Abstract
Radiolabelled small molecules for imaging prostate cancer have rapidly emerged over the last few years with gallium-68-labelled prostate-specific-membrane-antigen-11 (68Ga-PSMA11), the most widely used. However, the current evidence-based guidelines for management of prostate cancer were established using computed tomography (CT), magnetic resonance imaging (MRI) and bone scan, despite their limitations. Prostate-specific-membrane antigen (PSMA) positron-emission tomography (PET)/CT, however, has higher sensitivity and specificity and can lead to both upstaging and downstaging and subsequent changes in management of prostate cancer. The literature for PSMA PET/CT is mostly in the setting of biochemical recurrence and primary staging of intermediate-to-high-risk prostate cancer. Preliminary studies also suggest that there may be a role in nonmetastatic castrate-resistant prostate cancer (nmCRPC) and possibly response to therapy. Despite high sensitivity and specificity, PSMA PET/CT as a single modality for staging advanced prostate cancer is suboptimal, given the low PSMA expression in this subgroup and the complementary role of fluorodeoxyglucose (FDG) PET/CT is required. This is also true in early-stage prostate adenocarcinoma with neuroendocrine differentiation or small-/large-cell neuroendocrine tumours of the prostate. Lack of a globally accepted standardized reporting system for PSMA PET/CT is a current limitation. This is essential to pave the way to incorporating this invaluable molecular imaging modality in clinical trials to assess its impact on outcome, particularly when upstaging or downstaging conventionally imaged disease. This would then lead to recognition by healthcare providers, incorporation into guidelines for management of prostate cancer and routine use in clinical practice.
Keywords: management of prostate cancer, prostate cancer, PSMA PET/CT, sensitivity, specificity
Introduction
Novel modalities for imaging prostate cancer have rapidly emerged over the last few years. Foremost of these are radiolabelled small molecules, including gallium-68-labelled prostate-specific-membrane-antigen-11 (68Ga-PSMA11), 18F-DCFPyL and 18F-PSMA1007, that bind with high affinity to prostate-specific-membrane antigen (PSMA) and are imaged with positron-emission tomography (PET).1 Several other small molecules and radiotracer compounds have also been used both in preclinical and clinical research, to name a few: 99mTc-PSMA (for single-photon-emission computed tomography imaging), 125I-DClBzl, 18F-CTT1057 and 68Ga-THP-PSMA.2 The current evidence-base-guiding prostate cancer management, however, was established using conventional imaging such as computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy. PSMA PET appears more accurate and can lead to both upstaging and downstaging of disease status. This knowledge can lead to changes in prostate cancer management, although, whether this improves patient outcomes, is more difficult to assess.3 Most of the experience and evolving evidence base for PSMA PET involves either primary staging of intermediate-to-high-risk patients prior to curative-intent surgery or radiotherapy, or localization of disease in patients with biochemical recurrence. In this review, we will focus on the role of PSMA PET in guiding prostate cancer management.
Current standard of care for imaging prostate cancer
Initial staging of intermediate-to-high-risk prostate cancer and restaging at biochemical recurrence is of utmost importance for choosing the optimal treatment approach, be it localized or systemic treatment, or a combination of both.
International guidelines vary on recommendation for imaging for staging and biochemical recurrence. The European Association of Urology (EAU) and Prostate Cancer Working Group 2/3 guidelines (PCWG2/3) recommend cross-sectional imaging of abdomen and pelvis, as well as radionuclide bone scan for primary staging of intermediate-to-high-risk prostate cancer.4–6 In patients with biochemical recurrence after radical prostatectomy [prostate-specific antigen (PSA) ⩾ 0.2 ng/ml], the EAU guidelines were recently amended to perform PSMA PET/CT, if available, in patients for active treatment.4
Limitations of conventional imaging
Anatomical imaging relies primarily on size for detection of nodal metastasis. A large proportion of nodal metastases in prostate cancer, up to 80%, are smaller than 8 mm in size, and thus morphological imaging fails to recognize the vast majority of these nodes.7 Diffusion-weighted images (DWI) on MRI could potentially assist with distinguishing a normal from a metastatic node;8 however, a wide overlap of DWI has been observed between the benign and malignant lymph nodes.9 This subjects anatomical imaging to a high false-negative rate for nodal staging of primary intermediate-to-high risk or metastatic prostate cancer (Figure 1). On the other hand, enlarged lymph nodes on anatomical imaging could represent other pathologies such as reactive nodes, granulomatous disease, follicular lymphoma or nodal metastases from a synchronous primary, rendering these tests also a high false-positive rate (Figure 2).
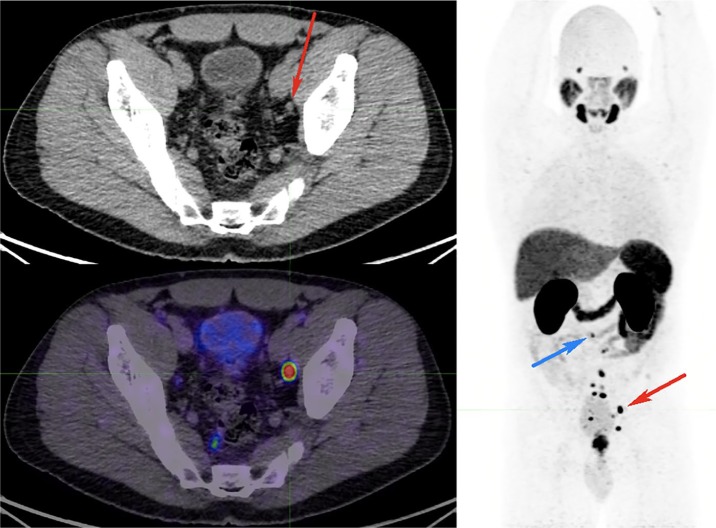
Figure 1.
68Ga-PSMA PET/CT for primary staging of Gleason score 5 + 4, PSA 12.9 prostate adenocarcinoma.
The MIP image shows multiple subcentimetre PSMA-avid pelvic and abdominal nodes (largest node 6 mm) in the left external iliac nodal station (red arrow on axial CT image), with the other smaller nodes up to the aortocaval nodal station as small as 2 mm (blue arrow on maximal-intensity projection image). All these nodes would have been missed by size criteria on CT. The patient also had a negative whole-body bone scan (not shown) at the time. Post PSMA PET/CT stage has migrated to M1a. The treatment strategy changed from a localized curative approach to a noncurative approach.
CT, computed tomography; PET, positron-emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific-membrane antigen; 68Ga-PSMA, gallium-68-labelled prostate-specific-membrane antigen; MIP, maximal intensity projection.
