Abstract
Background:
The efficacy of inhaled ciprofloxacin agents in the treatment of patients with bronchiectasis is controversial. The objective of the study was to review systematically the efficacy of inhaled ciprofloxacin agents in patients with bronchiectasis.
Methods:
We searched PubMed, EMBASE, and Cochrane Library databases for randomized controlled trials (RCTs) evaluating inhaled ciprofloxacin agents among patients with bronchiectasis. Data were pooled using a meta-analysis technique.
Results:
Two phase II and four phase III RCTs were included with a total of 1685 patients. Treatment durations of phase III studies were 48 weeks, while those of phase II studies were shorter. Pooled analysis of overall studies exhibited a statistically significant benefit of inhaled ciprofloxacin agents in three exacerbation outcome measures, including time to first exacerbation (hazard ratio 0.74, 95% confidence interval [CI] 0.63–0.86, I2 23%), exacerbation frequency (risk ratio [RR] 0.73, 95% CI 0.61–0.86, I2 42%), and exacerbation proportion (RR 0.85, 95% CI 0.76–0.96, I2 25%) without significant heterogeneity. Outcomes evaluating pulmonary function, quality of life, and adverse events were not significantly different between the two groups. Although eradication of respiratory pathogens was more frequently observed, the emergence of ciprofloxacin resistance was also significantly higher in the ciprofloxacin group.
Conclusions:
A meta-analysis of RCTs of inhaled ciprofloxacin agents showed clinical benefit in terms of pulmonary exacerbations in patients with bronchiectasis. Since a significant increase of resistance was also noticed, clinical trials with a longer study period are required for a conclusive assessment.
The reviews of this paper are available via the supplemental material section.
Keywords: bronchiectasis, inhaled ciprofloxacin, meta-analysis, systematic review
Introduction
Noncystic fibrosis bronchiectasis (henceforth referred to as bronchiectasis) is a heterogeneous chronic lung disease characterized by a recurrent inflammation, poor sputum clearance, and recurrent lung infection.1 Chronic lung infection was reported to be a major contributing factor to pulmonary exacerbation in bronchiectasis.2 Exacerbation of bronchiectasis can produce damage to the lung parenchyma, decrease quality of life, and eventually contribute to increased mortality.3–5 Among various pathogens of chronic lung infection in bronchiectasis, Pseudomonas aeruginosa is considered to be a major pathogen associated with poor clinical outcomes.6,7 European Respiratory Society and British Thoracic Society guidelines recommended eradication of P. aeruginosa in patients with bronchiectasis.2,8 Efficacy of inhaled antipseudomonal antibiotics was proven by clinical trials with patients with cystic fibrosis (CF)-associated bronchiectasis, and this approach was also tried in patients with bronchiectasis.9 However, the study results of inhaled antibiotics including tobramycin, colistin, and aztreonam lysinate in patients with bronchiectasis were not as satisfactory as those in patients with CF.10–12
Recently, efficacy of inhaled ciprofloxacin agents has been evaluated through multiple randomized controlled trials (RCTs).13–17 Ciprofloxacin dry powder for inhalation (DPI), BAYQ3939, has been tested for its clinical benefit in reducing exacerbation and bacterial loads in bronchiectasis through a phase II trial and two phase III trials, RESPIRE 1 and 2.14,15 ARD-3150, an inhaled antibiotic, which is a combination of liposomal ciprofloxacin and free ciprofloxacin, has also been tested for its efficacy in ORBIT-2, -3, and -4 trials.16,17 However, despite several positive outcomes in terms of exacerbation, the efficacy of inhaled ciprofloxacin in these RCTs was controversial. A systematic review with meta-analysis of these two inhaled ciprofloxacin agents will help better assess their effects and safety in the treatment of patients with bronchiectasis.
Methods
This study was conducted in accordance with the Cochrane Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on reporting systematic reviews and meta-analyses.18
Literature search and study selection
The following patient, intervention, comparison, outcome, and setting (PICOs) criteria were used for study selection: (a) patient population/problem: patients with bronchiectasis; (b) intervention: inhaled ciprofloxacin agents, including DPI and inhaled liposomal ciprofloxacin (ILC); (c) comparison: compare the clinical efficacy of inhaled ciprofloxacin agents with placebo; (d) outcome: pulmonary exacerbations; (e) setting: outpatients enrolled in clinical trials.
RCTs evaluating inhaled ciprofloxacin agents among patients with bronchiectasis were screened, regardless of region, age groups, or type of study drugs. All data formats including published article, conference abstract, and registered study data were considered. EMBASE, Cochrane Library, and MEDLINE were searched up to January 2019 using the searching keyword of (bronchiectasis AND ciprofloxacin). We searched EMBASE through its own search engine (www.embase.com), Cochrane Library through the online search engine (www.cochranelibrary.com), and MEDLINE through PubMed (www.ncbi.nlm.nih.gov/pubmed/). In addition, we searched registered clinical trials through the US National Library of Medicine database of worldwide clinical trials (www.clinicaltrials.gov). The searching strategy was identically applied to the databases. Articles published in English were reviewed.
