Abstract
Short-term efficacy and safety of prednisone in herpes zoster and the effect on IL-6 and IL-10 were investigated. A total of 125 patients (aged 40–70 years) with acute infective herpes zoster who were admitted to Daqing Oilfield General Hospital were selected and divided into 3 groups according to different treatment methods: low-dose (n=44), middle-dose (n=42) and high-dose (n=39) groups. The therapeutic effect, visual analogue scale (VAS) pain score, pain relieving and disappearing time, herpes stopping and disappearing time, incrustation and decrustation time, and incidence of adverse reactions in the three groups were recorded. The changes of IL-6 and IL-10 levels in the peripheral blood of patients before and after treatment were detected by enzyme-linked immunosorbent assay (ELISA) in order to analyze their relationship with pain degree and the time of symptom remission and subsidence. There were no significant differences in cure rate, significant effective rate, effective rate, ineffective rate and total effective rate among the three groups (P>0.05). The pain relieving and disappearing time in the middle-dose group were shorter than those in the low- and high-dose groups (P<0.05). The levels of IL-6 and IL-10 showed no statistical differences in the 3 groups before treatment (P>0.05). Pearson correlation analysis showed that IL-6 was positively correlated with VAS pain score, pain relieving and disappearing time, herpes stopping and disappearing time, incrustation and decrustation time (P<0.05), while IL-10 was negatively correlated with the above indicators (P<0.05). In conclusion, middle-dose prednisone has similar short-term efficacy to high-dose prednisone in the treatment of herpes zoster, but with lower complication and higher safety. IL-6 and IL-10 are closely related to the pain degree and the time of symptom remission and subsidence, which may provide a reference for clinical evaluation of the therapeutic effect of patients with herpes zoster.
Keywords: prednisone, herpes zoster, therapeutic effect, IL-6, IL-10
Introduction
Herpes zoster is caused by reactivation of latent varicella-zoster virus (VZV) in ganglia and usually occurs decades after the primary infection. It is mainly characterized by vesicular rash distributed in unilateral skin and is often accompanied with pain (1,2). Herpes zoster is common in individuals aged over 50 years because of their declined immune function. Approximately 90% of adults are infected with VZV and are at risk of developing herpes zoster. The overall incidence rate of herpes zoster is 2.0–4.6 cases/1,000 person/year, and that in patients aged 80 or above is 10.0–12.8 cases/1,000 person/year. Moreover, the incidence rate of post herpetic neuralgia increases and the symptoms are more severe with age (3–7).
Currently, corticosteroid-containing regimens are the main treatments for herpes zoster. Corticosteroids, with strong anti-inflammatory effects, may effectively improve the levels of inflammatory factors such as IL-6 and IL-10, reduce nerve injury, and promote the regression of blisters as well as improve nerve pain (8). However, the dosage has been controversial among clinical workers in recent years. Levin (9) reported that corticosteroids caused immunosuppression, thus increasing or even aggravating the infection of patients with herpes zoster. A study also questioned that corticosteroids had no capacity to prevent post-herpetic neuralgia in patients with acute infective herpes zoster (10). However, in the study by Harpaz et al (1), it was found that the use of low to middle doses of short-acting systemic corticosteroids did not cause immunosuppression. A study of the respiratory tract also found that the use of high-dose glucocorticoid combined with cytochrome P450 enzyme inhibitor was not an important risk factor for the occurrence of herpes zoster, suggesting that glucocorticoid-induced immunosuppression was not severe (11).
In order to solve the above contradictions, a prospective analysis was carried out to explore the therapeutic effect and safety of different doses of prednisone in herpes zoster, so as to provide reference for clinical treatment.
