Abstract
It has been indicated that the combination of pancreatic and duodenal homeobox 1 (Pdx1), MAF bZIP transcription factor A (MafA) and neurogenin 3 (Ngn3) was able to reprogram various cell types towards pancreatic β-like cells (pβLCs). Paired box 4 (Pax4), a transcription factor, has a key role in regulating the maturation of pancreatic β-cells (pβCs). In the present study, it was investigated whether Pax4 is able to synergistically act with Pdx1, Ngn3 and MafA to induce human umbilical cord mesenchymal stem cells (HuMSCs) to differentiate into functional pβCs in vitro. HuMSCs were isolated, cultured and separately transfected with adenovirus (Ad) expressing enhanced green fluorescence protein, Pax4 (Ad-Pax4), Pdx1+MafA+Ngn3 (Ad-3F) or Ad-Pxa4 + Ad-3F. The expression of C-peptide, insulin and glucagon was detected by immunofluorescence. The transcription of a panel of genes was determined by reverse transcription-quantitative PCR, including glucagon (GCG), insulin (INS), NK6 homeobox 1 (NKX6-1), solute carrier family 2 member 2 (SLC2A2), glucokinase (GCK), proprotein convertase subtilisin/kexin type 1 (PCSK1), neuronal differentiation 1 (NEUROD1), ISL LIM homeobox 1 (ISL 1), Pax6 and PCSK type 2 (PCSK2). Insulin secretion stimulated by glucose was determined using ELISA. The results suggested that, compared with Ad-3F alone, cells co-transfected with Ad-Pax4 and Ad-3F expressed higher levels of INS and C-peptide, as well as genes expressed in pancreatic β precursor cells, and secreted more insulin in response to high glucose. Furthermore, the expression of GCG in cells transfected with Ad-3F was depressed by Ad-Pax4. The present study demonstrated that Pax4 was able to synergistically act with the transcription factors Pdx1, Ngn3 and MafA to convert HuMSCs to functional pβLCs. HuMSCs may be potential seed cells for generating functional pβLCs in the therapy of diabetes.
Keywords: diabetes, Pax4, human umbilical cord mesenchymal stem cells, cell differentiation, pancreatic β-cells
Introduction
Diabetes, including type 1 and type 2 diabetes mellitus, is one of the most serious health-threatening diseases worldwide. Transplantation of pancreatic β-cells (pβCs), which are responsible for producing insulin, is an effective strategy for the treatment of type 1 and type 2 diabetes (1). However, the availability of pβCs is limited and therefore, the production of more pβCs is urgently required. The technology of differentiating stem cells into certain cell types holds great promise. Previous studies have focused on inducing embryonic stem cells (ESCs) to differentiate into pancreatic β-like cells (pβLCs) (2). However, due to several concerns, including ethics, immunological rejection and tumorigenesis potential, the suitability and safety of pβLCs derived from ESCs remain under debate (3).
Human umbilical cord mesenchymal stem cells (HuMSCs) isolated from human umbilical cord express the surface markers of stem cells, including CD29, CD44, CD59 and CD105 (4,5). Studies from our and other groups have demonstrated that HuMSCs were able to be induced into several types of cell, including osteocytes, chondrocytes, lipocytes, neuron-like cells, cardiomyocyte-like cells and spermatogonium-like cells (6–13). Furthermore, a previous study by our group indicated that vector-mediated overexpression of pancreatic and duodenal homeobox 1 (Pdx1), MAF bZIP transcription factor A (MafA) and neurogenin 3 (Ngn3) in HuMSC significantly promoted the expression of pancreatic genes (13). Therefore, HuMSCs have multi-lineage differentiation potential and may be used for generating pβLCs.
The differentiation of stem cells is tightly regulated by the temporal and spatial expression of transcription factors; in other words, stem cell differentiation is able to be determined by the transcription factors introduced. In addition to stem cells, terminally differentiated cells may be reprogramed to other cell types (14). Numerous cell types have been trans-differentiated into pβLCs by forcing the expression of three transcription factors, Pdx1, Ngn3 and MafA, which have important roles in promoting the development of the pancreas (15–17). Pax4 is also an indispensable transcription factor for the generation, differentiation, development and survival of pβCs, evidenced by the observation that Pax4-knockout mice lack a pancreas and die 1–2 days after birth (18). Recent studies highlight the important role of the Pax4 gene in driving the formation of pβLCs from other cell types, including pancreatic δ- and α-cells (19,20). Based on these results, it may be proposed that the Pax4 gene synergistically acts with the transcription factors Pdx1, Ngn3 and MafA to promote the development of pβCs. To this end, the present study aimed to determine whether co-transfecting the above-mentioned 4 transcription factors in HuMSC increases the efficiency of pβLC formation.
In the present study, HuMSCs were cultured and co-transfected with Pdx1, Ngn3, MafA and Pax4, and the function of the resulting pβLCs was assessed. It was demonstrated that Pax4 synergistically acts with Pdx1, Ngn3 and MafA to enhance the differentiation of HuMSCs to pβLCs.