Data extraction and management
Two independent authors (JUL and JHK) examined the search results meeting the PICOs criteria. Details of each study were extracted and tabulated, including study design, phase, geographic region, type of medication, treatment schedule, number of study patients (both ciprofloxacin and placebo), observation period, primary endpoints, secondary endpoints, and funding source. Data for both the primary and secondary endpoints were extracted from the published article or registered database. The Cochrane Collaboration’s tool for assessing the risk of bias was used for quality evaluation of the included studies.19
Outcomes and definitions
For primary outcomes, the outcome measures related to exacerbation were evaluated: time to first exacerbation, frequency of exacerbation, and proportion of exacerbated patients during the study period. Included studies had adopted different definitions of exacerbation: exacerbation of bronchiectasis was defined as (a) worsening of at least three signs or symptoms (i.e. dyspnea, wheezing, cough, 24-h sputum volume or sputum purulence) beyond normal day-to-day variation for at least 2 consecutive days, (b) fever (body temperature > 38.0°C) or malaise/fatigue, and (c) systemic antibiotic treatment in studies by De Soyza et al. and Aksamit et al.,14,15 while in studies by Serisier et al. and Haworth et al.,16,17 a pulmonary exacerbation was defined as the concurrent presence of four or more of the abnormal respiratory signs, symptoms, or laboratory findings (Supplementary Table 1).
Secondary outcomes were pathogen eradication, emergence of resistance, pulmonary function (defined as changes of forced expiratory volume in 1 s [FEV1]), quality of life (using St George’s Respiratory Questionnaire [SGRQ] score, or quality of life-bronchiectasis respiratory symptoms domain score [QOL-B RSS]), and adverse events (AEs), including any treatment-emergent AEs [TE-AEs] and serious TE-AEs). Pathogen eradication was defined as negative culture of a prespecified pathogen, while emergence of resistance was heterogeneously defined according to each study (Supplementary Table 2).
Statistical analysis
All analyses were performed based on the intention-to-treat population if available. Hazard ratio (HR) was calculated for time to first exacerbation, while rate ratio was used for frequency of exacerbation. For the change of continuous variables, mean difference (MD) was calculated. Generic inverse variance methods were used for the calculation of pooled HR, rate ratio, and MD with 95% confidence intervals (CIs). As the phase III studies of DPI provided 97.5% or 99.9% CIs for the primary endpoints,14,15 we converted these values into 95% CI for the pooled analyses, based on the calculation equation of CI. For binary outcomes, risk ratio (RR) was calculated using the Mantel–Haenszel method. Subgroup analyses were performed to evaluate separate pooled effects of each agent, DPI and ILC. Statistical heterogeneity between studies was evaluated using a chi-square test (p < 0.10 was defined to indicate significant heterogeneity), and I2 was used to denote the degree of heterogeneity (0–25% low heterogeneity, 25–50% moderate heterogeneity, 50–75% substantial heterogeneity, 75–100% considerable heterogeneity). As studies of two different formulations of inhaled ciprofloxacin agents were included, random-effects models were used regardless of statistical heterogeneity between the studies. Sensitivity analysis was considered if a significant heterogeneity was observed in the subgroup analyses of each agent. Publication bias was assessed by the funnel plot method and Egger’s test. Review Manager for Windows, version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used for meta-analysis.
Results
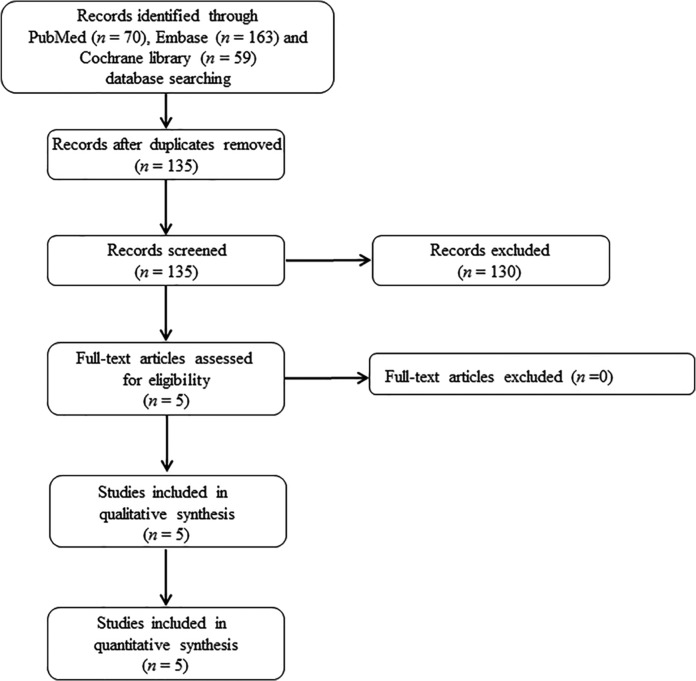
From the 135 articles initially screened, 5 publications on 6 RCTs were eligible and included in the analysis (Figure 1).13–17 Among the five publications with full text, one article consisted of results of two RCTs (ORBIT-3 and ORBIT-4 trials).17 Since RESPIRE 1 and RESPIRE 2 trials consisted of two different regimens of DPI (14 days on/off and 28 days on/off with separate study populations) for each RCT, we regarded the results of these different regimens as separate study values for meta-analysis.14,15 For several secondary outcomes including pathogen eradication, emergence of resistance, TE-AEs, and serious TE-AEs pooled data of these regimens were used, because the included studies provided only the data comparing with pooled placebo. For the evaluation of pulmonary function, emergence of resistance, pooled data of ORBIT-3 and ORBIT-4 was used for the same reason. We requested additional data from the authors by email when the original publication did not contain sufficient information, but we did not receive replies.