Patients and methods
Study objects
A total of 125 patients (aged 40–70 years) with acute infective herpes zoster who were admitted to Daqing Oilfield General Hospital from June 2016 to September 2017 were selected and divided into 3 groups according to different treatment methods: low-dose (n=44), middle-dose (n=42) and high-dose (n=39) groups. Inclusion criteria were: Patients meeting the diagnostic criteria for herpes zoster as per the Expert Consensus on Diagnosis and Treatment of Neuropathic Pain, established by the International Association for the Study of Pain (IASP) in 2008; patients with complete clinical data; patients with no past or present tumors; patients with no clinical features of motor nerve involvement; patients with no corticosteroid use within 3 months. Exclusion criteria were: patients with: history of drug allergy and abnormal bleeding; hypertension, diabetes, systemic inflammatory response, autoimmune diseases; severe defects of liver, kidney and heart lung functions; pregnant or nursing women; and patients with mental and cognitive dysfunctions. This study was approved by the Ethics Committee of the Daqing Oilfield General Hospital, and the patients and their families signed informed consent forms.
Methods
The patients were treated with famciclovir at a dose of 0.25 g/time, three times a day. In addition, according to medication guidelines for glucocorticoids and instructions for prednisone, 10–40 mg/day is generally recommended as the dose of prednisone, and it can be increased to 60 mg/day, if necessary. Therefore, we divided the patients into three groups: low-, middle- and high-dose groups. The patients in the low-dose group were given prednisone 15 mg/day, orally, three times a day, and the patients in the the middle-dose group were given prednisone 25 mg/day, 5 times a day, whereas those in the high-dose group were given prednisone 40 mg/day, 4 times a day, for two weeks.
Outcome measures
The therapeutic effect, visual analogue scale (VAS) pain score, pain relieving and disappearing time, herpes stopping and disappearing time, incrustation and decrustation time, and incidence of adverse reactions in the three groups were recorded. The changes of IL-6 and IL-10 levels in the peripheral blood of patients before and after treatment were detected by enzyme-linked immunosorbent assay (ELISA) in order to analyze their relationship with pain degree and the time of symptom remission and subsidence.
Efficacy evaluation criteria
Referring to Clinical Dermatology (12): cure: Herpes scab shedding area was >90%, and the pain did not affect life and sleep quality; significant effective: Herpes scab shedding area was ≥60% - <90%, VAS pain score decreased by >50% compared with that before treatment; effective: Herpes scab shedding area was ≥30% - <60%, and the pain score of VAS decreased by >25% compared with that before treatment; ineffective: Herpes scab shedding area was <30%, VAS pain score had no obvious change or even increased compared with that before treatment. The total effective rate of treatment was calculated as: (cure + significant effective + effective)/total number ×100%.
ELISA
Both IL-6 and IL-10 were detected by ELISA, and the detection kits were purchased from Wuhan Elabscience Biotechnology Co., Ltd., with the item numbers of E-EL-H0102c and E-EL-H0103c, respectively. Refer to the kit instructions for specific detection steps.
Statistical analysis
SPSS 19.0 (Asia Analytics Formerly SPSS) was used to analyze the data of this study. Enumeration data were expressed as rates, and the comparison of rates was carried out using the χ2 test. Measurement data were expressed as mean ± SD. Comparisons of the three groups were carried out using analysis of variance (ANOVA) and the least significant difference (LSD) test was used for pairwise comparison afterwards. Comparisons before and after treatment in the group employed the paired t-test. Pearson correlation analysis was used to analyze the correlation between the levels of IL-6 and IL-10 before and after treatment and VAS pain score, as well as the difference between IL-6 and IL-10 before and after treatment and the pain relieving and disappearing time, herpes stopping and disappearing time, the time of incrustation and decrustation. P<0.05 indicated statistical significance.
Results
General information
The study comprised 44 patients in the low-dose group, including 25 males and 19 females, with an age of 52.25±8.36 years; 42 patients in the middle-dose group, including 23 males and 19 females, with an age of 53.71±7.83 years; 39 patients in the high-dose group, including 21 males and 18 females, with an age of 50.62±8.04 years. Therefore, there was no statistical difference in sex ratio and age among the three groups (P>0.05). Moreover, there were no statistical differences in the course of disease, herpes distribution, and other basic data among the three groups (P>0.05) (Table I).
Table I.
