Abstract
Introduction:
The mechanism of tumorigenesis and metastasis of ovarian cancer has not yet been elucidated. This study aimed to investigate the role and molecular mechanism of cytosolic nonspecific dipeptidase 2 in tumorigenesis and metastasis.
Methods:
Cytosolic nonspecific dipeptidase 2 expression in human ovarian cancer tissues and cell lines was assessed with methyl thiazolyl tetrazolium (MTT), clone formation, and transwell assays performed to evaluate the ability of ovarian cancer cells to proliferate and migrate. Nude mice tumor formation experiments were also performed by subcutaneously injecting cells with stable cytosolic nonspecific dipeptidase 2 knockdown and control SKOV3 cells into BALB/c female nude mice to detect changes in PI3K/AKT pathway-related proteins by Western blotting.
Results:
Cytosolic nonspecific dipeptidase 2 was highly expressed in human ovarian cancer tissues, with its expression associated with pathological data, including ovarian cancer metastasis. A cytosolic nonspecific dipeptidase 2 stable knockdown or ectopic expression ovarian cancer cell model was established and demonstrated that cytosolic nonspecific dipeptidase 2 could promote the proliferation of ovarian cancer cells. Transwell cell migration and invasion assays confirmed that cytosolic nonspecific dipeptidase 2 enhanced cell metastasis in ovarian cancer. Furthermore, in vivo xenograft experiments demonstrated that cytosolic nonspecific dipeptidase 2 can promote the development and progression of ovarian cancer, increasing the expression of phosphorylated PI3K and AKT.
Conclusions:
Cytosolic nonspecific dipeptidase 2 promotes the occurrence and development of ovarian cancer through the PI3K/AKT signaling pathway.
Keywords: ovarian cancer, CNDP2, growth, metastasis
Introduction
Ovarian cancer is one of the most common malignant tumors in women, with more than 220 000 cases of newly diagnosed ovarian cancer globally every year.1,2 Ovarian cancer is also the most fatal gynecological malignancy, with a mortality of approximately 140 000 cases per year.3 Although the prognosis of early ovarian cancer is better, most women are already at an advanced stage when they visit hospital, and the 5-year survival rate is only approximately 40%.4 The molecular pathogenesis of ovarian cancer has not been fully elucidated, and there is currently no early screening method with good sensitivity and specificity to control tumor progress in the early stages of ovarian cancer treatment.5,6 Moreover, although there are many emerging molecular treatment options, there have been none with efficacy superior to traditional chemotherapy methods so far.
The PI3K/AKT signaling pathway is an important signal transduction pathway for cellular metabolism and is antiapoptotic.7 As the main type of PI3K, PI3K class IA is a heterodimer consisting of the regulatory subunit p85α/β/γ and the catalytic subunit p110α/β/δ/γ, which is the most relevant to tumors.8 PI3K has multiple activation pathways, the most important of which is the binding of the upstream tyrosine receptor kinase to the SH2 region on p85α, phosphorylating the 458 tyrosine of p85α, causing a conformational change in PI3K IA dimer and activating PI3K.9,10 AKT is a downstream target molecule of PI3K, with AKT Thr308 phosphorylated by PI3K-dependent kinase (PDK) 1 and Ser473 of the C-terminal hydrophobic region by PDK2.11,12 The activated AKT is then transferred to the cytoplasm or nucleus where it promotes cell growth and protein synthesis by phosphorylating mammalian target of rapamycin (mTOR).13 Previous studies found that abnormal activation of PI3K/AKT signaling pathway promoted ovarian cancer cell proliferation, invasion, metastasis, and inhibited cell apoptosis.
Cytosolic nonspecific dipeptidase 2 (CNDP2), also known as carboxypeptidase of glutamate like-B, is a metal peptidase present in the cytosol that catalyzes the hydrolysis of carnosine and several dipeptides.14 The CNDP2 is commonly expressed in human tissues but selectively expressed in different pathological tissues. In hepatocellular carcinoma (HCC), CNDP2 expression was downregulated, being involved in regulating growth, colony formation, and invasion of HCC cells.14 Cytosolic nonspecific dipeptidase 2 has been shown to act as a tumor suppressor gene in pancreatic cancer, inhibiting pancreatic cell proliferation, cell migration, and inducing cell cycle arrest.15 However, CNDP2 expression is not reduced in all tumors, and its specific molecular function is not yet clear. For example, quantitative proteomic studies have showed that CNDP2 was highly expressed in renal cell carcinoma,16,17 breast cancer, and colon cancer.18
Therefore, the aim of this study was to first analyze the expression of CNDP2 in ovarian cancer, exploring the correlation between CNDP2 differential expression and the clinical parameters of ovarian cancer. In addition, the specific molecular function of CNDP2, modifying the PI3K/AKT signaling pathway, was clarified in ovarian cancer providing a new molecular basis for the study of the mechanism of ovarian cancer.