Patients and methods
Plasmids, human umbilical cord and reagents
Adenovirus plasmid carrying Pdx1, Ngn3 and MafA (Ad-3F) and Pax4 (Ad-Pax4) were kindly provided by Professor Chiju Wei from the Multidisciplinary Research Center of Shantou University (Shantou, China) and were originally obtained from the Beta Cell Biology Consortium. Adenovirus plasmid carrying only EGFP was used as a transfection control.
The umbilical cord was obtained from normal full-term pregnant women with cesarean section at the Second Affiliated Hospital of Shantou University (Shantou, China). Maternal donors and their families provided written informed consent and the present study was approved by the ethics committee of Shanghai Children's Hospital (Shanghai, China; ethical approval no. SHMC201707561) and the ethics committee of the Second Affiliated Hospital of Shantou University Medical College (Shantou, China).
H-Dulbecco's modified Eagle's medium (DMEM)/F12 and fetal bovine serum (FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.); human epidermal growth factor (EGF) and human basic fibroblast growth factor (bFGF) were purchased from Invitrogen (Thermo Fisher Scientific, Inc.); goat anti-human insulin polyclonal antibody (cat. no. sc-7839) was obtained from Santa Cruz Biotechnology; mouse anti-human glucagon monoclonal antibody (cat. no. BM1621) was from Boster Biotechnology; rabbit anti-human C-peptide polyclonal antibody (cat. no. ab82696) was procured from Abcam; cyanine 3 (CY3)-labeled donkey anti-goat IgG (cat. no. A0502) was purchased from Beyotime Institute of Biotechnology; CY3-labeled goat anti-mouse IgG (cat. no. AP181C) was obtained from Sigma-Aldrich (Merck KGaA); and horseradish peroxidase-labeled goat anti-rabbit IgG (cat. no. G-21234) was from Molecular Probes (Thermo Fisher Scientific, Inc.). Primers were synthesized by Nanjing Kingsley Biotechnology Co., Ltd. The kit for reverse transcription (RT; HiScript II Q Select RT SuperMix for qPCR; cat. no. R233-01) and real-time PCR (AceQ qPCR SYBR Green Master Mix; cat. no. Q141-02) was purchased from Vazyme Biotechnology Co. Human insulin ELISA kit (cat. no. EZHI-14K) was purchased from Sigma-Aldrich (Merck KGaA).
HuMSC culture
Umbilical cords of healthy fetuses from full-term pregnancies were obtained and washed with PBS. Amniotic membrane and blood vessels were removed and the remaining tissue was cut into pieces (1.0 mm3). The pieces were cultured in DMEM/F12 containing 10% FBS, EGF (5 ng/ml) and Bfgf (5 ng/ml), and maintained at 37°C with 5% CO2 in a humidified atmosphere. The culture medium was replaced every 2 days. When the cells reached 80–90% confluence, the cells were digested with 0.25% trypsin-EDTA (Sigma-Aldrich; Merck KGaA) and passaged.
Cell transfection
HuMSCs were seeded into 12-well plates at a density of 2×105 cells/well. After culture for 12 h, Ad-3F and/or Ad-Pxa4 (2 µl; multiplicity of infection, 10) were added to the respective wells and served as the 3F, Pax4 or 3F/Pax4 group. After incubation for 12 h, the cells were washed three times with PBS and cultured in H-DMEM containing 1% FBS for 4 days. The culture medium was replaced every 2 days.
Immunofluorescence
HuMSCs of the 4th generation were digested and seeded in a 96-well plate. After reaching 60–70% confluence, the cells were transfected with various Ad plasmids as mentioned above. The cells were fixed with 4% paraformaldehyde at room temperature for 5 min and permeabilized with Triton X-100 at room temperature for 30 min. Subsequently, cells were incubated with primary antibodies, including goat anti-human insulin (dilution, 1:100), mouse anti-human glucagon (dilution, 1:100) and rabbit anti-human C-peptide (dilution, 1:100) at 37°C for 2 h. Cells were then incubated with CY3-labeled donkey anti-goat IgG (dilution, 1:1,000), CY3-labeled goat anti-mouse IgG (dilution, 1:1,000) and CY3-labeled goat anti-rabbit IgG (dilution, 1:1,000) at room temperature for 1 h in the dark. Following staining with DAPI, the fluorescence was examined with a fluorescence microscope (DMi8 Fluorescence Imaging; Leica Microsystems GmbH). The percentage of insulin-, C-peptide- or glucagon-positive cells was calculated as the ratio of positive cells among total cells. The percentage of insulin- or C-peptide-positive cells was used to evaluate the differentiation efficiency of HuMSCs into pβCs.
