Abstract
Objective:
The association between stress and eating remains unclear in children potentially due to factors that may moderate the association. We examined whether weight status or sex moderated associations between response to a stress induction and eating in the absence of hunger (EAH), among low-income children.
Method:
Children (n = 223; M age = 7.8 years, SD = 0.7 years) participated in a stress induction protocol (modified Trier Social Stress Test for Children [TSST-C]) during which behavioral coding of observed anxiety and change in self-reported distress were measured. Afterwards, participants completed a standardized EAH protocol where they were offered palatable foods. Total kilocalories consumed during the EAH protocol was calculated. Weight and height were measured and weight status calculated as overweight (BMI > 85th percentile for age and sex) vs. not overweight. Multivariate linear regression models adjusting for covariates were conducted to test whether child weight status or sex moderated the stress response-EAH association, for both stress response variables.
Results:
Weight status moderated the association between observed stress response and EAH such that children with overweight engaged in more EAH as observed anxiety increased, whereas children without overweight engaged in less EAH as observed anxiety increased (βinteraction = .48; p = .010). Weight status did not moderate associations between self-reported distress and EAH. Child sex was not a significant moderator.
Conclusions:
After exposure to stress, children with overweight in middle childhood may eat more palatable food compared to children without overweight, possibly due to hypersensitization to food cues or weight stigma experienced by youth with overweight. It may be helpful to encourage youth with overweight to engage in stress-management techniques that do not involve eating as a response to stress.
Keywords: Eating Behavior, Eating in the Absence of Hunger, Stress Induction, Observational study, Overweight, Observed Anxiety
Introduction
Overeating in response to stress has been hypothesized as a pathway to excessive weight gain (Groesz et al., 2012). Eating in the absence of hunger (EAH), or consuming food past the point of satiation, is a specific eating behavior that has been implicated as promoting risk for overweight and obesity over time (Lansigan, Emond, & Gilbert-Diamond, 2015). Chronic stress, which is defined as stress experienced over a prolonged period, and lab-induced stress have been associated with EAH in numerous studies of adults (Born et al., 2010; Lemmens, Rutters, Born, & Westerterp-Plantenga, 2011; Rutters, Nieuwenhuizen, Lemmens, Born, & Westerterp-Plantenga, 2009) as well as in some studies of children (Francis, Granger, & Susman, 2013; Michels et al., 2015; Miller et al., 2018). Stress-induction procedures have been shown to increase EAH and consumption of palatable food in adults (Born et al., 2010; Klatzkin, Baldassaro, & Hayden, 2018; Lemmens et al., 2011; Rutters et al., 2009), but few studies have examined this in children. Furthermore, individual factors such as weight status and sex have also been identified as shaping stress-eating associations in adults (Grunberg & Straub, 1992; Lemmens et al., 2011; Pérusse-Lachance et al., 2013; Slochower, Kaplan, & Mann, 1981), but these associations have not been well characterized in children (Nguyen-Rodriguez, Chou, Unger, & Spruijt-Metz, 2008). Understanding early-emerging associations between stress and excessive food consumption, and potential moderators of this association, is important both to identify youth most at risk for stress-related obesity and to inform intervention targets. Therefore, the goal of the current study was to examine child response to a stress-induction protocol in relation to EAH, and to assess child weight status and sex as potential moderators of the association between stress response and EAH.
In adults, stress has been shown to alter food consumption patterns, with studies finding increased caloric intake, particularly of palatable foods, in response to stress (O’Connor, Jones, Conner, McMillan, & Ferguson, 2008; Rutters et al., 2009). It has been suggested that stress may either result in less reward signaling and reward sensitivity, thus prompting increased consumption of such foods to achieve the desired effect (Born et al., 2010), or that stress neurobiology may directly promote cravings for palatable foods (Sinha, 2018). Both processes may lead to consumption of highly palatable food in the absence of hunger (Bellisle, Drewnowski, Anderson, Westerterp-Plantenga, & Martin, 2012).
Although multiple studies have examined associations between stress and eating behaviors, findings are somewhat inconsistent and methods used to assess stress have varied. Specifically, studies have found that individuals both over- and under-eat in response to stress (e.g., Emond et al., 2016; Epel et al 2004; Kandiah, Yake, Jones, & Meyer, 2006; Pérusse-Lachance et al., 2013; Zellner, 2006). A 2012 review suggested that 40–70% of people ate less when stressed, and that 30–50% of individuals ate more when stressed (Gibson, 2012). Many studies report overeating in response to stress in adults, with most of these examining lab-induced stress (Born et al., 2010; Chaput, Drapeau, Poirier, Teasdale, & Tremblay, 2008; Epel, Lapidus, McEwen, & Brownell, 2001; Lemmens et al., 2011; Rutters et al., 2009) rather than chronic stress or stress experienced throughout the day (i.e., daily stress).
Children and adolescents are likely also susceptible to the effects of stress on eating behaviors, yet have been studied less often. This is a significant gap because habits developed during early life, such as eating in response to stress, may track into adulthood (Baidal & Taveras, 2012); identifying early-emerging patterns of eating in response to stress is critical in order to interrupt such associations. As in adults, chronic stress in children has been linked to self-reported unhealthy dietary practices such as lower fruit and vegetable intake, increased frequency of fatty food consumption (Cartwright et al., 2003), sweet food consumption (Michels et al., 2015) and overall lower dietary quality (De Vriendt et al., 2012). Early life stress may also alter eating behaviors, or the manner in which a child consumes food, later in life. A longitudinal study found that low-income children exposed to more chronic stress at 3 to 5 years of age were at greater risk for emotional overeating and EAH at 7 to 10 years of age (Miller et al., 2018).
To date, seven studies have evaluated food consumption in response to stress exposures in children. As in research with adults, these studies find a mix of overeating and undereating in response to stress (Balantekin & Roemmich, 2012; Farrow, Haycraft, & Blissett, 2015; Francis et al., 2013; Jeong & Kim, 2007; Miller et al., 2018; Roemmich, Lambiase, Lobarinas, & Balantekin, 2011; Roemmich et al., 2002), with the majority of the studies examining response to acute stress (Balantekin & Roemmich, 2012; Farrow et al., 2015; Francis et al., 2013; Roemmich et al., 2011; Roemmich et al., 2002). One possible reason for such mixed findings is that the methods used to assess eating have varied, with some studies examining observed EAH (Farrow et al., 2015; Francis et al., 2013; Miller et al., 2018), others examining food consumption and preferences, (Balantekin & Roemmich, 2012; Roemmich et al., 2011; Roemmich et al., 2002) and still others using measures of self-reported eating (Jeong & Kim, 2007).