Materials and Methods
Cell Lines and Materials
The ovarian cancer cell lines SKOV3, A2780, OVCAR3, HRA, A2780, ISEO80, and COC1 were all from the American Type Culture Collection (Manassas, Virginia). Cell lines were cultured in minimal essential medium, Dulbecco modified Eagle medium, or Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum (FBS) and grown at 37°C in 5% CO2. The antibodies CNDP2 (Abcam, ab204351, Cambridge, Massachusetts), p-AKT(Ser473; Cell Signaling Technology, #4060, Danvers, Massachusetts), AKT (Cell Signaling Technology, #9272, Danvers, Massachusetts), p-PI3K p85(Tyr458; Cell Signaling Technology, #4228, Danvers, Massachusetts), PI3K p85 (Cell Signaling Technology, #4292, Danvers, Massachusetts), and β-actin (Abcam, ab8227, Cambridge, Massachusetts) were used.
Patient and Tumor Samples
All experiments using clinical samples were approved by the ethics committee of Taizhou Central Hospital (ID: 2016 no.27). All patients gave their full consent to participate in the present study, and written consent was obtained from each patient. In total, 100 cases of primary ovarian cancer were randomly selected, and samples were surgically resected in the Taizhou Central Hospital, including the corresponding paraneoplastic tissue. All ovarian cancer tissue samples were immediately fixed in 4% paraformaldehyde for preparation of immunohistochemistry, and a small portion was frozen in liquid nitrogen.
Immunohistochemistry
All paraformaldehyde-fixed ovarian cancer tissue samples were paraffin-embedded and sectioned. Sections were deparaffinized in xylene for 20 minutes, and endogenous peroxidase was quenched with 3% hydrogen peroxide. Next, the slides were rehydrated with ethanol, and antigen retrieval was performed in sodium citrate buffer. The tissue was blocked with blocking solution for 5 minutes at room temperature before incubation with CNDP2 antibody at a final concentration of 10 μg/mL (1:100 dilution) for 1 hour at room temperature. Then, the slides were incubated with horse radish peroxidase polymerization reagents, visualized with diaminobenzidine dye, and hematoxylin counterstained. The staining results were evaluated by semiquantitative methods including staining intensity (0: negative, 1: low, 2: moderate, 3: strong, 4: strong) and percentage of stained cells (0: 0%, 1: 1%-10%, 2: 11%-35%, 3: 26%-50%, 4: 51%-80%, 5: 81%-100%). The scores of the percentage of staining intensity and percentage of stained cells were added to obtain the final score, with a value of 4 or less regarded as low expression and 5 or more considered as high expression in pathological statistics.
Cytosolic Nonspecific Dipeptidase 2 Knockdown and Overexpression
The short hairpin RNA (shRNA) sequence of CNDP2 was 5′-CCGGCCGGAAAGACACATTCTTTAACTCGAGTTAAAGAATGTGTCTTTCCGGTTTTTG-3′. The CNDP2-shRNA and the control shRNA were separately packaged in the lentivirus, then SKOV3 and OVCAR3 cells were infected, and stable knockdown cell lines were obtained by puromycin screening. CNDP2 complementary DNA (cDNA) was amplified by polymerase chain reaction (PCR) from the cDNA of ovarian cancer cells SKOV3. Constructs encoding CNDP2 were subcloned into pcDNA3 vector (Addgene, Watertown, Massachusetts), and the overexpression vector was transfected into cells using lip2000 (Invitrogen, Carlsbad, California).
Real-Time Quantitative PCR
Total RNA was extracted using the Trizol LS reagent (Invitrogen, Carlsbad, California) and messenger RNA (mRNA) was reverse-transcribed using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, California). The PCR amplification was performed using the following primers: CNDP2, 5′-CCCTCTGTTGTTACCTGCCAT-3′ (sense) and 5′-GGGAACCAGTTAGAGGCTGG-3′ (antisense); and β-actin, 5′-TTAGTTGCGTTACACCCTTTC-3′ (sense) and 5′-ACCTTCACCGTTCCAGTTT3′ (antisense). Real-time quantitative PCR was performed following a standard SYBR Green PCR protocol using SYBR Premix Ex Taq (Takara, Dalian, China). Relative eIF3d mRNA expression was normalized to the housekeeping gene β-actin.
Cell Proliferation Assay
Cell proliferation was assessed using an Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions. The absorbance at 450 nm was detected using a microplate reader (Bio-Rad, Hercules, California), and the growth curve was generated based on the absorbance values at 450 nm. The results reflect the average of 3 replicates under the same conditions.
Colony Formation Assay
Cytosolic nonspecific dipeptidase 2 knockdown or overexpression cells (2 × 103) and control groups were seeded in 6-well plates and cultured in complete medium for 2 weeks. The media were discarded and then cells were fixed with 4% paraformaldehyde and stained with 0.05% Crystal violet. Images were taken, and the number of colonies were counted in each well.
Migration and Invasion Assay
Stable CNDP2-depleted or overexpression cells (105) and control cells were washed twice with phosphate-buffered saline, resuspended in 600 µL of serum-free medium, and cultured in the upper chamber of the transwell plate. Complete medium (600 µL) containing 10% FBS was added to the lower chamber of the transwell, and the system was incubated at 37°C for 24 hours. Then, noninvasive cells in the upper chamber were gently removed with a cotton swab, and invading cells on the lower surface of the transwell membrane were stained with 0.05% Crystal violet. Cells were photographed under a microscope. Experiments were performed in triplicate.