RT-quantitative (q)PCR
The total RNA was extracted with a TRIzol kit (Invitrogen; Thermo Fisher Scientific, Inc.). The total RNA was used as template to synthesize complementary (c)DNA by using the commercial RT kit. The expression of target genes was detected by qPCR. The thermocycling protocol was as follows: Denaturation at 95°C for 1 min; followed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 45 sec, and then a final elongation at 72°C for 10 min. RT-qPCR was performed in a 7300 Real-time PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) and standardized by the value of β-actin in the same sample. Data were quantified by using the 2−ΔΔCq method (21). The sequences of primers were as follows: PDX1 (GenBank ID, NM_000209.4; product length, 193 bp; forward (F)-5′-ATCTCCCCATACGAAGTGCC-3′, reverse (R)-5′-CGTGAGCTTTGGTGGATTTCAT-3′; NGN3 (XM_017016280.1; product length, 86 bp; F-5′-CTAAGAGCGAGTTGGCACTGA-3′, R-5′-GAGGTTGTGCATTCGATTGCG-3′); MAFA (NM_201589.4; product length, 150 bp; F-5′-GTACAGGACGTGGACACCAG-3′, R-5′-AATCACCGTTCTCCGCTCAA-3′); PAX4 (NM_001366111.1; product length, 127 bp; F-5′-ATACCCGGCAGCAGATTGTG-3′, R-5′-AAGACACCTGTGCGGTAGTAA-3′); glucagon (GCG; NM_002054.5; product length, 108 bp; F-5′-AGCTGCCTTGTACCAGCATT-3′, R-5′-TGCTCTCTCTTCACCTGCTC-3′); insulin (INS; NM_001291897.1; product length, 130 bp; F-5′-CAATGCCACGCTTCTGC-3′, R-5′-TTCTACACACCCAAGACCCG-3′); neuronal differentiation 1 (NEUROD1; NM_002500.4; product length, 92 bp; F-5′-ATCAGCCCACTCTCGCTGTA-3′, R-5′-GCCCCAGGGTTATGAGACTAT-3′); NK6 homeobox 1 (NKX6.1; NM_006168.2; product length, 106 bp; F-5′-CGAGTCCTGCTTCTTCTTGG-3′, R-5′-GGGGATGACAGAGAGTCAGG-3′); ISL LIM homeobox 1 (ISL1; NM_002202.3; product length, 108 bp; F-5′-TCACGAAGTCGTTCTTGCTG-3′, R-5′-CATGCTTTGTTAGGGATGGG-3′); PAX6 (NM_001310161.1; product length, 101 bp; F-5′-TCCGTTGGAACTGATGGAGT-3′, R-5′-GTTGGTATCCGGGGACTTC-3′); SLC2A2 (NM_001278658.1; product length, 93 bp; F-5′-GACAGTGAAAACCAGGGTCC-3′, R-5′-TGTGCCACACTCACACAAGA-3′); glucokinase (GCK; NM_000162.5; product length, 97 bp; F-5′-CCTTCTTCAGGTCCTCCTCC-3′, R-5′-ATGCTGGACGACAGAGCC-3′); PCSK1 (NM_000439.5; product length, 101 bp; F-5′-CGGGTCATACTCAGAGGTCC-3′, R-5′-CTCTGGCTGCTGGCATCT-3′); proprotein convertase subtilisin/kexin type 2 (PCSK2; NM_002594.5; product length, 99 bp; F-5′-TTTCGGTCAAATCCTTCCTG-3′, R-5′-TGCAAAGGCCAAGAGAAGAC-3′); β-ACTIN (NM_001101.5; product length, 93 bp; F-5′-GTTGTCGACGACGAGC-3′, R-5′-GCACAGAGCCTCGCCTT-3′).
ELISA
Transfected cells were incubated with Krebs-Ringer buffer (KRB) medium containing 2.8 or 20 mM glucose (Beijing Maichen Science and Technology) at 37°C for 3 h. The KRB medium was collected for detection of secreted insulin. The remaining cells were scraped off in 35% ethanol solution containing 0.18 M hydrochloric acid. The cells were then homogenized by ultrasound (20 kHZ, ultrasound utilized for 10 sec with 60 sec intervals. The procedure was repeated 6 times) and put under vortex in a shaker (100 times/min) at 4°C overnight. The homogenate was centrifuged at 300 × g for 30 min at 4°C. The supernatant was collected to determine the intracellular insulin content and insulin secretion was normalized to it. The insulin assay was performed by using the commercial ELISA kit.
Statistical analysis
Values are expressed as the mean ± standard deviation. Student's t-test was used to determine the statistical significance of differences between two groups, and for more than two groups, one-way analysis of variance followed by a least-significant difference post-hoc test was used. P<0.05 was considered to indicate statistical significance. All data were analyzed by SPSS 20.0 (IBM Corp.).