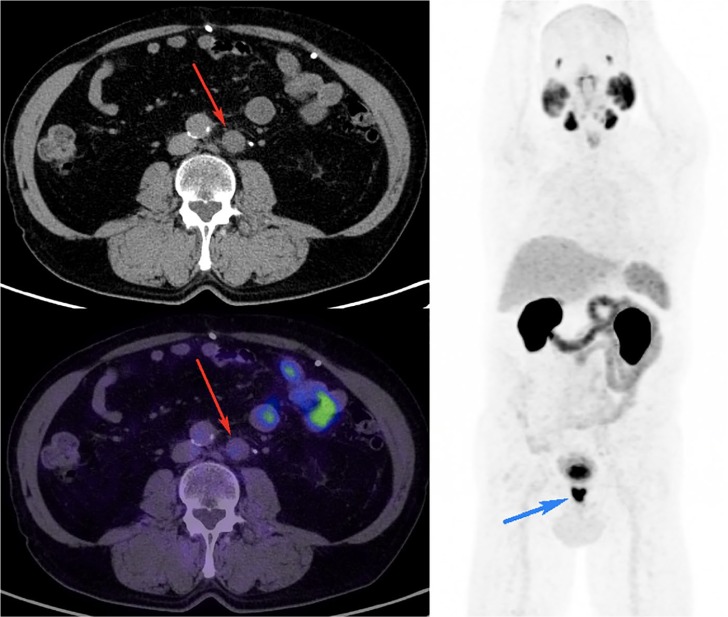
Figure 2.
68Ga-PSMA PET/CT performed for primary staging of Gleason score 4 + 3 prostate cancer.
Staging CT performed prior to PSMA PET showed multiple enlarged left para-aortic nodes suspicious for metastasis. 68Ga-PSMA PET/CT did not show any PSMA expression in these nodes (red arrow on axial fused PET/CT image showing the largest node) despite intense PSMA expression in the prostate primary (blue arrow on MIP image), suggesting another pathology. Biopsy of this node confirmed the diagnosis of large B-cell lymphoma. PSMA PET CT down-staged the disease from M1a to N0M0.
CT, computed tomography; MIP, maximal-intensity projection; PET, positron-emission tomography; PSMA, prostate-specific-membrane antigen; 68Ga-PSMA, gallium-68-labelled prostate-specific-membrane antigen.
Bone marrow is a common site of distant metastasis in prostate cancer. Conventional imaging with CT has a very low sensitivity for early detection, as marrow lesions are generally invisible until there is a reactive marrow response and progressive sclerosis. Radionuclide bone scan has the advantage of staging whole body for skeletal metastases but lacks specificity, as it images the osteoblastic activity rather than the tumour. Osteoblastic activity on the bone scan has a wide range of differential diagnoses including degenerative, benign or malignant. Even when confirming widespread osseous metastases, bone scan often underestimates the true disease burden throughout the ‘marrow’ compared with molecular imaging with PET when tracers used that image the tumour directly, such as PSMA (Figure 3) or fluorodeoxyglucose (FDG).
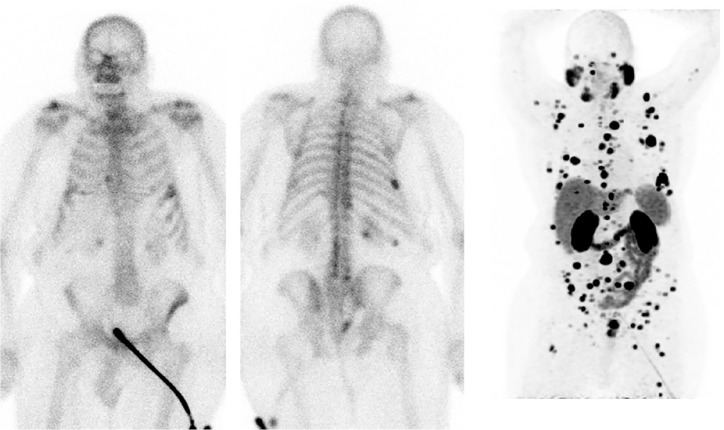
Figure 3.
Contemporaneous whole-body bone scan and 68Ga-PSMA PET/CT in a case of mCRPC progressing on abiraterone and zoledronic acid (rising PSA).
Restaging bone scan (above) showed stable osteoblastic metastases compared with the previous bone scans (not shown); however, 68Ga-PSMA PET/CT (MIP image shown below) demonstrated a significantly higher metastatic disease burden in the axial and appendicular skeleton/marrow explaining the PSA rise and sites of disease progression, although confounded, given the lack of a prior comparative scan.
CT, computed tomography; mCRPC, metastatic castration-resistant prostate cancer; MIP, maximal-intensity projection; PET, positron-emission tomography; PSA, prostate-specific antigen; 68Ga-PSMA, gallium-68-labelled prostate-specific-membrane antigen.
Sclerotic bone lesions detected on CT staging, particularly when solitary or not in the typical pattern of widespread metastases, have a large list of differential diagnosis from benign to malignant and pose a diagnostic and treatment dilemma for the reporting physician and the treating clinicians. Radionuclide bone scan can potentially assist narrowing differential diagnoses but lacks specificity as it images the osteoblastic activity of the lesion not the underlying pathology (Figure 4). This principle stands true for suspected visceral metastases as well. Lesions detected on CT or MRI in the liver or lung usually have a range of differential diagnoses which can often be narrowed down by their imaging characteristics or additional imaging but ultimately, these imaging modalities do not offer the specificity provided by molecular imaging that targets the tumour cells directly.
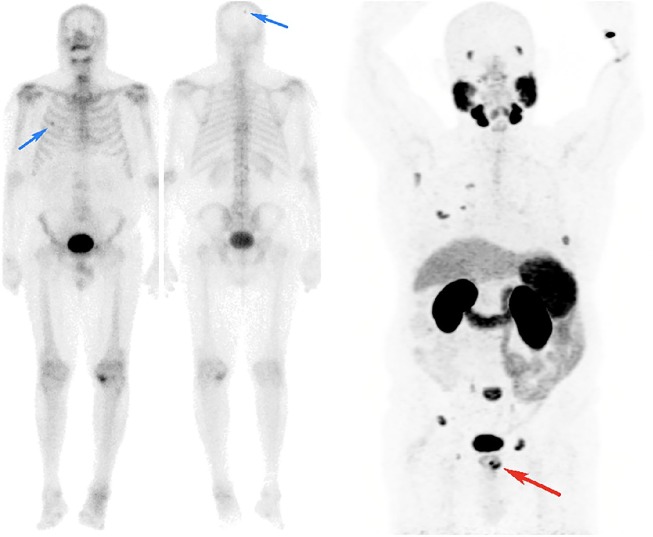
Figure 4.