Another possible reason for the lack of consistent findings may be that individual factors such as weight status or sex may moderate the associations between stress and eating (Lemmens et al., 2011; O’Connor et al., 2008; Pérusse-Lachance et al., 2013). For example, Pérusse-Lachance et al. (2013) found that men decreased their energy intake after a stress task, whereas women increased their energy intake after the stress task. Similarly, Grunberg and Straub (1992) found that men decreased energy intake after an acute stress, but did not find a significant stress-eating association for women. Weight status has also been found to be an important moderator, with studies showing that adults with overweight eat more in response to stress, whereas the eating patterns of adults without overweight do not seem to be affected by stress (Lemmens et al., 2011; Slochower et al., 1981).
Unfortunately, research examining moderators of the stress-eating association among children remains sparse. Studies with both children 5 to 12 years of age (Michels et al., 2015) and adolescents 15 to 19 years of age (Michaud et al., 1990) have concluded that stress is linked to obesogenic eating behaviors in Western European females, but not in males. In contrast to studies of adults, no studies to our knowledge have considered weight status as a moderator of associations between stress and observed eating behavior in children. However, Nguyen-Rodriguez et al. (2008) examined weight status as a moderator of perceived stress in the past month and emotional eating, but found no differences in the association between middle school students with overweight and the students without overweight.
Therefore, the purpose of the current study was to determine whether child sex (male vs. female) and child weight status (i.e., normal weight vs. overweight/obese) have a moderating effect on the association between child response to a stress induction protocol (i.e., a modified version of the Trier Social Stress Test for Children) and amount of kilocalories consumed during EAH. The current study specifically investigates the associations in a sample of low-income children, since these children are at a greater risk for overweight (Shrewsbury & Wardle, 2008) and tend to experience high levels of chronic stress (Evans & Kim, 2013). We hypothesized that there would be a positive association between stress and EAH, and that the association would be stronger among females and children with overweight.
Methods
Participants and Recruitment
Participants were low-income children from the Midwest United States. Children were originally recruited from Head Start, a federally-funded preschool program free to children in poverty, to participate in an observational study of stress and eating behavior (Lumeng et al., 2014). Data for the current study were drawn from a follow up study of the cohort that was conducted during middle childhood. This report is based on 223 children who participated in the stress induction protocol and the EAH protocol.
This study was approved by the University of Michigan Institutional Review Board. Parents/legal guardians (typically mothers) provided written informed consent, and children provided verbal assent. Inclusion criteria at enrollment were that: the child was enrolled in Head Start, not in foster care, born at ≥35 weeks gestation without serious perinatal/neonatal complications, and did not have food allergies or serious medical problems; the parent and child were able to communicate in English; and neither parent had ≥ a 4-year college degree.
Measures
Mothers or guardians completed questionnaires to assess child age, sex, race/ethnicity, pubertal development, and maternal education (categorized for analysis as high school diploma or more vs. not).
Stress Induction Protocol
Children played calm games (e.g., board games, puzzles, and coloring) for a minimum of 20 minutes prior to the stress induction protocol. The stress induction protocol was based on a modified version of the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., 1997) and has been described previously (Doom et al., 2018). Briefly, the protocol was designed to elicit social-evaluative stress and it included approximately 10 minutes of cognitive testing and interactions with a female examiner who had been trained to interact with the child without giving positive feedback on the child’s performance and to use a neutral (though not harsh) tone. This individual was characterized to the child as a “strict teacher” prior to her entry into the room by the first research assistant, who had been working with the child on other tasks. When the strict teacher entered the room, she took charge and instructed the first research assistant to make several slight adjustments to the equipment to enforce that she was picky about rules. The child was also told that if he/she performed better than other children tested, he/she would win a prize at the end.
The first research assistant then left the room and the strict teacher administered the testing battery. The battery consisted of forward and backward digit span tasks and the oral word fluency task from the Wechsler Individual Achievement Test-Third Edition (WIAT-III, 2009), and a TSST-C storybook task (Buske-Kirschbaum et al., 1997; Doom et al., 2018).
Observed Stress Response: Behavioral Coding of Anxiety Displays
Children’s observed stress response was operationalized as behavioral and facial indicators of anxiety during the stress induction protocol. Displays of anxiety were used to measure stress response due to the overlapping neuronal circuits of stress and anxiety (Shin & Liberzon, 2009), which yield recognizable facial and behavioral cues (Giannakakis et al., 2017). These behaviors were coded from video using a coding system that was adapted for the current study (Miller & Olson, 2000). Undergraduate research assistants (who did not administer protocols) were trained to achieve interrater reliability (κ > 0.70) prior to assessing child anxiety displays. To avoid coder drift and ensure ongoing reliability, 20% of the videos were double-coded (κ = 0.84). Coders rated the degree of anxiety displayed during five segments: when the child was alone in the room, when the child was in the room with the research assistant anticipating the arrival of the strict teacher, when the strict teacher entered the room, during the oral word fluency task, and during the digit span task. Anxiety was assessed at 30-second intervals as either: none (0), mild or minimal (1; maximum of two mild instances, e.g., looking down to avoid eye contact, nervously moving eyes back and forth), or moderate to intense (2; one stronger display, or more than two mild instances, e.g., weeping, being on the verge of tears, or a dejected demeanor). The proportion of intervals with any observed anxiety (i.e., those rated as 1 or 2) was calculated for each of the five segments. Mean proportion of observed anxiety was calculated across all segments to reflect the child’s observed response to the stress induction.
Subjective Stress Response: Self-Reported Distress Rating
Children’s subjective stress response during the protocol was operationalized as the change in self-reported distress from baseline to post-stress induction. Each child was asked by the first research assistant to self-report their perceived distress directly before the stress induction (baseline), two minutes after the stress induction (stress), and approximately 45 minutes after the stress induction (recovery). Children were given visual aids (i.e., drawn faces) and asked to rate how they were feeling on a 5-point Likert scale ranging from very calm and relaxed (1) to very nervous, scared, or stressed (5). Specifically, as the research assistant indicated each corresponding scale point, children were asked, “How are you feeling right now? Very nervous, scared, or stressed out; a little bit nervous, scared or stressed out; kind of in the middle; a little bit calm or relaxed; or very calm or relaxed?” A score was calculated to reflect the change in self-reported distress from baseline to stress (higher score = greater increase) to determine the child’s subjective response to the stress induction.
