Abstract
Objective:
To examine the effect of High-intensity interval training (HIIT) on body fat mass and distribution in cardiac rehabilitation (CR) patients with myocardial infarction (MI).
Patients and Methods:
We retrospectively screened 391 consecutive MI patients enrolled in CR between September 1st, 2015 and February 28th, 2018. We included 120 patients who completed 36 CR sessions and pre-post dual-energy X-ray absorptiometry (DXA); ninety engaged in HIIT and 30 engaged in moderate-intensity continuous training (MICT). HIIT included 4–8 alternating intervals of high- (30–60 seconds at RPE 15–17 [Borg 6–20]) and low-intensity (1–5 min at RPE <14), and MICT performed 20–45 min of exercise at RPE 12–14. Body weight, fat mass, and lean mass were measured via DXA with lipid profile measured via clinical procedures.
Results:
HIIT and MICT groups were similar age (67 vs. 67 yrs), sex (27% vs. 27%) and BMI (30.3 vs.29.5 kg/m2) at baseline. HIIT group demonstrated greater reductions in body fat percentage (−2.3±1.8 vs. −0.4±2.0 %, P<.001), fat mass (−2.5±2.2 vs. −0.5±2.0 kg, P<.001), abdominal fat percentage (−3.3±3.2 vs. −0.1±3.2 %, P<.001), and waist circumference (−3±5 vs. −0±5 cm, P=.01). HIIT group demonstrated greater reductions in total cholesterol (P=.002), low-density lipoproteins (P<.001), and triglycerides (P=.006). Improvements in total body mass and BMI were not different across groups. After matching exercise duration, intensity, and energy expenditure; HIIT induced improvements in total fat mass (P=.02), body fat percentage (P=.01) and abdominal fat percentage persisted (P=.02).
Conclusion:
Our data suggest supervised HIIT results in significant reductions in total fat mass and abdominal fat percentage, and improved lipid profile in CR patients with MI.
INTRODUCTION
Each year more than 7 million people experience acute myocardial infarction (MI) worldwide1, and with more effective treatment, substantial reductions in mortality have been reported in recent decades.1,2 Despite consistent improvements in mortality following first MI, among patients who do survive a MI, 20% suffer a second cardiovascular event in the first year and ~50% present with major coronary events when having previously been discharged from the hospital following diagnosis with MI.3
Increasing evidence suggest that body fat and abdominal fat percentage are independent risk factors for long-term cardiovascular events and mortality in patients with4 and those without coronary heart disease (CHD),5 and may be better predictors than body weight and BMI.6,7 For example, in patients with established CHD who are in the highest quartile of body fat percentage, the risk of cardiovascular events is double when compared with those in the lowest quartile.4 Exercise training is considered an adjunctive therapy in patients with MI based on current guidelines.8 However, there remains no universal agreement on the most effective exercise prescription to improve body composition and fat distribution for patients with CHD. Traditional moderate-intensity continuous training (MICT) is generally used to halt weight gain for individuals who are overweight or obese,9 and has demonstrated sex-based differences in substrate metabolism during MICT, women have a larger depot of intramyocellular lipid available to support MICT fuel needs, suggestive of a greater capacity to use intramyocellular lipid.10 Recent studies have demonstrated that high-intensity interval training (HIIT) may be more effective at reducing subcutaneous and abdominal body fat,11–14 increasing cardiopulmonary fitness, and improving insulin resistance15 than MICT in apparently healthy overweight or obese individuals.11–15 There is a paucity of research which examines the sex-based difference in effects of HIIT on body composition. Townsend et al. reported that the total post-exercise oxygen consumption, a key mechanism of exercise reducing body fat, was not significantly different between men and women,16 which suggest that HIIT may be able to elicit same health benefit to both men and women.
The purpose of this study was to quantify the efficacy of HIIT on body composition and adipose distribution in patients undergoing CR after MI. We hypothesized that HIIT will be associated with greater improvements in total body fat and abdominal adipose tissue for patients with MI compared to MICT.
METHODS
Study Design and Participants
This study was a retrospective cohort review of consecutive patients enrolled in cardiac rehabilitation after MI at Mayo Clinic, Rochester, MN, between September 1st, 2015 and February 28th, 2018. A total of 391 consecutive MI patients enrolled in early outpatient (phase II) cardiac rehabilitation (CR) were retrospectively screened. We included all 120 patients who completed all 36 prescribed CR sessions and underwent both pre and post dual-energy X-ray absorptiometry (DXA). Of the 120 patients, 90 engaged in HIIT with 30 MICT. All aspects of this study conformed to the principles outlined in the Declaration of Helsinki and were approved by the Mayo Clinic Institutional Review Board. All participants agreed to the use of their medical records for research purposes.
