Key Points
Question
Is birth weight associated with type 2 diabetes and glycemic traits?
Findings
This mendelian randomization study found that a 1-SD decrease in birth weight due to the genetic risk score was associated with a higher risk of type 2 diabetes among European and East Asian populations. In addition, a 1-SD decrease in birth weight was associated with a 0.189-SD increase in fasting glucose concentration, but not with fasting insulin, 2-hour glucose, or hemoglobin A1c level.
Meaning
A genetic predisposition to lower birth weight was associated with an increased risk of type 2 diabetes and increased fasting glucose, suggesting potential mechanisms through which perturbation of the antenatal and early-life environment affect predisposition to diabetes in later life.
This mendelian randomization study examines the association of birth weight with type 2 diabetes and glycemic traits.
Abstract
Importance
Observational studies have shown associations of birth weight with type 2 diabetes (T2D) and glycemic traits, but it remains unclear whether these associations represent causal associations.
Objective
To test the association of birth weight with T2D and glycemic traits using a mendelian randomization analysis.
Design, Setting, and Participants
This mendelian randomization study used a genetic risk score for birth weight that was constructed with 7 genome-wide significant single-nucleotide polymorphisms. The associations of this score with birth weight and T2D were tested in a mendelian randomization analysis using study-level data. The association of birth weight with T2D was tested using both study-level data (7 single-nucleotide polymorphisms were used as an instrumental variable) and summary-level data from the consortia (43 single-nucleotide polymorphisms were used as an instrumental variable). Data from 180 056 participants from 49 studies were included.
Main Outcomes and Measures
Type 2 diabetes and glycemic traits.
Results
This mendelian randomization analysis included 49 studies with 41 155 patients with T2D and 80 008 control participants from study-level data and 34 840 patients with T2D and 114 981 control participants from summary-level data. Study-level data showed that a 1-SD decrease in birth weight due to the genetic risk score was associated with higher risk of T2D among all participants (odds ratio [OR], 2.10; 95% CI, 1.69-2.61; P = 4.03 × 10−5), among European participants (OR, 1.96; 95% CI, 1.42-2.71; P = .04), and among East Asian participants (OR, 1.39; 95% CI, 1.18-1.62; P = .04). Similar results were observed from summary-level analyses. In addition, each 1-SD lower birth weight was associated with 0.189 SD higher fasting glucose concentration (β = 0.189; SE = 0.060; P = .002), but not with fasting insulin, 2-hour glucose, or hemoglobin A1c concentration.
Conclusions and Relevance
In this study, a genetic predisposition to lower birth weight was associated with increased risk of T2D and higher fasting glucose concentration, suggesting genetic effects on retarded fetal growth and increased diabetes risk that either are independent of each other or operate through alterations of integrated biological mechanisms.
Introduction
Type 2 diabetes (T2D) has become a worldwide epidemic, with more than 422 million patients in 2014.1 However, the etiology of T2D is not fully understood. Identifying potentially causal risk factors would help guide prevention of the disease.
The thrifty phenotype hypothesis postulates that fetal growth and nutrition play important roles in influencing susceptibility to T2D in later life.2 In observational studies, low birth weight, a widely used indicator for fetal growth restriction, has been consistently associated with higher risk of T2D3,4 and adverse glycemic traits5 in later life. However, both maternal socioeconomic status and unmeasured lifestyle factors might confound these associations; therefore, the causality of these observations remains to be determined. We hypothesized that birth weight may be causally associated with T2D risk and related traits such as fasting glucose concentration, insulin level, insulin resistance, and insulin sensitivity.
Mendelian randomization (MR) analysis has become widely used to assess the potential causal associations of environmental risk factors with disease.6,7,8,9,10,11 This method is analogous to a randomized clinical trial where randomization to genotype takes place at conception, and it is less likely to be affected by confounding and reverse causation.7,12 Previous analyses have provided compelling evidence that fetal genotype has substantial impact on early growth, as measured by birth weight.13
Therefore, in this study, we used the genetic variants for birth weight as an instrumental variable14,15 to perform an MR analysis to examine the association of birth weight with T2D and glycemic traits, using both study-level data and summary-level data.
Methods
Study Design
This study was conducted using summary association data generated by previous studies. Owing to the use of previously collected, deidentified, aggregated data, this study did not require institutional review board approval per the US Federal Policy for Protection of Human Research Subjects. Ethical approval was obtained for all original studies. Reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Observational studies are prone to reverse causation, confounding, and biases and can generate unreliable findings in relation to the causal effects of modifiable exposures on disease outcomes. Mendelian randomization is a method aimed at unbiased detection of causal effects and estimation of their magnitudes (eMethods in the Supplement). To consistently estimate the causal effects, the genetic variants used in an MR analysis must satisfy 3 assumptions (eFigure 1 in the Supplement):16 (1) the genetic variants used as instrumental variables (IV) are associated with the exposure (birth weight); (2) the genetic variants are not associated with any confounder of the exposure-outcome association; and (3) the genetic variants are conditionally independent of the outcome (T2D and glycemic traits) given the exposure and confounders. The second and third assumptions are known as independence from pleiotropy.16
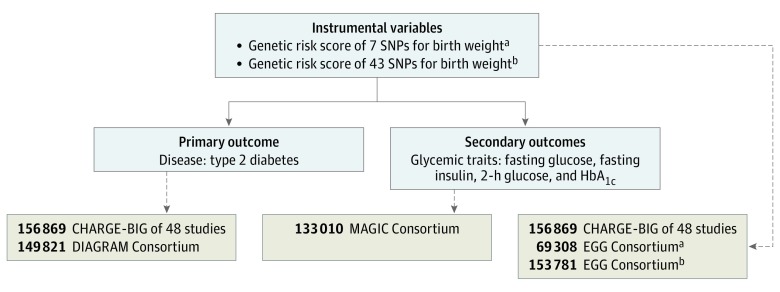
The study design of this MR analysis consisted of 2 components17,18,19,20,21,22,23 (Figure 1). First, we explored the association of birth weight with risk of T2D using study-level data, including 49 cross-sectional and prospective cohort studies with a total of 180 056 participants, including 41 155 patients with T2D from the Cohorts for Heart and Aging Research in Genomic Epidemiology—Birth Gene Study (CHARGE-BIG). The primary IV was a genetic risk score (GRS) for birth weight using 7 single-nucleotide polymorphisms (SNPs) (P < 5 × 10−8) from a genome-wide association study (GWAS) in the Early Growth Genetics (EGG) Consortium.23 We analyzed the data within each study using standardized analytic methods. The IV estimator is calculated as the pooled β coefficient from the GRS-T2D association divided by the pooled β coefficient from the GRS–birth weight association. Second, we tested the association of birth weight with T2D and glycemic traits using summary-level data from the EGG Consortium (n = 153 781),13,23 the Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium (n = 149 821),17 and the Meta-analyses of Glucose and Insulin-Related Traits (MAGIC) Consortium (n = 133 010).18,19,20,21,22 In this study, the 7-SNP score23 was used as the main IV because the new GWAS that identified 60 SNPs for birth weight was published after the study-level results had already been run. Therefore, we used the 43 SNPs available in this analysis, a subset of the 60 SNPs,13 as the IV for birth weight in summary-level analyses.