Results
Morphology of cultured HuMSCs and cell transfection
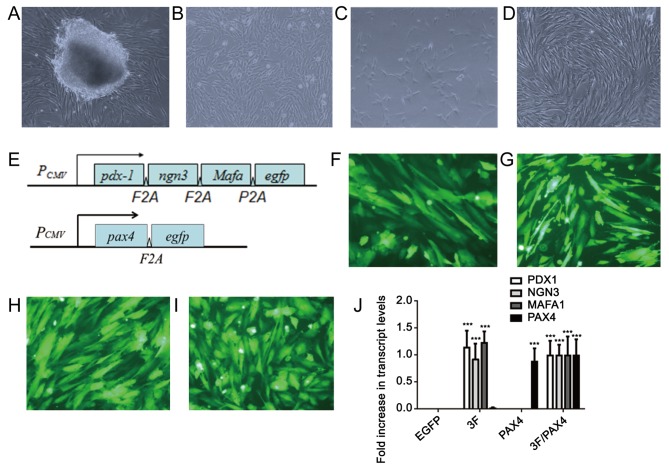
After the pieces of umbilical cord tissues had been cultured for 5–7 days, cells that migrated out from the surrounding tissues were fibroblast-like (Fig. 1A). After culture for 10–14 days, the cells reached 80–90% confluence and were passaged (Fig. 1B). After the second generation, the cells grew rapidly and were subjected to subculture once every 3–5 days. In the 3rd-5th generation, cells had a stable fibroblast-like morphology (Fig. 1C and D), and a previous study by our group demonstrated that these cells highly expressed adult stem cell markers, including CD29, CD44 and CD59 (2). However, after the 10th generation, the cells tended to grow slowly and become wide. Therefore, cells in the 3rd-5th generation were used in the present study. A diagram of the Ad-3F and Ad-Pax4 construct is presented in Fig. 1E. The results of EGFP immunofluorescence revealed that the adenovirus plasmid transfection efficiency was >80% (Fig. 1F-I). Furthermore, the results from RT-qPCR suggested that the transfected genes were efficiently expressed in HuMSCs (Fig. 1J).
Figure 1.
Morphology of cultured HuMSCs and cell transfection. (A) Primary culture of HuMSCs at day 7. Fibroblasts of HuMSCs migrated out of the tissue block. (B-D) Fibroblast-like HuMSCs of the (B) 1st, (C) 3rd and (D) 5th passage (magnification, ×100). (E) Diagram of the Ad-3F and Ad-Pax4 constructs. (F-I) EGFP immunofluorescence of HuMSCs transfected with (F) Ad-EGFP, (G) Ad-Pax4, (H) Ad-3F and (I) Ad-Pxa4/Ad-3F at 48 h (magnification, ×200). (J) Expression of genes, including PDX1, NGN3, MAFA and PAX4, in HuMSCs transfected with Ad-EGFP, Ad-Pax4, Ad-3F and Ad-Pax4/Ad-3F at 48 h. ***P<0.001 vs. EGFP. Pdx1, pancreatic and duodenal homeobox 1; MafA, MAF bZIP transcription factor A; Ngn3, neurogenin 3; Pax4, paired box 4; Ad-Pax4, adenovirus expressing Pax4; Ad-3F, Ad-Pdx1/MafA/Ngn3; HuMSCs, human umbilical cord mesenchymal stem cells; PCMV, cytomegalovirus plasmid; EGFP, enhanced green fluorescence plasmid.
Effect of Ad-3F in combination with Ad-Pax4 on the differentiation of HuMSCs to pβLCs by assessing crucial genes associated with differentiation. To investigate whether Ad-Pax4 further promotes the differentiation of Ad-3F-transfected HuMSCs to pβLCs, a panel of genes involved in converting the progenitors of pβCs into pβCs were detected, including NKX6-1, SLC2A2, GCK, PCSK1, NEUROD1, ISL1, PAX6 and PCSK2. The results demonstrated that compared with that in the control group, transfection with Ad-Pax4 alone had little effect on the expression of the above-mentioned genes, while transfection with Ad-3F or Ad-3F/Pax-4 increased the expression of these genes. Of note, Ad-Pax4 further promoted the expression of NKX6-1, SLC2A2, GCK and PCSK1 in Ad-3F-transfected cells (Fig. 2).
Figure 2.
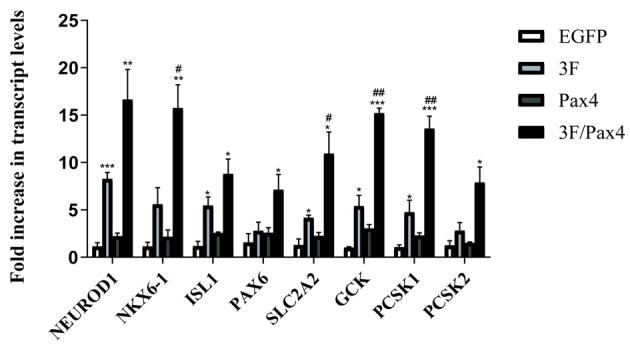
Effect of 3F plasmid in combination with Pax4 overexpression on the expression of genes associated with the differentiation of human umbilical cord mesenchymal stem cells to pancreatic β-like cells. The genes assessed included NKX6-1, SLC2A2, GCK, PCSK1, NEUROD1, ISL1, PAX6 and PCSK2. Values are expressed as the mean ± standard deviation (n=5). *P<0.05, **P<0.01, ***P<0.001 vs. EGFP group; #P<0.05, ##P<0.01 vs. 3F group. Groups: EGFP, cells transfected with EGFP plasmid; 3F, cells transfected with Pdx1/MafA/Ngn3 plasmid; Pax4, cells transfected with Pax4 plasmid; 3F/Pax4, cells transfected with Pdx1/MafA/Ngn3 plasmid and Pax4 plasmid. Pdx1, pancreatic and duodenal homeobox 1; MafA, MAF bZIP transcription factor A; Ngn3, neurogenin 3; Pax4, paired box 4; EGFP, enhanced green fluorescence plasmid; GCK, glucokinase; NKx6.1, NK6 homeobox 1; SLC2A2, glucokinase; PCSK1, proprotein convertase subtilisin/kexin type 1; NEUROD1, neuronal differentiation 1; ISL1, ISL LIM homeobox 1; PCSK2, proprotein convertase subtilisin/kexin type 2.