Xenograft Tumor Experiments
Nude mice tumor formation experiments were performed by subcutaneously injecting 5 × 106 cells with stable knockdown of CNDP2, and control SKOV3 cells were injected into BALB/c female nude mice. Each group of experiments consisted of 4 replicate nude mice. The sizes of the tumors were recorded every 3 to 4 days. After 4 weeks, the nude mice were killed and each tumor was weighed and photographed.
Statistical Methods
SPSS version 19.0 was used for statistical analysis. The t test was used for statistical analysis of the categorical data, and P values <.05 were considered significant.
Results
High Expression of CNDP2 in Ovarian Cancer Is Related to Poor Prognosis
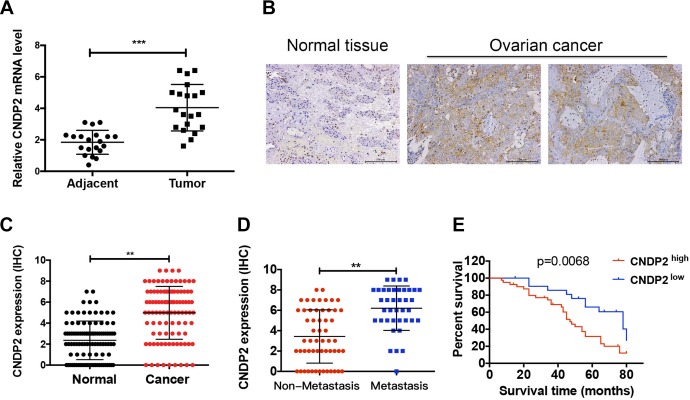
In order to explore the potential role of CNDP2 in ovarian cancer, the expression of CNDP2 mRNA was evaluated in 19 cases of ovarian cancer tissue and its adjacent tissue. The RT-PCR results showed that the expression of CNDP2 in ovarian cancer tissues was significantly higher than that in normal adjacent tissues (Figure 1A; P < .001). The CNDP2 protein was also highly expressed in 100 pairs of ovarian cancer tissues in comparison to their adjacent nontumor tissues (Figure 1B and C; P < .01). The 100 cases of ovarian cancer tissue were divided into a CNDP2 high-expression group (66%) and low-expression group (34%) according to staining intensity and density. By comparing the correlation between CNDP2 expression and clinicopathological data in patients with ovarian cancer, it was found that the expression of CNDP2 was related to lymph node metastasis and Tumor-Node-Metastasis (TNM) staging (Table 1). Furthermore, the expression of CNDP2 in metastatic ovarian cancer was significantly increased compared to nonmetastatic ovarian cancer (Figure 1D; P < .01). Kaplan-Meier analysis showed that patients with high CNDP2 expression had a shorter overall survival than patients with low CNDP2 expression (Figure 1E). These results indicate that CNDP2 may lead to metastasis and malignant progression of ovarian cancer, and high expression of CNDP2 predicts a poor prognosis.
Figure 1.
Cytosolic nonspecific dipeptidase 2 (CNDP2) is highly expressed in ovarian cancer and indicates poor prognosis. A, The messenger RNA (mRNA) expression of CNDP2 in 19 cases of ovarian cancer and adjacent tissues. B, Low expression of normal tissues (negative staining) and high expression of ovarian cancer tissues (positive staining). C, Expression of CNDP2 in 100 cases with ovarian cancer and adjacent normal tissues. D, Expression of CNDP2 in metastatic and nonmetastatic ovarian cancer tissues. E, The survival curves of 34 low CNDP2 expression patients and 66 high CNDP2 expression patients. The CNDP2 immunohistochemical (IHC) staining scores for ovarian cancer tissues obtained from patients. **P < .01, ***P < .001, Mann-Whitney U test. Kaplan-Meier plots of the overall survival of patients with ovarian cancer based on CNDP2 high- or low-level expression.
Table 1.
Correlation of CNDP2 Expression With Clinicopathological Data of Patients With Ovarian Cancer.
| Clinicopathological Patient Variable | CNDP2 Expression | P | ||
|---|---|---|---|---|
| −/+ | ++/+++ | |||
| Age | .7345 | |||
| <50 years | 32 | 11 | 21 | |
| >50 years | 68 | 23 | 45 | |
| Histology | .3763 | |||
| Serious | 75 | 20 | 45 | |
| Nonserious | 25 | 14 | 21 | |
| Nodal metastasis | .0021 a | |||
| 0 | 58 | 28 | 30 | |
| 1 | 42 | 6 | 36 | |
| Tumor differentiation | .4135 | |||
| Well/moderate | 40 | 11 | 29 | |
| Poor | 60 | 25 | 38 | |
| Tumor-Node-Metastasis (TNM) stage | .006 a | |||
| I/II | 26 | 15 | 11 | |
| III/IV | 74 | 19 | 55 | |
Abbreviation: CNDP2, cytosolic nonspecific dipeptidase 2.
a P < .05.