Figure 1. Study Design.
Sources of data for analysis included study-level data from the Cohorts for Heart and Aging Research in Genomic Epidemiology Birth Gene (CHARGE-BIG) Study (49 studies, n = 180 056 participants) and summary-level data from the Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium (n = 149 821 participants),17 the Meta-analyses of Glucose and Insulin-Related Traits (MAGIC) Consortium (n = 133 010 participants),18,19,20,21,22 and the Early Growth Genetics (EGG) Consortium (n = 153 781 participants).13,23 HbA1c indicates hemoglobin A1c; and SNP, single-nucleotide polymorphism.
aEstimates of 7 SNPs for birth weight were extracted from the EGG Consortium (n = 69 308 participants).23
bEstimates of 43 SNPs for birth weight were extracted from the EGG Consortium (n = 153 781 participants).13
Study Populations and Data Sources
Study-Level Data
Study-level data including 49 cross-sectional and prospective cohort studies with up to 180 056 participants from the CHARGE-BIG were used (eTable 1 in the Supplement). Descriptions of each participating study are shown in the eAppendix in the Supplement. All participants provided written, informed consent, and ethical approval was granted by local ethics committees for participating studies (eTable 2 in the Supplement). Birth weight was collected by self-reported questionnaires or medical records in each study. Detailed information on the study-specific data collection methods is provided in eTable 2 in the Supplement. Covariates were measured using direct measurement or self-reported using questionnaire data from each study (eTable 2 in the Supplement). The primary outcomes were prevalence or incidence of T2D, defined based on report of T2D or current use of antidiabetes medication. Participants with missing values or those lost to follow-up were excluded. Precise information on the outcome for each study is reported in eTable 3 in the Supplement.
Selection of SNPs and GRS Calculation
Seven SNPs were identified as being associated with birth weight by a previous GWAS.23 All studies used direct genotype information on SNPs for birth weight from previously genotyped array data. Whenever a SNP was not genotyped directly, we used either (1) the HapMap II CEU (European) reference panel-imputed genetic information from GWAS or (2) genotype information from a predefined list of proxies that are in high linkage disequilibrium with the SNP (r2 > 0.8). Genotyping platforms, genotype frequencies, Hardy-Weinberg equilibrium P values, and call rates for the 7 SNPs are listed in eTable 4 and eTable 5 in the Supplement. To estimate the genetic predisposition to low birth weight, a GRS for low birth weight was calculated on the basis of these 7 well-established SNPs (eTable 6 in the Supplement).23 We assumed that each SNP in the panel acts independently in an additive manner, and the GRS was calculated using a weighted method (eAppendix in the Supplement).
Summary-Level Data
Summary-level data from the EGG Consortium,13,23 DIAGRAM Consortium,17 and MAGIC Consortium18,19,20,21,22 were used. For IV, both the 7-SNP GRS (explained between 0.32% and 1.52% of variance in birth weight) (eTable 6 and eTable 7 in the Supplement)23 and the 43-SNP GRS (explained 2.0% of variance in birth weight) (eTable 8 and eTable 9 in the Supplement)13 for birth weight were used from 2 previous GWAS studies in the EGG Consortium with up to 153 781 individuals. For T2D, data were obtained from the DIAGRAM Consortium; this study included 149 821 individuals of European descent.17 In addition to the primary outcomes of T2D, secondary outcomes of glycemic traits such as fasting glucose, fasting insulin, 2-hour glucose, and hemoglobin A1c concentrations were examined (eTable 7 and eTable 9 in the Supplement). Data from the MAGIC Consortium with up to 133 010 individuals were used for glycemic traits. Informed consent was obtained from all participants of contributing studies. Contributing studies received ethical approval from their respective institutional review boards.
Statistical Analysis
Study-Level Data
For study-level data from the CHARGE-BIG study, a standard analytic protocol was applied to each individual study to produce comparable results. Logistic regression was used to test the association of birth weight with risk of T2D after adjustment for age, sex, and other baseline covariates, where available (smoking status, physical activity, total energy intake, and alcohol intake). Linear regression was used to test the association of the GRS with birth weight after adjustment for age, sex, and principal components for population stratification (principal components analysis [PCA]). Logistic regression was used to test the association of the GRS with risk of T2D after adjustment for age, sex, and PCA. The inclusion of PCA as covariates is commonly used to correct for population stratification according to ancestral background.24
To validate assumption 1, that the GRS for birth weight was a strong IV for birth weight (eTable 10 in the Supplement), an F statistic for the IV was calculated in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) cohorts as a measure of the strength of IV for prediction of the birth weight, controlling for covariates (age, sex, PCA). An F statistic greater than 10 is evidence of a strong IV.25
To examine assumption 2, that GRS for birth weight was not associated with potential confounders, the association of the GRS with age, body mass index, smoking, alcohol use, and total energy intake was determined among individuals in the NHS and HPFS cohorts (eTable 11 in the Supplement).