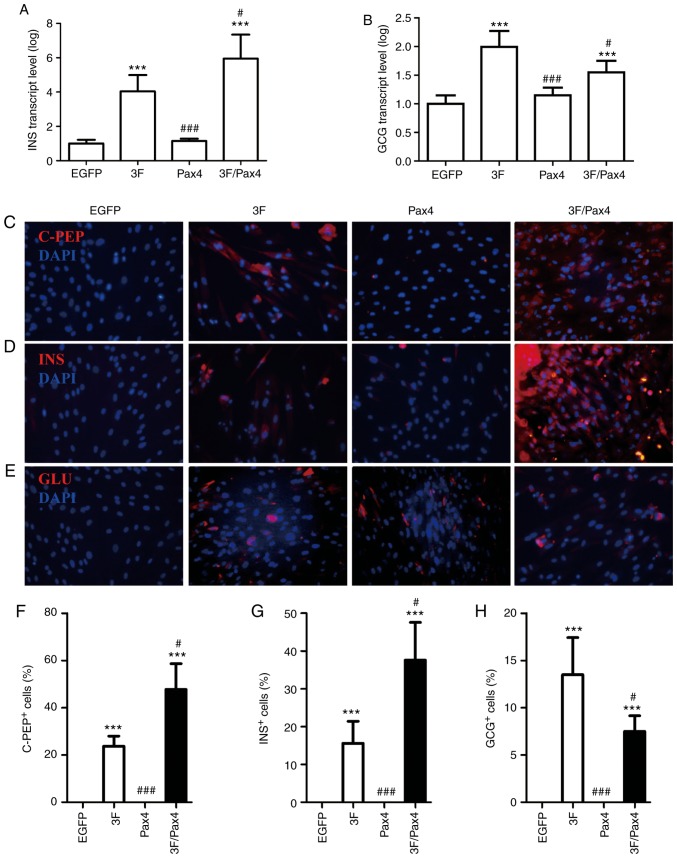
Effect of Ad-3F in combination with Ad-Pax4 on the expression of C-peptide, insulin and glucagon. To investigate the effect of Ad-3F in combination with Ad-Pax4 on the expression of C-peptide, insulin and glucagon, RT-qPCR and immunofluorescence analyses were performed. The RT-qPCR results indicated that compared with that in the control group, the gene expression of INS and GCG was significantly enhanced in the Ad-3F and Ad-3F/Pax-4 group (Fig. 3A and B). Compared with that in the Ad-3F group, the gene expression of INS was significantly increased, while that of GCG was significantly reduced in the Ad-3F/Pax-4 group (Fig. 3A and B). The immunofluorescence results indicated that compared with those in the control group, the glucagon-, insulin- and C-peptide-positive cells were significantly increased in the Ad-3F and Ad-3F/Pax4 groups (Fig. 3B-H). Compared with those in the Ad-3F group, insulin- and C-peptide-positive cells were significantly increased, while glucagon-positive cells were significantly reduced in Ad-3F/Pax4 group (Fig. 3B-H). Furthermore, the differentiation efficiency of HuMSCs co-transfected with Ad-3F and Ad-PAX4 into pβLCs was 30–40%, as reflected by the insulin- or C-peptide-positive cells (Fig. 3C, D, F and G).
Figure 3.
Effect of 3F plasmid in combination with Pax4 overexpression on the expression of C-PEP, INS and glucagon. (A and B) Gene expression of (A) INS and (B) GCG in the different groups compared with the EGFP group. (C-E) Immunofluorescence detection of (C) C-PEP (red), (D) INS and (E) GCG (red) (magnification, ×200). (F-H) Quantification of (F) C-PEP- (G) INS- and (H) GCG-positive cells from C-E, respectively. Values are expressed as the mean ± standard deviation (n=5). ***P<0.001 vs. EGFP group; #P<0.05, ###P<0.001 vs. 3F group. Groups: EGFP, cells transfected with EGFP plasmid; 3F, cells transfected with Pdx1/MafA/Ngn3 plasmid; Pax4, cells transfected with Pax4 plasmid; 3F/Pax4, cells transfected with Pdx1/MafA/Ngn3 plasmid and Pax4 plasmid. Pdx1, pancreatic and duodenal homeobox 1; MafA, MAF bZIP transcription factor A; Ngn3, neurogenin 3; Pax4, paired box 4; EGFP, enhanced green fluorescence plasmid; C-PEP, C-peptide; INS, insulin; GCG, glucagon.
