Abstract
Several effective interventions are available for preventing HIV in women. Targeting interventions requires understanding their risk of acquiring HIV. We used surveillance data to estimate risks of HIV acquisition for 13–59-year-old women following a diagnosis of syphilis, gonorrhoea or chlamydia in Florida during 2000–2009. We excluded women reported with HIV before their STI, and measured HIV reported subsequent to STI (through 2011). Rates were compared to women with no reported STI. A total of 328,456 women had: syphilis (3325), gonorrhoea (67,784) or chlamydia (257,347). During 2,221,944 person-years of follow-up, 2118 of them were diagnosed with HIV. For women with no STI reported, during 64,763,832 person-years, 19,531 were reported with HIV. The crude rate of subsequent HIV diagnosis (per 100,000 person-years) was higher for women diagnosed with syphilis (597.9), gonorrhoea (171.3) or chlamydia (66.3) than women with no STI (30.2). Annual rates of HIV decreased over-all by 61.8% between 2001 and 2011. Women with syphilis or gonorrhoea were at highest risk for HIV and therefore might benefit from intensive counselling. However, they represented only a small fraction of the women who acquired HIV. Most cases of HIV infection among women occurred among the large group of women who were not at highest risk.
Keywords: HIV, AIDS, sexually transmitted infection, HIV incidence, syphilis, gonorrhoea, chlamydia, women, epidemiology, prevention, North America
Introduction
Several interventions are now available that have evidence of effectiveness for preventing HIV infection,1 including counselling,2 HIV testing,3 condom use,4 treatment of infected persons,5 post-exposure prophylaxis6 and pre-exposure prophylaxis.7,8 Appropriate targeting of these interventions requires understanding of a woman’s risk of acquiring HIV infection.9
In the United States, the rate of new HIV diagnosis for all women was 8.0 per 100,000 in 2010.10 Prospective studies of women in some African countries have documented HIV incidence rates as high as 2000–4000 per 100,000 person-years (relative risk = 500 compared to all US women).1,7,8 Women considered ‘at-risk’ in the United States will likely have a risk that lies between these two extremes, but there are not many studies that provide good estimates. Studies that estimate risks for women have usually been cross-sectional or case–control studies; they estimate the risk of having HIV infection.11–18 There are not many studies in the United States that have followed HIV-negative women to see how many acquire HIV, and studies that are done often show rates that are lower than expected.13,19 In one study of 2099 high-risk women, recent infections detected at baseline suggested an annual incidence of 2520 per 100,000 person-years, but when the uninfected women were followed for a year only four acquired HIV, an incidence of 240 per 100,000 person-years.20,21 Persons who acquire other sexually transmitted infections (STIs) have a high relative risk for acquiring HIV, suggesting they should be a priority for interventions,11,15,17,19 but their absolute level of risk is less clear.
Infection with HIV is an important health problem in Florida, where HIV was the second leading cause of death for 25–44 year-old black women in 2010 (23.2 per 100,000).22 Since 1998, the Florida Department of Health has routinely matched STI and HIV surveillance databases (using an algorithm based on name, age, race and date of birth) and added HIV diagnoses (for persons who have had syphilis, gonorrhoea or chlamydia) to the STI dataset. We used that database to determine the risk of being diagnosed with HIV infection following diagnosis of an STI, as an estimate of the risk of acquiring HIV.
Methods
We analysed all records of 13–59-year-old women in Florida who had reportable STIs (syphilis, gonorrhoea or chlamydia) that were reported between 1 January 2000 and 31 December 2009. We excluded late latent syphilis diagnoses if non-treponemal antibody was undetectable at dilutions of ≥ 1:32 because they were likely to be old infections. We then took the first diagnosis of syphilis, gonorrhoea or chlamydia that was recorded after 1 January 2000 for each woman. Women with more than one infection when the first diagnosis was made were categorised according to the first infection on this hierarchy: (1) syphilis; (2) gonorrhoea; and (3) chlamydia. We then excluded women who were reported as having HIV at or before the time of their first STI diagnosis (and within 60 days afterwards because they were likely already infected at baseline), and searched the database to see if the remaining women had subsequently been newly reported as having HIV infection. Follow-up for HIV began 60 days after the STI diagnosis and included all HIV infections diagnosed before 31 December 2011 that were reported by 19 March 2012. Person-years-at-risk for HIV began 60 days after the date of diagnosis for the STI and ended on the date the HIV infection was diagnosed, or on 31 December 2011 if no HIV was reported.