General information.
| Characteristics | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | χ2/t value | P-value |
|---|---|---|---|---|---|
| Sex [n (%)] | 0.079 | 0.961 | |||
| Male | 25 (56.82) | 23 (54.76) | 21 (53.85) | ||
| Female | 19 (43.18) | 19 (45.24) | 18 (46.15) | ||
| Age (years) | 52.25±8.36 | 53.71±7.83 | 50.62±8.04 | 1.477 | 0.232 |
| Course of disease (days) | 3.83±1.91 | 3.95±2.32 | 4.02±2.11 | 0.086 | 0.918 |
| Distribution [n (%)] | 2.907 | 0.940 | |||
| Limbs | 4 (9.09) | 3 (7.14) | 4 (10.26) | ||
| Waist and abdomen | 10 (22.73) | 9 (21.43) | 8 (20.51) | ||
| Chest and back | 19 (43.18) | 17 (40.48) | 15 (38.46) | ||
| Head and face | 10 (22.73) | 13 (30.95) | 12 (30.77) | ||
| Perineum | 1 (2.27) | 0 (0.00) | 0 (0.00) | ||
| Vaccination rate | 4 (9.09) | 5 (11.90) | 4 (10.26) | 0.184 | 0.912 |
| Immune function [n (%)] | 0.709 | 0.701 | |||
| Normal | 30 (68.18) | 28 (66.67) | 27 (69.23) | ||
| Suppressed | 14 (31.82) | 14 (33.33) | 12 (30.77) |
Clinical efficacy
The cure rate, significant effective rate, effective rate and ineffective rate in the three groups had no statistical difference (P>0.05), neither the total effective rate (P>0.05) (Table II).
Table II.
Clinical efficacy.
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | χ2/t value | P-value |
|---|---|---|---|---|---|
| Cure | 10 (22.73) | 8 (19.05) | 8 (20.51) | 0.180 | 0.914 |
| Significant effective rate | 12 (27.27) | 13 (30.95) | 10 (25.64) | 0.301 | 0.860 |
| Effective rate | 15 (34.09) | 12 (28.57) | 12 (30.77) | 0.310 | 0.857 |
| Ineffective rate | 7 (15.91) | 9 (21.43) | 9 (23.08) | 0.745 | 0.689 |
| Total effective rate | 37 (84.09) | 33 (78.57) | 30 (76.92) | 0.745 | 0.689 |
VAS pain score
There were no significant differences in VAS pain scores among the three groups before treatment (P>0.05), while the scores were all decreased after treatment (P<0.05). Moreover, the score in the middle-dose group was lower than that in the low-dose group and the high-dose group (P<0.05), and there was no significant difference between the low-dose group and the high-dose group (P>0.05) (Table III).
Table III.
VAS pain score.
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | F value | P-value |
|---|---|---|---|---|---|
| Before treatment | 7.16±1.25 | 7.33±1.33 | 7.66±1.35 | 1.543 | 0.217 |
| After treatment | 3.42±1.42a | 2.26±1.15 | 3.38±1.52a | 9.695 | <0.001 |
| t value | 12.565 | 17.376 | 12.896 | ||
| P-value | <0.001 | <0.001 | <0.001 |
P<0.05 compared with the middle-dose group.
Pain relieving and disappearing time
The pain relieving and disappearing time in the middle-dose group were shorter than those in the low-dose group and the high-dose group (P<0.05). There were no statistical differences in pain relieving time between the low-dose group and the high-dose group (P>0.05), while the pain disappearing time in the high-dose group was shorter than that in the low-dose group (P<0.05) (Table IV).
Table IV.
Pain relieving and disappearing time (days).
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | F value | P-value |
|---|---|---|---|---|---|
| Pain relieving time | 3.1±1.5a | 2.2±1.1 | 2.8±1.3a | 5.193 | 0.007 |
| Pain disappearing time | 13.7±3.8a | 9.32±3.2 | 11.4±2.7a,b | 19.286 | <0.001 |
P<0.05 compared with the middle-dose group
P<0.05 compared with low-dose group.