HIIT and MICT Protocols
Supervised exercise training was performed on a treadmill, cycle ergometer or recumbent stepper on average three times per week at the Mayo Clinic CR center. Three days of self-guided home based exercise when not at the CR center was also recommended. All patients performed MICT during the first week of CR, after which, if patients were able to exercise for ≥ 20 minutes at a rating of perceived exertion (RPE: Borg 6–20) of 12–14, they chose to continue MICT or transition to HIIT. Patients were excluded if they presented with impaired cognition, language barriers, angina at low workload and musculoskeletal limitations including injuries, frailty, and weakness. The HIIT sessions included brief, intermittent bouts of high intensity exercise, interspersed with periods of low intensity exercise (active recovery). Since the majority of patients are prescribed rate modulating pharmacotherapy after MI, the intensity of HIIT in the present study was prescribed using RPE in an effort to maximize generalizability and ensure real-world applicability. These patients began with 4 high intensity intervals of 30–60 seconds at a RPE of 15–17 interspersed with 1–5 minutes of low intensity intervals at a RPE<14, and progressed to 5–8 high intensity intervals of 2–4 minutes at RPE 15–17 during 20–45 minutes of training. The exercise training modality was intentionally patient selected to maximize adherence to the protocol. HIIT was performed only during supervised sessions with the frequency not to exceed 3 days per week (non-consecutive days). MICT was performed for 20–45 min at a RPE of 12–14. Exercise intensity and duration of every session of HIIT and MCIT were documented. For all patients, sessions included a 5–10 minute warm-up and cool-down at an RPE<12. Heart rate (HR) and rhythm was continuously monitored via a Q-Tel tele-monitoring system (Welch Allyn, USA) during the training sessions with blood pressure (systolic [SBP] and diastolic [DBP]) measured by manual sphygmomanometry at rest, after the second or third interval (in the HIIT group) or after 15 minutes (in the MICT group), and at the end of each exercise training session. Metabolic equivalent (MET) was calculated according to American College of Sports Medicine (ACSM)’ metabolic calculation equations.17 Energy expenditure (EE) was calculated according to the physical activity calculation equation from the ACSM guidelines, Kcal· min−1 = [(METs × 3.5 mL· kg−1· min−1 × body weight in kg) ÷1,000] × 518.
Anthropometric Measurement
Body composition was measured using DXA scans (Lunar iDXA Series X, Madison, WI, USA). Scans were performed by trained radiology technicians using standardized procedures recommended by GE-Healthcare as described previously.19 The DXA scans were performed within one week prior to the start and within one week after the completion of 36 CR sessions. These scans were used to measure total body mass, body fat percentage, body fat mass, total body lean mass, and abdominal region fat percentage (defined as the area between the ribs and the pelvis by GE-Healthcare systems). Percentages of total were calculated accordingly. The scanner was calibrated daily against a standard calibration block supplied by the manufacturer to control for possible baseline drift. Height was measured using a stadiometer. Body mass index (kg/m2) was calculated using the formula: body weight (kg)/height (m)2. Waist and hip circumference were manually measured according to standard procedures of ACSM guidelines.18
Lipid Profile
Total cholesterol, high-density lipoprotein cholesterol (HDL-c) and triglycerides were measured within one week prior to the start and within one week after the completion of 36 sessions of CR. Low-density lipoprotein cholesterol (LDL-c) was estimated using the Friedewald equation as described previously.20 Non-HDL cholesterol (Non-HDL-c) was calculated as total cholesterol minus HDL cholesterol. Blood samples were analyzed and reported to the medical record using standard clinical procedures of Mayo Clinic.
Dietary Assessment
Diet was assessed using the Rate-Your-Plate (RYP) questionnaire developed by Gans et al.21 in the late 1980s (periodically updated thereafter) and is recommended as a tool to assess patient’s dietary quality by the American Association of Cardiovascular and Pulmonary Rehabilitation. The RYP tool consists of 24 questions, each response corresponding with a point value for the total RYP score. Total scores range from 24 to 72, with higher scores indicating better diet quality.21 The RYP tool has been validated and shown to have a strong relationship with body adiposity with higher RYP diet quality scores being associated with lower measures of body adiposity.22 All patients performed an interviewer‐administered RYP survey during the first and last CR session.
Statin Therapy Intensity Assessment
Statin therapy intensity was assessed within one week prior to the start and within one week after the completion of 36 sessions of CR. Statin therapy intensities were stratified in high-, moderate-, and low-intensity using ACC/AHA guidelines on the treatment of blood cholesterol as described previously.23 Statin intensity therapy is defined as a pseudo continuous variables, 3 = high intensity, 2 = moderate intensity, 1 = low intensity, and 0 = none.
Exercise Capacity
Exercise capacity was routinely assessed at the start of CR via using exercise stress test, cardiopulmonary exercise test (CPET) or 6 minute walk test (6 MWT) appropriately. The present study collected the patients’ maximal oxygen consumed (VO2peak) data that was determined by CPET to assess patients’ exercise capacity.
Depressive Symptoms Assessment
Depressive symptoms were evaluated using the Patient Health Questionnaire (PHQ-9) over the course of 36 sessions of CR as prescribed previously.24
Statistical Analysis
Baseline data are presented as frequency and percentage for categorical variables and compared across groups using Pearson chi-square test or Fisher exact test. Continuous data are presented as mean and standard deviation and compared across groups using two-sample t-test or non-parametric Wilcoxon rank-sum test, as appropriate depending on distribution. Changes from baseline to 36 sessions of CR are summarized using mean and standard deviation and compared within group using paired t-test. Normality was evaluated and changes looked approximately normally distributed. In order to compare change in each parameter of interest across groups, analysis of covariance (ANCOVA) was used. In this analysis, change was modelled as the outcome in a linear regression model and group effects were tested with adjustment for the baseline value of the parameter being tested. These analyses were repeated in a matched subset of each group to control for potential differences in exercise duration, exercise intensity, and exercise energy expenditure. In order to assess differences over time across groups, a repeated measures analysis of variance (ANOVA) was used. In this analysis, measurements at each week were used the interaction of time and group was tested to examine differences in effects over time across group. Energy expenditure and METs was log transformed in these analyses to approximate normality prior to analysis. Means and confidence intervals were back-transformed to the original scale for presentation. Comparisons at each week used the method of Scheffe to adjust p-values for multiple comparisons. SAS version 9.4 (Cary, NC) was used for analyses and Two-sided p-values ≤ .05 were considered to be statistically significant.