Eating Behavior Protocol
Eating behavior assessments occurred after the stress induction protocol. Given that both appetite and stress can fluctuate during the day and that eating and stress-elicitation protocols are often administered in the afternoon (Fisher & Birch, 2000; Kudielka & Wüst, 2010), we conducted our protocols in the afternoon (mean start time for the EAH = 6:25pm). EAH was measured using a standardized protocol described in Miller et al. (2018) immediately after a dinnertime family meal. For the dinnertime meal, each of the present family members were served a 12-inch deli meat sandwich, baked potato chips, fruit cup, apple sauce, condiments (i.e., mustard and mayonnaise), and bottled water. Upon completion of the meal, children were asked to report their level of fullness. The child was then moved to a separate dessert room where the research assistant told the child, “You can have dessert. You can’t take it with you, but you can eat as much as you like here for five minutes. If you are ready to be done before that, all you have to do is let me know. I’m going to do some work now.” The child remained in the room for five minutes, with free access to pre-measured bowls of desserts including: four Little Debbie Oatmeal Cream Pies (152g; 680 kilocalories; kcals), two Little Debbie Cosmic Brownies (124g; 560 kcals), eight Chips Ahoy! Chewy Chocolate Chip Cookies (124g; 560 kcals), eight Keebler Fudge Stripe Cookies (108g; 560 kcals), eight Little Debbie Mini Powdered Donuts (100g; 440 kcals), and three Kellogg’s Original Rice Krispy Treats (66g; 270 kcals). After the five-minute period, the remaining dessert was weighed. The remaining dessert weight was subtracted from the initial dessert weight to calculate the total kcals consumed. More kcals consumed indicated a higher degree of EAH.
Anthropometry
The weight and height of children were measured by trained research staff using a Detecto DR-550C scale (calibrated weekly) and Seca 213/217 stadiometer. Children were measured twice and if measurements differed by more than 0.1 kg (for weight) or 0.5 cm (for height) two more measurements were taken. Body mass index (BMI) was calculated, and weight status calculated as overweight (BMI ≥ 85% for age and sex) vs. not overweight (BMI < 85% for age and sex) based on the US Centers for Disease Control reference growth curves (Kuczmarski et al., 2002).
Covariates
Pubertal development.
Pubertal development was assessed to control for potential effects of puberty on the stress response. During the middle childhood visit, mothers used a visual rating scale based on Tanner staging (Morris & Udry, 1980) to report their child’s pubertal development. The visual scale contained photos and short descriptions for each number ranging from 1 (no development) to 5 (fully developed). Parents of boys rated their child’s pubic hair development, while parents of girls rated their child’s breast and pubic hair development. For boys, the pubic hair development score was used for analyses. The average of the breast and pubic hair development scores was used for girls.
Fullness rating.
To account for potential effects of satiety on EAH kcals consumed, fullness rating was examined as a potential covariate using an instrument from Fisher and Birch (2000). Once the child finished eating dinner with their family, the child was asked to report their satiety level. The research assistant asked the child, “How full do you feel right now? Are you hungry, full, or in-between?” While stating the three fullness options, the research assistant pointed to cartoon figures representing each level of satiety. Once the child selected their level of fullness, their response was recorded as 1 (hungry), 2 (in-between), or 3 (full).
Statistical Analyses
Data were analyzed using SPSS (Version 24.0). Descriptive statistics were conducted to analyze characteristics of the sample including child demographics, pubertal development, fullness rating, weight status, observed anxiety, self-reported distress, EAH kcal consumed, and maternal education. An alpha level of p < .05 was used for bivariate and regression analyses, with an alpha level of p < .15 for interaction terms.
Bivariate analyses were conducted using t-tests (for categorical independent variables) and correlations (for continuous variables). Bivariate analyses were used to examine whether observed anxiety, self-reported distress, overweight status, and sex were related to EAH, and to test associations of potential covariates (pubertal development, fullness rating) with these key study variables in order to determine which covariates to include in the regression models.
Two multivariate linear regression models were conducted to determine whether child weight status moderated the association between stress response and EAH; one model utilized observed anxiety as the measure of stress response while the other model used self-reported distress as the measure of stress response. Both models controlled for child sex, pubertal development, and fullness rating. Two multivariate linear regression models were next conducted to determine whether child sex moderated the stress response-EAH association; similarly, one model used observed anxiety as the measure of stress response and the other model used self-reported distress. These models controlled for child weight status, pubertal development, and fullness rating.
Results
Descriptive Statistics
Descriptive statistics of the sample are displayed in Table 1. The 223 children had a mean age of 7.8 years (SD = 0.7) and the sample was mostly non-Hispanic White (54.9%). Approximately 48% of participants had overweight.
Table 1.
Characteristics of the sample (n=223)
| Variable | N (%) or M (SD) | Range |
|---|---|---|
| Child | ||
| Age (years) | 7.8 (0.7) | 7.0 to 10.5 |
| Sex | ||
| Female | 111 (49.6%) | -- |
| Male | 113 (50.4%) | -- |
| Race/Ethnicity | ||
| White, Non-Hispanic | 123 (54.9%) | -- |
| Black | 33 (14.7%) | -- |
| Biracial | 44 (19.6%) | -- |
| Hispanic, any race | 22 (9.8%) | -- |
| Other | 2 (0.9%) | -- |
| Pubertal Development (1=none to 5=fully developed) | 1.4 (0.3) | 1.0 to 2.4 |
| Fullness Rating (1=hungry to 3=full) | 2.46 (0.60) | 1.0 to 3.0 |
| Weight Status | ||
| Overweight/Obese | 108 (48.4%) | -- |
| Not Overweight | 115 (51.6%) | -- |
| EAH Kcal consumed | 352.3 (185.5) | 0 to 1125 |
| Proportion Observed Anxiety during Stress | 0.59 (0.23) | 0 to 1 |
| Self-Reported Distress: Baseline (1=low to 5=high) | 1.36 (0.77) | 1 to 5 |
| Self-Reported Distress: Stress (1=low to 5=high) | 3.53 (1.35) | 1 to 5 |
| Self-Reported Distress: Recovery (1=low to 5=high) | 1.10 (0.37) | 1 to 4 |
| Self-Reported Distress Change from Baseline to Stress | 2.19 (1.43) | −3 to 4 |
| Maternal Education | ||
| High school or less | 99 (44.2%) | -- |
| More than high school | 124 (55.8%) | -- |
Bivariate Analysis Results
Results of bivariate analyses are presented in Table 2. Observed anxiety and reported distress were positively associated (p = .045). Pubertal development was associated with more kcal consumed in EAH (p < .001). Girls reported more distress (p = .037), children with overweight consumed more kcal in EAH (p = .002), and child weight status and sex were both associated with pubertal development such that girls and children with overweight were more developed (both p ‘s < .001). Finally, child sex and overweight were associated (χ2 = 4.55, p = .022), such that a larger percentage of girls had overweight compared to boys (27% of girls vs. 21% of boys).
Table 2.