Primary staging of Gleason score 4 + 3, PSA 13 prostate adenocarcinoma.
CT scan of the abdomen and pelvis (not shown) was normal and the whole-body bone scan showed suspicious osteoblastic activities in the right 4th rib and the right parietal skull (blue arrows). Equivocal bone scan findings triggered imaging with 68Ga-PSMA PET/CT (MIP image shown above) which showed intense uptake in the left lobe of the prostate (red arrow), no PSMA expression in the parietal skull, suggesting a false-positive site on the bone scan, but avid uptake in the right 4th rib in addition to multiple other sites of skeletal metastases, confidently staging the patient as M1b.
CT, computed tomography; MIP, maximal-intensity projection; PET, positron-emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific-membrane antigen; 68Ga-PSMA, gallium-68-labelled prostate-specific-membrane antigen.
In the setting of response to treatment, evaluation of bone metastases is of utmost importance, as this is a major contributor to disease-related morbidity and mortality.10 However, a well-recognized major limitation of bone scan in this context is its inability to distinguish bone healing, also called the ‘flare’ phenomenon, following initiation of an effective therapy from disease progression. Ongoing healing process results in longstanding osteoblastic activity on bone scan and permanent sclerotic changes on CT despite a good clinical and biochemical response. Hence, these imaging modalities are not a true reflection of disease status, unlike PSMA PET/CT (Figures 5 and 6).
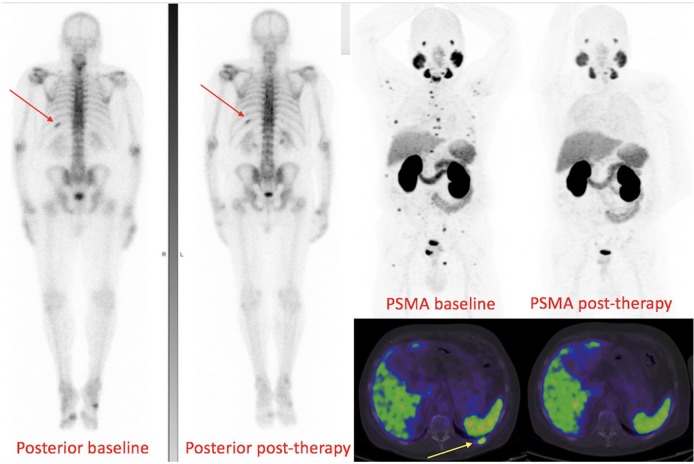
Figure 5.
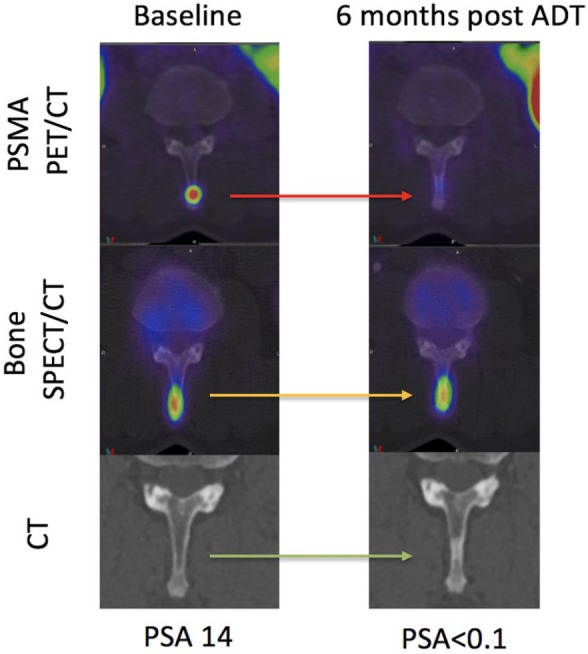
Baseline and restaging 6 months following ADT in a patient with grade group IV prostate cancer. 68Ga-PSMA PET/CT (axial fused), SPECT/CT bone scan (axial fused) and CT scans centred on a spinous process osseous metastasis are shown. At baseline, the metastasis is seen on PSMA PET/CT and bone SPECT/CT but not CT. At 6 months, a complete biochemical response (PSA < 0.1 ng/ml) was achieved correlating with complete response on PSMA PET/CT. The bone SPECT/CT, however, was stable and the CT demonstrated a ‘new’ sclerotic lesion. The bone scan and CT are not true reflective of disease status at 6 months.
ADT, androgen-deprivation therapy; CT, computed tomography; PET, positron-emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific-membrane antigen; 68Ga-PSMA, gallium-68-labelled prostate-specific-membrane antigen; SPECT, single-photon-emission computed tomography.
Figure 6.
Baseline and post-therapy whole-body bone scan and 68Ga-PSMA PET/CT (MIP and axial fused image above) of a man with newly diagnosed Gleason score 4 + 3 PSA 36 prostate cancer. Baseline bone scan showed focal uptake in the left 10th rib posteriorly (red arrow) and faint uptake in the right pubic body and possibly left inferior pubic ramus. Baseline PSMA PET showed intense PSMA uptake in the right lobe of the prostate, as well as all those osseous sites (yellow arrow showing the left 10th rib on axial fused image) in addition to multiple other PSMA-avid metastases in the axial and appendicular skeleton. At 6 months following external-beam radiotherapy to the prostate, ADT and six cycles of docetaxel with associated PSA response (down to 0.6), the PSMA PET became negative at all sites of disease while the bone scan remained unchanged. This highlights again that PSMA PET scan is a true reflection of the disease status, unlike bone scan and CT.
ADT, androgen-deprivation therapy; CT, computed tomography; MIP, maximal-intensity projection; PET, positron-emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific-membrane antigen; 68Ga-PSMA, gallium-68-labelled prostate-specific-membrane antigen.
The PCWG2/3 recommendations require documentation of two new osteoblastic lesions in two subsequent bone scans at a minimum of 8 weeks apart to confirm the diagnosis of disease progression; this approach of assessing temporal change over time enables differentiation of bone healing versus progression.6,10 While useful for clinical trials, especially when randomized and comparing between two treatments, for an individual patient this might equal to at least a 2-month delay in discontinuation of an ineffective therapy, also often subjecting the patient to its possible adverse effects, and switching to the next line of potentially more effective therapy.
Response assessment in lymph nodes or other organs is measured using the RECIST criteria and limitations are increasingly recognized in the PET era.11 These include inability to define target lesions at baseline when below size criteria (e.g. subcentimetre metastasis) or erroneously labelling enlarged but benign lesions. Changes in size are only a surrogate of true response, as size may increase or remain unchanged as tumours become fibrotic, cystic or myxoid. Change in size occurs slowly, potentially mandating a longer trial of ineffective therapy. Size change can also result from differences in contrast enhancement due to technique or different equipment. Lastly, measurement can also be subject to substantial reporter variability.