RESULTS
Study Participants
Baseline characteristic were not different for MICT and HIIT groups, although three medications were not well balanced across groups: antiplatelet drugs, diuretics, and Digoxin (Table 1). Mean age (67±16 vs. 67±12 y, P=.84), body weight (86.6±3.0 vs. 87.5±1.7, kg, P=.80), and BMI (30.3±4.5 vs. 29.5±4.8, kg/m2, P=.42) were not different across groups at baseline. Only 27% of the patients were women in both groups. Percentage of participants who underwent PCI (47% vs. 43%, P=.75) and CABG (30% vs. 19%, P=.20) was not significantly different across MICT and HIIT groups. The number of days between patients’ MI event and beginning of CR was also not significantly different across MICT and HIIT groups (14±6 vs. 14±10, days, P =.15).
TABLE 1.
| MICT (n=30) | HIIT (n=90) | p-value | |
|---|---|---|---|
| Age, mean ± SD, y | 67±16 | 67±12 | P=.84 |
| Female sex, n (%) | 8 (27) | 24 (27) | P>.99 |
| BMI, mean ± SD, kg/m2 | 30.3±4.5 | 29.5±4.8 | P=.42 |
| Height, mean ± SD, cm | 169±2 | 172±1 | P=.08 |
| Body weight, mean ± SD, kg | 86.6±3.0 | 87.5±1.7 | P=.80 |
| Days between CR and MI, mean ± SD, days | 14±6 | 14±10 | P=.15 |
| Co-morbidities, n (%) | |||
| PCI | 14 (47) | 39 (43) | P=.75 |
| CABG | 9 (30) | 17 (19) | P=.20 |
| Hyperglycemia | 3 (10) | 10 (11) | P>.99 |
| Hypertension | 24 (80) | 78 (87) | P=.38 |
| Dyslipidemia | 12 (40) | 41 (46) | P=.60 |
| Metabolic syndrome | 10 (33) | 33 (36.7) | P=.74 |
| Ever Smoker | 12 (40) | 38 (42) | P=.83 |
| Medications, n (%) | |||
| ACEIs/ARBs | 15 (50) | 49 (54) | P=.67 |
| Antiplatelet drugs | 24 (80) | 88 (98) | P=.003 |
| Anticoagulants | 1 (3) | 3 (3) | P>.99 |
| Beta receptor blockers | 23 (77) | 74 (82) | P=.50 |
| CCBs | 4 (13) | 18 (20) | P=.59 |
| Diuretics | 7 (23) | 6 (7) | P=.02 |
| Digoxin | 3 (10) | 0 | P=.01 |
| Nitrates | 5 (17) | 12 (13) | P=.65 |
| Statins | 27 (90) | 85 (94) | P=.41 |
| VO2peak, ml·kg−1·min−1 | 23.03±7.33 | 22.82±5.85 | P=.90 |
ACEIs = indicates angiotensin-converting enzyme inhibitors; ARBs = angiotensin II receptor blockers; BMI = body mass index; CABG = coronary artery bypass grafting; CCBs = Calcium channel blockers; HIIT = high-intensity interval training; MICT = moderate-intensity continuous training, PCI = percutaneous coronary intervention; VO2peak = maximal oxygen consumed.
Data are reported as Mean ± Standard deviation or number and percent population (%). Values were analyzed with two-sample t-test or Wilcoxon rank-sum test for continuous variables and Chi-square or Fisher exact test for categorical variables.
Resting Heart Rate and Blood Pressures
Prior to exercise training, there were no differences across groups at rest for HR (P=.05), SBP (P=.65), or DBP (P=.88). After 36 sessions of CR, the HIIT group demonstrated a significant lower resting HR (P=.003) whereas the MICT group had no change in resting HR. Patients both in MICT and HIIT groups demonstrated significantly lower DBP (P=.02, P<.001), but not in SBP after CR (Table 2).
TABLE 2.