Figure 1.
Cochrane Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the selection of eligible studies.
Characteristics of included studies
Table 1 shows the characteristics of the six included studies. All studies were published between 2013 and 2019. A total of 1685 patients were evaluated for their outcomes in the included studies and all of them were involved in the meta-analysis: 1094 patients were included in the inhaled ciprofloxacin arm, and 591 patients were included in the placebo arm. Study size ranged from 42 patients to 304 patients. All studies were designed as RCTs: two phase II trials 13,16 and four phase III trials.14,15,17 Five studies were conducted in multiple geographic regions,13–15,17 while one was conducted only in Australia and New Zealand.16 Each agent (DPI and ILC) was evaluated by one phase II trial and two phase III trials, respectively.13–17 Studies of DPI included patients infected with predefined respiratory pathogens including P. aeruginosa,13–15 while those of ILC only included patients infected with P. aeruginosa.16,17 Detailed microbiologic inclusion criteria of individual studies are presented in Supplementary Table 3. The duration of treatment ranged from 8 weeks to 48 weeks, including on- and off-periods. Three studies which evaluated DPI had a 4–8 weeks follow-up period after the end of treatment,13–15 while three studies on ILC did not.16,17 Two phase II studies set microbiologic outcomes as primary endpoints,13,16 while four phase III studies set pulmonary exacerbation measures as primary endpoints.14,15,17 Secondary endpoints of individual studies are presented in Supplementary Table 1.
Table 1.
Characteristics of included studies.
| Author | Study design, phase, name | Region | Drug | Treatment cycle, treatment duration, follow up after treatment | Patients no., drug/placebo | Primary endpoint |
|---|---|---|---|---|---|---|
| Wilson et al.13* | RCT, phase II, n/a | 1 AP, 4 EU, 1 NA | DPI, BAYQ3939 | 28-day on/off, 8 weeks (1 cycle), 4 weeks | 60/64 | Sputum bacterial density (respiratory pathogens) |
| De Soyza et al.14* | RCT, phase III, RESPIRE 1 | 3 AP, 9 EU, 1 NA, 1 SA | DPI, BAYQ3939 | 14-day on/off, 48 weeks (12 cycles), 8 weeks 28-day on/off, 48 weeks (6 cycles), 8 weeks |
137/68 141/70 |
Time to first exacerbation Frequency of exacerbations |
| Aksamit et al.15* | RCT, phase III, RESPIRE 2 | 7 AP, 13 EU, 1 NA, 2 SA, 1 AF | DPI, BAYQ3939 | 14-day on/off, 48 weeks (12 cycles), 8 weeks 28-day on/off, 48 weeks (6 cycles), 8 weeks |
176/88 171/86 |
Time to first exacerbation Frequency of exacerbations |
| Serisier et al.16$ | RCT, phase II, ORBIT-2 | 2 AP | ILC, ARD 3150 | 28 days on/off, 24 weeks (3 cycles), none | 20/22 | Sputum bacterial density (P. aeruginosa) |
| Haworth et al.17$ | RCT, phase III, ORBIT-3 | 3 AP, 10 EU, 2 NA, 1 AF | ILC, ARD 3150 | 28-day on/off, 48 weeks (6 cycles), none | 183/95 | Time to first exacerbation |
| Haworth et al.17$ | RCT, phase III, ORBIT-4 | 3 AP, 10 EU, 2 NA, 1 SA | ILC, ARD 3150 | 28-day on/off, 48 weeks (6 cycles), none | 206/98 | Time to first exacerbation |
Funded by Bayer Pharma AG, Wuppertal, Germany. †Funded by Aradigm Corporation, Newark, CA, USA.
AF, Africa; AP, Asian-Pacific; DPI, dry powder for inhalation; EU, Europe; ILC, inhaled liposomal ciprofloxacin; n/a, not available; NA, North America; RCT, randomized controlled trial; SA, South America.