High Expression of CNDP2 in Ovarian Cancer Cell Lines
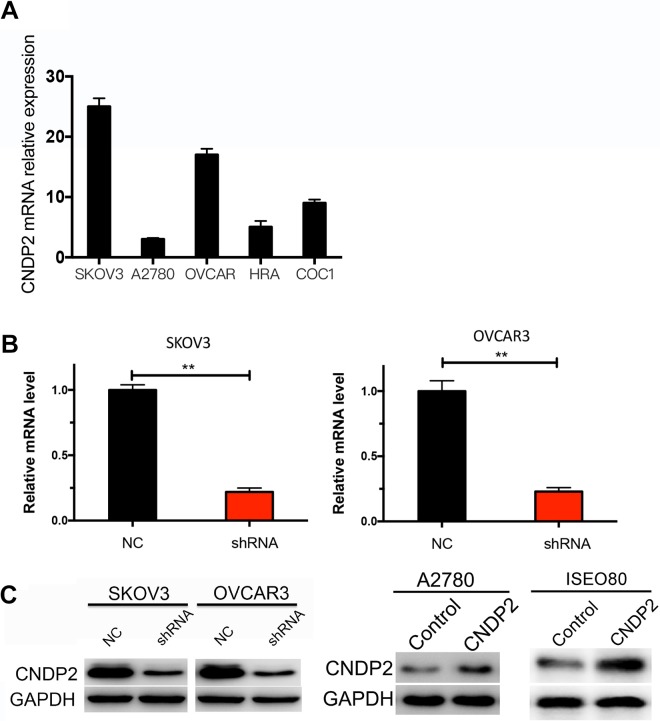
Next, the function of CNDP2 was investigated in ovarian cancer cell lines, SKOV3, A2780, OVCAR3, HRA, and COC1 by RT-PCR. The results showed that CNDP2 was highly expressed in SKOV3 and OVCAR3 cells but relatively low in A2780 cell and other cell lines (Figure 2A). Therefore, SKOV3, OVCAR3, and A2780 cells were selected for further analysis, and shRNA and overexpression vectors were constructed to establish stable CNDP2 knockdown SKOV3 and OVCAR3 cell lines or CNDP2 overexpression A2780 cell line. In addition, normal ovarian cells (ISEO80) was transfected with CNDP2 expression vector to evaluate the function of CNDP2 in ovarian cancer cell. The knockdown efficiency of CNDP2-shRNA in SKOV3 and OVCAR3 (Figure 2B) was determined by RT-PCR. Western blot analysis was performed to detect CNDP2 protein levels in CNDP2 knockdown in SKOV3 and OVCAR3 cells or CNDP2 overexpression in A2780 and ISEO80 cells (Figure 2C).
Figure 2.
Cytosolic nonspecific dipeptidase 2 (CNDP2) is highly expressed in ovarian cancer cell lines. A, The messenger RNA (mRNA) expression of 5 ovarian cancer cell lines was determined by real-time polymerase chain reaction (RT-PCR). B, RT-PCR detection of CNDP2 knockdown efficiency by shRNA in SKOV3 and OVCAR3 cells. C, Western blot detection of CNDP2 knockdown efficiency by shRNA in SKOV3 and OVCAR3 cells or overexpression efficiency by CNDP2 expression vector in A2780 and ISEO80. The graphed data represent the mean ± standard deviation (SD) of 3 independent experiments. **P < .01.
The Promotion of CNDP2 in Ovarian Cancer Cell Proliferation
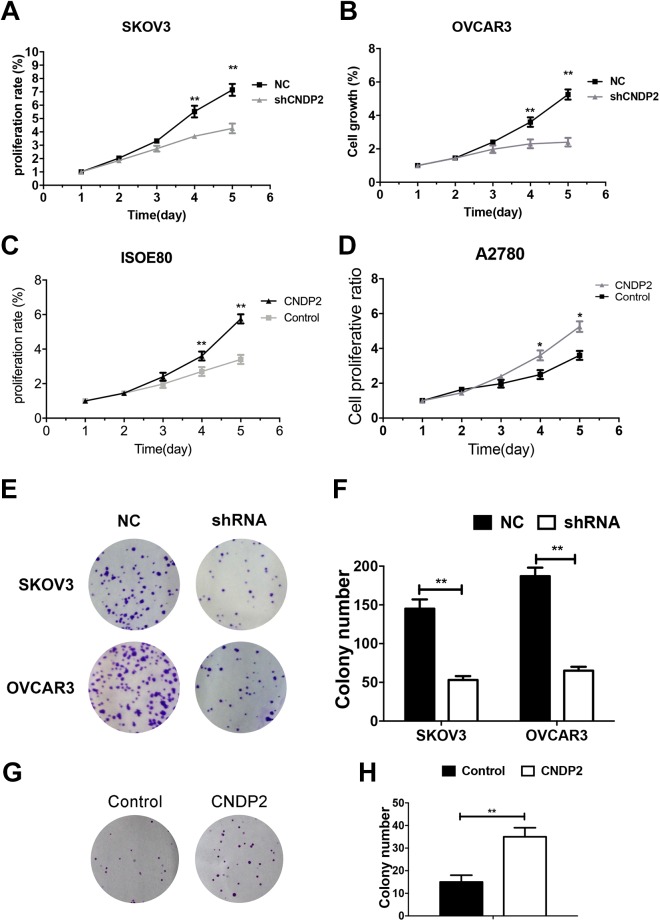
Cell Counting Kit-8 assays and colony formation assays were performed to study the effect of CNDP2 on the proliferation of ovarian cancer cells. In the CCK-8 assay, the proliferation of CNDP2-shRNA SKOV3 or OVCAR3 cells was significantly reduced compared to normal control (NC)-shRNA cells (Figure 3A and B; P < .01). In the colony formation assays, the number of colonies in SKOV3 or OVCAR3 cells was significantly reduced compared to NC-shRNA on CNDP2 knockdown (Figure 3E and F; P < .01). In contrast, cell proliferation and colony formation were significantly enhanced by overexpression of CNDP2 in ovarian cancer cells (Figure 3C, D, G, and H). Taken together, these results indicate that CNDP2 promoted ovarian cancer cell proliferation.