For comparison, we calculated the rate of HIV infection diagnosed among women with no reported preceding STI. This group includes women with HIV diagnoses before (or within 60 days after) their first STI diagnosis. To calculate the numerator, we took the number of HIV diagnoses for 13–59-year-old women in the Florida HIV/AIDS Surveillance database for 2000–2011, and subtracted the number of HIV diagnoses reported for women following an STI (measured above). The person-years-at-risk for this group includes risk time contributed by women before (and 60 days after) they were reported as having an STI. It was estimated by: (1) taking the number of 13–59-year-old women living in Florida in 2005, (5,582,148 according to the census); (2) multiplying by 12 years (to account for 2000–2011); and (3) subtracting the person-years-at-risk calculated for the women who had an STI (above).
Estimates were compared for various subgroups by STI, race/ethnicity, age, location and year of first STI. Multiple risk factors were combined to identify smaller subgroups at high risk. Age is not entirely comparable between groups because age was recorded at the time of STI diagnosis for women with an STI, and at the time of HIV diagnosis for other women. Location was classified as the residence at the time of the STI diagnosis even if the HIV infection was later reported from another location in Florida. ‘High-risk’ areas were defined as ZIP codes where at least 100 women aged 13–59 were reported with newly diagnosed HIV infection between 2000 and 2011, and there was an average annual rate of at least 65 HIV diagnoses per 100,000 women. Population estimates by combined age/race/ZIP code were only available for 2000 and 2010, so person-years-at-risk were calculated by assuming that the age/race proportions of women living in high-risk areas were the same in 2005 as they were in 2010.
Changes in HIV risk over time were assessed by plotting cumulative HIV diagnoses by time since the STI diagnosis for women grouped according to the year of their STI diagnosis (2000–2009).
To estimate risks by year of HIV diagnosis, we combined all women from the STI database and compared them to women with no STI reported. Risks for women with no STI reported were estimated by taking the yearly number of HIV diagnoses and census estimates for women living in Florida, and subtracting the corresponding women who have had STI.
We did not calculate confidence intervals for our estimates or p values for our comparisons because we included all surveillance data from Florida, not a sample. Our numbers are very large, and most of our errors would be expected to be due to variations in testing and reporting of STI or HIV and not due to chance. Centers for Disease Control and Prevention (CDC) staff did not have access to personal identifiers. Secondary analyses of routinely collected surveillance data do not involve human research and therefore do not require approval by the CDC Institutional Review Boards (IRB).
Results
Between January 2000 and December 2009, there were 328,456 women aged 13–59 years living in Florida who were reported as having an STI. The first infections reported for these women were: syphilis (3325), gonorrhoea (67,784) or chlamydia (257,347) (Table 1). Most of the women were young; 133,531 (40.7%) were diagnosed with an STI at the age of 13–19 years, and another 155,093 (47.2%) were aged 20–29 years. Race/ethnicity was categorised as black 136,975 (41.7%), white 84,255 (25.7%), Hispanic 35,946 (10.9%), other 1308 (0.4%) and unknown 69,972 (21.3%). Nineteen per cent (63,483) of the women with an STI lived in one of the 43 ZIP code areas considered to be at high risk for HIV.
Table 1.
Rate of HIV diagnosis for 13–59 year-old women in Florida 2000–2011, comparing women with and without a history of a preceding sexually transmitted infection in 2000–2009.