Strengths of conventional imaging
The major strength of conventional imaging is its wide availability. Thanks to decades of exposure and experience with CT, MRI and bone scan, both reporting physicians and the referring clinicians are confident with interpreting their results despite their limitations. Another major advantage of these tests is their standardization and incorporation into clinical trial designs and guidelines such as RECIST and PCWG. Last but not least, these scans, unlike PSMA PET/CT, are funded by healthcare providers for both staging and restaging prostate cancer (Table 1).
Table 1.
Summary of strengths of conventional imaging (PCWG2/3) and advantages and limitations of PSMA PET/CT.
| Advantages of conventional imaging (PCWG2/3) | Advantages of PSMA PET/CT | Limitations of PSMA PET/CT |
|---|---|---|
| • CT, MRI and bone scan are widely available • Decades of experience in reporting and standardization (such as RECIST criteria for CT and PCWG criteria for progression on bone scan) despite limitations • Funded by healthcare providers in most countries |
• Detection rate in prostate or prostatectomy bed comparable or
higher than MRI • Detection of nodal metastasis not limited by size criteria • Higher detection rate for metastatic disease particularly in lower PSA range • Lower false-positive rate (nodal, osseous and visceral) • High negative predictive value for enlarged but not metastatic nodes • Nodal, osseous and visceral metastases measurable separately by volume which can be used for monitoring therapy response • Detection of primary or local recurrence, nodal, osseous and visceral disease on a single-imaging modality ‘one-stop shop’ with higher degree of confidence than conventional imaging • Detection of marrow disease before visible on bone scan or CT • Direct visualization of tumour rather than its secondary effect (osteoblastic activity or sclerosis), closing the lag time between (a) PSA progression and a positive scan and (b) PSA response and resolution of lesions on imaging |
• Lesions (including prostatic, nodal or visceral) smaller than
4 mm could potentially be below PET resolution • No standardized criteria for reporting are widely recognized including measurement of total disease burden (nodal, osseous or visceral) • Possible ‘PSMA upregulation’ immediately following initiation of ADT or novel antiandrogens in mCRPC; timing and significance of these changes are not yet well defined • May not be ideal as a single modality in very advanced disease as PSMA expression may be lost; complementary role of FDG PET/CT is needed • Not yet funded by healthcare providers; cost is highly variable by jurisdictions (but not necessarily higher than conventional imaging) • Several similar but slightly different radiopharmaceuticals currently in use; results likely comparable |
ADT, androgen-deprivation therapy; CT, computed tomography; FDG, fluorodeoxyglucose; mCRPC, metastatic castration-resistant prostate cancer; MRI, magnetic resonance imaging; PCWG2/3, Prostate Cancer Working Group 2/3; PET, positron-emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific-membrane antigen.
Strengths of PSMA PET/CT
In a recent metanalysis of 37 studies including 4790 patients, for patients with biochemical recurrence, the rate of positive PSMA PET/CT scans increased with higher pre-PET PSMA levels.12 PSMA PET/CT particularly improved the detection rate of metastatic disease at low PSA levels of <0.2 ng/ml (33%) and 0.2–0.5 ng/ml (45%). A total of 5/37 studies reported sensitivity and specificity, and the summary sensitivity and specificity on per-node analysis were 75% and 99%, respectively. For primary staging studies, the pooled estimate of positivity in the prostate region was 90% (under the random-effect assumption) with very low proportions for sites outside the pelvis (affected by small study effects). In biochemical recurrence studies, the overall estimates of positivity were 28% in the prostate bed, 38% in pelvic lymph nodes, 13% in extrapelvic lymph nodes, 22% in bone and 5% in distant viscera.12
Multiple studies have shown that PSMA PET/CT has a moderate sensitivity but very high specificity for detection of nodal metastasis in intermediate-to-high-risk prostate cancer. A retrospective study of 130 patients with intermediate-to-high-risk prostate cancer demonstrated a sensitivity of 66% and a specificity of 99%; the missed metastatic nodes in these patients were either metastasis from a PSMA negative primary or a single micrometastatic node.13 A small prospective study of 30 intermediate-to-high-risk prostate cancer patients also showed a sensitivity of 64% in patient-based analysis and 56% in lymph node region-based analysis. Although the mean size of the missed metastatic lymph nodes in this study was 2.7 mm, PSMA PET was able to detect disease in the 3–10 mm range, below the size criteria for lymph node detection on CT.14 Sensitivity and specificity in both studies were determined by histological confirmation following prostatectomy and template pelvic lymph node dissection after imaging.
Retrospective studies have shown that PSMA PET/CT significantly outperforms whole-body bone scan for detection of bone/marrow metastases.15,16 Bone scintigraphy or sodium fluoride (NaF) PET/CT have a higher sensitivity and can visualize bone metastases before they are seen on CT.17 Since PSMA or FDG image tumour directly, they have a significantly higher sensitivity and specificity for detection of bone marrow involvement than CT or bone scan (Figure 4).
PSMA PET/CT also has the advantage of diagnosing M1c disease on a single-imaging modality or confirming or excluding suspected visceral metastasis seen on anatomical imaging. This can potentially save the patient time and risk of adverse events from additional procedures (biopsy), as well as reducing the cost for healthcare providers. Lack of PSMA expression in such lesions on PET, in the presence of PSMA-avid disease in the primary, excludes prostate cancer metastases with a high negative predictive value, while PSMA expression in those lesions confirms the presence of metastatic disease confidently.
Although the high sensitivity of PSMA PET/CT for detection of nodal, skeletal or visceral metastases is clearly an advantage at the diagnostic level, its impact on patient outcome needs to be better understood. As already mentioned earlier in this review, some patients who would have been otherwise staged as M0 and deemed suitable for localized definitive treatment, would migrate to stage M1a or M1b (Figure 1) which would alter their management path from localized to systemic therapy. While it is rational to assume that management decisions based on more accurate staging translates to better patient outcomes, this remains an open question which ideally is addressed in a randomized, controlled, prospective trial with outcome measures.