Figure 3.
Cytosolic nonspecific dipeptidase 2 (CNDP2) promotes ovarian cancer cell proliferation. Cell proliferation was assessed by CCK8 assay. A and B, Proliferation of SKOV3 and OVCAR3 cells transfected with CNDP2 short hairpin RNA (shRNA). C and D, Proliferation of A2780 and ISEO80 cells overexpressing CNDP2. E, Colony formation assays of SKOV3 and OVCAR3 cells transfected with CNDP2-shRNA and (F) count and statistics of the number of colonies. G, Colony formation assays of A2780 cells overexpressing CNDP2 and (H) count and statistics of the numbers of colonies. The graphed data represent the mean ± standard deviation (SD) of 3 independent experiments. *P < .05; **P < .01. CCK-8 indicates Cell Counting Kit-8.
Cytosolic Nonspecific Dipeptidase 2 Promotes Ovarian Cancer Tumorigenesis In Vivo
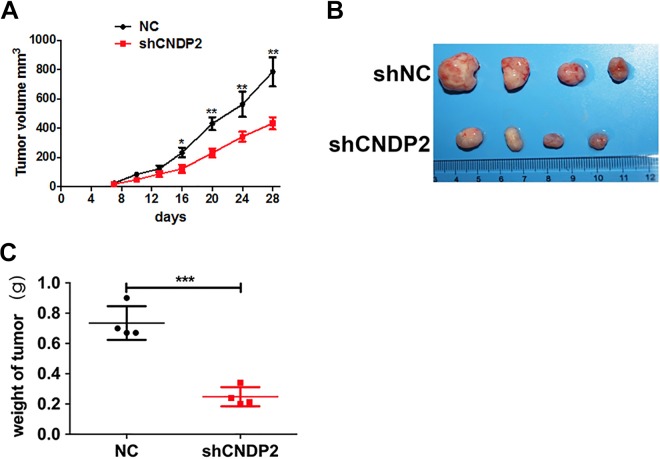
Subcutaneous tumorigenesis in nude mice using a xenograft tumor model was performed to study the role of CNDP2 in the development of ovarian cancer in vivo. In vitro cultured stable CNDP2 knockdown and control SKOV3 cells were injected subcutaneously into nude mice (4 mice per group), showing that compared to the control group, knockdown of CNDP2 (CNDP2-shRNA group) significantly slowed tumorigenesis of ovarian cancer cells (Figure 4A). Moreover, tumor size (Figure 4B) and weight (Figure 4C) of nude mice bearing CNDP2-shRNA cells were also significantly decreased compared to the control group, confirming the role of CNDP2 in promoting ovarian cancer in vivo.
Figure 4.
Cytosolic nonspecific dipeptidase 2 (CNDP2) promotes ovarian cancer tumorigenesis in vivo. A, Tumor volumes of normal control (NC) and CNDP2-shRNA SKOV3 cells in tumor-bearing nude mice. B, Photographs of the tumor from mice injected with CNDP2 short hairpin RNA (shRNA) after 7 weeks. C, Tumor weight after 4 weeks. The data shown represent the mean ± standard deviation (SD). **P < .01, ***P < .001.