| No sexually transmitted infection reported 2000–2009 | Sexually transmitted infection reported 2000–2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reported with HIV | Reported with HIV | Relative ratea | ||||||||
| Characteristic | n | Person-years | n | Rateb | Characteristic | n | Person-years | n | Rateb | |
| Total | 5,582,148 | 64,763,832 | 19,531 | 30.2 | Total | 328,456 | 2,221,944 | 2,118 | 95.3 | 3.2 |
| Syphilis | 3,325 | 24,251 | 145 | 597.9 | 19.8 | |||||
| Gonorrhoea | 67,784 | 490,929 | 841 | 171.3 | 5.7 | |||||
| Chlamydia | 257,347 | 1,706,764 | 1,132 | 66.3 | 2.2 | |||||
| Agec | ||||||||||
| 13–19 | 804,073 | 8,746,605 | 399 | 4.6 | 133,531 | 902,271 | 734 | 81.3 | 17.8c | |
| 20–29 | 1,080,370 | 11,909,433 | 4,142 | 34.8 | 155,093 | 1,055,007 | 896 | 84.9 | 2.4 | |
| 30–39 | 1,152,980 | 13,630,242 | 6,231 | 45.7 | 30,642 | 205,518 | 341 | 165.9 | 3.6 | |
| 40–49 | 1,357,905 | 16,244,885 | 5,840 | 35.9 | 7,658 | 49,975 | 126 | 252.1 | 7.0 | |
| 50–59 | 1,186,820 | 14,232,667 | 2,820 | 19.8 | 1,532 | 9,173 | 21 | 228.9 | 11.6 | |
| Raced | ||||||||||
| Black | 981,681 | 10,803,207 | 13,519 | 125.1 | 136,975 | 976,965 | 1,605 | 164.3 | 1.3 | |
| White | 3,314,053 | 39,177,187 | 2,938 | 7.5 | 84,255 | 591,449 | 348 | 58.8 | 7.8 | |
| Hispanic | 1,130,446 | 13,336,584 | 2,687 | 20.1 | 35,946 | 228,768 | 96 | 42.0 | 2.1 | |
| Other | 155,968 | 1,864,784 | 387 | 20.8 | 1,308 | 6,832 | 4 | 58.6 | 2.8 | |
| Unknown | 0 | 0 | 0 | 69,972 | 417,929 | 65 | 15.6 | |||
| Areae | ||||||||||
| High risk | 470,633 | 5,205,682 | 7,973 | 153.2 | 63,483 | 441,914 | 861 | 194.8 | 1.3 | |
| Low risk | 5,111,515 | 59,558,150 | 11,241 | 18.9 | 264,973 | 1,780,030 | 1,257 | 70.6 | 3.7 | |
Relative rate compares women with an STI to women on the same row who have no STI.
Rate is per 100,000 person-years.
Age is age at diagnosis of HIV for persons with no STI, and age at diagnosis of STI for others.
Race/ethnicity information is available for all women in the census, but unknown for 69,972 women with STI.
Area is missing for 317 HIV-infected women with no history of an STI.
The 328,456 women with an STI had 2,221,944 person-years of follow-up in the database; 2118 were subsequently diagnosed as having HIV 61 days–11.6 years after the STI was diagnosed (mean follow-up 6.8 years, mean time to HIV 3.6 years). The 5,582,148 women (ages 13–59) in Florida with no STI reported in the interval had 64,763,832 person-years of follow-up; 19,531 were diagnosed as having HIV between 2000 and 2011. The rate of a subsequent diagnosis of HIV infection (per 100,000 person-years) was higher for women diagnosed with syphilis (597.9), gonorrhoea (171.3) or chlamydia (66.3) than women with no STI reported (30.2) (Table 1). The age group at highest risk of a reported STI was 13–19 year-olds (133,531 had STIs with a population size of 804,073 in 2005) whereas the group with the highest number of HIV diagnoses (6231) was 30–39-year-olds with no STI reported (45.7 per 100,000 person-years). Among all women diagnosed with one of these STIs, the rate of subsequent HIV diagnosis (per 100,000 person-years) was higher for blacks (164.3) than whites (58.8) or Hispanics (42.0). In contrast, the relative rate for HIV, comparing women with versus without an STI, was higher for white women (7.8) than for black women (1.3) or Hispanic women (2.1).
Rates were higher for subgroups of women with multiple risk factors (Table 2). Among 30–59-year-old black women living in high-risk areas who were diagnosed as having syphilis, the rate of subsequent diagnosis of HIV was 1625.3 per 100,000 person-years. Rates were also very high for other groups of black women with syphilis or gonorrhoea. However, the groups with the highest rates were also relatively small groups. For women with syphilis, the rate of a subsequent HIV diagnosis was 597.9 per 100,000 person-years, 19.8 times the rate for women with no STI (Table 1). However, the 145 women who developed HIV after a diagnosis of syphilis comprise only 0.67% of the 21,649 women diagnosed with HIV between 2000 and 2011.
Table 2.