Meta-analyses were conducted using study-level data from each study; we then pooled the β coefficients across studies, using random-effects or fixed-effects meta-analysis. Meta-analyses were conducted in Stata statistical software version 13.0 (StataCorp). All P values reported are 2-sided. We assessed heterogeneity with the I2 statistic. We assessed between-study heterogeneity via the Cochrane Q statistic and I2 statistics.26,27,28 For the proposed cutoff of I2 > 0.25, we found nonnegligible heterogeneity between studies, in particular among the birth weight–T2D associations, but also for the association between GRS and birth weight or T2D (I2 > 0.25). As a consequence, we used random-effects meta-analysis throughout. After meta-analysis, we used the IV estimators to quantify the strength of the association of birth weight with risk of T2D.29 The IV estimator, which is identical to that derived by the widely used 2-stage least-squares method,30 was calculated as the β of the regression coefficients for GRS-T2D and GRS–birth weight associations (eMethods in the Supplement).
Summary-Level Data
For the summary-level data from the EGG, DIAGRAM, and MAGIC consortia, the estimates of the association of birth weight with T2D risk and glycemic traits were pooled using the inverse-variance weighted, MR-Egger, and weighted-median methods for multiple genetic variants (eMethods in the Supplement). Detailed information on this MR method has been described previously.31,32,33
To examine assumption 3, that the IV for birth weight affects risk of T2D only through birth weight, but not through other pathways, the MR-Egger method was used (eMethods in the Supplement). Egger regression is a tool to detect small study bias in meta-analysis and it can be adapted to test for bias from type I pleiotropy, which is problematic for the interpretation of MR. Type I pleiotropy occurs when a single locus directly influences multiple phenotypes and is more pronounced at the level of the gene than at the level of single SNPs.6 Under the assumption that the association of each genetic variant with the exposure is independent of the pleiotropic effect of the variant (not via the exposure), the MR-Egger test gives a valid test of the null causal hypothesis.16 Using the MR-Egger method, the effect of the IV on the exposure is plotted against its effect on the outcome, and an intercept distinct from the origin provides evidence for pleiotropic effects. Additionally, the slope of the MR-Egger can provide pleiotropy-corrected causal estimates under a weaker assumption (the instrument strength independent of direct effect assumption).16
For analyses of both study-level data and summary-level data, the effect size for each meta-analysis is reported in the main results as the effect of a 1-SD change in birth weight or glycemic quantitative traits, as this metric is more interpretable than an arbitrary difference. Absolute risk increase (ARI) per 1000 participant-years for T2D was also calculated (eMethods in the Supplement). P < .05 was considered statistically significant. Analyses were performed using Stata statistical software version 13 (StataCorp) and R statistical software version 3.2.3 (R Project for Statistical Computing).
Results
Characteristics of the 49 Participating Studies
The characteristics of the 49 participating studies with up to 180 056 participants, including 41 155 patients with T2D, are presented in eTable 1 in the Supplement. Twenty-two studies reported the genetic association between GRS and birth weight, and 33 studies reported the genetic association between GRS and risk of T2D. A total of 41 155 patients with T2D and 80 008 control individuals without T2D provided study-level data. Data from the DIAGRAM Consortium included 34 840 patients with T2D and 114 981 control individuals, overwhelmingly of European descent.17 The MAGIC Consortium included 133 010 participants,18,19,20,21,22 and the EGG Consortium included 153 781 participants (Figure 1).13,23
Results for Testing MR Assumptions
To validate MR assumptions 1 and 2, the NHS and HPFS cohorts were used to examine the associations of GRS with birth weight and potential confounders. We found that the GRS for birth weight was a strong IV (F > 18) (eTable 10 in the Supplement), thus validating assumption 1. In addition, no associations between the GRS and age, body mass index, smoking, alcohol use, and total energy intake were observed in the NHS and HPFS cohorts (eTable 11 in the Supplement), thus validating assumption 2.
Association of Birth Weight With Risk of T2D
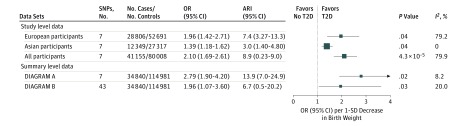
Study-level data showed that each 1-SD decrease in birth weight due to the GRS was associated with higher risk of T2D among all participants (odds ratio [OR], 2.10; 95% CI, 1.69-2.61; and ARI per 1000 participant-years, 8.9; 95% CI, 0.2-9.0; P = 4.03 × 10−5), among European participants (OR, 1.96; 95% CI, 1.42-2.71; and ARI per 1000 participant-years, 7.48; 95% CI, 3.27-13.34; P = .04)34 (Table 1), and among East Asian participants (OR, 1.39; 95% CI, 1.18-1.62; and ARI per 1000 participant-years, 3.04; 95% CI, 1.40-4.84; P = .04) (Figure 2; eFigure 2 and eFigure 3 in the Supplement). We did not find a significant difference in OR for T2D between MR estimates and conventional observational results (OR, 1.41 per 1-SD lower birth weight; 95% CI, 1.16-1.66) from 11 studies of the CHARGE-BIG (P = .86) (eFigure 4 in the Supplement).
Table 1. Mendelian Randomization of Birth Weight and Risk of Type 2 Diabetes.
| MR Estimatesa | Summary Data Ab | Summary Data Bb | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Simple median–based methodc | 1.57(1.24 to 2.00) | 2.0 × 10-4 | 1.24(1.09 to 1.41) | .001 |
| Weighted median–based methodc | 1.52(1.24 to 1.86) | 1.1 × 10-4 | 1.29(1.13 to 1.47) | 6.0 × 10-4 |
| Inverse-variance–weighted methodc | 1.69(1.12 to 2.55) | .045 | 1.36(1.14 to 1.62) | .001 |
| MR-Egger methodc | 2.79(1.90 to 4.20) | .02 | 1.96(1.07 to 3.60) | .03 |
| MR-Egger regressiond | 0.007 (−0.081 to 0.095) | .94 | 0.011 (−0.002 to 0.02) | .22 |
Abbreviations: MR, mendelian randomization; OR, odds ratio.