Herpes stopping and disappearing time
The herpes stopping and disappearing time in the middle-dose group were shorter than those in the low-dose group and the high-dose group (P<0.05), and there were no statistical differences between the low-dose group and the high-dose group (P>0.05) (Table V).
Table V.
Herpes stopping and disappearing time (days).
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | F value | P-value |
|---|---|---|---|---|---|
| Herpes stopping time | 3.6±1.1a | 2.9±1.7 | 3.3±1.1a | 3.519 | 0.033 |
| Herpes disappearing time | 13.4±1.3a | 10.5±2.1 | 14.0±1.8a | 49.832 | <0.001 |
P<0.05 compared with the middle-dose group.
Incrustation and decrustation time
The incrustation time in the middle-dose group was shorter than that in the low-dose group and high-dose group (P<0.05), and there were no statistical differences between the low-dose group and the high-dose group (P>0.05). The decrustation time in the low-dose group and the middle-dose group was shorter than that in the high-dose group (P<0.05), and there were no statistical differences between the low-dose group and the middle-dose group (P>0.05) (Table VI).
Table VI.
Incrustation and decrustation time (days).
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | F value | P-value |
|---|---|---|---|---|---|
| Incrustation time | 4.8±0.7a | 3.2±1.1 | 4.2±0.8a | 35.889 | <0.001 |
| Decrustation time | 9.6±5.7 | 9.7±2.9 | 12.4±3.4a,b | 5.686 | 0.004 |
P<0.05 compared with the middle-dose group
P<0.05 compared with low-dose group.
Incidence of post herpetic neuralgia
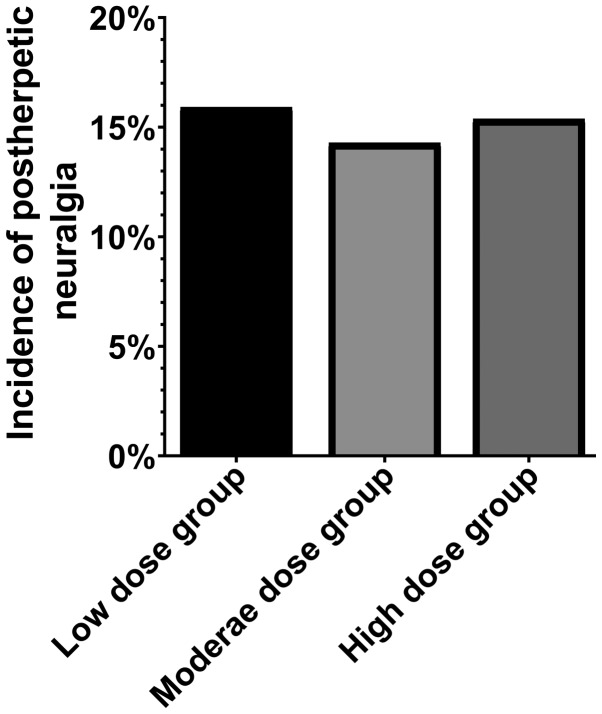
The incidence of post herpetic neuralgia in the low-, middle- and high-dose groups was 15.91% (7 cases), 14.29% (6 cases), 15.38% (6 cases), respectively. Therefore, there were no statistical differences in the incidence of post-herpetic neuralgia among the three groups (P>0.05) (Fig. 1).
Figure 1.
Incidence of post herpetic neuralgia. There were no significant differences in the incidence of post-herpetic neuralgia among the three groups.
Incidence of adverse reactions
There were no statistical differences in the total incidence of adverse reactions among the three groups (P>0.05). However, the incidence of mild edema in the high-dose group was higher than that in the low-dose group and the middle-dose group (P<0.05), and there were no statistical differences in the incidence of other adverse reactions (P>0.05) (Table VII).
Table VII.
Incidence of adverse reactions [n (%)].
