of P815 cells was detected by ELISA. *P<0.01 vs. 0 h group. IL-13, interleukin 13; SCF, stem cell factor.
SCF promotes the production of IL-13 in MCs by MEK-ERK signaling pathway
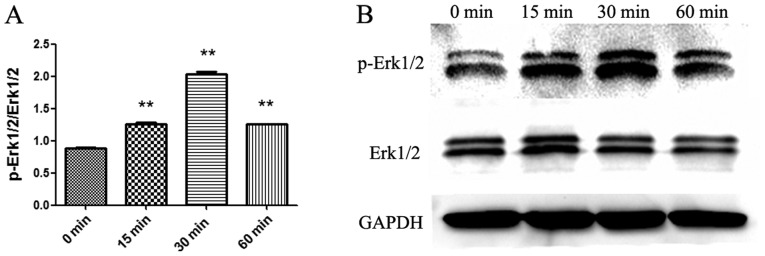
After pretreated 30 min with MEK/ERK pathway inhibitor U0126 (10 µM) or JAK/STAT3 pathway inhibitor JSI-124 (100 nM) or PI3K/Akt pathway inhibitor wortmannin (1 µM), P815 cells were stimulated 6 h with SCF (50 ng/ml), then the content of IL-13 in the culture supernatant of P815 cells was detected by ELISA. The results are shown in Fig. 5. U0126 completely blocked the effect of SCF on promotion IL-13 production in P815 cells, but JSI-124 and Wortmannin had no effect on this role of SCF. To demonstrate the activation of the MEK-ERK signaling pathway, cytosolic proteins was extracted from P815 cells stimulated with SCF (50 ng/ml) at different times and the activation of Erk1/2 was detected by Western blot (Fig. 6). Erk1/2 phosphorylation was the maximum in P815 cells stimulated 30 min with SCF. So it can be proved that the SCF signal can promote the production of IL-13 by activating the MEK-ERK signaling pathway in P815 cells.
Figure 5.
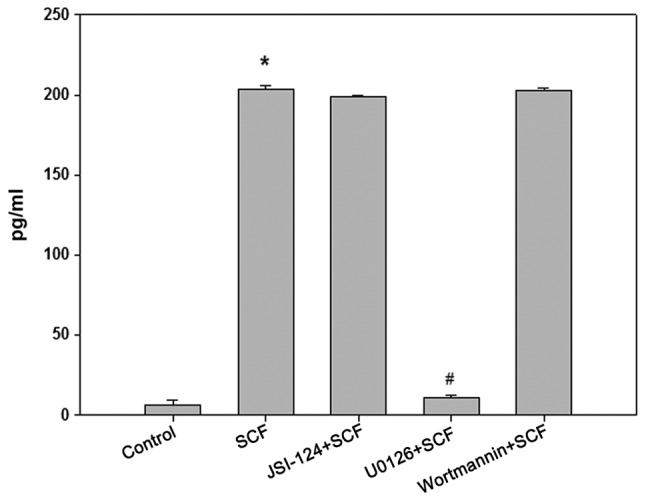
SCF signaling promotes P815 cells to secrete IL-13 via the MEK-ERK signaling pathway. P815 cells (cell density, 5×105 cells/ml) were seeded in 24-well plates. After pretreatment for 30 min with U0126 (10 µM), JSI-124 (100 nM) or Wortmannin (1 µM), P815 cells were stimulated for 6 h with SCF (50 ng/ml), then the content of IL-13 in the culture supernatant of P815 cells was detected by ELISA. ‘Control’ means P815 cells stimulated with dissolution medium. ‘SCF’ refers to P815 cells stimulated with 50 ng/ml SCF. ‘JSI-124/U0126/Wortmannin’+SCF’ means P815 cells pretreated for 30 min with JSI-124, U0126 or Wortmannin, followed by stimulation with 50 ng/ml SCF. *P<0.01 vs. control; #P<0.01 vs. SCF. IL-13, interleukin 13; SCF, stem cell factor.
Figure 6.
Effect of SCF signaling on the phosphorylation of ERK in P815 cells. P815 cells (cell density, 5×105 cells/ml) were seeded in 24 well plates. P815 cells were stimulated 0, 15, 30 and 60 min with SCF (50 ng/ml). Subsequently, cytoplasmic proteins were extracted. The phosphorylation of ERK1/2 in cytoplasmic proteins of P815 cells was detected by western blotting. (A) Statistical graph based on densitometric analysis and (B) representative blot. **P<0.01 vs. 0 min. p-, phosphorylated. SCF, stem cell factor.