Effect of Ad-3F in combination with Ad-Pax4 on glucose-stimulated insulin secretion (GSIS). To investigate the effect of Ad-3F in combination with Ad-Pax4 on GSIS, the cells were stimulated with low (2.8 mM) and high (20 mM) glucose. The results suggested that compared with low glucose, the insulin secretion in cells stimulated with high glucose was significantly increased in the Ad-3F and Ad-3F/Pax4 groups (Fig. 4). Compared with that in the 3F group, the insulin secretion was significantly increased in the Ad-3F/Pax-4 group (Fig. 4).
Figure 4.
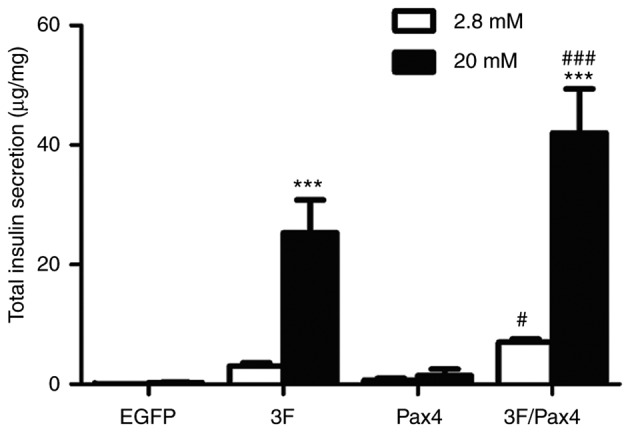
Effect of 3F plasmid in combination with Pax4 overexpression on insulin secretion in response to glucose. Transfected cells were stimulated either with low-glucose or high-glucose, and insulin secretion was determined by ELISA. Experiments were performed 4 days after transduction. Values are expressed as the mean ± standard deviation (n=5). ***P<0.001 vs. low-glucose group; #P<0.05, ###P<0.001 vs. 3F group. Groups: EGFP, cells transfected with EGFP plasmid; 3F, cells transfected with Pdx1/MafA/Ngn3 plasmid; Pax4, cells transfected with Pax4 plasmid; 3F/Pax4, cells transfected with Pdx1/MafA/Ngn3 plasmid and Pax4 plasmid. Pdx1, pancreatic and duodenal homeobox 1; MafA, MAF bZIP transcription factor A; Ngn3, neurogenin 3; Pax4, paired box 4; EGFP, enhanced green fluorescence plasmid.
Discussion
In the present study, cultured HuMSCs were successfully differentiated into functional pβLCs by introducing 4 transcription factors, Pdx1, Ngn3, MafA and Pax4. The results demonstrated that, compared to Ad-3F-transfected cells, those co-transfected with Ad-Pxa4 and Ad-3F expressed higher levels of insulin, c-peptide and genes expressed in pancreatic β-precursor cells, and secreted more insulin in response to glucose. Furthermore, Ad-Pax4 significantly decreased the expression of glucagon in Ad-3F-transfected HuMSC.
The embryonic pancreas development is regulated by the interactions of numerous signaling pathways that contribute to the proper initiation of the sequential expression of transcription factors (22–25). Several transcription factors have been demonstrated to be necessary for the development of a functional pancreas, including Pdx1, Ngn3 and MafA. Pdx1, a member of the ParaHox protein family, has an important role in driving the formation of the pancreatic bud (26,27). Ngn3, a member of the basic helix-loop-helix transcription factors, is required for the formation of a common precursor for the four pancreatic endocrine cell types. MafA, an eye-specific member of the Maf family, has been demonstrated to have a vital role in regulating insulin gene expression (28–30). It has been previously reported that the 3 transcription factors combined were able to reprogram other cell types into functional pβLCs (17). Pax4 has been demonstrated to have a critical role in converting the pancreatic β-precursors to pancreatic endocrine cell types (31). In a previous study, exocrine tissue was effectively converted to islet-like cells by using a cocktail of transcription factors, Pdx1, Ngn3, MafA and Pax4 (32). Consistent with that, the present study indicated that overexpression of Pax4 increased the expression of INS, C-PEP and genes expressed in pancreatic β-precursor cells, and enhanced glucose-stimulated insulin secretion (GSIS) in Ad-3F transfected HuMSCs, indicating a synergetic effect of Pax4 and Pdx1/Ngn3/MafA on converting HuMSCs to functional pβLCs.