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | χ2 value | P-value |
|---|---|---|---|---|---|
| Dizziness | 1 (2.27) | 1 (2.38) | 1 (2.56) | 0.008 | 0.996 |
| Sleepiness | 0 (0.00) | 1 (2.38) | 2 (5.13) | 2.321 | 0.313 |
| Mild edema | 0 (0.00) | 0 (0.00) | 3 (7.69)a,b | 6.778 | 0.034 |
| Stomach discomfort | 1 (2.27) | 2 (4.76) | 1 (2.56) | 0.504 | 0.777 |
| Total adverse reactions | 2 (4.55) | 4 (9.52) | 7 (17.95) | 4.038 | 0.133 |
P<0.05 compared with the middle-dose group
P<0.05 the compared with low-dose group.
Changes of IL-6 and IL-10 levels
The levels of IL-6 and IL-10 showed no statistical difference in the three groups before treatment (P>0.05). After treatment, the level of IL-6 decreased in the three groups (P<0.05), the middle-dose group and the high-dose group were lower than the low-dose group (P<0.05), and the high-dose group was lower than middle-dose group (P<0.05). By contrast, the level of IL-10 increased in the three groups (P<0.05), the middle-dose group and the high-dose group were higher than low-dose group (P<0.05), and the high-dose group was higher than the middle-dose group (P<0.05) (Table VIII).
Table VIII.
Changes of IL-6 and IL-10 levels.
| Variable | Low-dose group (n=44) | Middle-dose group (n=42) | High-dose group (n=39) | F value | P-value |
|---|---|---|---|---|---|
| IL-6 (pg/ml) | |||||
| Before treatment | 482.4±36.5 | 483.8±37.2 | 486.7±38.8 | 0.140 | 0.870 |
| After treatment | 308.7±28.3 | 226.3±21.8b | 118.7±21.7a,b | 635.062 | <0.001 |
| t value | 26.807 | 41.076 | 55.450 | ||
| P-value | <0.001 | <0.001 | <0.001 | ||
| IL-10 (µg/ml) | |||||
| Before treatment | 124.6±11.8 | 126.5±10.9 | 123.4±14.5 | 0.644 | 0.527 |
| After treatment | 142.5±21.2 | 168.9±20.8b | 211.6±24.5a,b | 101.621 | <0.001 |
| t value | 4.262 | 9.987 | 17.961 | ||
| P-value | <0.001 | <0.001 | <0.001 | ||
P<0.05 compared with the middle-dose group
P<0.05 compared with low-dose group.
Correlation analysis
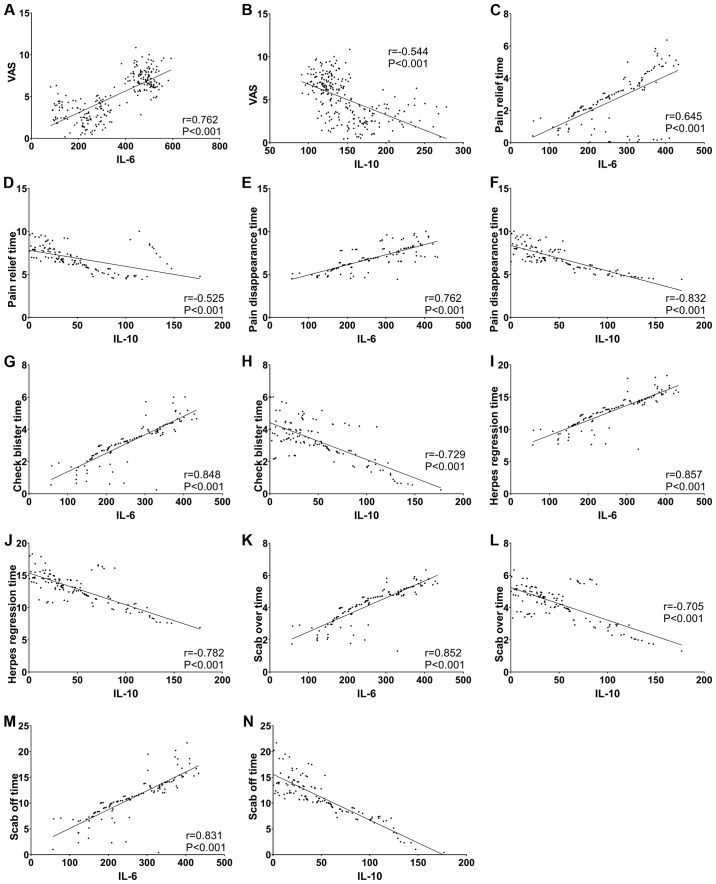
Pearson correlation analysis showed that IL-6 was positively correlated with VAS pain score, pain relieving and disappearing time, herpes stopping and disappearing time, and incrustation and decrustation time (all P<0.05), while IL-10 was negatively correlated with the above indicators (all P<0.05) (Fig. 2).