| MICT (n=30) | HIIT (n=90) | ANCOVA | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Change | p-value | Baseline | Change | p-value | p-value | |
| Resting HR, bpm | 70±12 | −2±10 | P=.36 | 66±13 | −4±12 | P=.003 | P=.03 |
| SBP, mmHg | 118±22 | 2±21 | P=.64 | 120±17 | −2±18 | P=.34 | P=.41 |
| DBP, mmHg | 70±10 | −6±13 | P=.02 | 70±11 | −6±13 | P<.001 | P=.93 |
| Rate-Your-Plate scores | 53±8 | 4±9 | P=.01 | 56±7 | 5±5 | P<.001 | P=.02 |
| Statin therapy intensity | 2.3±1.0 | 0.1±0.8 | P=.65 | 2.6±0.8 | −0.0±0.5 | P=.40 | P=.75 |
| Anthropometric measurements | |||||||
| Body weight, kg | 86.4±14.6 | −0.3±3.5 | P=.89 | 87.6±16.7 | −1.4±3.9 | P<.001 | P=.16 |
| BMI, kg/m2 | 30.3±4.5 | −0.2±1.8 | P=.60 | 29.5±4.8 | −0.5±1.2 | P<.001 | P=.15 |
| Body fat, % | 38.1±7.3 | −0.4±2.0 | P=.24 | 36.3±7.7 | −2.3±1.8 | P<.001 | P<.001 |
| Fat mass, kg | 32.2±8.7 | −0.5±2.0 | P=.14 | 31.2±10.1 | −2.5±2.2 | P<.001 | P<.001 |
| Lean mass, kg | 51.4±9.2 | 0.2±2.8 | P=.70 | 53.5±9.8 | 0.9±1.4 | P<.001 | P=.07 |
| Abdominal fat, % | 45.9±9.0 | −0.1±3.2 | P=.91 | 44.5±10.1 | −3.3±3.2 | P<.001 | P<.001 |
| Waist circum., cm | 106±12 | 0±5 | P=.70 | 104±14 | −3±5 | P<.001 | P=.01 |
| Hip circum., cm | 107±8 | −1±4 | P=.07 | 107±10 | −2±5 | P=.007 | P=.86 |
| W/H ratio | 1.0±0.1 | 0.00±0.1 | P>.99 | 1.0±0.1 | −0.00±0.1 | P=.03 | P=.15 |
| Lipid profile | |||||||
| TC, mg/dl | 165.3±49.6 | −6.8±48.2 | P=.54 | 167.8±46.1 | −35.1±41.4 | P<.001 | P=.002 |
| HDL-c, mg/dl | 43.4±9.1 | 2.7±8.2 | P=.16 | 47.5±13.4 | 2.0±7.3 | P=.02 | P=.88 |
| LDL-c, mg/dl | 90.7±44.7 | −9.9±39.7 | P=.28 | 93.5±38.9 | −33.5±36.5 | P<.001 | P<.001 |
| Triglycerides, mg/dl | 164.3±89.1 | −6.4±55.2 | P=.61 | 137.9±84.9 | −24.8±50.0 | P<.001 | P=.006 |
| Non-HDL-c, mg/dl | 121.9±44.8 | −9.5±46.2 | P=.37 | 120.3±44.8 | −37.1±40.2 | P<.001 | P<.001 |
| PHQ-9 scores | 4.1±2.3 | −1.2±3.8 | P=..07 | 3.9±3.3 | −1.7±3.8 | P<.001 | P=.24 |
DBP= diastolic blood pressure; HDL-c = high-density lipoprotein cholesterol; HR = heart rate; LDL-c = low-density lipoprotein cholesterol; SBP = systolic blood pressure; PHQ = patient health questionnaire-9; W/H = waist/hip circumference; TC = total cholesterol.
Values are reported as Mean ± Standard deviation or number and percent population (%). The changes within group from baseline to the completion of 36 sessions of cardiac rehabilitation (CR) are assessed with paired t-test; the difference of changes across groups from baseline to the completion of 36 CR sessions, and Rate-Your-Plate scores comparisons at baseline and the completion of 36 CR sessions are analyzed by ANCOVA.
Training Intensity and Energy Expenditure during 36 sessions of Cardiac Rehabilitation
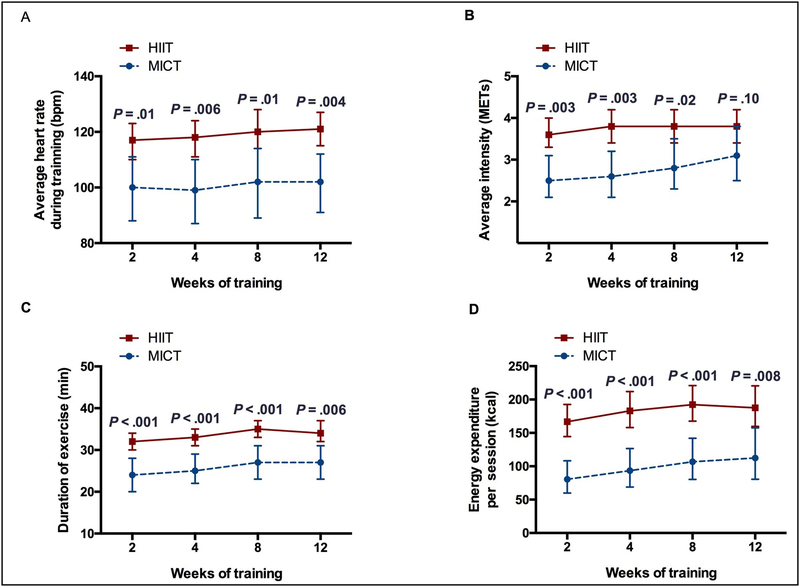
The HIIT group demonstrated significantly higher average HR, average exercise intensity, longer duration of exercise, and higher energy expenditure at each time point compared to MICT group (P<.05) with the exception of the average exercise intensity at the 12-weeks (P=.10). There were no differences in average HR (P=.98), duration of exercise (P=.99) and energy expenditure per session (P=.43) across groups over time. Evidence of a difference across groups in average exercise intensity was noted (P=.04) (Figure 1).