Quality assessment
The risk-of-bias items presented as percentage and summary for each included study are shown in Supplementary Figure 1. Blinding of participants and personnel and incomplete outcome data had the lowest risk of 0%, while random sequence generation had the highest risk of 25%. Two phase II studies had unclear risk of bias in several domains: The study by Serisier and colleagues had risk of bias in random sequence generation, blinding of outcome assessment, and selective reporting, and the study by Wilson and colleagues had risk of bias in random sequence generation and allocation concealment.13,16 All phase III studies showed low risk of bias in the entire domains.14,15,17
Primary outcomes
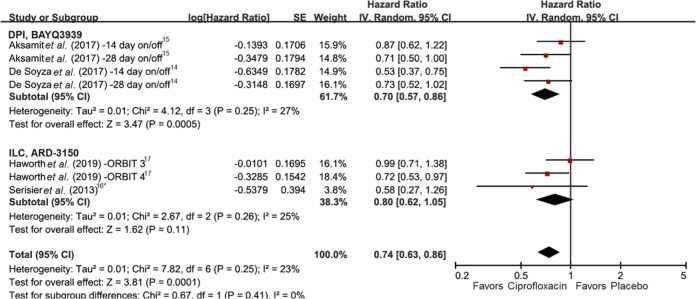
The meta-analysis results of three pulmonary exacerbation measures are summarized in Table 2. Results of seven studies involving 1561 patients were entered in evaluation of time to first exacerbation.14–17 Use of inhaled ciprofloxacin showed significant prolongation of time to first exacerbation with low heterogeneity (HR 0.74, 95% CI 0.63–0.86, I2 23%) (Figure 2). In subgroup ), while ILC did not show statistical significance.
Table 2.
Effect of inhaled ciprofloxacin in patients with bronchiectasis according to outcome measures and study drugs.
| Study drugs | Time to first
exacerbation |
Frequency of
exacerbations |
Exacerbation proportion |
|||
|---|---|---|---|---|---|---|
| No. of studies, no. of patients (drug versus control) | HR (95% CI), heterogeneity | No. of studies, no. of patients (drug versus control) | Rate ratio (95% CI), heterogeneity | No. of studies, no. of patients (drug versus control) | RR (95% CI), heterogeneity | |
| All drugs | 7, 1034 versus 527 | 0.74 (0.63–0.86), (I 2 23%) | 6, 1014 versus 505 | 0.73 (0.61–0.86), (I 2 42%) | 8, 500/1094 (45.7) versus 312/591 (52.8) | 0.85 (0.76–0.96), (I 2 25%) |
| DPI, BAYQ3939 | 4, 625 versus 312 | 0.70 (0.57–0.86), (I 2 27%) | 4, 625 versus 312 | 0.72 (0.56–0.94), (I 2 52%) | 5, 266/685 (38.8) versus 177/376 (47.1) | 0.81 (0.69–0.94), (I 2 12%) |
| ILC, ARD 3150 | 3, 409 versus 215 | 0.80 (0.62–1.05), (I2 25%) | 2, 389 versus 193 | 0.73 (0.55–0.98), (I 2 58%) | 3, 234/409 (57.2) versus 135/215 (62.8) | 0.90 (0.75–1.09), (I2 41%) |
Bold text indicates p < 0.05. Statistical heterogeneity between studies was assessed using a chi-square test, while none of analysis was significantly heterogeneous.
CI, confidence interval; DPI, dry powder for inhalation; HR, hazard ratio; ILC, inhaled liposomal ciprofloxacin; RR, risk ratio.
Figure 2.
Forest plot presenting the hazard ratios of time to first exacerbation among patients with bronchiectasis treated with inhaled ciprofloxacin versus placebo.
*Extracted from a Kaplan–Meier graph. CI, confidence interval; df, degrees of freedom; SE, standard error.
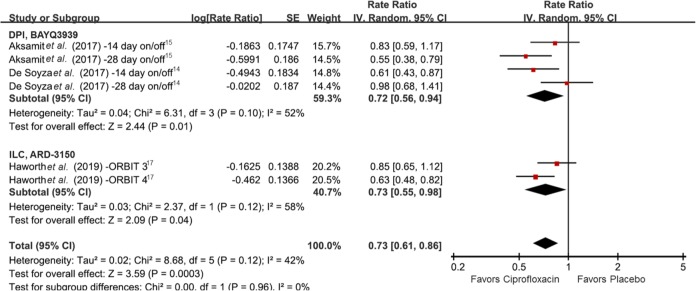
Frequency of exacerbations evaluated in the six RCTs was also significantly decreased in the inhaled ciprofloxacin group compared with the placebo group (rate ratio 0.73, 95% CI 0.61–0.86, I2 42%) (Figure 3).14,15,17 Both DPI and ILC showed statistical significance in subgroup analyses (rate ratio 0.72, 95% CI 0.56–0.94, I2 52% and rate ratio 0.73, 95% CI 0.55–0.98, I2 58%, respectively). Although moderate to substantial heterogeneity was observed, it was not statistically significant by chi-square test (p ⩾ 0.10).