Bivariate associations among key study variables and covariates
| 1 | 2 | 3 | 4 | 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
r or M (SD) |
p |
r or M (SD) |
p |
r or M (SD) |
p |
r or M (SD) |
p |
r or M (SD) |
p | |
| 1. Proportion Observed Anxiety | -- | -- | ||||||||
| 2. Self-Reported Distress (baseline to stress) | .13 | .045 | -- | -- | ||||||
| 3. EAH Kcal Consumed | .002 | .977 | .06 | .391 | -- | -- | ||||
| 4. Pubertal Developmenta | −.07 | .298 | .05 | .441 | .25 | .000 | -- | -- | ||
| 5. Fullness Ratingb | −.02 | .769 | −.10 | .124 | −.08 | .215 | −.07 | .298 | -- | -- |
| 6. Child Weight Status | ||||||||||
| Overweight/Obese | 0.58 (0.24) | .231 | 2.21 (1.44) | .868 | 393(204) | .002 | 1.49 (0.35) | .000 | 2.43 (0.63) | .455 |
| Not Overweight | 0.61 (0.22) | 2.18 (1.42) | 316 (158) | 1.30 (0.23) | 2.49 (0.57) | |||||
| 7. Child Sex | ||||||||||
| Male | 0.60 (0.22) | .935 | 1.99 (1.43) | .037 | 364 (200) | .333 | 1.33 (0.26) | .001 | 2.46 (0.65) | .992 |
| Female | 0.60 (0.23) | 2.38 (1.41) | 340 (167) | 1.45 (0.34) | 2.46 (0.54) | |||||
Note.
1=none to 5=fully developed;
1=hungry to 3=full.
Regression Analysis Results
Moderation by weight status.
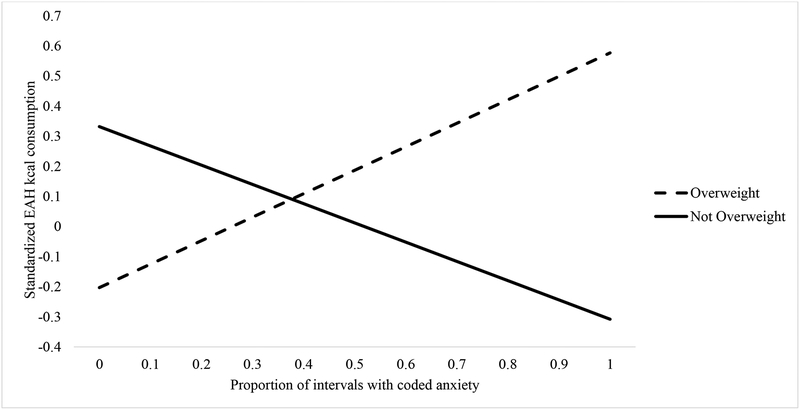
Results of the multivariate linear regression models testing child weight status as a moderator of the stress response-EAH association are presented in Table 3. These models were adjusted for child sex, pubertal development, and fullness rating. For the observed anxiety model, observed anxiety, child weight status and their interaction were entered in the model in addition to the covariates. The final model was significant F(5, 216) = 6.39, p < .001 (R2 = .13). In the final model, there was no main effect of observed anxiety (p =.119) or overweight (p = .13), but there was a trend for sex (p = .065), such that girls consumed fewer EAH kcal. The observed anxiety × weight status interaction term was found to be significant (β = .48, p = .010). As indicated by the slopes presented in Figure 1, this model suggests that children with overweight engaged in more EAH as observed anxiety increased, whereas children without overweight engaged in less EAH as observed anxiety increased. Analysis of the slopes for each group indicated that both slopes were marginally significant (βoverweight = .18, p = .053; βnot overweight = −.16, p = .093) and in opposite directions. Thus, the significant interaction is likely attributed to the different directions of association between observed anxiety and EAH among children with overweight compared to children without overweight.
Table 3.
Adjusted associations between child stress indicators, overweight or sex as a moderator, and EAH kcal consumed: Observed anxiety and self-reported distress models
| β (SE) | p | F (Model) | Model R2 | |
|---|---|---|---|---|
| Moderator: Child Overweight | ||||
| Observed Anxiety | F(6, 215)=5.31*** | .13 | ||
| Proportion Anxiety | −.15 (.09) | .119 | ||
| Overweight | −.28 (.18) | .130 | ||
| Anxiety × Overweight | .48 (.19) | .010 | ||
| Sex | −.12 (.07) | .065 | ||
| Fullness Rating | .01 (.06) | .880 | ||
| Pubertal Development | .24 (.07) | .001 | ||
| Self-Reported Distress | F(6, 215)=4.69*** | .12 | ||
| Increase in Distress | −.04 (.09) | .650 | ||
| Overweight | .00 (.12) | .990 | ||
| Distress × Overweight | .21 (.13) | .102 | ||
| Sex | −.16 (.07) | .021 | ||
| Fullness Rating | .01 (.07) | .905 | ||
| Pubertal Development | .23 (.07) | .002 | ||
| Moderator: Child Sex | ||||
| Observed Anxiety | F(6, 215)=4.07*** | .10 | ||
| Proportion Anxiety | .03 (.21) | .903 | ||
| Sex | −.15 (.18) | .419 | ||
| Anxiety × Sex | .01 (.27) | .979 | ||
| Overweight | .16 (.07) | .019 | ||
| Fullness Rating | .01 (.07) | .849 | ||
| Pubertal Development | .24 (.07) | .001 | ||
| Self-Reported Distress | F(6, 215)=4.23*** | .11 | ||
| Increase in Distress | −.03 (.21) | .899 | ||
| Sex | −.21 (.12) | .086 | ||
| Distress × Sex | .11 (.24) | .648 | ||
| Overweight | .17 (.07) | .016 | ||
| Fullness Rating | .01 (.07) | .862 | ||
| Pubertal Development | .23 (.07) | .002 |
Note. Standardized beta coefficients from multivariate linear regression models adjusting for child sex (in overweight as moderator models), child overweight (in sex as moderator models), child fullness rating, and child pubertal development.
p <.001.
Figure 1.
Proportion of Intervals with Anxiety and EAH Kcal Consumption in Overweight and Not Overweight Children
Note. Follow-up analysis of the slopes for each group indicated that both slopes were marginal (βoverweight = .18, p = .053; βnot overweight = −.16, p = .093); thus the significant interaction is likely attributed to the different directions of association between observed anxiety and EAH among children with overweight compared to children without overweight.