PSMA PET/CT in primary staging of intermediate-to-high-risk prostate cancer
A recent meta-analysis of six studies (including 298 patients) evaluating the diagnostic performance of PSMA PET/CT in primary staging of intermediate-to-high-risk prostate cancer showed pooled sensitivity of 71% and pooled specificity of 95%.18
In a retrospective study of 130 patients in this setting, 31% showed nodal metastases on PSMA PET/CT with sensitivity, specificity and accuracy of 66%, 99% and 88% (histologically proven) compared with 44%, 85% and 72% on anatomical imaging, respectively.13 In two other small retrospective cohorts of 15 patients (each) being evaluated for radiotherapy, PSMA PET/CT changed tumour, node and metastasis (TNM) staging in more than 50% of cases altering the radiotherapy treatment regimen and the target volume.19,20 In a prospective multicentre trial of mixed primary staging (108 patients) and biochemical recurrence, PSMA PET/CT led to a change in management intent in 21% of primary-staging patients.21
A prospective study of 30 patients with intermediate-to-high-risk prostate cancer who underwent PSMA PET/CT followed by radical prostatectomy and extended pelvic node dissection showed sensitivity, specificity, positive predictive value (PPV) and negative predictive value of 64%, 95%, 88% and 82%, respectively on a patient-based analysis.14 Therefore, given the moderate sensitivity of PSMA PET/CT for detection of lymph node metastasis in this context (although higher than size criteria on anatomical imaging), pelvic node dissection remains the gold-standard practice.
To summarize, the majority of the literature to date has been around the utility of PSMA PET/CT in the setting of biochemical recurrence in prostate cancer with lesser focus around the primary staging. However, the available evidence so far is promising and may further expand the role of this imaging modality in primary staging in the future. A multicentre (10 centres) randomized study comparing PSMA PET/CT with CT and bone scanning in primary staging is nearing completion and will provide high-level evidence of accuracy and outcomes.22
PSMA PET/CT in biochemical recurrence
In the largest retrospective cohort of biochemical recurrence including 1007 patients, 80% of patients (sensitivity) had at least one lesion suggestive of prostate cancer recurrence on PSMA PET/CT. Nodal metastasis detection rate was 46% in PSA < 0.2 ng/ml category; in the other PSA subcategories, the higher the PSA level, the greater the detection rate (86% for PSA between 2 and 3 ng/ml and 96% for PSA > 10 ng/ml). Hence, detection rate was clearly associated with PSA level; androgen-deprivation therapy (ADT) was also shown to be associated with tumour detection. There was an association without statistical significance between Gleason score and detection rate; PSA doubling time and velocity were not associated with tumour detection rate.23
In a prospective multicentre trial of 635 men with biochemical recurrence, on a patient-based analysis, the PPV of PSMA PET/CT was 84% by histological validation and 92% by a composite reference standard. PSMA PET/CT localized recurrent disease in 75% of patients and detection rates significantly increased with increasing PSA levels. PET-directed focal therapy alone led to a PSA drop of 50% or more in 80% the patients.24
In a retrospective analysis of 248 patients with biochemical recurrence after radical prostatectomy, PSMA PET/CT showed an overall detection rate (sensitivity) of 89%. The detection rates were higher for higher PSA levels, with the lowest rate being 58% in the PSA category of <0.2 ng/ml. No significant association was observed with PSA doubling time or ADT (within 6 months of PSMA PET/CT scan) in this study but in the higher Gleason score category (⩾8) the detection efficacy was significantly increased. Compared with CT, PSMA PET revealed additional sites of metastases in 25% of cases.25 Another retrospective study included 532 men with biochemical recurrence, 425 post radical prostatectomy and 107 post definitive radiotherapy. In the PSA category < 0.5 ng/ml, the detection rate for the radical prostatectomy group and definitive radiotherapy groups were 38% and 33%, respectively. A sum of 71% of the postradiotherapy group showed evidence of local recurrence on PSMA PET/CT. This study also showed a relatively high rate of detection of metastasis outside pelvis at low PSA levels, influencing the decision for salvage therapy.26
In a multicentre prospective trial of mixed primary staging (108) and biochemical recurrence (312 patients), overall PSMA PET/CT led to a change in the management plan in 51% of the patients with the greatest impact amongst the biochemical recurrence group (62% change in management intent). PSMA PET/CT revealed unsuspected metastases in the prostate bed in 27% of the patients, locoregional nodal metastases in 39% and distant metastases in 16%.21
PSMA PET/CT in nonmetastatic castrate-resistant prostate cancer (nmCRPC)
nmCRPC or M0 CRPC is characterized by rising PSA level, castrate testosterone levels and no evidence of distant metastasis by conventional imaging. These patients are presumed to have microscopic distant metastatic disease below imaging resolution. Evolving evidence is demonstrating improved survival from early treatment intensification in these patients. The SPARTAN trial (Selective Prostate Androgen Receptor Targeting with ARN-509) has shown significant efficacy of apalutamide in men with nmCRPC with a PSA doubling time of less than 10 months (median metastasis-free survival of 40.5 months versus 16.2 months with placebo).27 Similarly, the PROSPER trial (patient-reported outcomes following enzalutamide in men with nmCRPC), in men with PSA doubling time of 10 months or less, has also shown significant improvement in metastasis-free survival in men on enzalutamide (36.6 months) compared with men on placebo (14.7 months), as well as improvement in health-related quality of life.28
In a retrospective international collaborative study, 200 patients with PSMA PET/CTs were selected from a large cohort using a ‘SPARTAN-like’ inclusion criteria. PSMA PET/CT detected N1 and M1 disease in almost all (98%) of these patients. PSMA PET/CT detection rate for M1 disease was similar to PSA doubling time < 10 months and the Gleason score > 8 subgroup.29 Although these patients will benefit from androgen-receptor inhibitors, as shown in SPARTAN trial, whether local salvage therapy would have additional benefit in this high-risk cohort remains questionable and would be best answered in the setting of a prospective, multicentre, randomized controlled trial.
Limitations of PSMA PET
No standardized reporting system or criteria is currently used widely for reporting PSMA PET/CT in clinical day-to-day practice. In the context of clinical trial design, this is a major disadvantage. Nevertheless, literature is evolving in this domain, including an international collaborative work promoted by the European Association of Nuclear Medicine, which provides a valuable framework for standardized reporting.30 Upon successful clinical application of prostate MRI reporting system (PIRADS), there is now a proposal published on a PSMA-RADS system for reporting PSMA PET scans.31 Another proposed criteria for molecular imaging TNM (miTNM) staging on PSMA PET/CT ‘Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE)’ has been published through an international collaborative work.32 None of the above has yet been incorporated into the daily clinical practice. Currently, any degree of PSMA uptake (above the adjacent background uptake) in a region without physiological PSMA expression is considered abnormal and would be suggestive of ‘recurrent’ or ‘metastatic’ disease and interpreted as such in the absence of a clear alternative explanation.30
The use of the word ‘specific’ in PSMA implies lack of PSMA expression in other malignancies. PSMA expression, however, has been shown and reported in the literature in multiple extraprostatic, benign and nonprostatic, malignant lesions, although this is usually characterized by a lower-intensity uptake.33,34 This emphasizes the high degree of vigilance and careful interpretation required by reporting physicians when unexpected PSMA expression is observed in lesions out of context with the patient’s PSA, Gleason score or clinical presentation.