Figure 2.
Correlation analysis. IL-6 is positively correlated with VAS pain score, pain relieving and disappearing time, herpes stopping and disappearing time, incrustation and decrustation time (all P<0.05), while IL-10 is negatively correlated with the above indicators (all P<0.05).
Discussion
At present, there are few studies on the therapeutic effect and safety of different doses of corticosteroids in herpes zoster. However, previous studies found that the use of corticosteroids increased the risk of herpes zoster, with a risk ratio of 1.75 (13,14). Moreover, the risk increased with the increase of the dose, especially for patients whose dose exceeded 10 mg/day (13,14). Therefore, it is speculated that the immunosuppression of corticosteroids may affect the therapeutic effect and safety of patients with herpes zoster.
This study established three groups of patients receiving different doses of prednisone. We grouped the subjects by dose, and found that there were more patients treated with doses of 15, 25 and 40 mg/time. Relevant literature was also reviewed. The administered dose of prednisone was 30 mg/time in the study of Anderson and Janoff (15). By contrast, in the study of Clemmensen and Andersen (16), the dose was 60 mg/time initially, 30 mg/time after one week and 15 mg/time in the third week. Whitley et al (17) set the initial dose of prednisone at 45 mg/time, which was reduced to 30 mg/time after one week, and was discontinued in the third week. Therefore, wedivided the patients into the low-dose, middle-dose and high-dose groups according to relevant statistics. The results of the present study showed that there were no significant differences in the therapeutic efficacy among the three groups. However, the VAS score demonstrated that, the higher the dose of prednisone, the lower the VAS pain score of the patients after two weeks of treatment, and the greater the improvement in pain. Interestingly, we found that among the three doses, the middle-dose prednisone had the shortest pain relieving and disappearing time, herpes stopping and disappearing time, and incrustation and decrustation time. Only pain disappearing time and decrustation time in the high-dose group were shorter than those in the low-dose group. The high-dose group had the highest incidence of mild edema, while there was no mild edema in the low-dose group and the middle-dose group. Moreover, there was no difference for the incidence of post-herpetic neuralgia in the three groups of patients with herpes zoster, which was similar to the results reported in previous studies that corticosteroids had no ability to prevent the occurrence of post-herpetic neuralgia (18,19).
From the above results, middle-dose prednisone can achieve the best therapeutic effect and higher safety in treating herpes zoster. In studies on different doses of corticosteroid therapy, Rygård et al (20) revealed that small-dose corticosteroid effectively reduced the shock duration, mechanical ventilation and ICU stay in treating septic shock. Despite increased adverse reactions, it did not affect the short-term and long-term mortality of patients. Sugimoto et al (21) reported that for patients who need lung transplantation after hematopoietic stem cell transplantation, preoperative low-dose corticosteroid was more effective than high-dose corticosteroid in reducing postoperative complications. Izquierdo and Cosio (22) demonstrated a similar point of view; that for patients with chronic obstructive pulmonary diseases, low-dose corticosteroid showed significantly higher efficacy and lower incidence of corticosteroid-related pneumonia than high-dose corticosteroid. The above studies reported that it was beneficial to reduce the use of corticosteroids in the treatment of the diseases. It is well known that inhaled corticosteroids are more effective than oral ones. In the study by Daley-Yates (23), it was reported that the binding affinity of glucocorticoid receptor was exponentially related to the dose of inhaled glucocorticoids, suggesting that low doses of inhaled corticosteroids also provided the same effective molecules to achieve therapeutic effects similar to those at high doses, and reduced systemic exposure and improved the therapeutic index. However, this treatment is not advocated in the treatment of herpes zoster, but a historical equivalent dose method.