Figure 1:
Exercise parameters over the course of cardiac rehabilitation. HIIT = high-intensity interval training; MICT = moderate-intensity continuous training
A. Average heart rate during training. HIIT vs. MICT, 2-weeks: 117 vs. 100 bpm, P=.01; 4-weeks: 118 vs. 99 bpm, P=.006; 8-weeks: 121 vs. 102 bpm, P=.01; 12-weeks: 121 vs. 102 bpm, P=.004, respectively. There was no evidence of a difference across groups over time (P=.98).
B. Average intensity. 2-weeks: 3.6 vs. 2.5 METs, P=.003; 4-weeks: 3.8 vs. 2.6 METs, P=.003; 8-weeks: 3.8 vs. 2.8 METs, P=.02; 12-weeks: 3.8 vs. 3.1 METs, P=.10, respectively. Evidence of a difference across groups over time was noted (P=.04).
C. Duration of exercise. 2-weeks: 32 vs. 24 mins, P<.001; 4-weeks: 33 vs. 25 mins, P<.001; 8-weeks: 35 vs. 27 mins, P<.001; 12-weeks: 35 vs. 27 mins, P=.006, respectively. There was no evidence of a difference across groups over time (P=.99).
D. Energy expenditure per session. 2-weeks: 166 vs. 80 kcal, P<.001; 4-weeks: 183 vs. 93 kcal, P<.001; 8-weeks: 192 vs. 107 kcal, P<.001; 12-weeks: 188 vs. 112 kcal, P=.008, respectively. There was no evidence of a difference across groups over time (P=.43). Results presented are mean and 95% confidence limits based on repeated measures ANOVA. Comparisons at each time point are adjusted for multiple comparisons using the method of Scheffe.
Dietary Quality
The RYP scores were not different across MICT and HIIT groups at baseline (P=.06). Changes were different across groups after the completion of 36 CR sessions (P=.02).
Exercise Capacity and Depressive Symptoms
There were 26 (87%) patients in MICT group and 74 (82%) patients in HIIT performed CPET at the start of CR. There were no differences for VO2peak across groups (P = .90). The PHQ-9 scores were significant different from baseline to the completion of 36 CR sessions in HIIT group (P < .001), not different in MICT group (P=.07). Change were not different across groups after the completion of 36 CR sessions (P=.24).
Statin Therapy Intensity Assessment
The average statin therapy intensities were not different from baseline to the completion of 36 CR sessions within each MICT (P=.65) and HIIT (P=.40). Changes were different across groups after the completion of 36 CR sessions (P=.75)
Anthropometric Outcomes
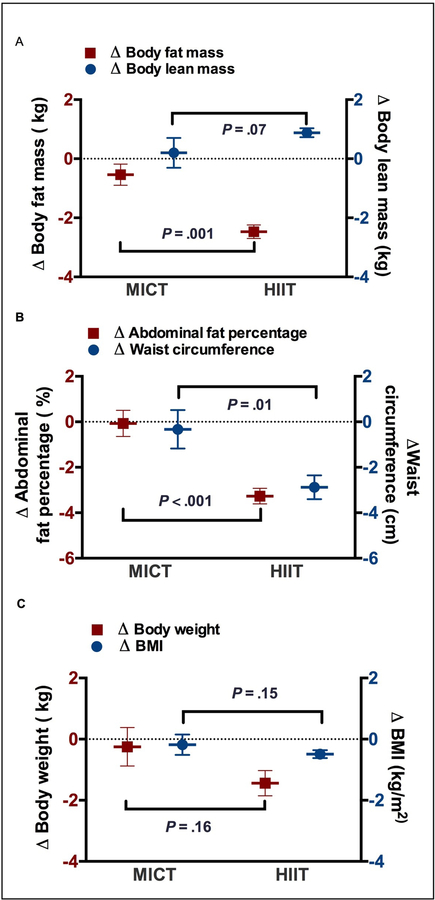
Body weight (P<.001), BMI (P<.001), body fat percentage (P<.001), fat mass (P<.001), abdominal fat percentage (P<.001), waist circumference (P<.001), hip circumference (P=.007) and waist/hip circumference ratio (P=.03) showed a significant decrease, with an increase in lean mass from baseline to completion of CR in the HIIT group (P<.001) (Table 2). Patients in MICT demonstrated no significant difference from baseline to completion of CR in body weight, BMI, body fat percentage, fat mass, lean mass, abdominal fat percentage, waist circumference, hip circumference and waist/hip ratio. The changes in body weight, BMI, lean mass, hip circumference, and W/H ratio were no significant across MICT and HIIT groups (P>.05). However, patients in the HIIT group demonstrated greater reductions in body fat percentage (P<.001), total fat mass (P<.001) and abdominal fat percentage (P<.001), waist circumference (P=.01). (Table 2 and Figure 2)
Figure 2:
Changes in body composition and abdominal fat distribution within and across MICT and HIIT groups. BMI = body mass index; HIIT = high-intensity interval training; MICT= moderate-intensity continuous training.
A. Changes in body weight and BMI from baseline to the completion of 36 sessions of cardiac rehabilitation (CR) across groups.
B. Changes in total body fat mass and lean mass from baseline to the completion of 36 CR sessions across groups. C. Changes in abdominal fat percentage and waist circumference from baseline to the completion of 36 CR sessions across groups.