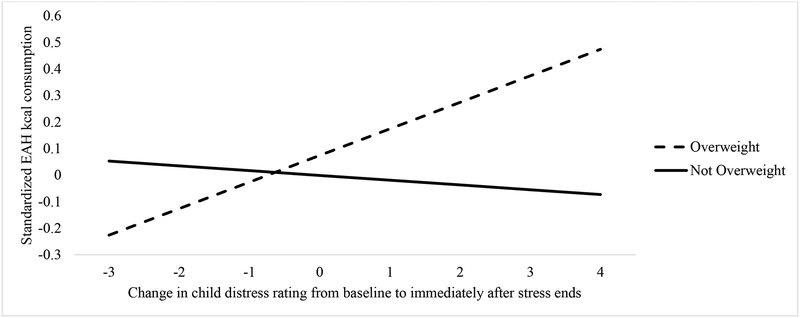
For the regression model using self-reported distress, the change in self-reported distress from baseline to post-induction, child weight status, and their interaction were entered in the model in addition to the covariates. The final model was significant (F (5, 216) = 5.65, p < .001, R2 = .12). In the final model, there was no main effect of self-reported distress (p = .650) or overweight (p = .99), but there was a main effect of sex (p = .021) such that girls consumed fewer EAH kcal. The interaction between self-reported distress and weight status was marginally significant (β = .22, p = .100). To follow up on the hypothesized interaction, the slopes for the interaction between self-reported distress and weight status in relation to EAH were calculated and are presented in Figure 2. Analysis of the slopes for each group indicated that the association was non-significant in children without overweight (βnot overweight = −.03, p = .752) and marginal in children with overweight (βoverweight = .15, p = .100), suggesting a similar pattern as for observed anxiety such that children with overweight engaged in more EAH as self-reported distress increased, but only at a trend level.
Figure 2.
Change in Child Distress Rating and EAH Kcal Consumption in Overweight and Not Overweight Children
Note. Analysis of the slopes for each group indicated that the association was non-significant in children without overweight (βnot overweight = −.03, p = .752) and marginal in children with overweight (βoverweight = .15, p = .100), suggesting a similar pattern as for observed anxiety such that children with overweight engaged in more EAH as self-reported distress increased, but at a trend level.
Moderation by sex.
Parallel multivariate linear regression models were conducted to test child sex as a moderator of the stress response-EAH association adjusting for child weight status, pubertal development, and fullness rating (see Table 3). Although the final models examining child sex as a moderator using observed anxiety (F (5, 216) = 4.89, p < .001; R2 = .10) and self-reported distress (F (5, 216) = 5.09, p < .001; R2 = .11) were significant, we found no moderating effect of sex (p = .979 for observed anxiety; p = .648 for self-reported distress). There were no main effects for stress indicators (p=.903 for observed anxiety, p=.899 for self-reported distress). The effect of child sex on EAH was not significant in the observed anxiety model (p=.419), and was marginal in the self-reported distress model (p=.086) such that girls consumed fewer EAH kcal (see Table 3). There was a main effect of overweight such that children with overweight consumed more kcal in the EAH protocol (p = .019 for observed anxiety model; p = .016 for self-reported distress model).
Discussion
The current study sought to investigate whether child weight status and child sex moderated the association between child stress response in a stress-induction protocol and subsequent EAH in a sample of low-income youth. There were three main findings of this study. First, patterns of EAH and stress response varied by weight status, such that children with overweight engaged in more EAH when they displayed greater anxiety, whereas children without overweight engaged in less EAH when they displayed greater anxiety. Second, although the direction of the associations was the same for observed anxiety and self-reported distress, the interaction between weight status and self-reported distress was not significant. Third, child sex did not moderate the stress response – EAH association.
Similar findings regarding weight status as a moderator of the association between stress response and eating have been shown in studies of adults. To our knowledge, the only other study to examine weight status as a moderator of stress-eating associations in children found no difference in the association between perceived stress and emotional eating in children with overweight as opposed to children without overweight (Nguyen-Rodriguez et al., 2008). Our findings may differ from Nguyen-Rodriguez et al. (2008) since the current study assessed eating behavior as an outcome rather than emotional eating, which is an eating style. The lack of moderation by weight status found in Nguyen-Rodriguez et al. (2008) may also be due to their use of self-report measures to assess both emotional eating and stress.
We found significant results for our observed, but not self-reported measures of stress response in children, suggesting that differences between studies may in part be driven by method of assessment. Child self-reported distress, particularly of feelings like worry and anxiety that may have been elicited by our protocol, may not be highly reliable at young ages (Kazdin & Peiti, 1982). It may also be that the pathway from stress exposure to eating is physiological rather than psychological so the self-reported distress scale may be unable to capture stress-eating associations. When considering stress response in children, it may be important to use multiple measures of assessment, including observational and physiological measures if possible.
Type of stress exposure may also explain differences in findings across studies. Measures of acute stress use lab-induced stress, while chronic stress scales typically measure exposure to external, contextual and ongoing or daily stressors. Nguyen-Rodriguez et al. used the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1994), which is a measure of chronic stress exposure. It is possible that inducing stress in a lab-setting leads to different results. It could also be that weight status acts as a moderator of the association between acute stress response, rather than chronic stress, and eating behavior. Indeed, when Roemmich et al. (2011) examined acute stress and eating behaviors in a small, experimental study of school-aged children, they also found an interaction between dietary restraint and adiposity (percent body fat) on stress-induced eating. Similar to our findings, this work found that children who had low dietary restraint and lower adiposity consumed fewer kilocalories in response to acute stress, whereas children with low dietary restraint and higher adiposity consumed more kilocalories in response to acute stress.
Other studies examining weight status, acute stress, and eating in adults have found that overweight, compared to non-overweight individuals, consumed more kilocalories during EAH in a stress-manipulation versus control condition (Lemmens et al., 2011) and women with obesity prepared larger portions after a stress-induction compared to women without obesity (Klatzkin, Gaffney, Cyrus, Bigus, & Brownley, 2018). Of note, Roemmich et al. (2011), Lemmens et al. (2011), and Klatzkin, Gaffney, et al. (2018) were all small lab-based studies so their generalizability may be limited. Our study, which also found main effects of overweight on EAH, therefore adds to the literature in this area, and suggests that weight status may to be an important variable to consider when assessing stress-eating behavior associations, particularly under acute stress conditions, in children.
Our finding that children with overweight engaged in more EAH particularly when they exhibited a greater stress response supported our hypothesis, and may be explained by multiple factors. Potential mechanisms underlying differences in stress-responsive eating by weight status are not yet well-understood, but may involve both biological and psychological pathways. There is evidence that individuals with overweight (vs. lean) are more sensitized to food cues after stress, displaying higher activation in brain reward regions following stress (Jastreboff et al., 2012). Individuals with overweight may over-eat palatable foods as a coping strategy that they have developed over time, one that serves to reduce physiological distress (Dallman, 2010). In animal models, it has been observed that obesity-related and stress biology processes interact in complex ways to shape stress-related eating behavior, with certain types of stress exposure and metabolic conditions resulting in excessive eating (hyperphagia), and others resulting in reduced eating (hypophagia) (see Razzoli & Bartolomucci [2016] for further discussion). Future studies could vary stress exposure type in order to articulate these processes.