Corticosteroids are widely used for their powerful anti-inflammatory effects (24). Inflammation and changes in cytokines accompany the process of herpes zoster (25), and the abnormal secretions of inflammatory cytokines and immunoglobulins caused by humoral immunity and cellular immune response disorders, such as IL-6 and IL-10, are related to the occurrence of post herpetic neuralgia in acute herpes zoster (26). Our analysis revealed that after treatment, IL-6 and IL-10 in the three groups of patients were effectively improved, and the improvement degree was dose-dependent. This study analyzed the relationship between IL-6, IL-10 and the pain degree and the duration of symptom improvement, and the results showed that IL-6 was positively correlated with VAS pain score, pain relieving and disappearing time, herpes stopping and disappearing time, incrustation and decrustation time, while IL-10 was negatively correlated with them. Huang et al (27) reported that treadmill exercise and therapeutic ultrasound controlled pain associated with nerve injury in rats by improving IL-6 and IL-10. In addition, Leung et al (28) also believed that exogenous administration of IL-10 reduced hyperalgesia in mice. These studies verified the accuracy of some of our results, which increased the credibility of our other results.
There are some deficiencies in this study. Firstly, prospective analysis was used in this study, so there were inevitably some biases in the inclusion of patients, as well as in the treatment methods. Secondly, the subjects included had a small size and narrow scope, which may increase some accidental errors. Therefore, multi-center clinical experiments should be conducted to further confirm our results. The long-term efficacy of different doses of prednisone in the treatment of herpes zoster needs to be further followed up. In this study, the number of patients in each group exceeded 30, so the sample size requirement was statistically met. For the determination of adverse reactions, some adverse reactions may be caused by fanciclovir. However, the statistical difference in the incidence of adverse reactions was considered to be caused by prednisone, because only the doses of prednisone were different in this study.
In conclusion, middle-dose prednisone has similar short-term efficacy to high-dose prednisone in the treatment of herpes zoster, but with less complication and higher safety. IL-6 and IL-10 are closely related to the pain degree and the time of symptom remission and subsidence, which may provide a reference for clinical evaluation of the therapeutic effect of patients with herpes zoster.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
LP conceived the study and wrote the manuscript. BD was responsible for ELISA. LS analyzed and interpreted the patients' data. YZ and XZ helped with statistical analysis. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Daqing Oilfield General Hospital. Patients who participated in this research, signed informed consent and had complete clinical data. Signed informed consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control Prevention (CDC) Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. quiz CE2-CE4. [PubMed] [Google Scholar]
- 2.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369:255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, Rentier B, Rümke H, Sadzot-Delvaux C, Senterre J, et al. Varicella vaccination in Europe - taking the practical approach. BMC Med. 2009;7:26–26. doi: 10.1186/1741-7015-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9(Suppl):21–26. doi: 10.1586/erv.10.30. [DOI] [PubMed] [Google Scholar]
- 5.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502–502. doi: 10.1186/s12879-015-1262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: Results from a systematic literature review. BMC Infect Dis. 2013;13:170–170. doi: 10.1186/1471-2334-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordgaard-Lassen I, Dahlerup J F, Belard E, Gerstoft J, Kjeldsen J, Kragballe K, Ravn P, Sørensen IJ, Theede K, Tjellesen L. Danish Society for Gastroenterology. Guidelines for screening, prophylaxis and critical information prior to initiating anti-TNF-alpha treatment. Dan Med J. 2012;59:C4480. [PubMed] [Google Scholar]