In an MR framework, genetic variants for birth weight were assumed to influence type 2 diabetes only through birth weight, not through other pathways. In the present study, we used MR-Egger regression to assess for the presence of pleiotropy.16 This approach is based on Egger regression, which was used to assess publication bias in the meta-analysis.34 Using the MR-Egger method, the β coefficient of the MR-Egger regression provides pleiotropy-corrected causal estimates and an intercept distinct from the origin provides evidence for pleiotropic effects.16
Sample sizes of patients with type 2 diabetes and control individuals were 12 171 and 56 862 for both summary data A and summary data B. Number of single-nucleotide polymorphisms used of summary data A and summary data B are 7 and 43, respectively. Number of participants with birth weight in summary data A and summary data B are 69 308 and 153 781, respectively.
We used simple median–based method, weighted median–based method, inverse-variance–weighted method, and MR-Egger method to provide consistent results for causal effect of birth weight on type 2 diabetes.
Values in this row are intercept (95% CI).
Figure 2. Mendelian Randomization of Birth Weight and Risk of Type 2 Diabetes (T2D).
For type 2 diabetes, the data were analyzed from 49 studies from the Cohorts for Heart and Aging Research in Genomic Epidemiology Birth Gene Study where standardized analytic methods were used in individual study. This study included 41 155 patients with T2D and 80 008 controls. Data from the Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium included 34 840 patients with T2D and 114 981 controls, overwhelmingly of European descent. Summary results of 7 single-nucleotide polymorphisms (SNPs) for birth weight identified in genome-wide association studies were extracted from the Early Growth Genetics Consortium.23 Summary results for risk of T2D were extracted from the DIAGRAM Consortium.17 Summary results of 43 SNPs for birth weight were extracted from the Early Growth Genetics birth weight genome-wide association study.13 Summary results for risk of T2D were extracted from the DIAGRAM Consortium.17 We used the standard deviation value (543 g) from the birth weight genome-wide association study of the EGG Consortium.13 Results are standardized to a 1-SD lower birth weight owing to genetic risk score. ARI indicates absolute risk increase; OR, odds ratio
We further conducted stratified analyses of estimated causality by age, sex, body mass index, ethnic group, sample size, study design, and number of SNPs included. An association of birth weight with T2D was observed among both men and women, both obese and normal-weight participants, and both European and East Asian participants. However, evidence for a causal association was not observed in the subsample of individuals younger than 50 years (Table 2).
Table 2. Stratified Analyses of Estimated Causality Between Birth Weight and Risk of Type 2 Diabetes.
| Subgroup | Genetic Association of Birth Weight per SDa | Genetic Association of Type 2 Diabetes | Estimated Causalityb | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | β (95% CI) | P Value | No. of Studies | β (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | 23 | |||||||
| ≥50 | 0.04 (0.03 to 0.05) | 3.6 × 10−4 | 28 | 0.03 (0.01 to 0.05) | .0004 | 2.12 (1.70 to 2.64) | .0006 | |
| <50 | 5 | 0.04 (−0.10 to 0.02) | .18 | 1.67 (0.87 to 5.65) | .18 | |||
| Sex | ||||||||
| Male | 17 | 0.04 (0.02 to 0.05) | 8.4 × 10−4 | 24 | 0.03 (0.01 to 0.05) | .006 | 1.89 (1.46 to 2.46) | .02 |
| Female | 16 | 0.04 (0.01 to 0.06) | 9.4 × 10−4 | 23 | 0.03 (0.01 to 0.04) | .002 | 2.10 (1.49 to 2.97) | .03 |
| Body mass indexc | 23 | |||||||
| ≥25 | 0.04 (0.03 to 0.05) | 3.6 × 10−4 | 25 | 0.02 (0.00 to 0.04) | .02 | 1.81 (1.39 to 2.37) | .03 | |
| <25 | 8 | 0.04 (0.02 to 0.06) | <.001 | 2.82 (2.20 to 3.60) | 3.1 × 10−5 | |||
| Ethnic group | ||||||||
| European | 22 | 0.04 (0.03 to 0.05) | 3.6 × 10−4 | 24 | 0.03 (0.01 to 0.05) | .02 | 1.96 (1.42 to 2.71) | .04 |
| East Asian | 1 | 0.09 (0.00 to 0.18) | 5.1 × 10−3 | 9 | 0.03 (0.02 to 0.04) | <.001 | 1.39 (1.18 to 1.62) | .04 |
| Sample size, No. | 23 | |||||||
| ≥1500 | 0.04 (0.03 to 0.05) | 3.6 × 10−4 | 27 | 0.03 (0.01 to 0.04) | .001 | 1.96 (1.58 to 2.44) | .002 | |
| <1500 | 6 | 0.07 (0.03 to 0.12) | <.001 | 3.45 (2.41 to 6.19) | .003 | |||
| Study design | 23 | |||||||
| Cohort | 0.04 (0.03 to 0.05) | 3.6 × 10−4 | 26 | 0.03 (0.01 to 0.05) | <.001 | 2.06 (1.64 to 2.60) | .002 | |
| Case-control | 5 | 0.02 (−0.03 to 0.06) | .47 | 1.55 (0.85 to 2.84) | .47 | |||
| Cross-sectional | 2 | 0.06 (−0.01 to 0.16) | .19 | 3.26 (0.89 to 7.02) | .19 | |||
| No. of single-nucleotide polymorphisms | 23 | |||||||
| 7 | 0.04 (0.03 to 0.05) | 3.6 × 10−4 | 27 | 0.03 (0.01 to 0.05) | .003 | 2.17 (1.65 to 2.87) | .005 | |
| <7 | 6 | 0.03 (0.01 to 0.04) | .0004 | 1.91 (1.58 to 2.31) | .0007 | |||
Abbreviation: OR, odds ratio.
Results were standardized to a 1-SD decrease in birth weight due to genetic risk score. The standard deviation was 543 g from the Early Growth Genetics Consortium.13
The estimates were derived from 49 studies from the Cohorts for Heart and Aging Research in Genomic Epidemiology Birth Gene Study where standardized analytic methods adjusted for confounders such as age, body mass index, sex, and the first 3 principal components for population stratification were used in individual study. In a mendelian randomization framework, the association between genetic risk score and type 2 diabetes is assumed to be independent of confounding factors. In our study, the instrumental variable estimator is calculated as the β coefficient from the association of genetic risk score with type 2 diabetes divided by the β coefficient from the association of genetic risk score with birth weight. These results are supportive of a causal, nonconfounded association.