Values are reported as Mean ± Standard deviation, the change from baseline to the completion of 36 CR sessions across groups was analyzed by ANCOVA:
Lipid Profile
The HIIT group demonstrated a significant decrease in total cholesterol (P<.001), low-density cholesterol (P<.001), triglycerides (P<.001), and Non-HDL-c (P<.001), with increased high-density cholesterol from baseline to completion of the CR program (P=.02). Patients in MICT had no significant changes in the lipid values. Compared to the MICT group, the HIIT group showed significantly greater decreases in total cholesterol (P=.002), low-density cholesterol (P<.001), triglycerides (P=.006), and Non-HDL-c (P<.001). (Table 2)
Sub-Group Analysis
Due to differences in total exercise duration, exercise intensity, and energy expenditure per session over the course of the CR intervention we selectively matched a group of HIIT and MICT participants based specifically on these metrics. After matching, the HIIT group continued to demonstrate a significantly greater reduction in total body fat mass (P=.02), body fat percentage (P=.01) and abdominal fat percentage (P=.02), as well as in total cholesterol (P=.04), low-density cholesterol (P=.04) and Non-HDL-c (P=.03) compared to MICT, with a significant difference in Rate-Your-Plate scores across groups (P=.02) as well as no difference in statin therapy intensity within each group from baseline to the completion of 36 CR sessions (P=.46) (Table 3).
TABLE 3.
Outcomes after sub-group matching for differences in exercise duration, exercise intensity and exercise energy expenditure per session over the course of the 36 sessions CR a,b
| MICT (n=15) | HIIT (n=15) | ANCOVA | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Change | p-value | Baseline | Change | p-value | p-value | |
| Rate-Your-Plate scores | 56±6 | 2±5 | P=.19 | 52±6 | 8±5 | P<.001 | P=.02 |
| Statin therapy intensity | 2.1±1.3 | 0.3±0.7 | P=.16 | 2.8±0.6 | −0.1±0.3 | P=.33 | P=.46 |
| Anthropometric measurements | |||||||
| Body weight, kg | 86.4±12.3 | −0.3±3.7 | P=.55 | 86.4±18.2 | −0.6±1.9 | P=.43 | P=.74 |
| BMI, kg/m2 | 30.5±4.2 | −0.6±1.8 | P=.25 | 30.2±4.8 | 0.0±1.4 | P=.99 | P=.33 |
| Body fat, % | 39.0±6.0 | 0.1±1.9 | P=.89 | 38.9±6.1 | −1.9±2.0 | P=.003 | P=.01 |
| Fat mass, kg | 32.6±7.3 | −0.1±1.6 | P=.83 | 32.7±9.2 | −1.7±1.9 | P=.003 | P=.02 |
| Lean mass, kg | 50.6±7.9 | −0.2±3.0 | P=.82 | 51.0±10.7 | 1.1±1.6 | P=.02 | P=.15 |
| Abdominal fat, % | 45.5±6.9 | 0.4±3.1 | P=.62 | 47.5±8.1 | −2.6±3.3 | P=.008 | P=.02 |
| Waist circum., cm | 106±10 | −0±5 | P=.80 | 108±17 | −2±5 | P=.13 | P=.36 |
| Hip circum., cm | 107±7.4 | −1±4 | P=.63 | 106±9 | −0±10 | P=.87 | P=.97 |
| W/H ratio | 1.0±0.1 | 0.0±0.1 | P=.71 | 1.0±0.1 | −0.0±0.1 | P=.61 | P=.66 |
| Lipid profile | |||||||
| TC, mg/dl | 166.4±37.7 | 0.9±47.6 | P=.96 | 174.7±40.1 | −38.9±39.5 | P=.006 | P=.04 |
| HDL-c, mg/dl | 44.1±5.5 | 3.0±8.5 | P=.39 | 51.3±16.6 | 0.2±10.2 | P=.96 | P=.81 |
| LDL-c, mg/dl | 89.4±27.8 | −5.3±35.3 | P=.71 | 97.2±34.7 | −38.1±35.3 | P=.005 | P=.04 |
| Triglycerides, mg/dl | 187.7±97.5 | −7.6±53.2 | P=.72 | 158.3±104.4 | −28.8±60.0 | P=.13 | P=.08 |
| Non-HDL-c, mg/dl | 122.3±35.9 | −2.1±48.0 | P=.91 | 123.3±38.6 | −39.1±42.2 | P=.008 | P=.03 |
HDL-c = high-density lipoprotein cholesterol; LDL-c = low-density lipoprotein cholesterol; W/H = waist/hip circumference; TC = total cholesterol.
Values are reported as Mean ± Standard deviation or number and percent population (%). The changes within group from baseline to the completion of 36 sessions of cardiac rehabilitation are assessed with paired t-test, and the difference of changes across groups was analyzed by ANCOVA.
DISCUSSION
This study evaluated the impact of HIIT on body composition and adiposity in MI patients enrolled in early outpatient CR. The present study has, for the first time, demonstrated that HIIT can reduce total body, abdominal fat and waist circumference in patients with MI, to a greater extent than MICT.