The role of PSMA PET/CT in response assessment is a less explored territory and perhaps a current limitation. However, the literature is developing gradually with small primary and preliminary studies showing promising results in both metastatic castrate-resistant prostate cancer (mCRPC) and metastatic castrate-sensitive prostate cancer (mCSPC) in the setting of docetaxel chemotherapy, as well as mCRPC in the context of Lutetium-177 (177Lu)-PSMA therapy.35 In a retrospective study of 142 patients with biochemical recurrence, a subgroup of 23 patients had undergone PSMA PET/CT before and after therapy (either external-beam radiation, ADT or docetaxel). Whole-body total lesion on PSMA PET (TL-PSMA) was shown to have a higher agreement with PSA level than the CT-based response evaluation using RECIST 1.1 criteria.36
ADT with either gonadotropin-releasing-hormone (GnRH) analogues or GnRH antagonists, first-generation antiandrogens (bicalutamide, nilutamide and flutamide) and the new antiandrogens (abiraterone and enzalutamide) are commonly employed at different spectrums of prostate cancer. The effect of these therapies on PSMA expression and the maximum standardized uptake value (SUVmax) of the lesions on PSMA PET/CT has become the subject of a few small studies due its important clinical implications on the interpretation of PSMA PET scans and also timing and sequencing of PSMA targeted therapies.
In a small retrospective study of 10 patients with 31 prostate cancer lesions on PSMA PET/CT, it was shown that continuous long-term ADT significantly reduced the visibility of castrate-sensitive prostate cancer on subsequent PSMA PET scans. Therefore, if the objective of imaging is visualization of the maximum disease burden, the PSMA PET/CT should be performed prior to ADT initiation.37 In a prospective study of two small cohorts of hormone-sensitive and castrate-resistant prostate-cancer patients, there was rapid dichotomous response (as early as day 9 post initiation of androgen blockade) on PSMA PET/CT imaging, with the hormone-sensitive cohort showing a median 30% reduction in SUVmax from baseline while the castrate-resistant cohort demonstrated a median 45% increase in SUVmax.38
Early-spectrum prostate adenocarcinoma is invariably PSMA avid. However, there is a small subgroup (approximately 15%) of high-grade but low-PSA prostate cancers such as prostate adenocarcinoma with neuroendocrine differentiation or small- or large-cell neuroendocrine carcinomas with Gleason score of 8 or above that behave aggressively.39,40 These subgroups, given their aggressive features, show high metabolic activity on FDG PET/CT but no or low PSMA expression on PSMA PET. So, PSMA PET/CT as a single-staging modality will not be perfect without the complementary information provided by FDG PET/CT, even in the early spectrum of prostate cancer diagnosis.
Loss of PSMA expression in patients with advanced disease
PSMA PET/CT as a single-imaging modality in restaging biochemical recurrence has its own restraints. This is particularly true in the cohort of progressive mCRPC who have been previously exposed to several lines of therapy where tumour heterogeneity develops (Figure 7). We observed this in screening patients for our phase II trial of 177Lu-PSMA-617.41 Sixteen patients (24%) were screened and excluded on the basis of low PSMA expression or sites of FDG-avid, PSMA-negative disease on PET/CT scans. These patients had a poor outcome with a median survival of 2.5 months.42 This highlights how FDG PET/CT provides complementary information to PSMA PET/CT by identifying sites of disease that are aggressive with low PSMA expression.
Figure 7.
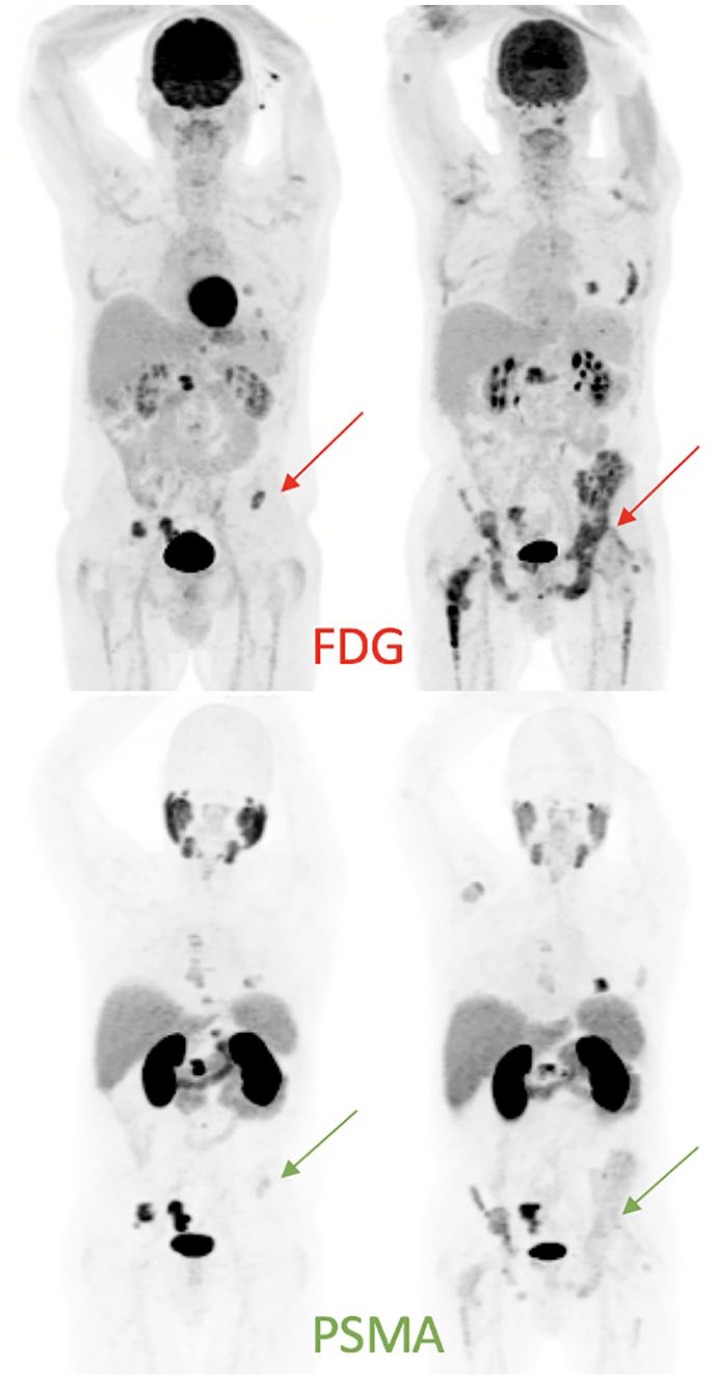
Patient with progressive mCRPC despite ADT and abiraterone was referred for 177Lu-PSMA therapy.