Calculated as weight in kilograms divided by height in meters squared.
Summary-level data showed a similar association of low birth weight with risk of T2D when using the 7 SNPs (OR, 2.79; 95% CI, 1.90-4.20; and ARI per 1000 participant-years, 13.96; 95% CI, 7.02-24.96; P = .02) and when using 43 SNPs (OR, 1.86; 95% CI, 1.07-3.60; and ARI per 1000 participant-years, 6.70; 95% CI, 0.55-20.28; P = .03) (Figure 2). We further excluded previously reported loci for T2D such as CDKAL1, ADCY5, BCAR1, HHEX/IDE, GCK, MTNR1B, and ANK1, and low birth weight remained associated with risk of T2D (OR, 1.75; 95% CI, 1.05-3.16; P = .04).
Association of Birth Weight With Glycemic Quantitative Traits
Using the weighted median–based method, we found that a 1-SD lower birth weight due to the GRS was associated with 0.189 SD higher fasting glucose concentration (β = 0.189; SE = 0.060; P = .002) at the Bonferroni-adjusted level of significance (P < .01). Consistently, the inverse-variance–weighted analysis also showed an association of birth weight with fasting glucose concentration (0.207 SD higher fasting glucose concentration per 1-SD lower birth weight; β = 0.0.207; SE = 0.073; P = .03) (Table 3). These findings were replicated using the 43 SNPs as an IV, suggesting robustness of our findings. However, there was no evidence for an association of birth weight with other glycemic traits such as fasting insulin, 2-hour glucose, or hemoglobin A1c concentrations (Table 3).
Table 3. Mendelian Randomization Analyses of Birth Weight and Glycemic Quantitative Traitsa.
| Data Source | SD | No. | MR Estimates, Units of SD per 1-SD Decrease in Birth Weight | MR-Egger Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weighted Median–Based Method | Inverse-Variance–Weighted Method | MR-Egger Method | |||||||||
| SNPs | Participants | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | Intercept (SE) | P Value | ||
| Fasting glucose, mg/dL | |||||||||||
| Summary data Ab | 13.1 | 7 | 133 010 | 0.189 (0.060) | .002 | 0.207 (0.073) | .03 | 0.113 (0.341) | .74 | 0.005 (0.017) | .78 |
| Summary data Bc | 13.1 | 43 | 133 010 | 0.109 (0.049) | .03 | 0.415 (0.105) | .04 | 0.031 (0.099) | .23 | −0.018 (0.010) | .07 |
| Fasting insulin, log (pmol/L) | |||||||||||
| Summary data Ab | 0.44 | 7 | 108 557 | 0.089 (0.096) | .36 | 0.021 (0.108) | .86 | 0.131 (0.502) | .79 | −0.006 (0.026) | .82 |
| Summary data Bc | 0.44 | 43 | 108 557 | 0.033 (0.082) | .69 | 0.050 (0.060) | .41 | −0.027 (0.213) | .90 | 0.002 (0.006) | .70 |
| 2-h glucose, mg/dLd | |||||||||||
| Summary data Ab | 10.1 | 7 | 42 854 | 0.494 (0.352) | .16 | 0.563 (0.411) | .22 | −0.584 (1.851) | .75 | 0.060 (0.094) | .52 |
| Summary data Bc | 10.1 | 43 | 42 854 | 0.406 (0.254) | .11 | 0.319 (0.203) | .12 | 0.378 (0.727) | .60 | −0.002 (0.022) | .93 |
| Hemoglobin A1c, % of total hemoglobin | |||||||||||
| Summary data Ab | 0.54 | 7 | 46 368 | 0.118 (0.072) | .10 | 0.186 (0.084) | .07 | 0.135 (0.390) | .73 | 0.003 (0.020) | .89 |
| Summary data Bc | 0.54 | 43 | 46 368 | 0.038 (0.063) | .55 | 0.086 (0.069) | .22 | 0.158 (0.242) | .51 | −0.002 (0.007) | .76 |
Abbreviations: HbA1c, hemoglobinA1c; MR, mendelian randomization; SNP, single-nucleotide polymorphism.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555; HbA1c to proportion of total hemoglobin, multiply by 0.01.
Results were standardized to a 1-SD decrease in birth weight due to genetic variants. For birth weight, 1-SD was assumed to correspond to 543 g, the pooled results from the Early Growth Genetics (EGG) Consortium.23 The Meta-analyses of Glucose and Insulin-Related Traits (MAGIC) Consortium did not report estimates of variants in units of standard deviations. β values from this consortium were standardized so that the association of birth weight with glycemic traits could be uniformly expressed in terms of standard deviations. For fasting glucose, 2-hour glucose, and HbA1c from the MAGIC Consortium, 1 SD was assumed to correspond to 13.1 mg/dL, 10.1 mg/dL, and 0.535%, respectively, the pooled SD of studies included in a previous report from the MAGIC Consortium.18 The threshold of significance was at the Bonferroni-adjusted level P < .01 (0.05 / 4 = 0.01).
Estimates of 7 SNPs for birth weight were extracted from EGG Consortium.23 For glycemic traits, estimates were derived from the MAGIC Consortium (n = 133 010 participants).18,19,20,21,22
Estimates of 43 SNPs for birth weight were extracted from EGG Consortium.13 For glycemic traits, estimates were derived from the MAGIC Consortium (n = 133 010 participants).18,19,20,21,22
Two-hour glucose refers to measured blood glucose concentration 2 hours after consumption of dissolved glucose.
Sensitivity Analyses of MR
In sensitivity analyses, we used 4 different methods (simple median based, weighted median based, inverse-variance weighted, and MR-Egger) to estimate the association of birth weight with risk of T2D using summary-level data. The results showed consistent associations (Table 2), indicating robustness of our findings. We further conducted a sensitivity analysis of association of birth weight with risk of T2D using 8 studies providing both GRS–birth weight and GRS-T2D associations (eFigure 5 in the Supplement) in the CHARGE-BIG study. Similarly, we found that each 1-SD lower birth weight due to the GRS was associated with higher risk of T2D (OR, 2.66; 95% CI, 1.30-4.02; P = 6.76 × 10−4), providing further evidence of finding robustness.