Figure 3.
Forest plot presenting the rate ratios of exacerbation frequency among patients with bronchiectasis treated with inhaled ciprofloxacin versus placebo. CI, confidence interval; df, degrees of freedom; SE, standard error.
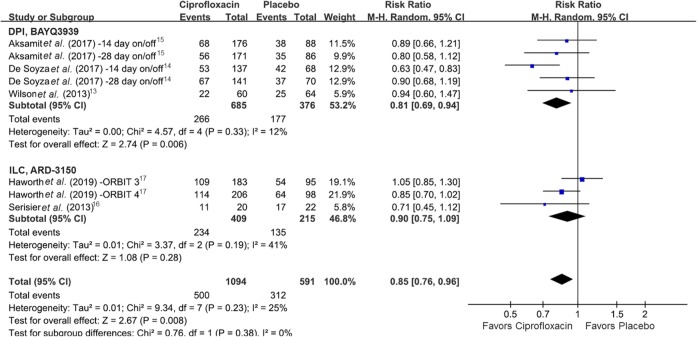
A total of eight studies were included in the pooled analysis of exacerbation proportion, and inhaled ciprofloxacin was associated with a significant decrease of exacerbation proportion when compared with placebo (RR 0.85, 95% CI 0.76–0.96, I2 25%) (Figure 4).13–17 In the subgroup analysis, the proportion was significantly less in the DPI group (RR 0.81, 95% CI 0.69–0.94, I2 12%), while statistical significance was not observed in the ILC group (RR 0.90, 95% CI 0.76–1.09, I2 41%). Sensitivity analysis was not performed to assess the primary outcomes since no significant heterogeneity was noticed using chi-square test. Publication bias was evaluated by the funnel plot method and Egger’s test (Supplementary Figure 2). Although publication bias was suggested for time to first exacerbation, it was not significant by Egger’s test (p = 0.362).
Figure 4.
Forest plot presenting the risk ratios of exacerbation proportion among patients with bronchiectasis treated with inhaled ciprofloxacin versus placebo. CI, confidence interval; df, degrees of freedom.
Secondary outcomes
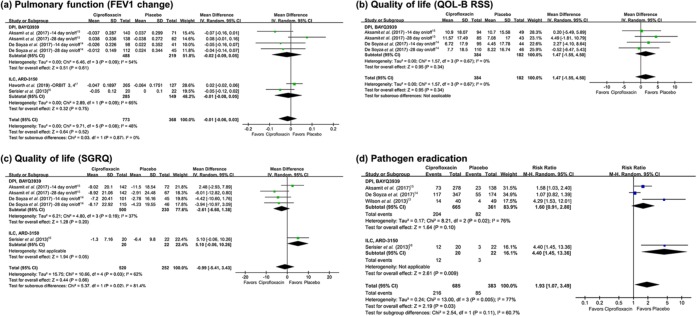
Forrest plots of secondary outcomes are presented in Figures 5 and 6. Seven studies evaluated the effect of inhaled ciprofloxacin on pulmonary function as changes in FEV1.14–17 Analysis involving overall studies showed no significant difference in FEV1 changes between the ciprofloxacin group and the placebo group, and subgroup analyses also showed no significant differences (Figure 5(a)). Since a significant heterogeneity was noticed in both subgroup analyses, we additionally performed sensitivity analysis excluding an outlier study by Aksamit and colleagues (28-day on/off regimen).15 In the sensitivity analysis, the heterogeneity became nonsignificant, and the change of pulmonary function was still not significantly different (MD –0.03, 95% CI –0.07–0.001, I2 34%).
Figure 5.
Forest plots presenting pooled analyses of secondary outcomes among patients with bronchiectasis treated with inhaled ciprofloxacin versus placebo. (a) Pulmonary function (forced expiratory volume in 1 s [FEV1] change). (b) Quality of life (quality of life-bronchiectasis respiratory symptoms domain score [QOL-B RSS]). (c) Quality of life (St George’s Respiratory Questionnaire [SGRQ]). (d) Pathogen eradication.
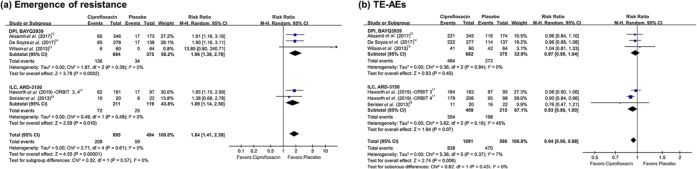
Figure 6.
Forest plots presenting pooled analyses of resistance emergence and adverse effects among patients with bronchiectasis treated with inhaled ciprofloxacin versus placebo.(a) Emergence of resistance. (b) Treatment-emergent adverse events (TE-AEs).