Overweight and obesity have become increasingly common worldwide, with overweight and obesity defined conventionally as having a BMI of >25 kg/m2 and >30 kg/m2, respectively. Overweight and obesity are associated with increased likelihood of developing cardiovascular disease and all-cause mortality.25,26 In contrast to the general population, patients with CHD demonstrate an inverse relationship between BMI and mortality, which has been termed the obesity paradox.27 However, it is now apparent that the obesity paradox is more specifically related to the preservation of lean muscle mass as there is no apparent paradox either in the general population28 or patients with CHD4 when measuring body fat percentage in place of BMI. Body fat mass and percentage are associated with a higher risk of cardiovascular events and all-cause mortality,4,28 whereas higher amounts of fat-free mass is related to a lower risk of cardiovascular events.4 Body fat percentage is considered to be a more powerful predictor of cardiovascular disease risk than body weight and BMI.6,7 Several other studies have examined the efficacy of HIIT on reducing subcutaneous and abdominal fat in overweight adolescents,29 young women30 and type 2 diabetic men.31 For example, Trapp et al. compared HIIT and MICT and found that individuals in the HIIT group showed greater decreases in subcutaneous fat.30 In contrast, Haifeng Zhang et al.14 demonstrated both MICT and HIIT significantly reduced total and abdominal fat mass in young female university students (age, 18–22), but with no differences across groups. The difference of outcomes between Haifeng’s study and the present study were likely due to differences in participant characteristics, including age (22 vs. 67 yrs), medical history (health university student vs. patients with MI), and sex (all women vs. combination of women and men). Combined, these data suggest that HIIT has a greater impact on reducing fat mass in patients with MI compared to MICT.
The effect of exercise on reducing body fat is influenced by both energy intake and expenditure. The present study initially found that both MICT and HIIT significantly improved RYP scores (dietary quality), and HIIT induced more energy expenditure (EE) during exercise compared with MICT in patients with MI. However, after matching groups for exercise EE, exercise duration, and exercise intensity the HIIT group continued to demonstrate a significantly greater reduction in body fat and abdominal fat percentage, and in total cholesterol, low-density cholesterol compared to MICT, with no difference in RYP scores across groups as well as no difference in statin therapy intensity within each group from baseline to the completion of 36 CR sessions (Table 3).
Mechanisms underlying the HIIT-induced effect on fat loss remain unclear. These findings may be related to exercise induced alterations in mitochondrial function, allowing for greater utilization of adipose tissue as a fuel source. For example, we have previously demonstrated that 4-week aerobic exercise can enhance skeletal muscle and myocardium mitochondrial biogenesis and function in male mice.32 Motta et al. also showed that HIIT ameliorated the fructose-induced metabolic dysfunction in male mice through affecting markers of mitochondrial biogenesis and β-oxidation, irisin and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1 α), in liver, white adipose tissue and skeletal muscle.33 Moreover, several more recent trials have demonstrated that 3–12 weeks HIIT can significantly increase resting metabolic rate,34 post-exercise oxygen consumption in healthy adults,35 and fat oxidation in obese adolescents.36 On the basis of our findings and previous studies, future work is needed to examine the effects of HIIT on mitochondrial function and its relationship with resting metabolic rate and post-exercise oxygen consumption in patients with MI.
Improvements in lipid profile have been shown after exercise training in overweight or obese individuals.37–39 The present study demonstrated a significant improvement in the lipid profile for patients in the HIIT group, but not in the MICT group while the statin therapy intensities were not different from baseline to the completion of 36 CR sessions within each MICT and HIIT group. It has recently been reported that HIIT has also shown a lower plasma triglyceride concentrations to a greater extent than MICT in young males.40 These finding are clinically significant because the majority of patients with MI have dyslipidemia and are administered lipid lowering pharmacotherapy as is consistent in the present study. It is important to note that several studies have demonstrated favorable effects on the lipid profile only in individuals who are overweight or obese,37,38 but not in normal weight individuals. The underlying mechanism(s) responsible for the impact of HIIT on the lipid profile remain unclear; however, our results indicate that HIIT should be considered as an important adjunct treatment strategy to improve dyslipidemia in patients with MI, especially for those who are overweight or obese.
A variety of HIIT protocols have been developed and used for CHD patients,41–43 which vary in intensity, high intensity interval duration, low intensity interval (recovery) duration, and number of intervals. Further, the exercise intensity is generally prescribed using a percent of maximal oxygen consumption, percent of maximal heart rate, percent of maximal power, or RPE (Borg scale).41,42 Buchheit et al.44 and Levinger et al.45 demonstrated that the RPE has shown a great correlation with HR, ventilation, and VO2 in individuals with and without CAD, and the correlation is not impacted by beta blocker medication, a commonly used HR modulating medication by patients with MI.45 Therefore, the present study used patient perception of effort via the RPE scale to prescribe the intensity of the stimulus and recovery periods with accompanying HR monitoring in attempting to solve the limit that HR of patients with MI who are prescribed HR modulating pharmacotherapy is not greatly associated with exercise intensity. Utilizing combination of RPE and HR as a guide for prescribing exercise intensity may be more appropriate than HR or maximal oxygen consumption alone. Further, this strategy may be more generalizable and ensure real-world applicability since many patients may not be able to reliably monitor HR when undergoing exercise outside the conventional CR setting.