At baseline, paired FDG and PSMA PET/CT (MIP images on the left) showed concordant osseous metastases, except for a small left-iliac metastasis with high FDG uptake and only faint PSMA expression. During 177Lu-PSMA therapy, the patient developed increasing left hip pain, with no corresponding abnormality seen on post-therapy imaging (not shown). Restaging scans 2 months post four cycles of 177Lu PSMA showed progression of osseous metastases in a heterogeneous pattern, significantly more prominent on FDG than PSMA (MIP images on the right).
ADT, androgen-deprivation therapy; CT, computed tomography; FDG, fluorodeoxyglucose; mCRPC, metastatic castration-resistant prostate cancer; MIP, maximal-intensity projection; PET, positron-emission tomography; PSMA, prostate-specific-membrane antigen.
This observation highlights that PSMA PET/CT, as a standalone imaging modality, for staging or restaging prostate cancer may not be a robust tool, as loss or reduction of PSMA expression could either be interpreted as ‘response’ or dedifferentiation into a more aggressive disease phenotype indicating ‘progression’. So, can this be extrapolated that paired PSMA and FDG PET/CT provide the best ‘overall’ assessment of disease status in prostate cancer regardless of stage? Is this feasible in routine clinical practice to employ both modalities? We believe the answer to the first question, at least in the progressive mCRPC cohort and the high-grade low-PSA subgroup, is yes; however, the feasibility of the paired PET approach in routine clinical practice is a question which would be best addressed in prospective trials with complex survival outcomes and cost–benefit analysis.
FDG PET/CT is also a powerful prognostic tool for survival in prostate cancer. In a study of men with mCRPC, the sum SUVmax of all lesions on FDG PET/CT was an independent prognostic factor of overall survival.43 In another study of mCSPC patients, the sum SUVmax of all lesions and the total number of lesions on FDG PET/CT were independent prognostic indicators of time-to-hormonal-treatment failure, which was defined as treatment change to chemotherapy or death.44
Future direction: quantitative PET
In a large phase III prospective trial of mCRPC, automated Bone Scan Index (aBSI) was a better prognosticating indicator of overall survival than manual lesion counting.45 It is a relatively easy task to automatically quantify total disease burden on PSMA and FDG PET scans analogous to Quantitative Total Bone Imaging (QTBI) on NaF PET/CT or aBSI on whole body-bone scan.46 In patients with extensive metastatic disease, such volumetric measurements of ‘total disease burden’ provide invaluable additional information that could result in a better understanding of the symptoms and assist with management and treatment individualization.47 We believe that developing such computer software that is standardized and cross validated through multiple high-volume international PET centres, enabling us to perform this task automatically and time efficiently is a much-needed priority.
High-burden metastatic disease (versus low burden) is well established on conventional imaging. For instance, in the CHAARTED and STAMPEDE clinical trials, high-burden disease was defined as four or more bone metastases on whole-body bone scan, CT or MRI with one or more outside the vertebral bodies or pelvis, or visceral metastases, or both.48,49 However, this could not be extrapolated into the molecular imaging, given first, the higher sensitivity of these scans and second, the high rate of detection of nodal metastases relative to conventional imaging. Therefore, an agreed consensus definition of high-burden disease on PSMA PET/CT is another subject in need of attention by molecular imaging and uro-oncology societies.
Conclusion
There has been a rapid explosion of molecular imaging, in particular PSMA PET/CT, in prostate cancer in recent years; a PubMed search filtered by date for ‘PSMA PET prostate’ revealed 5 publications in 2012 and 282 in 2018. This, in addition to its rapid incorporation into clinical use outside clinical trials, has resulted in a relatively slow emergence of high-quality prospective data. These are needed to better define the role of this highly valuable diagnostic test at different spectrums of prostate cancer from primary staging to restaging following biochemical recurrence alongside other clinical aspects such as response to therapy, whether it be hormonal, cytotoxic chemotherapy, external-beam radiation or novel targeted therapies. All of the above questions will be better answered when new PET tracers, including PSMA, are routinely embedded into prospective clinical trials.
Footnotes
Funding: Dr Hofman acknowledges research support in the form of a clinical fellowship award from the Peter MacCallum Foundation. Dr Hofman also receives research support from Movember Australia, Prostate Cancer Foundation of Australia (PCFA) and Prostate Cancer Foundation (PCF).
Conflict of interest statement: Dr Alipour reports no conflicts of interest. Dr Azad reports personal fees from Janssen, grants, personal fees, nonfinancial support and other from Astellas, personal fees from Novartis, grants and nonfinancial support from Merck Serono, personal fees from Tolmar, personal fees, nonfinancial support and other from Amgen, personal fees and other from Pfizer, personal fees from Bayer, personal fees and other from Telix Pharmaceuticals, personal fees and other from Bristol-Myers Squibb, personal fees and other from Sanofi, outside the submitted work. Dr Hofman reports personal fees for lectures from Janssen, Ipsen, Sanofi-Genzyme, and research support from Endocyte (a Novartis company).
ORCID iD: Ramin Alipour  https://orcid.org/0000-0002-1248-0967
https://orcid.org/0000-0002-1248-0967
Contributor Information
Ramin Alipour, Molecular Imaging and Therapeutic Nuclear Medicine, Peter MacCallum Cancer Centre, Level 5, 305 Grattan Street, Melbourne, VIC 3000, Australia.
Arun Azad, Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia; Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia.
Michael S. Hofman, Molecular Imaging and Therapeutic Nuclear Medicine, Peter MacCallum Cancer Centre, Melbourne, Australia Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia.
References
- 1. Hofman MS, Iravani A, Nzenza T, et al. Advances in urologic imaging: prostate-specific membrane antigen ligand PET imaging. Urol Clin North Am 2018; 45: 503–524. [DOI] [PubMed] [Google Scholar]
- 2. Wester HJ, Schottelius M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin Nucl Med 2019; 49: 302–312. [DOI] [PubMed] [Google Scholar]
- 3. Vapiwala N, Hofman MS, Murphy DG, et al. Strategies for evaluation of novel imaging in prostate cancer: putting the horse back before the cart. J Clin Oncol 2019; 37: 765–769. [DOI] [PubMed] [Google Scholar]
- 4. European Association of Urology. Guidelines on prostate cancer, https://uroweb.org/guideline/prostate-cancer/#5. Accessed August 1, 2019.