Rate of HIV diagnosis between 2000 and 2011 for select subgroups of black women in Florida.
| No sexually transmitted infection reported 2000–2009 | Sexually transmitted infection reported 2000–2009 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Reported with HIV | Characteristic | Reported with HIV | Relative ratea | |||||||
| Age | Area | n | Person-years | n | Rateb | STI | n | Person-years | n | Rateb | |
| 30–59 | High risk | 165,573 | 1,953,096 | 5,139 | 263.1 | Syphilis | 434 | 3,199 | 52 | 1625.3 | 6.2 |
| Gonorrhoea | 1,456 | 10,745 | 53 | 493.3 | 1.9 | ||||||
| 30–59 | Low risk | 420,093 | 4,973,013 | 4,942 | 99.4 | Syphilis | 493 | 3,833 | 24 | 626.1 | 6.3 |
| Gonorrhoea | 3,192 | 23,729 | 76 | 320.3 | 3.2 | ||||||
| 20–29 | High risk | 65,205 | 648,259 | 1,544 | 238.2 | Syphilis | 290 | 2,111 | 12 | 568.5 | 2.4 |
| Gonorrhoea | 4,969 | 38,307 | 137 | 357.6 | 1.5 | ||||||
| 20–29 | Low risk | 157,469 | 1,579,096 | 1,430 | 90.6 | Syphilis | 332 | 2,521 | 15 | 594.9 | 6.6 |
| Gonorrhoea | 11,187 | 87,627 | 140 | 159.8 | 1.8 | ||||||
Relative rate compares women with an STI to women of the same age and area group who have no STI.
Rate is per 100,000 person-years.
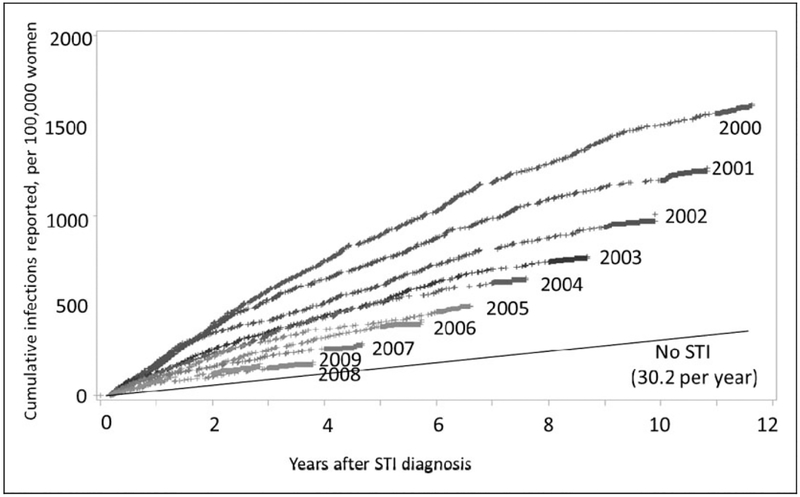
The likelihood of being newly diagnosed with HIV was highest in the years immediately following the STI diagnosis, but even 11 years after their diagnosis, women who had an STI were diagnosed with HIV at a higher rate than women who had not had an STI (Figure 1). The risk of HIV for women diagnosed with an STI in 2000 was substantially higher than the risk for women first diagnosed in subsequent years. Six years after their STI, the cumulative number of HIV diagnoses was 1020 per 100,000 women who had an STI in 2000 compared to only 463 per 100,000 women diagnosed in 2005. Women in the 2005 cohort could not have an STI in the previous five years, or they would have been assigned to the cohort-year of their first STI. When we removed that restriction and allowed women to enter a cohort every year they had an STI, we still saw a decrease in risk over time, though the decrease in risk for years after 2000 was attenuated by an average of 16 per 100,000 per year of follow-up (data not shown). There were also changes in the initial STI diagnosed for women over the years in our STI cohorts. Comparing initial STI diagnoses in our data-set, in the years 2000 and 2009: syphilis decreased (629 to 327); gonorrhoea decreased (9154 to 5508); and chlamydia increased (22,506 to 32,675).
Figure 1.
Cumulative number of HIV infections reported following the diagnosis of an STI, per 100,000 women, by year of STI diagnosis.