Acute, compared to chronic stress exposure, may affect eating behavior in different ways. It has been suggested that acute stress promotes the release of corticotropin-releasing hormone (CRH), a hormone which inhibits appetite (Richard, Lin, & Timofeeva, 2002). When individuals are exposed to long-term stress, however, the body produces excessive glucocorticoids (Lupien, McEwen, Gunnar, & Heim, 2009), which in turn can stimulate appetite (Sominsky & Spencer, 2014). Although our study found no differences in children’s observed anxiety (p = .231) or self-reported distress (p = .868) by weight status, children who demonstrated a stress response reacted differently in their eating behavior as a function of weight status, for example, with a 10% increase in the proportion of observed anxiety associated with 11.9 fewer kcals consumed in the not overweight group, but 14.5 more kcals consumed in the overweight group; and a 1-point increase in self-reported distress from baseline to stress associated with 3.3 fewer kcals consumed in the not overweight group, but 18.6 more kcal consumed in the overweight group. It is plausible that acute stress induced from the protocol led to some appetite suppression in children without overweight. In contrast, children with overweight may have responded to the stress of the protocol differently due to a tendency to engage in eating behavior as a psychological coping mechanism. Thus, although these effect sizes are small, their impact may add up over time and with repeated exposures to stress.
It has been proposed that individuals with overweight are already managing weight stigma that can be activated under social stress conditions such as the TSST-C and may deplete self-regulation capacity (Major, Eliezer, & Rieck, 2012). In the current study, despite not reporting feeling stressed, children with overweight may have expended excessive self-regulatory resources attempting to regulate their stress during the protocol, and thus had reduced capacity to control their eating behavior when the EAH food was presented (Johns, Inzlicht, & Schmader, 2008). Alternatively, children with overweight may also have needed to consume more of the palatable food to feel soothed after the stress, compared to leaner children, due to reduced reward sensitivity in the brain (Born et al., 2010), or may have had more intense craving for the food (Sinha, 2018). The precise mechanisms of association will be important to disentangle in future work.
Unique contributions of the current study are that it is the first relatively large-scale study of children to examine EAH in response to a behavioral stress-induction protocol and to assess stress response using multiple measures. Prior lab-based work in small samples has assessed objective eating behavior, but assessed self-reported stress (Roemmich et al., 2011). Larger-scale studies have typically relied on measures of self-reported stress as well as self-reported eating behavior (Michels et al., 2015; Nguyen-Rodriguez et al., 2008). We were able to examine how both self-reported and observed displays of stress relate to objectively-measured eating behavior. Self-reported and observed eating behaviors can yield remarkably different results, as most adult studies finding overeating in response to stress have observed eating behaviors (Born et al., 2010; Chaput et al., 2008; Epel et al., 2001; Lemmens et al., 2011; Rutters et al., 2009), whereas the majority of adult studies finding undereating in response to stress have used self-reported eating (Berg-Beckhoff & El Ansari, 2015; Popper, Smits, Meiselman, & Hirsch, 1989; Stone & Brownell, 1994; Wallis & Hetherington, 2009). Further research using multiple measures of child eating behaviors in relation to multiple measures of stress is needed to better understand the association.
In addition to the multiple measurements and relatively large sample size, the low-income sample was another strength. In comparison to wealthier children, low-income children are at greater risk for overweight (Shrewsbury & Wardle, 2008) and tend to experience high levels of chronic stress, both physical and psychosocial (Evans & Kim, 2013). Due to the high levels of chronic stress experienced by low-income families, understanding stress response-eating associations in this sample is critically important for mitigating risk for overweight among children growing up in low-income families. We expect that investigating stress-eating associations in a moderate- to high-income sample, who may experience low to moderate levels of chronic stress, could yield different results, as these individuals may have greater self-regulatory resources available. Examining factors such as socioeconomic status as a proxy for early life stress may also prove useful for understanding stress response-eating associations during middle childhood.
The finding that child sex did not moderate the stress response-EAH association was counter to our hypothesis. Sex differences in stress-eating have been found in studies such as Pérusse-Lachance et al. (2013) which found that men decreased energy intake, whereas women increased energy intake after a stress task. Although we observed some main effects of sex such that girls tended to consume less overall in the EAH protocol than boys, our primary findings that sex did not moderate the association between stress response and eating in the absence of hunger during middle childhood may suggest that males and females are similarly susceptible to eating in response to stress during this developmental period. The findings are consistent with Roemmich et al. (2002), who found that sex had no significant effect on the association between induced stress and eating behaviors in children the age of those in our study. Since stress responses develop throughout puberty, it is plausible that the participants in the current study were too early in their pubertal development to show significant sex differences in stress-eating patterns (Hess & Richards, 1999; Spear, 2009). Future longitudinal work on stress-eating across the developmental period of early to mid and later adolescence will be important in order to detect when the association first emerges.
As with all studies, the current study had limitations. First, we examined only one type of stress exposure, a lab-based acute stress induction procedure. As children may respond differently to real-life versus lab-induced stress, it would be important to replicate this lab-based study in a more naturalistic setting, as well as consider different stressor types (Emond et al., 2016). The stress reactivity measures used in the study were also assessed across different time intervals, as observed anxiety was coded throughout the entire TSST-C protocol, while self-reported distress was only assessed at baseline and post-stress induction. Furthermore, although we assessed observed stress response based on an established coding system and reliable independent raters, we assessed child self-rated distress using a novel measure. It will be important to examine the psychometric properties of this measure in future work.
The EAH protocol used may also present a limitation. A standardized dinner meal was served to avoid introducing variability in foods. The dinner meal which was served was familiar to most families and most children consumed at least part of the meal, however, the selected meal may have affected each child’s intake. Afterwards, desserts were the only food type offered, and some work has shown differences in eating behavior in response to stress as a function of food type, with increased consumption of sweet foods and decreased consumption of healthy options (Zellner et al., 2006). Some individuals may have a preference to eat other high-calorie palatable foods, rather than sweet foods, when stressed. In the current study children were also not offered other activities such as screen time or physical activity as in other work (Balantekin & Roemmich, 2012), and so it is unknown whether children may have engaged in these activities, instead of EAH, if given these alternate options. Finally, the results may not be generalizable beyond this population since the sample was recruited from the Midwest United States and consisted entirely of children who had attended Head Start as preschoolers, a population that can experience chronic stress.