- 9.Levin MJ. Varicella-zoster virus and virus DNA in the blood and oropharynx of people with latent or active varicella-zoster virus infections. J Clin Virol. 2014;61:487–495. doi: 10.1016/j.jcv.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Zhang J, Chen N, He L, Zhou M, Zhu C. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2013;1:CD005582. doi: 10.1002/14651858.CD005582.pub4. [DOI] [PubMed] [Google Scholar]
- 11.Ernst P, Dell'Aniello S, Mikaeloff Y, Suissa S. Risk of herpes zoster in patients prescribed inhaled corticosteroids: A cohort study. BMC Pulm Med. 2011;11:59. doi: 10.1186/1471-2466-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B. Jiangsu Science and Technology Press; 2010. Clinical Dermatology in China; pp. 613–618. [Google Scholar]
- 13.Marra F, Lo E, Kalashnikov V, Richardson K. Risk of herpes zoster in individuals on biologics, DMARDS and/or corticosteroids for autoimmune diseases: A systematic review and meta-analysis. Open Forum Infect Dis. 2016;3:ofw205. doi: 10.1093/ofid/ofw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108,604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420–429. doi: 10.1111/apt.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DJ, Janoff EN. Herpes zoster infection in a patient on methotrexate given prednisone to prevent post-herpetic neuralgia. Ann Intern Med. 1987;107:783–783. doi: 10.7326/0003-4819-107-5-783_1. [DOI] [PubMed] [Google Scholar]
- 16.Clemmensen OJ, Andersen KE. ACTH versus prednisone and placebo in herpes zoster treatment. Clin Exp Dermatol. 1984;9:557–563. doi: 10.1111/j.1365-2230.1984.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 17.Whitley RJ, Weiss H, Gnann JW, Jr, Tyring S, Mertz GJ, Pappas PG, Schleupner CJ, Hayden F, Wolf J, Soong SJ, The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. Ann Intern Med. 1996;125:376–383. doi: 10.7326/0003-4819-125-5-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Jeon YH. Herpes zoster and postherpetic neuralgia: Practical consideration for prevention and treatment. Korean J Pain. 2015;28:177–184. doi: 10.3344/kjp.2015.28.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heatrice A, II, Alavi M, Han PP, Enciso R. Oral corticosteroid therapy for preventing postherpetic neuralgia: A systematic review and meta-analysis. Open J Dent Oral Med. 2017;5:47–57. [Google Scholar]
- 20.Rygård SL, Butler E, Granholm A, Møller MH, Cohen J, Finfer S, Perner A, Myburgh J, Venkatesh B, Delaney A. Low-dose corticosteroids for adult patients with septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018;44:1003–1016. doi: 10.1007/s00134-018-5197-6. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto S, Miyoshi K, Kurosaki T, Otani S, Yamane M, Kobayashi M, Oto T. Favorable survival in lung transplant recipients on preoperative low-dose, as compared to high-dose corticosteroids, after hematopoietic stem cell transplantation. Int J Hematol. 2018;107:696–702. doi: 10.1007/s12185-018-2417-3. [DOI] [PubMed] [Google Scholar]
- 22.Izquierdo JL, Cosio BG. The dose of inhaled corticosteroids in patients with COPD: When less is better. Int J Chron Obstruct Pulmon Dis. 2018;13:3539–3547. doi: 10.2147/COPD.S175047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daley-Yates PT. Inhaled corticosteroids: Potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80:372–380. doi: 10.1111/bcp.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, Saag KG, Singh J, Curtis JR. Risk of herpes zoster in autoimmune and inflammatory diseases: Implications for vaccination. Arthritis Rheumatol. 2016;68:2328–2337. doi: 10.1002/art.39670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi HJ, Cui ZQ. Correlation of serum inflammatory cytokine and immunoglobulin content with post-herpetic neuralgia in patients with acute herpes zoster. Hainan Yixueyuan Xuebao. 2017;23:97–100. (In Chinese) [Google Scholar]
- 27.Huang PC, Tsai KL, Chen YW, Lin HT, Hung CH. Exercise combined with ultrasound attenuates neuropathic pain in rats associated with downregulation of IL-6 and TNF-α, but with upregulation of IL-10. Anesth Analg. 2017;124:2038–2044. doi: 10.1213/ANE.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 28.Leung A, Gregory NS, Allen LAH, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157:70–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.