To examine MR assumption 3, we further tested whether any of the selected SNPs were influenced by linkage disequilibrium and pleiotropy. We found that none of the SNPs were in linkage disequilibrium with each other (r2 > 0.05). In addition, the intercept term estimated from MR-Egger was centered at the origin with a confidence interval including the null (0.007; 95% CI −0.081 to 0.095; P = .94) (Table 1), suggesting the results were not influenced by pleiotropy. For glycemic traits, the intercept (SE) from MR-Egger regression also suggested that the observed results were not influenced by pleiotropy (Table 3).
Discussion
In the largest MR study thus far, to our knowledge, we investigated a potential causal role of birth weight in the development of T2D and regulation of glycemic traits using study-level data and summary-level data. Our results show that genetically determined lower birth weight was associated with increased risk of T2D and elevated fasting glucose concentration, supporting an association between lower birth weight and development of T2D.
Compelling observational studies have shown that lower birth weight is associated with a higher T2D risk.3,4,35,36,37,38,39,40 For example, data from a meta-analysis of 30 studies found an inverse birth weight–T2D association; the pooled OR of T2D was 1.13 (95% CI, 1.10-0.1.17) per kilogram decrease in birth weight.3 However, in most of the observational studies included in this meta-analysis, birth weight was associated with potential confounders. Therefore, residual confounding may have contributed to the observed associations, illustrating a major limitation of observational studies in inference of causality. In the present study, we used MR analysis to minimize the potential confounding effect. The GRS used in our study was not correlated with potential confounders, and was validated as a strong and reliable IV for birth weight.15 Therefore, our findings concur with a previous study15 and lend genetic support to prior evidence of observational association between birth weight and risk of T2D.
Our findings suggest that birth weight may be a useful target for a prevention strategy to mitigate T2D risk in later life. According to the thrifty phenotype hypothesis,2 the observed associations may originate in utero where intrauterine growth restriction affects epigenetic alterations and alters intracellular insulin-signaling pathways.41,42 Such permanent alterations in structure, physiology, and metabolism are thought to result in key disruptions to the endocrine system.42,43 It has been suggested that the public health implications of the inverse birth weight–T2D association depend on the precise nature of the underlying causal exposure and its amenability to change.3 Our MR results demonstrate that birth weight itself is a causal exposure, implying the public health impact of birth weight modification. Interestingly, previous interventions for increasing birth weight through changes in maternal nutrition have increased birth weight by up to 200 g in populations.44 Such an increase in birth weight could translate into a reduction in T2D risk of up to 10%.3 Therefore, our findings highlight the potential importance of improving fetal growth and nutrition in the prevention of T2D. In addition, ongoing research to understand the mechanistic links between the genetic loci that influence birth weight may lead to novel therapeutic strategies to modify birth weight and subsequently reduce the risk of T2D.45 Importantly, our findings are of public health significance and may help in understanding the mechanisms by which low birth weight increases risk of T2D.
The MR analysis used in this study satisfied 3 assumptions. Assumption 1 requires a strong link between the genetic variants used as an IV and birth weight. The GRS used in our study was demonstrated to be a strong IV with an F statistic greater than 18.31 For assumption 2, MR assumes the IV (GRS) was not associated with potential confounders. Study-level results showed that GRS was not associated with potential confounders; nevertheless, we could not exclude the possibility that our results might be affected by unmeasured confounders. For assumption 3, MR assumes that the IV for birth weight affects risk of T2D only through birth weight, but not through other pathways. To validate assumption 3,16 the intercept term estimated from MR-Egger regression was centered at the origin with a confidence interval including the null, suggesting that our results were not influenced by pleiotropy.
Our current study has several other strengths. First, to our knowledge, our study is the largest MR analysis assessing the association of birth weight with T2D risk and glycemic traits to date. The large sample size allowed us to assess the consistency of associations across studies and to gain sufficient power for conclusive estimation of associations. Second, sensitivity analyses of 2 different data sources (study-level and summary-level data sets) were conducted. The steps taken in this study reduced the risk of bias and pleiotropy. Importantly, the consistent associations estimated from complementary MR approaches, such as the weighted median regression method, inverse-variance–weighted method, and MR-Egger method, support the robustness of our findings. Finally, most of the studies included were homogeneous, and we used standardized methods and performed the analysis individually in each study. Therefore, the effect of population stratification on the instrumental results should be minimal.
Limitations
This study has some limitations, and the results should be interpreted with sufficient caution. Although the MR method is theoretically well established, we recognize that there are still many limitations in practice. First, we assumed that the associations of birth weight with T2D and glycemic traits were linear. Indeed, several observational studies suggested U-shaped associations.46,47,48,49 Therefore, further investigations employing a nonlinear MR approach are warranted to investigate the causality. In addition, we only used 7 SNPs in study-level analyses; this may lead to a weak IV, and thus introduce bias. Second, although the MR-Egger method suggested that our results were not affected by pleiotropy, it is possible that the shared genetic basis between birth weight and T2D may also contribute to the association. Third, although previous evidence indicated that variation in the fetal genome was the predominant driver of the birth weight associations,13 birth weight may be influenced by both fetal and correlated maternal genotypes. Given the correlation (r of approximately 0.5) between maternal and fetal genotype,13 we could not exclude the possibility that associations between fetal genotype and birth weight may result from indirect effects of the maternal genotype influencing birth weight via the intrauterine environment.13 In addition, recent GWAS identified several novel loci for offspring birth weight and highlighted maternal genetic effects that are independent of fetal genetics.50 Therefore, there are limitations in assuming causality on the basis of fetal genotype and fetal phenotype associations; dissecting maternal and fetal effects on adult T2D risk are also needed. In addition, maternal genetic variants could influence both offspring birth weight and other aspects of nurturing. Cross-generation MR studies are susceptible to such dynamic effects, which introduce potential biases. Therefore, future cross-generation MR studies should consider ways to reduce the biases that might result from these assumption violations.51
Fourth, the widely used GRS may not fit the assumptions for an IV well. Furthermore, most of the risk factors are not static, but dynamic, and this may only be captured by taking both genetic and environmental factors into account over time. Fifth, assumption 3, that genetic markers affect T2D only through birth weight, is sometimes referred to as the exclusion restriction. The MR assumptions are violated if the genetic marker affects T2D through pathways other than through birth weight, which may lead to substantial biases in MR analysis.52 We cannot exclude the possibility that genetic markers for birth weight were associated with other pathways that influence T2D risk. In the present study, we used only the MR-Egger method to examine assumption 3. Although we found that the results were not influenced by pleiotropy, the MR-Egger analysis has limitations and is not able to reliably detect a dose-response relationship in the genetic associations with birth weight and with T2D, and hence cannot distinguish between pleiotropy and causal effect.53 Sixth, even though many relevant covariates, including age, sex, ethnicity, region, total energy, and PCA were included in the statistical models, residual and unmeasured confounding cannot be ruled out. Many established confounders, such as maternal diet, lifestyle, and additional genetic markers, may confound the association of birth weight with T2D risk. Further investigation considering these confounders is needed. In addition, given that the studies involved are overwhelmingly in non-Hispanic white populations, testing for generalizability to other ethnic groups warrants further investigation.