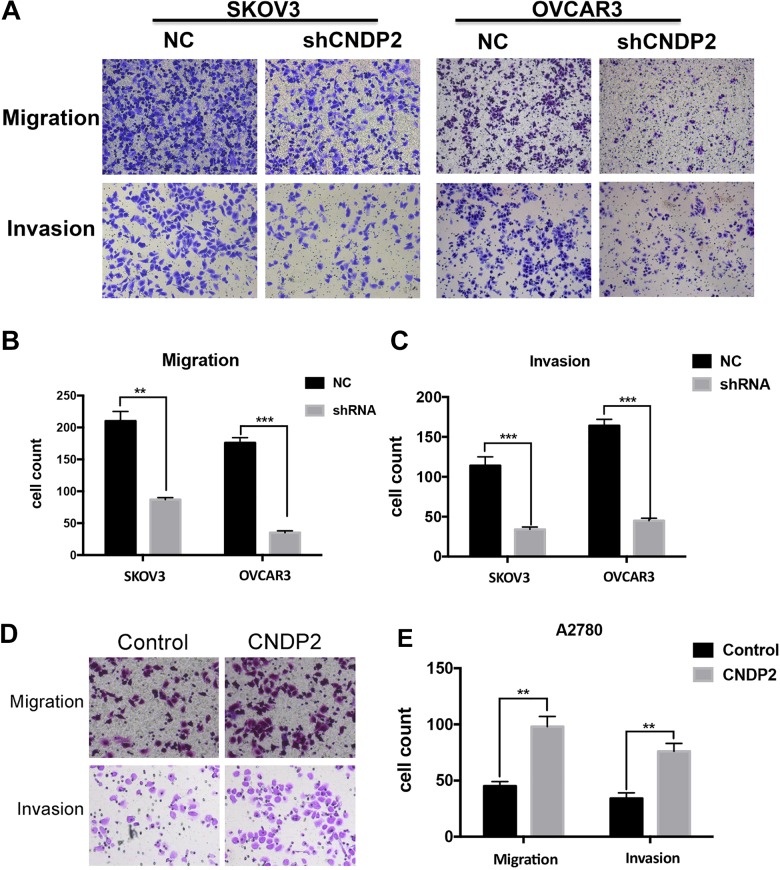
Cytosolic Nonspecific Dipeptidase 2 Promotes Ovarian Cancer Cell Migration and Invasion
Cytosolic nonspecific dipeptidase 2 also has an impact on the migration and invasion of ovarian cancer cells. In transwell cell invasion and metastasis assays, compared to the control cells, the migration and invasion ability in CNDP2 shRNA SKOV3 or OVCAR3 cells was significantly reduced (Figure 5A-C). However, overexpression of CNDP2 in A2780 promoted cell migration and invasion compared to vector control cells (Figure 5D and E). This indicates that CNDP2 could significantly promote the invasion and metastasis of ovarian cancer cells, which is consistent with the clinical data analysis (Figure 1D).
Figure 5.
Cytosolic nonspecific dipeptidase 2 (CNDP2) facilitates ovarian cancer cell migration and invasion. CNDP2 short hairpin RNA (shRNA) SKOV3 or OVCAR3 cells were plated into transwells and the migrated cells (A, top) and invasive cells (A, bottom) were photographed (A) and counted (B, C). A2780 cells overexpressing CNDP2 were plated into transwells, and the migrated cells (D, top) and invasive cells (D, bottom) were photographed (D) and counted (E). The graphed data represent the mean ± standard deviation (SD) of 3 independent experiments. **P < .01, ***P < .001.
The Activation of PI3K/AKT Pathway Under CNDP2-Positive Regulation
As the activation of PI3K/AKT pathway is common in ovarian cancer, and the PI3K/AKT pathway is closely associated with tumorigenesis and metastasis, the potential relationship between CNDP2 expression and PI3K/AKT pathway activation was investigated by Western blotting. The phosphorylation of PI3K p85 Tyr458 and AKT Ser473, respectively, represents the activation of PI3K and AKT proteins in the PI3K/AKT pathway. Western blot analysis showed that knockdown of CNDP2 by shRNA in SKOV3 or OVCAR3 cells suppressed the phosphorylation of PI3K p85 Tyr458 and AKT Ser473. Therefore, CNDP2 promoted the activation of PI3K/AKT pathway in ovarian cancer cells, which could be the molecular basis for its promotion of ovarian cancer cell proliferation and metastasis (Figure 6).
Figure 6.
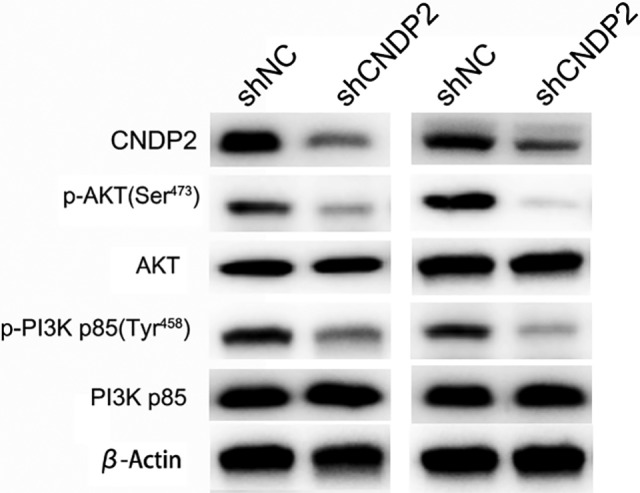
Cytosolic nonspecific dipeptidase 2 (CNDP2) positive regulates the activation of PI3K/AKT pathway. The protein levels of CNDP2, p-AKT(Ser473), AKT, p-PI3K p85, PI3K p85, and β-actin were detected by Western blotting in CNDP2 short hairpin RNA (shRNA) SKOV3 and OVCAR3 cells.