Four studies which evaluated the efficacy of DPI presented data of change in QOL-B RSS.14,15 Analysis of the included studies did not show a significant difference between the two groups (Figure 5(b)). For change in SGRQ score, five studies showed results.14–16 The ciprofloxacin group did not show significant improvement in SGRQ score (Figure 5(c)). Sensitivity analysis was not performed for SGRQ score since the subgroup analysis of each agent did not showed heterogeneity.
Pathogen eradication was also assessed in six studies involving 1068 patients, and all but one study had evaluated efficacy of ILC.13–16 Inhaled ciprofloxacin showed a positive effect in eradicating initially cultured respiratory pathogens (RR 1.93, 95% CI 1.07–3.49) (Figure 5(d)), but the analysis also showed a significant heterogeneity (p = 0.005, I2 77% in overall analysis; p = 0.02, I2 76% in subgroup analysis for DPI). Sensitivity analysis excluding two outlier studies by Wilson and colleagues and Serisier and colleagues13,16 showed reduced heterogeneity (p = 0.12, I2 58%), but the statistical difference was not noticed (RR 1.25, 95% CI 0.86–1.83).
Eight studies reported emergence of resistance.13–17 Pooled analysis showed that use of inhaled ciprofloxacin is likely to result in emergence of resistance without heterogeneity (RR 1.84, 95% CI 1.41–2.39, I2 0%) (Figure 6(a)). Subgroup analyses also showed that use of inhaled ciprofloxacin was significantly associated with emergence of resistance.
All eight studies reported any TE-AEs and serious TE-AEs, involving 1681 participants.13–17 Pooled analysis showed that use of inhaled ciprofloxacin is less likely to be associated with TE-AEs with low heterogeneity (RR 0.94, 95% CI 0.90–0.98, I2 7%) Figure 6(b)), while serious TE-AEs were not different between the ciprofloxacin group and placebo group (Supplementary Figure 3). Change in sputum density of bacteria was not evaluated due to disagreement in measurement methods and lack of data in the included studies.
Discussion
The present meta-analysis evaluated clinical efficacy of inhaled ciprofloxacin agents in patients with bronchiectasis, individual studies of which were controversial. Antibiotics for inhalation has a potential to produce higher concentrations in the airways, lower systemic concentration, and fewer systemic AEs compared with systemic administration,11,17,20 and the efficacy of some inhaled antibiotics was proven for management of CF bronchiectasis.9,21,22 While other antibiotics such as tobramycin, colistin, and aztreonam lysinate for inhalation had not been proven efficacious in patients with noncystic fibrosis bronchiectasis,10–12 inhaled ciprofloxacin agents showed promising results in recent RCTs.10,12,14,15 Two phase III trials of each agent, RESPIRE 1 and 2 for DPI and ORBIT-3 and -4 for ILC, were conducted respectively, but outcomes of individual studies were controversial. In RESPIRE 1, only the 14-day on/off regimen showed significant improvement in exacerbation compared with the placebo, while the 28-day/off regimen failed to prove its efficacy.14 RESPIRE 2 showed only trends in improving the two primary outcomes, but both regimens did not reach statistical significance.15 Statistical analysis for the two RESPIRE studies were almost the same, except that α corrections were differently applied (a significance level of 0.025 for each treatment arm in RESPIRE 1, and 0.049 for the 14-day on/off arm and 0.001 for the 28-day on/off arm in RESPIRE 2).15 Among trials evaluating ILC agents, only the ORBIT-4 trial exhibited benefits of inhaled ciprofloxacin compared with the placebo, while ORBIT-3 did not.17 In accordance with publication of each study, a pooled analysis of each agent was also performed: the pooled analysis of DPI showed a statistically significant benefit of inhaled ciprofloxacin in terms of exacerbation frequency,23 but that of ILC did not.17 Due to these controversial results, it was necessary to perform a meta-analysis including overall studies, for further evaluation of these agents.
Of note, the pooled analyses of included studies demonstrated a clinical benefit of inhaled ciprofloxacin agents in terms of pulmonary exacerbations. Two primary endpoints used in phase III studies evaluating efficacy of inhaled ciprofloxacin were as follows: time to first exacerbation for the approval of US Food and Drug Administration (FDA), and frequency of exacerbations for the European Medicines Agency (EMA)/other agencies.14,15 We additionally evaluated exacerbation proportion as one of primary outcomes since two phase II studies did not fully report exacerbation outcome measures.13,16 From our pooled analysis of both ILC and DPI, inhaled ciprofloxacin showed its benefits in prolongation of time to first exacerbation (HR 0.74, 95% CI 0.63–0.86, I2 23%; p = 0.0001) and reducing the frequency of exacerbations in bronchiectasis with low heterogeneity (rate ratio 0.73, 95% CI 0.61–0.86, I2 40%; p = 0.0003). Furthermore, exacerbation proportion at the end of study was significantly less in the ciprofloxacin group compared with the placebo group (RR 0.85, 95% CI 0.76–0.96, I2 25%; p = 0.008). Despite varying definitions of pulmonary exacerbation in the included studies, inhaled ciprofloxacin showed positive efficacy in reducing exacerbation of patients with bronchiectasis.