Conclusions
This study found that genetic predisposition to lower birth weight was associated with increased risk of T2D and impaired fasting glucose concentration. Our results suggest the presence of genetic effects on retarded fetal growth and increased diabetes risk that either are independent of each other or operate through alterations of integrated biological mechanisms.
eMethods. Mendelian Randomization Method
eFigure 1. Schematic Representation of a Mendelian Randomization Approach
eFigure 2. Genetic Association With Birth Weight
eFigure 3. Genetic Association With Risk of T2DM
eFigure 4. Association of Birth Weight With Risk of T2DM
eFigure 5. Causality Estimated From Individual Study
eTable 1. Baseline Characteristics of Included 49 Studies in the CHARGE-BIG Study
eTable 2. Assessment of Birth Weight and Covariates in the CHARGE-BIG Study
eTable 3. Assessment of Type 2 Diabetes in the CHARGE-BIG Study
eTable 4. Genotyping Information in the CHARGE-BIG Study
eTable 5. Distribution of Genotypes of Included 7 SNPs in the CHARGE-BIG Study
eTable 6. Associations Between Seven Loci Associated With Birth Weight and Various Anthropometric Measures Taken at Birth (Data From Summary Results)
eTable 7. Genetic Association of Birth Weight Genetic Variants With Glycemic Traits (Data From Summary Results)
eTable 8. Sixty Loci Associated With Birth Weight (P<5×10−8) in European Ancestry and/or Trans-Ancestry (Data From Summary Results)
eTable 9. Genetic Association of Birth Weight Related 60 Genetic Variants With Glycemic Traits (Data From Summary Results)
eTable 10. Association of the Genetic Risk Score With Birth Weight and F Statistic for the Instrumental Variable in the NHS, HPFS, and WHI Cohorts
eTable 11. Association of Birth Weight Genetic Risk Score With Confounders According to Quartiles of the GRS in the NHS, HPFS and WHI Studies
eAppendix. Description of Included Studies
eReferences
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):-. doi: 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595-601. doi: 10.1007/BF00400248 [DOI] [PubMed] [Google Scholar]
- 3.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886-2897. doi: 10.1001/jama.2008.886 [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Ley SH, Tobias DK, et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. BMJ. 2015;351:h3672. doi: 10.1136/bmj.h3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawlor DA, Davey Smith G, Ebrahim S. Birth weight of offspring and insulin resistance in late adulthood: cross sectional survey. BMJ. 2002;325(7360):359. doi: 10.1136/bmj.325.7360.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-R98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 8.Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L; CHARGE Consortium . Dairy consumption, systolic blood pressure, and risk of hypertension: mendelian randomization study. BMJ. 2017;356:j1000. doi: 10.1136/bmj.j1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang T, Ren J, Huang J, Li D. Association of homocysteine with type 2 diabetes: a meta-analysis implementing mendelian randomization approach. BMC Genomics. 2013;14:867. doi: 10.1186/1471-2164-14-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng T, Smith CE, Li C, Huang T. Childhood BMI and adult type 2 diabetes, coronary artery diseases, chronic kidney disease, and cardiometabolic traits: a mendelian randomization analysis. Diabetes Care. 2018;41(5):1089-1096. doi: 10.2337/dc17-2141 [DOI] [PubMed] [Google Scholar]
- 11.Huang T, Ding M, Bergholdt HKM, et al. ; Mendelian Randomization of Dairy Consumption Working Group . Dairy consumption and body mass index among adults: mendelian randomization analysis of 184802 individuals from 25 studies. Clin Chem. 2018;64(1):183-191. doi: 10.1373/clinchem.2017.280701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16(4):309-330. doi: 10.1177/0962280206077743 [DOI] [PubMed] [Google Scholar]
- 13.Horikoshi M, Beaumont RN, Day FR, et al. ; CHARGE Consortium Hematology Working Group; Early Growth Genetics (EGG) Consortium . Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538(7624):248-252. doi: 10.1038/nature19806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au Yeung SL, Lin SL, Li AM, Schooling CM. Birth weight and risk of ischemic heart disease: a mendelian randomization study. Sci Rep. 2016;6:38420. doi: 10.1038/srep38420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Huang T, Li Y, et al. Low birthweight and risk of type 2 diabetes: a mendelian randomisation study. Diabetologia. 2016;59(9):1920-1927. doi: 10.1007/s00125-016-4019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahajan A, Go MJ, Zhang W, et al. ; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; Mexican American Type 2 Diabetes (MAT2D) Consortium; Type 2 Diabetes Genetic Exploration by Next-Generation Sequencing in Multi-Ethnic Samples (T2D-GENES) Consortium . Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46(3):234-244. doi: 10.1038/ng.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott RA, Lagou V, Welch RP, et al. ; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium . Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991-1005. doi: 10.1038/ng.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strawbridge RJ, Dupuis J, Prokopenko I, et al. ; DIAGRAM Consortium; GIANT Consortium; MuTHER Consortium; CARDIoGRAM Consortium; C4D Consortium . Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60(10):2624-2634. doi: 10.2337/db11-0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soranzo N, Sanna S, Wheeler E, et al. ; WTCCC . Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229-3239. doi: 10.2337/db10-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena R, Hivert MF, Langenberg C, et al. ; GIANT Consortium; MAGIC Investigators . Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42(2):142-148. doi: 10.1038/ng.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuis J, Langenberg C, Prokopenko I, et al. ; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC Investigators . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-116. doi: 10.1038/ng.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, et al. ; Meta-Analyses of Glucose- and Insulin-related traits Consortium (MAGIC); Early Growth Genetics (EGG) Consortium . New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45(1):76-82. doi: 10.1038/ng.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904-909. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 25.Burgess S, Thompson SG; CRP CHD Genetics Collaboration . Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755-764. doi: 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914-916. doi: 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 29.Wald A. The fitting of straight lines if both variables are subject to error. Ann Math Stat. 1940;11:284-300. doi: 10.1214/aoms/1177731868 [DOI] [Google Scholar]