Discussion
Cytosolic nonspecific dipeptidase 2 and its homologous protein CNDP1 are dipeptide metalloproteinases belonging to the M20 family and can cleave dipeptide p-alanyl-L-histidine.19 The CNDP2 gene encodes a nonspecific carnosine peptidase that has high affinity for Cys-Gly in the γ-glutamate cycle and is involved in the biosynthesis of glutathione.20 Studies have shown that CNDP2 is highly expressed in the nigrostriatal region and therefore may be involved in the degenerative process of Parkinson disease.21 However, there is increasing evidence that the dipeptidase CNDP2 has more important functions in addition to its enzyme activity. For example, CNDP2 was found to be highly expressed in breast cancer and renal cell carcinoma, and the protein has different expression states in different stages of renal cancer.17 However, there is a lack of information regarding the role and function of CNDP2 in tumors. It has been suggested that CNDP2 may play an important role in tumorigenesis as a tumor suppressor gene in HCC and pancreatic cancer.14,15,22 Although the function of CNDP2 is controversial, it is evident that CNDP2 plays an important role in the development of tumors; therefore, it is particularly important to clarify the exact role of CNDP2 in different tumors. This study has shown the differential expression of CNDP2 in ovarian cancer, suggesting that CNDP2 may play an oncogene role in the development of ovarian cancer.
Previous studies have found that PI3K/AKT signaling pathway activates mTOR and its downstream ribosomal protein p70S6K, promoting the reorganization of actin and cell motility in ovarian cancer.23-25 AKT can upregulate the transcriptional activity of nuclear factor-κB, increasing the priming function of ovarian cancer cells, thereby contributing their invasion.26,27 The PI3K/AKT signaling pathway can upregulate the expression of matrix metalloproteinases (MMP) 2 and MMP-9 mRNA and protein and degrade extracellular matrix, promoting the transfer of ovarian cancer cells.28-30 The present study showed that CNDP2 activates the AKT signaling pathway not only promoting ovarian cancer cell proliferation and inhibition of apoptosis but may also participate in the invasion and metastasis of ovarian cancer cells by regulating the transcription of MMP-2, MMP9, or vascular endothelial growth factor. Activation of CNDP2 may also promote the transcription of these molecules. Therefore, targeting CNDP2 and AKT can further explore the phenotypic regulatory mechanisms of ovarian cancer and provide an effective target for the screening of ovarian cancer therapy.
In conclusion, this study explored the role and possible mechanisms of CNDP2 in the development of ovarian cancer. The experimental data showed that CNDP2 can activate the PI3K/AKT pathway, further promoting growth and metastasis in ovarian cancer, suggesting that CNDP2 may promote the biological function of ovarian cancer cells through cascade signaling of CNDP2-PI3K-AKT.
Abbreviations
- CCK-8
Cell Counting Kit-8
- cDNA
complementary DNA
- CNDP2
cytosolic nonspecific dipeptidase 2
- FBS
fetal bovine serum
- HCC
hepatocellular carcinoma
- mRNA
messenger RNA
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- PCR
polymerase chain reaction
- PDK
PI3K-dependent kinase
- shRNA
short hairpin RNA
- RT-PCR
real-time PCR.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study did not receive any corporate funding and was supported by Medical Science Foundation of Zhejiang, China (Y16H160098).