However, while treatment of inhaled ciprofloxacin showed significant improvement in exacerbations, it did not lead to improvements in quality of life according to the outcome measures used (questionnaires). Although we could not include ORBIT-3 and ORBIT-4 trials in the pooled analysis due to lack of standard deviation or 95% CI values, increase of mean QoL-B RSS scores was higher in the placebo arms (8.0% versus 12.0% in ORBIT-3 and 13.4% versus 14.4% in ORBIT-4, respectively). Only the 14-day regimen of RESPIRE 1 showed a benefit of inhaled ciprofloxacin with adjusted difference, and other original publications on each study did not fully discuss this point. Since improvement of quality of life is one of the important treatment goals in managing bronchiectasis,2,24 this discrepancy needs to be evaluated in depth for each trial. Likewise, changes in pulmonary function were also not different between the two groups in the present analysis. To observe whether inhaled antibiotics may affect disease progression and pulmonary function, clinical studies with a longer observational period would be required.
Any TE-AEs were less likely to occur in the treatment group and serious TE-AEs were not statistically different between the two groups. This suggest noninferiority of the inhaled ciprofloxacin group in terms of antibiotics-associated AEs, which was to occur from systemic exposure and localized side effects such as bronchospasm; this tendency was also discussed in the earlier studies.14,15,17 For pathogen eradication, the pooled analysis including four RESPIRE studies and two phase II studies showed a benefit of ciprofloxacin treatment with a significant heterogeneity (p = 0.03, I2 77). However, the sensitivity analysis which excluded two outlying phase II trials showed statistical nonsignificance and it was not evaluated in the ORBIT trials. In addition, it should also be taken into consideration that definitions of eradication vary among the included studies, in terms of evaluation timing and targeted pathogens. It is difficult to conclude that inhaled ciprofloxacin resulted in higher eradication rates of respiratory pathogens. On the other hand, the pooled analysis showed that resistance emergence during the study period was significantly higher in the ciprofloxacin group than in the placebo group without heterogeneity (RR 1.84, 95% CI 1.41–2.39, I2 0%). This finding was consistent in the subgroup analyses. Treatment duration of most patients enrolled in the included RCTs was almost a year, and resistance emergence was observed in 23.2% of the pooled ciprofloxacin group. This proportion is nearly double that of the placebo group (11.9%). More frequent emergence of resistance in the inhaled antibiotic group was also noticed in the previous studies of CF bronchiectasis.9 However, the included studies had ‘off periods’ after each cycle of inhaled ciprofloxacin,14,17 and the ciprofloxacin is highly concentrated in sputum.25,26 Among the included studies, the difference in resistance between the two groups in the phase III studies (ciprofloxacin versus placebo, 23.6% versus 12.5%) was not higher than that in phase II (20% versus 9.3%), despite a doubling of the study period. A prospective study with a longer observation period will clarify the correlation between the use of inhaled ciprofloxacin, resistance induction, and long-term efficacy of the drugs. Although long-term use can result in increased risk of resistance, use of antibiotics is crucial for the management of bronchiectasis since chronic bacterial infection of the lung is associated with frequent pulmonary exacerbation.2,8,14
There were several limitations. First, the number of included RCTs was small and both drugs do not yet have approval from the FDA or EMA. While the FDA did not approve the New Drug Application of DPI (November 2017) and ILC (January 2018) due to controversial outcomes of the respective two phase III trials, each pharmaceutical company is still trying to achieve approvals. Although these drugs are not currently available, the present meta-analysis gave a summary of existing controversies and firstly, demonstrated the clinical efficacy of inhaled antibiotics in treating patients with bronchiectasis. This finding should provide the background for further studies on inhaled antibiotics for bronchiectasis. Second, there was heterogeneity of the included studies in terms of antibiotic formulation and microbiologic inclusion criteria. With regard to methodological heterogeneity, we applied a random-effect model in the pooled analyses regardless of statistical heterogeneity. In addition, it should be noted that the positive finding of the pooled analysis might not necessarily mean effectiveness of individual agents. Third, while the primary outcomes of included studies were focused on pulmonary exacerbations, other longitudinal outcomes such as overall survival, hospitalization, or admissions to intensive-care units were not evaluated. Also, among the secondary outcomes, it was not clear whether the questionnaires objectively reflected the effects of inhaled ciprofloxacin on quality of life. Fourth, as the 14-day on/off regimen was included only in the RESPIRE trials, we could not assess differences between the 14-day and 28-day on/off regimens. Since 14-day on/off regimen of the RESPIRE 1 trial showed the best outcomes among the included individual studies, evaluation of the optimal on/off period should also be carried out in further studies. Lastly, registration in the PROSPERO database was omitted in the present analysis, which should be necessarily performed for systematic reviews to avoid duplication and promote protocol-driven work.