The role of the transcriptional factors Ngn3, Pdx1, MafA and Pax4 in controlling the development of a functional pancreas has been well established. Ngn3 facilitates the formation of pancreatic endocrine cells. Pax4 directs the cell differentiation towards pβCs. Pdx1 and MafA are necessary for maintaining mature pβC function (31). The mechanism of Pax4 in promoting pβC formation may rely on its role to suppress aristaless related homeobox genes, and overexpression of Pax4 was sufficient to convert pancreatic α-cells to β-cells (20,33). In the present study, it was indicated that overexpression of Pax4 decreased glucagon expression in HuMSCs transfected with Ad-3F, suggesting that Pax4 restricts the differentiation of HuMSCs into paCs. However, glucagon-positive cells still existed in HuMSCs co-transfected with Ad-3F and Ad-Pax4.
NKX6-1 has a key role in regulating insulin secretion and β-cell proliferation (34). Overexpression of NKX6-1 in mature β-cells enhanced proliferation and GSIS (35). It has been demonstrated that Glu2 was involved in controlling insulin secretion (36,37). GCK, a key regulator of glucose metabolism, is crucial for GSIS and overexpression of GCK in β-cells restored GSIS in a mouse model of high-fat diet-induced diabetes (38). PCSK1 is necessary for processing pro-insulin (39). In the present study, it was indicated that Pax4 promoted the expression of all these genes in the Ad-3F-transfected cells. In line with this, overexpression of Pax4 increased GSIS of HuMSCs transfected with Ad-3F. These results indicate that Pax4 synergistically acts with Pdx1, Ngn3 and MafA to promote the expression of these genes, thereby improving GSID. However, the underlying mechanisms remain to be elucidated.
In conclusion, the present study demonstrated that the Pax4 gene was able to synergistically act with the transcription factors Pdx1, Ngn3 and MafA to convert HuMSCs to functional pβCs. HuMSCs may be potential seed cells for generating functional pβCs for the therapy of diabetes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81671525 and 81070478), the Science and Technology Project from the Science Technology and Innovation Committee of Shenzhen Municipality (grant no. JCYJ20170817170110940) and the Sanming Project of Medicine in Shenzhen (grant no. SZSM201512033).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
TZ, JS and LM designed the study and performed the experiments. TZ, HW, TW and JY collected the data. CW, HJ and SJ analyzed the data. TZ and HW prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the ethics committees of Shanghai Children's Hospital (Shanghai, China) and of the Second Affiliated Hospital of Shantou University Medical College (Shantou, China). Written informed consent was obtained from the maternal donors and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stanekzai J, Isenovic ER, Mousa SA. Treatment options for diabetes: Potential role of stem cells. Diabetes Res Clin Pract. 2012;98:361–368. doi: 10.1016/j.diabres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Bose B, Shenoy SP, Konda S, Wangikar P. Human embryonic stem cell differentiation into insulin secreting β-cells for diabetes. Cell Biol Int. 2012;36:1013–1020. doi: 10.1042/CBI20120210. [DOI] [PubMed] [Google Scholar]
- 3.Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166:1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Yang Y, Ho G, Lin X, Wu W, Li W, Lin L, Feng X, Huo X, Jiang J, et al. Programming of human umbilical cord mesenchymal stem cells in vitro to promote pancreatic gene expression. Mol Med Rep. 2013;8:769–774. doi: 10.3892/mmr.2013.1598. [DOI] [PubMed] [Google Scholar]
- 5.Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: Adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–1486. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto T, Ono H, Saito Y. Electron microscopic histochemical studies on the localization of hyaluronic acid in Wharton's jelly of the human umbilical cord. Nihon Sanka Fujinka Gakkai Zasshi. 1996;48:501–507. (In Japanese) [PubMed] [Google Scholar]
- 7.Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011;21:253–261. doi: 10.1111/j.1365-3148.2011.01076.x. [DOI] [PubMed] [Google Scholar]
- 8.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 9.Kadivar M, Khatami S, Mortazavi Y, Shokrgozar MA, Taghikhani M, Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH. Human umbilical cord Wharton's Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl) 2005;118:1987–1993. [PubMed] [Google Scholar]