ORCID iD: Ling P. Zhao  https://orcid.org/0000-0002-2493-0697
https://orcid.org/0000-0002-2493-0697
References
- 1. Janas P, Kucybala I, Radon-Pokracka M, Huras H. Telocytes in the female reproductive system: an overview of up-to-date knowledge. Adv Clin Exp Med. 2018;27(4):559–565. [DOI] [PubMed] [Google Scholar]
- 2. Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20(20):5150–5156. [DOI] [PubMed] [Google Scholar]
- 3. Davidson B, Trope CG. Ovarian cancer: diagnostic, biological and prognostic aspects. Women’s Health (Lond). 2014;10(5):519–533. [DOI] [PubMed] [Google Scholar]
- 4. Zapardiel I, Diestro MD, Aletti G. Conservative treatment of early stage ovarian cancer: oncological and fertility outcomes. Eur J Surg Oncol. 2014;40(4):387–393. [DOI] [PubMed] [Google Scholar]
- 5. Nezhat FR, Apostol R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267. [DOI] [PubMed] [Google Scholar]
- 6. Sakarya DK, Yetimalar MH, Ozbasar D. Novel directions in adjuvant chemotherapy for early stage epithelial ovarian cancer. Asian Pac J Cancer Prev. 2015;16(10):4157–4160. [DOI] [PubMed] [Google Scholar]
- 7. Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–383. [DOI] [PubMed] [Google Scholar]
- 8. Vanhaesebroeck B, Whitehead MA, Pineiro R. Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J Mol Med. 2016;94(1):5–11. [DOI] [PubMed] [Google Scholar]
- 9. Ito Y, Vogt PK, Hart JR. Domain analysis reveals striking functional differences between the regulatory subunits of phosphatidylinositol 3-kinase (PI3 K), p85alpha and p85beta. Oncotarget. 2017;8(34):55863–55876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito Y, Hart JR, Ueno L, Vogt PK. Oncogenic activity of the regulatory subunit p85beta of phosphatidylinositol 3-kinase (PI3 K). Proc Natl Acad Sci U S A. 2014;111(47):16826–16829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu T, Pang Q, Wang Y, Yan X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int J Mol Med. 2017;40(6):1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dan HC, Antonia RJ, Baldwin AS. PI3K/Akt promotes feedforward mTORC2 activation through IKKalpha. Oncotarget. 2016;7(16):21064–21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaisuparat R, Limpiwatana S, Kongpanitkul S, Yodsanga S, Jham BC. The Akt/mTOR pathway is activated in verrucous carcinoma of the oral cavity. J Oral Pathol Med. 2016;45(8):581–585. [DOI] [PubMed] [Google Scholar]
- 14. Zhang P, Chan DW, Zhu Y, et al. Identification of carboxypeptidase of glutamate like-B as a candidate suppressor in cell growth and metastasis in human hepatocellular carcinoma. Clin Cancer Res. 2006;12(22):6617–6625. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Giovannetti E, Hwang JH, et al. Loss of 18q22.3 involving the carboxypeptidase of glutamate-like gene is associated with poor prognosis in resected pancreatic cancer. Clin Cancer Res. 2012;18(2):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okamura N, Masuda T, Gotoh A, et al. Quantitative proteomic analysis to discover potential diagnostic markers and therapeutic targets in human renal cell carcinoma. Proteomics. 2008;8(15):3194–3203. [DOI] [PubMed] [Google Scholar]
- 17. Perroud B, Ishimaru T, Borowsky AD, Weiss RH. Grade-dependent proteomics characterization of kidney cancer. Mol Cell Proteomics. 2009;8(5):971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue C, Zhang Z, Yu H, et al. Up-regulation of CNDP2 facilitates the proliferation of colon cancer. BMC Gastroenterol. 2014;14:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahluwalia TS, Lindholm E, Groop LC. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia. 2011;54(9):2295–2302. [DOI] [PubMed] [Google Scholar]
- 20. Kaur H, Kumar C, Junot C, Toledano MB, Bachhawat AK. Dug1p Is a Cys-Gly peptidase of the gamma-glutamyl cycle of Saccharomyces cerevisiae and represents a novel family of Cys-Gly peptidases. J Biol Chem. 2009;284(21):14493–14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Licker V, Cote M, Lobrinus JA, et al. Proteomic profiling of the substantia nigra demonstrates CNDP2 overexpression in Parkinson’s disease. J proteomics. 2012;75(15):4656–4667. [DOI] [PubMed] [Google Scholar]
- 22. Negroni L, Taouji S, Arma D, et al. Integrative quantitative proteomics unveils proteostasis imbalance in human hepatocellular carcinoma developed on nonfibrotic livers. Mol Cell Proteomics. 2014;13(12):3473–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ip CK, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene. 2011;30(21):2420–2432. [DOI] [PubMed] [Google Scholar]
- 24. Zhang F, Xiang S, Cao Y, et al. EIF3D promotes gallbladder cancer development by stabilizing GRK2 kinase and activating PI3K-AKT signaling pathway. Cell Death Dis. 2017;8(6):e2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang F, Ma Q, Xu Z, et al. Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer Res. 2017;36(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, Li J, Li G, Wang S. Effects of celastrol on enhancing apoptosis of ovarian cancer cells via the downregulation of microRNA21 and the suppression of the PI3K/AktNFkappaB signaling pathway in an in vitro model of ovarian carcinoma. Mol Med Rep. 2016;14(6):5363–5368. [DOI] [PubMed] [Google Scholar]
- 27. Ghasemi A, Hashemy SI, Aghaei M, Panjehpour M. RhoA/ROCK pathway mediates leptin-induced uPA expression to promote cell invasion in ovarian cancer cells. Cell Signal. 2017;32:104–114. [DOI] [PubMed] [Google Scholar]
- 28. Ahn JH, Lee KT, Choi YS, Choi JH. Iloprost, a prostacyclin analog, inhibits the invasion of ovarian cancer cells by downregulating matrix metallopeptidase-2 (MMP-2) through the IP--dependent pathway. Prostag Oth Lipid M. 2018;134:47–56. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Shi L, Deng Y, et al. Inhibition of leucine aminopeptidase 3 suppresses invasion of ovarian cancer cells through down-regulation of fascin and MMP-2/9. Eur J Npharmacol. 2015;768:116–122. [DOI] [PubMed] [Google Scholar]
- 30. Ghosh S, Basu M, Roy SS. ETS-1 protein regulates vascular endothelial growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3. J Biol Chem. 2012;287(18):15001–15015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]