Conclusion
A meta-analysis of RCTs of inhaled ciprofloxacin agents showed clinical benefit in terms of pulmonary exacerbations in patients with bronchiectasis. Since a significant increase in resistance was also noticed, clinical trials with a longer study period are required for a conclusive assessment.
Supplemental Material
Supplemental material, Author_response_to_reviewer_comments_v.1 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Author_response_to_reviewer_comments_v.2 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Author_response_to_reviewer_comments_v.3 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Full_electronic_search_strategy for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.2 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.3 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, SUPPLEMENTARY_MATRIALS_rev_2nd for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Acknowledgments
We would like to thank Jinseob Kim (Zarathu Co., Ltd, Seoul, Republic of Korea) for statistical advice.
Footnotes
Author contributions: JUL, SWH, and J-HK developed the study concept. JUL and J-HK performed the literature review, study selection, and data extraction. JUL and SWH performed the statistical analysis. JUL and J-HK wrote the first and final manuscript drafts. All authors contributed to the writing of the manuscript and approved the final submitted version.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Jae-Hoon Ko  https://orcid.org/0000-0002-9490-6609
https://orcid.org/0000-0002-9490-6609
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Jeong Uk Lim, Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Republic of Korea, Department of Internal Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
Seung Wook Hong, Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Republic of Korea, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Republic of Korea.
Jae-Hoon Ko, Division of Infectious Diseases, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul, 06531, Republic of Korea.
References
- 1. Redondo M, Keyt H, Dhar R, et al. Global impact of bronchiectasis and cystic fibrosis. Breathe (Sheff) 2016; 12: 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50. [DOI] [PubMed] [Google Scholar]
- 3. Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J 2015; 45: 1446–1462. [DOI] [PubMed] [Google Scholar]
- 4. Olveira C, Olveira G, Gaspar I, et al. Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res 2013; 22: 597–605. [DOI] [PubMed] [Google Scholar]
- 5. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angrill J, Agusti C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002; 57: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogers GB, Zain NM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 2014; 11: 496–503. [DOI] [PubMed] [Google Scholar]
- 8. Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65: 577. [DOI] [PubMed] [Google Scholar]
- 9. Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 1999; 340: 23–30. [DOI] [PubMed] [Google Scholar]
- 10. Barker AF, Couch L, Fiel SB, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med 2000; 162: 481–485. [DOI] [PubMed] [Google Scholar]
- 11. Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014; 189: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barker AF, O’Donnell AE, Flume P, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014; 2: 738–749. [DOI] [PubMed] [Google Scholar]
- 13. Wilson R, Welte T, Polverino E, et al. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomised study. Eur Respir J 2013; 41: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Soyza A, Aksamit T, Bandel TJ, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51 pii: 1702052. [DOI] [PubMed] [Google Scholar]
- 15. Aksamit T, De Soyza A, Bandel TJ, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51 pii: 1702053. [DOI] [PubMed] [Google Scholar]
- 16. Serisier DJ, Bilton D, De Soyza A, et al. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax 2013; 68: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haworth CS, Bilton D, Chalmers JD, et al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med 2019; 7: 213–226. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bilton D, Henig N, Morrissey B, et al. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006; 130: 1503–1510. [DOI] [PubMed] [Google Scholar]
- 21. Ramsey BW, Dorkin HL, Eisenberg JD, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 1993; 328: 1740–1746. [DOI] [PubMed] [Google Scholar]
- 22. Elborn JS, Vataire AL, Fukushima A, et al. Comparison of inhaled antibiotics for the treatment of chronic Pseudomonas aeruginosa lung infection in patients with cystic fibrosis: systematic literature review and network meta-analysis. Clin Ther 2016; 38: 2204–2226. [DOI] [PubMed] [Google Scholar]
- 23. Chotirmall SH, Chalmers JD. RESPIRE: breathing new life into bronchiectasis. Eur Respir J 2018; 51: pii: 1702444. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Zijp TR, Bahar MA, et al. Effects of prophylactic antibiotics on patients with stable COPD: a systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2018; 73: 3231–3243. [DOI] [PubMed] [Google Scholar]
- 25. Stass H, Nagelschmitz J, Weimann B, et al. Safety, tolerability and pharmacokinetics of ciprofloxacin dry powder for inhalation in patients with mild to moderate chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2011; 183: abstract A3730. [Google Scholar]
- 26. Tran TT, Yu H, Vidaillac C, et al. An evaluation of inhaled antibiotic liposome versus antibiotic nanoplex in controlling infection in bronchiectasis. Int J Pharm 2019; 559: 382–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response_to_reviewer_comments_v.1 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_response_to_reviewer_comments_v.2 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_response_to_reviewer_comments_v.3 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Full_electronic_search_strategy for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.3 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease
Supplemental material, SUPPLEMENTARY_MATRIALS_rev_2nd for Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials by Jeong Uk Lim, Seung Wook Hong and Jae-Hoon Ko in Therapeutic Advances in Respiratory Disease