- 11.Bao CS, Li XL, Liu L, Wang B, Yang FB, Chen LG. Transplantation of Human umbilical cord mesenchymal stem cells promotes functional recovery after spinal cord injury by blocking the expression of IL-7. Eur Rev Med Pharmacol Sci. 2018;22:6436–6447. doi: 10.26355/eurrev_201810_16056. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Chen Z, Zhao X, Pan F, Cai M, Wang T, Zhang H, Lu JR, Lei M. Sphingosine-1-phosphate promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions. J Biomed Sci. 2011;18:37. doi: 10.1186/1423-0127-18-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang P, Lin LM, Wu XY, Tang QL, Feng XY, Lin GY, Lin X, Wang HW, Huang TH, Ma L. Differentiation of human umbilical cord Wharton's jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem. 2010;109:747–754. doi: 10.1002/jcb.22453. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Melton DA. Extreme makeover: Converting one cell into another. Cell Stem Cell. 2008;3:382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Cavelti-Weder C, Zumsteg A, Li W, Zhou Q. Reprogramming of pancreatic Acinar cells to functional beta cells by in vivo transduction of a polycistronic construct containing Pdx1, Ngn3, MafA in mice. Curr Protoc Stem Cell Biol. 2017;40:A.10.1–4A.10.12. doi: 10.1002/cpsc.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T, Cavelti-Weder C, Caballero F, Lysy PA, Guo L, Sharma A, Li W, Zhou Q, Bonner-Weir S, Weir GC. Reprogramming mouse cells with a pancreatic duct phenotype to insulin-producing β-like cells. Endocrinology. 2015;156:2029–2038. doi: 10.1210/en.2014-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinci E, Banga A, Tungatt K, Segal J, Eberhard D, Dutton JR, Slack JM. Reprogramming of various cell types to a beta-like state by Pdx1, Ngn3 and MafA. PLoS One. 2013;8:e82424. doi: 10.1371/journal.pone.0082424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 19.Druelle N, Vieira A, Shabro A, Courtney M, Mondin M, Rekima S, Napolitano T, Silvano S, Navarro-Sanz S, Hadzic B, et al. Ectopic expression of Pax4 in pancreatic delta cells results in beta-like cell neogenesis. J Cell Biol. 2017;216:4299–4311. doi: 10.1083/jcb.201704044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Fava GE, Wang H, Mauvais-Jarvis F, Fonseca VA, Wu H. PAX4 gene transfer induces α-to-β cell phenotypic conversion and confers therapeutic benefits for diabetes treatment. Mol Ther. 2016;24:251–260. doi: 10.1038/mt.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Hald J, Sprinkel AE, Ray M, Serup P, Wright C, Madsen OD. Generation and characterization of Ptf1a antiserum and localization of Ptf1a in relation to Nkx6.1 and Pdx1 during the earliest stages of mouse pancreas development. J Histochem Cytochem. 2008;56:587–595. doi: 10.1369/jhc.2008.950675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L, Han L, Li Y, Zhao J, He P, Zhang W. Neurogenin 3-directed cre deletion of Tsc1 gene causes pancreatic acinar carcinoma. Neoplasia. 2014;16:909–917. doi: 10.1016/j.neo.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babu DA, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx1 and BETA2/NeuroD1 participate in a transcriptional complex that mediates short-range DNA looping at the insulin gene. J Biol Chem. 2008;283:8164–8172. doi: 10.1074/jbc.M800336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L, Sosa-Pineda B. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev Biol. 2004;266:178–189. doi: 10.1016/j.ydbio.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- 27.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;277:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- 30.Sheets TP, Park KE, Park CH, Swift SM, Powell A, Donovan DM, Telugu BP. Targeted mutation of NGN3 gene disrupts pancreatic endocrine cell development in pigs. Sci Rep. 2018;8:3582. doi: 10.1038/s41598-018-22050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napolitano T, Avolio F, Courtney M, Vieira A, Druelle N, Ben-Othman N, Hadzic B, Navarro S, Collombat P. Pax4 acts as a key player in pancreas development and plasticity. Semin Cell Dev Biol. 2015;44:107–114. doi: 10.1016/j.semcdb.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Lima MJ, Muir KR, Docherty HM, McGowan NW, Forbes S, Heremans Y, Heimberg H, Casey J, Docherty K. Generation of functional beta-like cells from human exocrine pancreas. PLoS One. 2016;11:e156204. doi: 10.1371/journal.pone.0156204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- 34.Ray JD, Kener KB, Bitner BF, Wright BJ, Ballard MS, Barrett EJ, Hill JT, Moss LG, Tessem JS. Nkx6.1-mediated insulin secretion and β-cell proliferation is dependent on upregulation of c-Fos. FEBS Lett. 2016;590:1791–1803. doi: 10.1002/1873-3468.12208. [DOI] [PubMed] [Google Scholar]
- 35.Fueger PT, Schisler JC, Lu D, Babu DA, Mirmira RG, Newgard CB, Hohmeier HE. Trefoil factor 3 stimulates human and rodent pancreatic islet beta-cell replication with retention of function. Mol Endocrinol. 2008;22:1251–1259. doi: 10.1210/me.2007-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillam MT, Dupraz P, Thorens B. Glucose uptake, utilization and signaling in GLUT2-null islets. Diabetes. 2000;49:1485–1491. doi: 10.2337/diabetes.49.9.1485. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JH, Ogawa A, Chen L, Orci L, Newgard CB, Alam T, Unger RH. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990;250:546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- 38.Lu B, Kurmi K, Munoz-Gomez M, Jacobus Ambuludi EJ, Tonne JM, Rakshit K, Hitosugi T, Kudva YC, Matveyenko AV, Ikeda Y. Impaired β-cell glucokinase as an underlying mechanism in diet-induced diabetes. Dis Model Mech. 2018;11(pii):dmm033316. doi: 10.1242/dmm.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivoli M, Caulfield TR, Martinez-Mayorga K, Johnson AT, Jiao GS, Lindberg I. Inhibition of prohormone convertases PC1/3 and PC2 by 2,5-dideoxystreptamine derivatives. Mol Pharmacol. 2012;81:440–454. doi: 10.1124/mol.111.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.