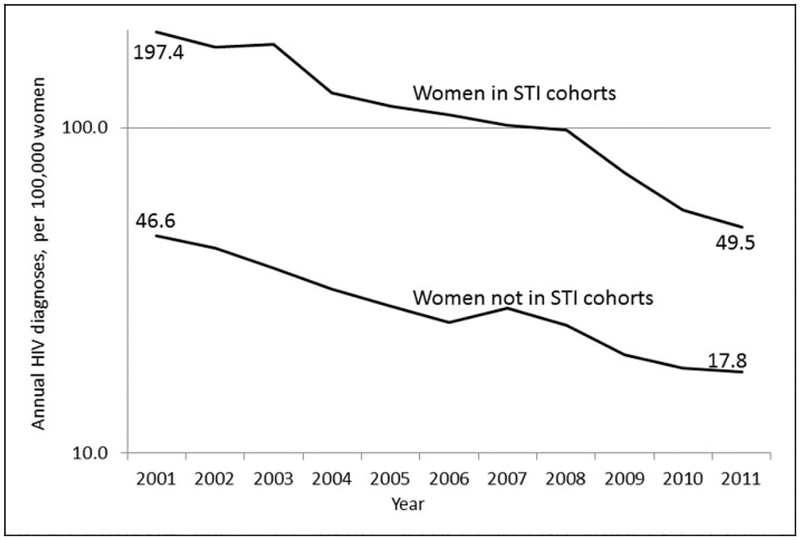
The annual risk of HIV for 13–59-year-old women in Florida who were not in the STI cohorts fell 61.8% (from 46.6 to 17.8 per 100,000 women) between the years 2001 and 2011 (Figure 2). The decrease in risk for women in the STI cohorts was larger (74.9%).
Figure 2.
Annual HIV diagnosis rates for 13–59 year-old women in the STI cohorts and women not in the STI cohorts, Florida, 2001–2011 (log scale).
Discussion
The Florida surveillance database allowed us to retrospectively identify over 328,000 women who had an STI reported and calculate their risk of a subsequent diagnosis and report of HIV. After having an STI, the rate of being diagnosed with HIV infection was 95 per 100,000 person-years, 3.2 times the rate for other women in the same age range in Florida and 10 times the rate for women of all ages in the United States. Rates were high for older women, black women and women who were diagnosed as having syphilis or gonorrhoea. Black women aged 30–59 years who lived in high-risk areas and had syphilis were subsequently diagnosed as having HIV infection at a rate of 1625.3 per 100,000 person-years.
There have been several approaches to estimating the rate of HIV infection for women. The incidence of HIV (per 100,000 person-years) has been estimated for 2006–2009 as 8.6–10.7 for all women (age ≥ 13 years) in the US and 39.7–50.8 for black women using HIV antibody titres to estimate which infections were recently acquired (the serologic testing algorithm for recent HIV seroconversion, STARHS).23 These rates are almost identical to rates of reported new HIV infections in the CDC national surveillance system in those same years (9.7–10.9 for all women and 47.4–52.9 for black women).10 The STARHS assay and newer approaches24 have been used to estimate the number of infections detected in cross-sectional studies that were recently acquired. However, these estimates may be too high if persons seek HIV testing due to recent high-risk behaviour.25 A few studies have followed cohorts of women in the United States to measure their rate of acquiring HIV, and each identified only a few new infections. One found four seroconversions among 449 women in Brooklyn, NY for an incidence rate of 621 per 100,000 person-years.13 Another found four serocon-versions among 2099 high-risk women recruited at several venues around the United States for a rate of 240 per 100,000 person-years.20,21 Several of the subgroups of women with STI that we studied had rates of new diagnosis in this range.
The rates of HIV diagnosis seen in our cohorts have decreased since 2000. One reason for this decrease is that persons who entered the first cohort (2000) could have multiple STI in previous years, whereas persons who entered in subsequent years could not have a previous STI (since 2000), or they would have been represented in an earlier cohort. This illustrates how the potential impact of a prevention programme will decrease if persons at the highest risk are recruited early, and persons at lower risk are recruited later. Florida also had an increase in diagnoses of chlamydia over time, so later cohorts included more young women who are at lower risk of HIV than older women. However, most of the decrease in risk for the STI cohorts appears to be related to the 61.8% decrease in risk of HIV for other 13–59-year-old women in Florida since 2000. This decrease is greater than that reported by most other states with stable HIV reporting and warrants further investigation to identify possible causes.23
The high level of risk seen in women with STI certainly merits counselling so that the women are aware of their risk and can take steps to reduce it. High-intensity counselling has been recommended for persons at risk by the US Preventive Services Task Force (at the ‘B’ level)26 and would therefore be covered by certain insurance plans under the Affordable Care Act.27,28 Women who are diagnosed with an STI should be advised to use condoms consistently and correctly. The high risks we found suggest some of the women might be in a relationship with an HIV-infected partner. Therefore, women with STIs should assure that when their partners are treated for the STI, they are also tested for HIV and, if they are HIV infected, they stay on therapy. The high risk for women in certain ZIP code areas is consistent with other studies,15,17,29,30 and suggests that community-level interventions could and should be evaluated.9,29 However, community intervention trials would require identification of interventions expected to have a substantial impact that could be measured at the community level.