- 30.Palmer TM, Sterne JA, Harbord RM, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in mendelian randomization analyses. Am J Epidemiol. 2011;173(12):1392-1403. doi: 10.1093/aje/kwr026 [DOI] [PubMed] [Google Scholar]
- 31.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665. doi: 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Class QA, Rickert ME, Lichtenstein P, D’Onofrio BM. Birth weight, physical morbidity, and mortality: a population-based sibling-comparison study. Am J Epidemiol. 2014;179(5):550-558. doi: 10.1093/aje/kwt304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lauzon-Guillain B, Balkau B, Charles MA, Romieu I, Boutron-Ruault MC, Clavel-Chapelon F. Birth weight, body silhouette over the life course, and incident diabetes in 91,453 middle-aged women from the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l’Education Nationale (E3N) Cohort. Diabetes Care. 2010;33(2):298-303. doi: 10.2337/dc09-1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovasc Diabetol. 2016;15(1):73. doi: 10.1186/s12933-016-0389-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaijser M, Bonamy AK, Akre O, et al. Perinatal risk factors for diabetes in later life. Diabetes. 2009;58(3):523-526. doi: 10.2337/db08-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Narváez EA, Palmer JR, Gerlovin H, et al. Birth weight and risk of type 2 diabetes in the black women’s health study: does adult BMI play a mediating role? Diabetes Care. 2014;37(9):2572-2578. doi: 10.2337/dc14-0731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann E, Gamborg M, Sørensen TI, Baker JL. Sex differences in the association between birth weight and adult type 2 diabetes. Diabetes. 2015;64(12):4220-4225. doi: 10.2337/db15-0494 [DOI] [PubMed] [Google Scholar]
- 41.Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol. 2008;32(3):213-218. doi: 10.1053/j.semperi.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71(5)(suppl):1344S-1352S. doi: 10.1093/ajcn/71.5.1344s [DOI] [PubMed] [Google Scholar]
- 43.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clin Obstet Gynecol. 2013;56(3):511-519. doi: 10.1097/GRF.0b013e31829cb9ca [DOI] [PubMed] [Google Scholar]
- 44.Olsen SF, Halldorsson TI, Willett WC, et al. ; NUTRIX Consortium . Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr. 2007;86(4):1104-1110. doi: 10.1093/ajcn/86.4.1104 [DOI] [PubMed] [Google Scholar]
- 45.Saad MJ, Carvalheira JB, Velloso LA. Birth weight and type 2 diabetes in adults. JAMA. 2009;301(15):1539. doi: 10.1001/jama.2009.485 [DOI] [PubMed] [Google Scholar]
- 46.Dyck RF, Klomp H, Tan L. From “thrifty genotype” to “hefty fetal phenotype”: the relationship between high birthweight and diabetes in Saskatchewan Registered Indians. Can J Public Health. 2001;92(5):340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308(6934):942-945. doi: 10.1136/bmj.308.6934.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130(4, pt 1):278-284. doi: 10.7326/0003-4819-130-4_Part_1-199902160-00005 [DOI] [PubMed] [Google Scholar]
- 49.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165(8):849-857. doi: 10.1093/aje/kwk071 [DOI] [PubMed] [Google Scholar]
- 50.Beaumont RN, Warrington NM, Cavadino A, et al. ; Early Growth Genetics (EGG) Consortium . Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum Mol Genet. 2018;27(4):742-756. doi: 10.1093/hmg/ddx429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawlor D, Richmond R, Warrington N, et al. Using mendelian randomization to determine causal effects of maternal pregnancy (intrauterine) exposures on offspring outcomes: sources of bias and methods for assessing them. Wellcome Open Res. 2017;2(11):11. doi: 10.12688/wellcomeopenres.10567.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanderWeele TJ, Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology. 2014;25(3):427-435. doi: 10.1097/EDE.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. doi: 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Mendelian Randomization Method
eFigure 1. Schematic Representation of a Mendelian Randomization Approach
eFigure 2. Genetic Association With Birth Weight
eFigure 3. Genetic Association With Risk of T2DM
eFigure 4. Association of Birth Weight With Risk of T2DM
eFigure 5. Causality Estimated From Individual Study
eTable 1. Baseline Characteristics of Included 49 Studies in the CHARGE-BIG Study
eTable 2. Assessment of Birth Weight and Covariates in the CHARGE-BIG Study
eTable 3. Assessment of Type 2 Diabetes in the CHARGE-BIG Study
eTable 4. Genotyping Information in the CHARGE-BIG Study
eTable 5. Distribution of Genotypes of Included 7 SNPs in the CHARGE-BIG Study
eTable 6. Associations Between Seven Loci Associated With Birth Weight and Various Anthropometric Measures Taken at Birth (Data From Summary Results)
eTable 7. Genetic Association of Birth Weight Genetic Variants With Glycemic Traits (Data From Summary Results)
eTable 8. Sixty Loci Associated With Birth Weight (P<5×10−8) in European Ancestry and/or Trans-Ancestry (Data From Summary Results)
eTable 9. Genetic Association of Birth Weight Related 60 Genetic Variants With Glycemic Traits (Data From Summary Results)
eTable 10. Association of the Genetic Risk Score With Birth Weight and F Statistic for the Instrumental Variable in the NHS, HPFS, and WHI Cohorts
eTable 11. Association of Birth Weight Genetic Risk Score With Confounders According to Quartiles of the GRS in the NHS, HPFS and WHI Studies
eAppendix. Description of Included Studies
eReferences