SCF activates CREB to promote the production of IL-13 in P815 cells
To further clarify the role of the downstream factor of MEK-ERK pathway in SCF promoting the IL-13 production in P815 cells. After pretreated 30 min with H-89 (20 µM, CREB blocker) or PDTC (50 µM, NF-κB inhibitor), P815 cells were stimulated with SCF (50 ng/ml) 6 h, and the content of IL-13 in supernatant was detected by ELISA (Fig. 7). PDTC had no effect on the SCF promoting P815 cells to produce IL-13, whereas H-89 completely inhibited the effect of SCF.
Figure 7.

SCF signal promotes the secretion of IL-13 from P815 cells via CREB. P815 cells (cell density, 5×105 cells/ml) were seeded in 24-well plates. After pretreatment for 30 min with H-89 (20 µM) or PDTC (50 µM), P815 cells were stimulated for 6 h with SCF (50 ng/ml), then the content of IL-13 in the culture supernatant of P815 cells was detected by ELISA. ‘Control’ means P815 cells stimulated with dissolution medium. ‘SCF’ refers to P815 cells stimulated with 50 ng/ml SCF. ‘PDTC or H-89+SCF’ refers to P815 cells pretreated for 30 min with PDTC or H-89, then stimulated with 50 ng/ml SCF. *P<0.01 vs. control; #P<0.01 vs. SCF. IL-13, interleukin 13; SCF, stem cell factor.
To demonstrate the activation of CREB, nuclear proteins of P815 cells stimulated 1 h by SCF (50 ng/ml) was extracted for EMSA. The results are shown as in Fig. 8. SCF signal induced significant CREB activation in P815 cells. These suggest that SCF signaling can regulate the production of IL-13 in P815 cells by activating transcription factor CREB.
Figure 8.
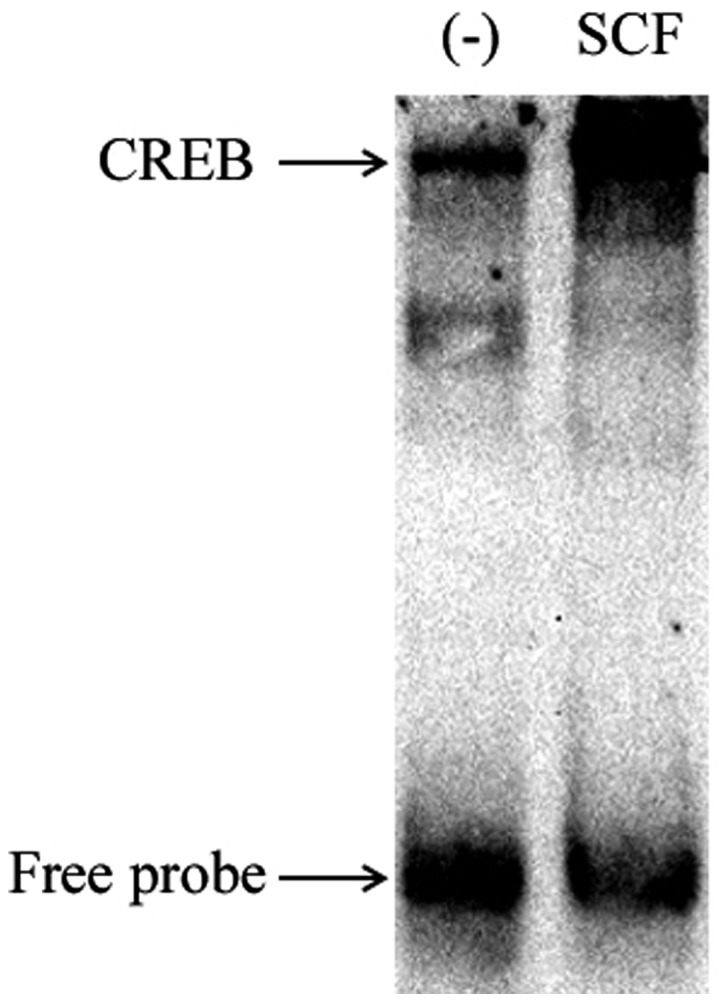
SCF signaling induces the activation of CREB in P815 cells. P815 cells (cell density, 5×105 cells/ml) were seeded in 24-well plates. P815 cells were stimulated for 1 h with SCF (50 ng/ml), then nuclear proteins were extracted. The activation of CREB in P815 cells was detected by EMSA. SCF, stem cell factor.