Electronic databases have the potential to track diseases in ways not possible with paper forms. Paper forms must be searched by hand, so surveillance in the past was typically reported as numbers of cases in a population. Electronic records are easily searched and, if the data are valid and complete, can add a new dimension to surveillance – patterns of infections within individuals over time. Others have merged disparate databases to identify intersection of populations at risk, but that approach is limited by the ability to match records.31 Florida, like most large jurisdictions, maintains separate HIV and STI surveillance systems. However, Florida routinely matches databases so that HIV diagnoses are added to the records of persons who have had an STI. That matched STI–HIV database used in this analysis appears to be quite accurate, perhaps because it has been used as a single database by the sexually transmitted diseases programme, and the primary users depend on the accuracy of the data. In the future, other programmes may have the capacity to do similar analyses that expand the use of surveil-lance data for different infections. Timely access to surveillance analyses at the local level will increase opportunities to target prevention interventions.
Our findings have limitations. We did not actively follow women who were all tested and found to be HIV-uninfected. We measured the risk of being reported as having HIV in Florida, not true HIV incidence. We do not know the date that HIV infection was acquired, or how many infections were undiagnosed. Our estimates would overestimate incidence if HIV-infected women were not tested for HIV at the time of their STI diagnosis. Our estimates would underestimate incidence if women later acquired HIV but were not detected by our database because they were not tested, or because they moved away from Florida and HIV infection was diagnosed elsewhere. We cannot be sure that the women who were diagnosed with HIV after their STI actually acquired HIV after their STI, but the sustained high rate of diagnosis for several years suggests that the women were at sustained high risk for acquiring HIV. We have slightly over-estimated risk for HIV by race/ethnicity for women with STI because race/ethnicity information was missing for 21% of women with STI (the denominator), but was less likely to be missing for those women who later acquired HIV (the numerator). Finally, the goal of our study was to identify women at risk for acquiring HIV, not to measure the impact of STIs on HIV transmission. Therefore, women who had HIV diagnosed at the same time as their STI were not included. Similarly, women who developed an STI elsewhere would not be considered STI-associated if they were reported with HIV in Florida.
Women who are diagnosed with syphilis or gonorrhoea can benefit from being counselled about their risk for HIV and the many approaches to reducing it. The women at highest risk should be considered for additional interventions; however, they represent only a small fraction of the women who will acquire HIV. Most HIV infections among women are occurring among the large group of women who are not at highest risk. Targeting them individually will be difficult.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Curran K, Baeten JM, Coates TJ, et al. HIV-1 prevention for HIV-1 serodiscordant couples. Curr HIV/AIDS Rep 2012; 9: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JS, Whitlock E, O’Connor E, et al. Behavioral counseling to prevent sexually transmitted infections: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2008; 149: 497–508. [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Crepaz N, Senterfitt JW, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States. Implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005; 39: 446–453. [DOI] [PubMed] [Google Scholar]
- 4.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young TN, Arens FJ, Kennedy GE, et al. Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure. Cochrane Database Syst Rev 2007; 1: CD002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–434. [DOI] [PubMed] [Google Scholar]
- 8.Baeton JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padian NS, McCoy SI, Abdool Karim SS, et al. HIV prevention transformed: the new prevention research agenda. Lancet 2011; 378: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. NCHHSTP Atlas, http://gis.cdc.gov/GRASP/NCHHSTPAtlas/main.html (accessed 27 April 2012). [Google Scholar]