- 5. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016; 34: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008; 63: 387–395. [DOI] [PubMed] [Google Scholar]
- 8. Eiber M, Beer AJ, Holzapfel K, et al. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol 2010; 45: 15–23. [DOI] [PubMed] [Google Scholar]
- 9. Akduman EI, Momtahen AJ, Balci NC, et al. Comparison between malignant and benign abdominal lymph nodes on diffusion-weighted imaging. Acad Radiol 2008; 15: 641–646. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz LH, Seymour L, Litiere S, et al. RECIST 1.1 - standardisation and disease-specific adaptations: perspectives from the RECIST working group. Eur J Cancer 2016; 62: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50(Suppl. 1): 122s–150s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. Epub ahead of print 14 February 2019. DOI: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 13. Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016; 195: 1436–1443. [DOI] [PubMed] [Google Scholar]
- 14. Van Leeuwen PJ, Emmett L, Ho B, et al. Prospective evaluation of 68gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int 2017; 119: 209–215. [DOI] [PubMed] [Google Scholar]
- 15. Thomas L, Balmus C, Ahmadzadehfar H, et al. Assessment of bone metastases in patients with prostate cancer-A comparison between (99m)Tc-bone-scintigraphy and [68Ga]Ga-PSMA PET/CT. Pharmaceuticals (Basel) 2017; 10pii: E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 2114–2121. [DOI] [PubMed] [Google Scholar]
- 17. Høilund-Carlsen PF, Hess S, Werner TJ, et al. Cancer metastasizes to the bone marrow and not to the bone: time for a paradigm shift! Eur J Nucl Med Mol Imaging 2018; 45: 893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SJ, Lee SW, Ha HK. Diagnostic performance of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for primary lymph node staging in newly diagnosed intermediate to high-risk prostate cancer patients: a systematic review and meta-analysis. Urol Int 2019; 102: 27–36. [DOI] [PubMed] [Google Scholar]
- 19. Dewes S, Schiller K, Sauter K, et al. Integration of 68Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: a retrospective study. Radiat Oncol 2016; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterzing F, Kratochwil C, Fiedler H, et al. 68Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging 2016; 43: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roach PJ, Francis R, Emmett L, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med 2018; 59: 82–88. [DOI] [PubMed] [Google Scholar]
- 22. Hofman MS, Murphy DG, Williams SG, et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int 2018; 122: 783–793. [DOI] [PubMed] [Google Scholar]
- 23. Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 2017; 44: 1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 2019; 5: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015; 56: 668–674. [DOI] [PubMed] [Google Scholar]
- 26. Meredith G, Wong D, Yaxley J, et al. The use of 68Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int 2016; 118(Suppl. 3): 49–55. [DOI] [PubMed] [Google Scholar]
- 27. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018; 378: 1408–1418. [DOI] [PubMed] [Google Scholar]
- 28. Tombal B, Saad F, Penson D, et al. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2019; 20: 556–569. [DOI] [PubMed] [Google Scholar]
- 29. Hadaschik BA, Iravani A, Hofman MS, et al. Prostate-specific membrane antigen positron emission tomography (PSMA-PET) in high-risk nonmetastatic castration-resistant prostate cancer (NMCRPC) SPARTAN-like patients negative by conventional imaging. Eur Urol Suppl 2009; 18: e698–e699. [Google Scholar]
- 30. Fanti S, Minozzi S, Morigi JJ, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging 2017; 44: 1622–1635. [DOI] [PubMed] [Google Scholar]
- 31. Rowe SP, Pienta KJ, Pomper MG, et al. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET imaging: PSMA-RADS version 1.0. J Nucl Med 2018; 59: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eiber M, Herrmann K, Calais J, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med 2018; 59: 469–478. [DOI] [PubMed] [Google Scholar]
- 33. Alipour R, Gupta S, Trethewey S. 68Ga-PSMA uptake in combined hepatocellular cholangiocarcinoma with skeletal metastases. Clin Nucl Med 2017; 42: e452–e453. [DOI] [PubMed] [Google Scholar]
- 34. Malik D, Kumar R, Mittal BR, et al. 68Ga-labeled PSMA uptake in nonprostatic malignancies: has the time come to remove “PS” from PSMA? Clin Nucl Med 2018; 43: 529–532. [DOI] [PubMed] [Google Scholar]
- 35. Seitz AK, Rauscher I, Haller B, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging 2018; 45: 602–612. [DOI] [PubMed] [Google Scholar]
- 36. Schmidkonz C, Cordes M, Schmidt D, et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging 2018; 45: 1862–1872. [DOI] [PubMed] [Google Scholar]
- 37. Afshar-Oromieh A, Debus N, Uhrig M, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging 2018; 45: 2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emmett LM, Yin C, Crumbaker M, et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga PSMA-11 PET in men with hormone sensitive and castrate resistant prostate cancer commencing androgen blockade. J Nucl Med. Epub ahead of print 14 December 2018. DOI: 10.2967/jnumed.118.223099. [DOI] [PubMed] [Google Scholar]
- 39. Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Euro Urol 2016; 70: 106–119. [DOI] [PubMed] [Google Scholar]
- 40. Mahal BA, Yang DD, Wang NQ, et al. Clinical and genomic characterization of low-prostate-specific antigen, high-grade prostate cancer. Euro Urol 2018; 74: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018; 19: 825–833. [DOI] [PubMed] [Google Scholar]
- 42. Thang SP, Violet J, Sandhu S, et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for 177Lu-labelled PSMA radioligand therapy. Euro Urol Oncol. Epub ahead of print 13 December 2018. DOI: 10.1016/j.euo.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 43. Jadvar H, Desai B, Ji L, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med 2013; 54: 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jadvar H, Velez E, Desai B, et al. Prediction of time to hormonal treatment failure in metastatic castrate-sensitive prostate cancer with 18F-FDG PET/CT. J Nucl Med 2019: pii: jnumed.118.223263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armstrong AJ, Anand A, Edenbrandt L, et al. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018; 4: 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gafita A, Bieth M, Kroenke M, et al. qPSMA: a semi-automatic software for whole-body tumor burden assessment in prostate cancer using 68Ga-PSMA11 PET/CT. J Nucl Med. 2019: pii: jnumed.118224055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mota JM, Armstrong AJ, Larson SM, et al. Measuring the unmeasurable: automated bone scan index as a quantitative endpoint in prostate cancer clinical trials. Prostate Cancer Prostatic Dis. Epub ahead of print 29 April 2019. DOI: 10.1038/s41391-019-0151-4. [DOI] [PubMed] [Google Scholar]
- 48. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018; 36: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018; 392: 2353–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]