Conclusion and Implications
During middle childhood, overweight may drive increased consumption of palatable foods in response to stress. Future research could consider examining the direction of effects by measuring these constructs longitudinally across development, or by experimentally testing whether changing weight status could reduce the stress-eating association. Findings from the study may have implications for obesity prevention. If children with overweight are more highly susceptible to stress-related eating, stress-management programs may serve as a valuable alternative or addition to traditional weight management approaches in youth. For example, a randomized controlled trial by Stavrou et al. (2016) applied a stress-management intervention and found it to be effective in reducing BMI of children with overweight and obesity. Furthermore, public health may benefit by establishing avenues (e.g., schools, healthcare settings, television commercials) where youth can become educated about stress-management techniques, which could have a positive impact across a broad array of child health outcomes.
What’s New:
Among school-aged children, weight status was found to moderate associations between distress response during a stress-induction task and eating in the absence of hunger (EAH). Children with overweight engaged in more EAH when distressed, whereas children without overweight engaged in less EAH when distressed.
Funding Sources:
This work was supported by the National Institutes of Health R01 DK098983 to A. Miller and J. Lumeng
Abbreviations:
- BMI
body mass index
- SD
standard deviation
- EAH
eating in the absence of hunger
- TSST-C
Trier Social Stress Test for Children
- g
grams
- kcal
kilocalories
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- Baidal J, & Taveras EM (2012). Childhood obesity: Shifting the focus to early prevention. Archives of Pediatrics & Adolescent Medicine, 166(12), 1179–1181. doi: 10.1001/2013.jamapediatrics.358 [DOI] [PubMed] [Google Scholar]
- Balantekin KN, & Roemmich JN (2012). Children’s coping after psychological stress. Choices among food, physical activity, and television. Appetite, 59(2), 298–304. [DOI] [PubMed] [Google Scholar]
- Bellisle F, Drewnowski A, Anderson GH, Westerterp-Plantenga M, & Martin CK (2012). Sweetness, Satiation, and Satiety. The Journal of Nutrition, 142(6), 1149S–1154S. doi: 10.3945/jn.111.149583 [DOI] [PubMed] [Google Scholar]
- Berg-Beckhoff G, & El Ansari W (2015). Do Egyptian university students eat more or eat less when perceiving stress? Paper presented at the European Congress of Epidemiology. [Google Scholar]
- Born JM, Lemmens SG, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, & Westerterp-Plantenga MS (2010). Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. International journal of obesity, 34(1), 172. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, & Hellhammer D (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic medicine, 59(4), 419–426. [DOI] [PubMed] [Google Scholar]
- Cartwright M, Wardle J, Steggles N, Simon AE, Croker H, & Jarvis MJ (2003). Stress and dietary practices in adolescents. Health Psychology, 22(4), 362. [DOI] [PubMed] [Google Scholar]
- Chaput J-P, Drapeau V, Poirier P, Teasdale N, & Tremblay A (2008). Glycemic instability and spontaneous energy intake: association with knowledge-based work. Psychosomatic medicine, 70(7), 797–804. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. Measuring stress: A guide for health and social scientists. [Google Scholar]
- Dallman MF (2010). Stress-induced obesity and the emotional nervous system. Trends in Endocrinology & Metabolism, 21(3), 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriendt T, Clays E, Huybrechts I, De Bourdeaudhuij I, Moreno LA, Patterson E, … Widhalm K (2012). European adolescents’ level of perceived stress is inversely related to their diet quality: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. British Journal of Nutrition, 108(2), 371–380. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cook SH, Sturza J, Kaciroti N, Gearhardt AN, Vazquez DM, … Miller AL (2018). Family conflict, chaos, and negative life events predict cortisol activity in low-income children. Developmental Psychobiology, 60(4), 364–379. doi:doi: 10.1002/dev.21602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond M, Ten Eycke K, Kosmerly S, Robinson AL, Stillar A, & Van Blyderveen S (2016). The effect of academic stress and attachment stress on stress-eaters and stress-undereaters. Appetite, 100, 210–215. doi: 10.1016/j.appet.2016.01.035 [DOI] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, & Niaura R (2004). Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci, 1032, 208–210. doi: 10.1196/annals.1314.022 [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, & Brownell K (2001). Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology, 26(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2013). Childhood Poverty, Chronic Stress, Self-Regulation, and Coping. Child Development Perspectives, 7(1), 43–48. doi: 10.1111/cdep.12013 [DOI] [Google Scholar]
- Farrow CV, Haycraft E, & Blissett JM (2015). Teaching our children when to eat: how parental feeding practices inform the development of emotional eating—a longitudinal experimental design. The American Journal of Clinical Nutrition, 101(5), 908–913. doi: 10.3945/ajcn.114.103713 [DOI] [PubMed] [Google Scholar]
- Fisher JO, & Birch LL (2000). Parents’ Restrictive Feeding Practices are Associated with Young Girls’ Negative Self-evaluation of Eating. Journal of the American Dietetic Association, 100(11), 1341–1346. doi: 10.1016/S0002-8223(00)00378-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis L, Granger D, & Susman E (2013). Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite, 64, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakis G, Pediaditis M, Manousos D, Kazantzaki E, Chiarugi F, Simos PG, … Tsiknakis M (2017). Stress and anxiety detection using facial cues from videos. Biomedical Signal Processing and Control, 31, 89–101. doi: 10.1016/j.bspc.2016.06.020 [DOI] [Google Scholar]
- Gibson EL (2012). The psychobiology of comfort eating: implications for neuropharmacological interventions. Behavioural Pharmacology, 23(5 and 6), 442–460. doi: 10.1097/FBP.0b013e328357bd4e [DOI] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, … Epel E (2012). What is eating you? Stress and the drive to eat. Appetite, 58(2), 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, & Straub RO (1992). The role of gender and taste class in the effects of stress on eating. Health Psychology, 11(2), 97–100. doi: 10.1037/0278-6133.11.2.97 [DOI] [PubMed] [Google Scholar]
- Hess RS, & Richards ML (1999). Developmental and gender influences on coping: Implications for skills training. Psychology in the Schools, 36(2), 149–157. [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, & Potenza MN (2012). Neural Correlates of Stress- and Food-Cue–Induced Food Craving In Obesity. Association with insulin levels. doi: 10.2337/dc12-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong E-Y, & Kim K-N (2007). Influence of stress on snack consumption in middle school girls. Nutrition research and practice, 1(4), 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M, Inzlicht M, & Schmader T (2008). Stereotype threat and executive resource depletion: examining the influence of emotion regulation. Journal of Experimental Psychology: General, 137(4), 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah J, Yake M, Jones J, & Meyer M (2006). Stress influences appetite and comfort food preferences in college women. Nutrition Research, 26(3), 118–123. doi: 10.1016/j.nutres.2005.11.010 [DOI] [Google Scholar]