- 11.LaLota M, Beck DW, Metch LR, et al. HIV seropositivity and correlates of infection among heterosexually active adults in high-risk areas in South Florida. AIDS Behav 2011; 15: 1259–1263. [DOI] [PubMed] [Google Scholar]
- 12.Jennes SM, Neaigus A, Murrill CS, et al. Estimated HIV incidence among high-risk heterosexuals in New York City, 2007. J Acquir Immune Defic Syndr 2011; 56: 193–197. [DOI] [PubMed] [Google Scholar]
- 13.Chirgwin KD, Feldman J, Dehovitz JA, et al. Incidence and risk factors for heterosexually acquired HIV in an inner-city cohort of women: temporal association with pregnancy. J Acquir Immune Defic Syndr 1999; 20: 295–299. [DOI] [PubMed] [Google Scholar]
- 14.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA 2008; 300: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adimora AA, Schoenbach VJ, Martinson FE, et al. Heterosexually transmitted HIV infection among African Americans in North Carolina. J Acquir Immune Defic Syndr 2006; 41: 616–623. [DOI] [PubMed] [Google Scholar]
- 16.Nash D, Bennani Y, Ramaswamy C, et al. Estimates of HIV incidence among persons testing for HIV using the sensitive/less sensitive enzyme immunoassay, New York City, 2001. J Acquir Immune Defic Syndr 2005; 39: 102–111. [DOI] [PubMed] [Google Scholar]
- 17.Towe VL, Sifakis F, Gindi RM, et al. Prevalence of HIV infection and sexual risk behaviors among individuals having heterosexual sex in low income neighborhoods in Baltimore, MD: the BESURE study. J Acquir Immune Defic Syndr 2010; 53: 522–528. [DOI] [PubMed] [Google Scholar]
- 18.Magnus M, Kuo I, Shelly K, et al. Risk factors driving the emergence of a generalized heterosexual HIV epidemic in Washington, District of Columbia networks at risk. AIDS 2009; 23: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 19.Padian NS, Shiboski SC, Glass SO, et al. Heterosexual transmission of human immunodeficiency virus (HIV) in Northern California: results from a ten-year study. Am J Epidemiol 1997; 146: 350–357. [DOI] [PubMed] [Google Scholar]
- 20.Hodder S, Justman J, Hughes J, et al. The HPTN 064 (ISIS Study)—HIV incidence in women at risk for HIV: US. Poster # 1048. In: 19th conference on retroviruses and opportunistic infections, Seattle, Washington, 5–8 March 2012. [Google Scholar]
- 21.Hodder SL, Justman J, Hughes JP, et al. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florida Death Rate Query System, http://www.florida-charts.com/FLQUERY/Death/DeathRate.aspx (accessed 23 July 2012).
- 23.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS ONE 2011; 6: e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laeyendecker O, Brookmeyer R, Cousins MM, et al. HIV incidence determination in the United States: a multiassay approach. J Infect Dis 2013; 207: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remis RS and Palmer RWH. Testing bias in calculating HIV incidence from the serologic testing algorithm for recent HIV seroconversion. AIDS 2009; 23: 493–503. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Preventive Services Task Force. Behavioral counseling to prevent sexually transmitted infections, http://www.uspreventiveservicestaskforce.org/uspstf/uspsstds.htm (accessed 16 January 2013).
- 27.Patient Protection and Affordable Care Act of 2010, Pub. L. No. 111–148, 124 Stat. 119, amended by Health Care and Education Reconciliation Act of 2010, Pub. L. No. 111–152, 124 Stat. 1029 (codified as amended in scattered sections of 42 U.S.C.).
- 28.Centers for Medicare & Medicaid Services. Decision memo for screening for sexually transmitted infections (STIs) and high-intensity behavioral counseling (HIBC) to prevent (STIs) (CAG-00426N). November 8, 2011. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=250 (accessed 26 March 2014).
- 29.Farley TA. Sexually transmitted diseases in the southeastern United States: location, race, and social context. Sex Transm Dis 2006; 33: S58–S64. [DOI] [PubMed] [Google Scholar]
- 30.Denning PH, DiNenno EA and Weigand RE. Characteristics associated with HIV infection among heterosexuals in urban areas with high AITS prevalence—24 cities, United States, 2006—2007. Morbid Mortal Wkly Rep 2011; 60: 1045–1049. [PubMed] [Google Scholar]
- 31.Stenger M, Kerani R, Golden M, et al. Factors associated with HIV seroconversion among men diagnosed with gonorrhea in Washington state: an evidence base for targeting integrated partner services. In: Presented at the National STD Prevention Conference, Atlanta GA, 9 March 2010. [Google Scholar]