Discussion
In the present study we demonstrate that there is the presence of c-kit expression on the P815 cells membrane surface, and SCF signaling can induce MEK-ERK-CREB signaling pathway activation in P815 cells, blocking the pathway inhibits the effect of SCF promoting P815 cells to produce IL-13.
SCF, also known as c-Kit ligand, is an important growth factor in the body, including soluble and membrane-bound two forms. SCF is mainly produced by fibroblasts and endothelial cells and it can promote the proliferation, migration, survival and differentiation of hematopoietic precursor cells, melanocytes, germ cells and other cells. Studies have indicated that SCF-c-Kit signaling also plays an important role in the survival, growth, adhesion and other biological activities of MCs (15,16). Since MCs play a key role in allergic reactions, SCF-c-Kit signaling is an important research target in the study of allergic reactions. SCF start its effect by binding to c-Kit, the combination of SCF led to c-Kit dimerization and open its protein kinase activity. Activation of c-Kit activates multiple intracellular signaling pathways, such as Src kinase, PI3K, PLC-γ, MAPK and so on (11,17). SCF induced activation of Src family is associated with the gene transcription and chemical chemotaxis of MCs (18). The PI3K pathway activation induced by SCF is associated with the development of MCs (19). MacNeil's results showed that SCF signal induced mast cell to produce IL-6 by P38MAPK and JNK signaling (20). In this study, we found that the SCF signal can activate the MEK-ERK-CREB signaling pathway, and the activation of this pathway is related to the production of IL-13 in MCs. Therefore, activation of each signal pathway induced by SCF is associated with specific biological functions.
Previous studies have suggested that the content of SCF in sputum and alveolar lavage fluid of asthma patients was increased. SCF was strongly correlated with IgE levels and the state of lung function in patients with allergic and non-allergic asthma patients (21,22). But the exact relationship between SCF and asthma is still not very clear. Asthma is a chronic airway inflammation, which involves a variety of cells and cytokines (23). Epidemiological studies have shown that there is an increasing trend in all parts of the world (including China) about the morbidity and mortality of asthma (24). MCs as the key effector cells in asthma, hay fever and other allergic reaction has been known to everyone, but more and more studies show the product of mast cells after activation can regulate the adaptive immune response intensity, duration and dynamics. Mast cells can also be used as immune regulatory cells to play an important role in a variety of biological processes (25). Once activated, MCs can secrete lipid products, cytokines and chemokines three types of chemical media. This study found that the production of IL-13 was increased in mast cells stimulated by SCF signal. Li's results showed that SCF signal induced mast cell to produce IL-13 through the early growth response factor-1 (26), which is consistent with our research results.
IL-13, molecular weight of 12 KDa, is a pleiotropic cytokine which regulates IgE synthesis, mucus hypersecretion, subepithelial fibrosis, eosinophil tissue infiltration, chemokine receptors (eg CCR5) expression, which is also closely linked with airway inflammation and bronchial remodeling. Current studies suggest that IL-13 plays a central role in the pathogenesis of asthma (27,28). Thus, SCF signaling may play an important role in the pathogenesis of asthma by affecting the production of IL-13 in mast cells.