- Kazdin AE, & Peiti TA (1982). SELF-REPORT AND INTERVIEW MEASURES OF CHILDHOOD AND ADOLESCENT DEPRESSION. Journal of Child Psychology and Psychiatry, 23(4), 437–457. doi:doi: 10.1111/j.1469-7610.1982.tb00089.x [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Baldassaro A, & Hayden E (2018). The impact of chronic stress on the predictors of acute stress-induced eating in women. Appetite, 123, 343–351. doi: 10.1016/j.appet.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Gaffney S, Cyrus K, Bigus E, & Brownley KA (2018). Stress-induced eating in women with binge-eating disorder and obesity. Biol Psychol, 131, 96–106. doi: 10.1016/j.biopsycho.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL (2002). 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11(246), 1–190. [PubMed] [Google Scholar]
- Kudielka BM, & Wüst S (2010). Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress, 13(1), 1–14. doi: 10.3109/10253890902874913 [DOI] [PubMed] [Google Scholar]
- Lansigan RK, Emond JA, & Gilbert-Diamond D (2015). Understanding eating in the absence of hunger among young children: A systematic review of existing studies. Appetite, 85, 36–47. doi: 10.1016/j.appet.2014.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens SG, Rutters F, Born JM, & Westerterp-Plantenga MS (2011). Stress augments food ‘wanting’and energy intake in visceral overweight subjects in the absence of hunger. Physiology & behavior, 103(2), 157–163. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Miller A, Peterson KE, Kaciroti N, Sturza J, Rosenblum K, & Vazquez DM (2014). Diurnal cortisol pattern, eating behaviors and overweight in low-income preschool-aged children. Appetite, 73, 65–72. doi: 10.1016/j.appet.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Major B, Eliezer D, & Rieck H (2012). The psychological weight of weight stigma. Social Psychological and Personality Science, 3(6), 651–658. [Google Scholar]
- Michaud C, Kahn JP, Musse N, Burlet C, Nicolas JP, & Mejean L (1990). Relationships between a critical life event and eating behaviour in high-school students. Stress Medicine, 6(1), 57–64. doi : doi : 10.1002/smi.2460060112 [DOI] [Google Scholar]
- Michels N, Sioen I, Boone L, Braet C, Vanaelst B, Huybrechts I, & De Henauw S (2015). Longitudinal association between child stress and lifestyle. Health Psychology, 34(1), 40–50. doi: 10.1037/hea0000108 [DOI] [PubMed] [Google Scholar]
- Miller AL, Gearhardt AN, Retzloff L, Sturza J, Kaciroti N, & Lumeng JC (2018). Early Childhood Stress and Child Age Predict Longitudinal Increases in Obesogenic Eating among Low-Income Children. Academic Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, & Olson SL (2000). Emotional expressiveness during peer conflicts: A predictor of social maladjustment among high-risk preschoolers. Journal of Abnormal Child Psychology, 28(4), 339–352. [DOI] [PubMed] [Google Scholar]
- Morris NM, & Udry JR (1980). Validation of a self-administered instrument to assess stage of adolescent development. Journal of youth and adolescence, 9(3), 271–280. [DOI] [PubMed] [Google Scholar]
- Nguyen-Rodriguez ST, Chou C-P, Unger JB, & Spruijt-Metz D (2008). BMI as a moderator of perceived stress and emotional eating in adolescents. Eating behaviors, 9(2), 238–246. doi: 10.1016/j.eatbeh.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DB, Jones F, Conner M, McMillan B, & Ferguson E (2008). Effects of daily hassles and eating style on eating behavior. Health Psychology, 27(1S), S20. [DOI] [PubMed] [Google Scholar]
- Pérusse-Lachance E, Brassard P, Chaput J-P, Drapeau V, Teasdale N, Sénécal C, & Tremblay A (2013). Sex differences in the effects of mental work and moderate-intensity physical activity on energy intake in young adults. ISRN nutrition, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper R, Smits G, Meiselman HL, & Hirsch E (1989). Eating in combat: a survey of US Marines. Military medicine, 154(12), 619–623. [PubMed] [Google Scholar]
- Razzoli M, & Bartolomucci A (2016). The Dichotomous Effect of Chronic Stress on Obesity. Trends in Endocrinology & Metabolism, 27(7), 504–515. doi: 10.1016/j.tem.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Lin Q, & Timofeeva E (2002). The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur JPharmacol, 440(2–3), 189–197. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Lambiase MJ, Lobarinas CL, & Balantekin KN (2011). Interactive effects of dietary restraint and adiposity on stress-induced eating and the food choice of children. Eating behaviors, 12(4), 309–312. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Wright SM, & Epstein LH (2002). Dietary Restraint and Stress-Induced Snacking in Youth. Obesity, 10(11), 1120–1126. [DOI] [PubMed] [Google Scholar]
- Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, & Westerterp-Plantenga MS (2009). Acute Stress-related Changes in Eating in the Absence of Hunger. Obesity, 17(1), 72–77. [DOI] [PubMed] [Google Scholar]
- Shin LM, & Liberzon I (2009). The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology, 35, 169. doi: 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrewsbury V, & Wardle J (2008). Socioeconomic Status and Adiposity in Childhood: A Systematic Review of Cross-sectional Studies 1990–2005. Obesity, 16(2), 275–284. doi:doi: 10.1038/oby.2007.35 [DOI] [PubMed] [Google Scholar]
- Sinha R (2018). Role of addiction and stress neurobiology on food intake and obesity. Biol Psychol, 131, 5–13. doi : 10.1016/j.biopsycho.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slochower J, Kaplan SP, & Mann L (1981). The effects of life stress and weight on mood and eating. Appetite, 2(2), 115–125. [DOI] [PubMed] [Google Scholar]
- Sominsky L, & Spencer SJ (2014). Eating behavior and stress: a pathway to obesity. Frontiers in psychology, 5, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2009). Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and psychopathology, 21(1), 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Nicolaides NC, Papageorgiou I, Papadopoulou P, Terzioglou E, Chrousos GP, … Charmandari E (2016). The effectiveness of a stress-management intervention program in the management of overweight and obesity in childhood and adolescence. Journal of molecular biochemistry, 5(2), 63–70. [PMC free article] [PubMed] [Google Scholar]
- Stone AA, & Brownell KD (1994). The stress-eating paradox: Multiple daily measurements in adult males and females. Psychology & Health, 9(6), 425–436. doi: 10.1080/08870449408407469 [DOI] [Google Scholar]
- Wallis DJ, & Hetherington MM (2009). Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite, 52(2), 355–362. [DOI] [PubMed] [Google Scholar]
- The Wechsler Individual Achievement Test-Third Edition (WIAT III). (2009).
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, & Wolf A (2006). Food selection changes under stress. Physiology & behavior, 87(4), 789–793. doi: 10.1016/j.physbeh.2006.01.014 [DOI] [PubMed] [Google Scholar]