In conclusion, this study suggests that SCF signaling can induce mast cells P815 to produce IL-13, and this effect is related to the MEK-ERK-CREB signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Science Foundation of China (grant no. 81273273), Anhui Provincial Natural Science Foundation (grant no. 1708085MH218) and the Scientific Research Innovation Team Project of Anhui Colleges and Universities (grant no. 2016-40).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
CS and SG designed the experiments. YW, HM, XT, YL, HW and JH performed the experiments. QF, SG and CS analyzed the data. CS wrote the manuscript. CS and SG revised the manuscript. The manuscript has been read and approved by each author, and all authors believe that the manuscript represents honest work.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Bengbu Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Morita H, Saito H, Matsumoto K, Nakae S. Regulatory roles of mast cells in immune responses. Semin Immunopathol. 2016;38:623–629. doi: 10.1007/s00281-016-0566-0. [DOI] [PubMed] [Google Scholar]
- 2.Huang H, Li Y, Liu B. Transcriptional regulation of mast cell and basophil lineage commitment. Semin Immunopathol. 2016;38:539–548. doi: 10.1007/s00281-016-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeckxstaens G. Mast cells and inflammatory bowel disease. Curr Opin Pharmacol. 2015;25:45–49. doi: 10.1016/j.coph.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 5.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 6.Arthur G, Bradding P. New developments in mast cell biology: Clinical implications. Chest. 2016;150:680–693. doi: 10.1016/j.chest.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett. 2016;178:10–14. doi: 10.1016/j.imlet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130:1123–1129.e2. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson C, Tufvesson E, Diamant Z, Bjermer L. Revisiting the role of the mast cell in asthma. Curr Opin Pulm Med. 2016;22:10–17. doi: 10.1097/MCP.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 10.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 11.Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiol Rev. 2012;92:1619–1649. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- 12.Lorentz A, Bischoff SC. Regulation of human intestinal mast cells by stem cell factor and IL-4. Immunol Rev. 2001;179:57–60. doi: 10.1034/j.1600-065X.2001.790106.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Azzam N, Kondeti V, Duah E, Gombedza F, Thodeti CK, Paruchuri S. Modulation of mast cell proliferative and inflammatory responses by leukotriene d4 and stem cell factor signaling interactions. J Cell Physiol. 2015;230:595–602. doi: 10.1002/jcp.24777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Faber TW, Pullen NA, Fernando JF, Kolawole EM, McLeod JJ, Taruselli M, Williams KL, Rivera KO, Barnstein BO, Conrad DH, Ryan JJ. ADAM10 is required for SCF-induced mast cell migration. Cell Immunol. 2014;290:80–88. doi: 10.1016/j.cellimm.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smrž D, Bandara G, Beaven MA, Metcalfe DD, Gilfillan AM. Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells. Eur J Immunol. 2013;43:1873–1882. doi: 10.1002/eji.201243214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankov K, Popovic S, Mikov M. C-KIT signaling in cancer treatment. Curr Pharm Des. 2014;20:2849–2880. doi: 10.2174/13816128113199990593. [DOI] [PubMed] [Google Scholar]
- 18.O'Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa, DeBerry C, Metcalfe DD, Zhou M, Lowell C, Linnekin D. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–350. doi: 10.1182/blood.V98.2.343. [DOI] [PubMed] [Google Scholar]
- 19.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 20.MacNeil AJ, Junkins RD, Wu Z, Lin TJ. Stem cell factor induces AP-1-dependent mast cell IL-6 production via MAPK kinase 3 activity. J Leukoc Biol. 2014;95:903–915. doi: 10.1189/jlb.0713401. [DOI] [PubMed] [Google Scholar]
- 21.Moaaz M, Abo El-Nazar S, Abd El-Rahman M, Soliman E. Stem cell factor and interleukin-31 expression: Association with IgE among Egyptian patients with atopic and Nonatopic bronchial asthma. Immunol Invest. 2016;45:87–106. doi: 10.3109/08820139.2015.1089890. [DOI] [PubMed] [Google Scholar]
- 22.Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang GM, Feng ZH, Huang B. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 23.Samitas K, Delimpoura V, Zervas E, Gaga M. Anti-IgE treatment, airway inflammation and remodelling in severe allergic asthma: Current knowledge andfuture perspectives. Eur Respir Rev. 2015;24:594–601. doi: 10.1183/16000617.00001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65:152–167. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 25.Bulfone-Paus S, Bahri R. Mast cells as regulators of T cell responses. Front Immunol. 2015;6:394. doi: 10.3389/fimmu.2015.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Berman J, Tang JT, Lin TJ. The early growth response factor-1 is involved in stem cell factor (SCF)-induced interleukin 13 production by mast cells, but is dispensable for SCF-dependent mast cell growth. J Biol Chem. 2007;282:22573–22581. doi: 10.1074/jbc.M610859200. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell J, Dimov V, Townley RG. IL-13 and the IL-13 receptor as therapeutic targets for asthma and allergic disease. Curr Opin Investig Drugs. 2010;11:527–534. [PubMed] [Google Scholar]
- 28.Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep. 2013;13:415–420. doi: 10.1007/s11882-013-0373-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.