Compared to MICT, increasing research suggests that HIIT has the capacity to induce changes in numerous physiological and health related markers,46 including greater improvement in cardiopulmonary fitness,47,48 quality of life48,49 and skeletal muscle myopathy50 in CHD patients. However, there are controversial concerns regarding the potential for increased adverse events in clinical populations when undergoing HIIT where the likelihood of an untoward episode is already increased. It must be noted that HIIT protocols employed by studies for clinical populations have generally been modified to be less strenuous for greater tolerance and applicability.43,46 Current HIIT protocols used in clinical practice are usually characterized by a lower absolute intensity but with a longer duration of work and shorter rest periods compared with the more traditional sprint interval training protocols used in athletes.51,52 These adapted HIIT protocols have been found to be well tolerated and safe to practice in clinical populations with CVD. Although the present study is not powered to determine the safety of HIIT, 90 participants in this study completed at least two hundred and sixteen sessions of HIIT (each patient completing at least 24 sessions) without a single major adverse event.
Study Limitations
The patients in this study were overweight and therefore future studies focused on MI patients with normal body weight are encouraged. Although no major adverse events were registered, the currently study was not designed to establish the safety of HIIT in patients with MI. However, the safety of supervised HIIT in CR is generally well documented in patients with established coronary artery disease.53,54 The ideal dose of exercise training in patient with MI with regards to the intensity and duration is still not known. Furthermore, future studies focused on recruitment of women should be conducted as only 27% of the patients in this study were women. Although not unusual in similar studies, this sex bias was an unintended consequence of our clinical population but constitutes a limitation of the generalizability of the results.
CONCLUSION
The present study showed that 36 sessions of supervised HIIT was superior to MICT for reducing total body fat mass and abdominal fat percentage, and improving the lipid profile in patients after MI. These findings support the hypothesis that supervised HIIT should be considered an important treatment strategy for outpatient CR patients with MI.
ACKNOWLEDGEMENTS
We thank the patients and staff of Mayo Clinic Cardiac Rehabilitation Program, in Rochester, MN, for their valuable contributions to the study.
Grant Support: The present study was supported in part by grants from the National Institutes of Health (HL-126638 to TPO) and Hunan Development and Reform Commission Foundation of China (Grant no. [2012] 1521).
Abbreviations and Acronyms:
- CHD
coronary heart disease
- CR
cardiac rehabilitation
- DXA
dual-energy X-ray absorptiometry
- HIIT
high-intensity aerobic interval training
- MICT
moderate-intensity continuous training
- MI
myocardial infarction
- BMI
body mass index
- RPE
rating of perceived exertion
- RYP
Rate-Your-Plate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Competing Interests: The authors report no competing interests.
REFERENCES
- 1.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet 2017;389(10065):197–210. [DOI] [PubMed] [Google Scholar]
- 2.Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ 2012;344(5):d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36(19):1163–1170. [DOI] [PubMed] [Google Scholar]
- 4.Medina-Inojosa JR, Somers VK, Thomas RJ, et al. Association between adiposity and lean mass with long-term cardiovascular events in patients with coronary artery disease: No paradox. J Am Heart Assoc 2018;7(10):e007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padwal R, Leslie WD, Lix LM, Majumdar SR. Relationship among body fat percentage, body mass index, and all-cause mortality: A cohort study. Ann Intern Med 2016;164(8):532–541. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Q, Dong SY, Sun XN, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz J Med Biol Res 2012;45(7):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurniawan LB, Bahrun U, Hatta M, Arif M. Body Mass, total body fat percentage, and visceral fat level predict insulin resistance better than waist circumference and body mass index in healthy young male adults in Indonesia. J Clin Med 2018;7(5):E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126(25):e354–471. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly JE, Honas JJ, Smith BK, et al. Aerobic exercise alone results in clinically significant weight loss for men and women: midwest exercise trial 2. Obesity (Silver Spring) 2013;21(3):E219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devries MC. Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol 2016;101(2):243–249. [DOI] [PubMed] [Google Scholar]
- 11.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev 2017;18(6):635–646. [DOI] [PubMed] [Google Scholar]
- 12.Turk Y, Theel W, Kasteleyn MJ, et al. High intensity training in obesity: a Meta-analysis. Obes Sci Pract 2017;3(3):258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes 2011;2011(11):e868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Tong TK, Qiu W, et al. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res 2017;2017:5071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy S, Thoma C, Houghton D, Trenell MI. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia 2017;60(1):7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend LK, Couture KM, Hazell TJ. Mode of exercise and sex are not important for oxygen consumption during and in recovery from sprint interval training. Appl Physiol Nutr Metab 2014;39(12):1388–1394. [DOI] [PubMed] [Google Scholar]
- 17.Glass S, Dwyer GB, eds. ACSM Metabolic Calculations Handbook Third ed; 2007:10–17. [Google Scholar]
- 18.Riebe D, Ehrman JK, Liguori G, Magal M, eds. ACSM’s Guidelines for Exercise Testing and Prescription Tenth ed: Wolters Kluwer; 2018:197. [Google Scholar]
- 19.Imboden MT, Welch WA, Swartz AM, et al. Reference standards for body fat measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS One 2017;12(4):e0175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Gans KM, Hixson ML, Eaton CB, Lasater TM. Rate Your Plate: A dietary assessment and educational tool for blood cholesterol control. Nutr Clin Care 2001(3):163–169. [Google Scholar]
- 22.Ganguzza L, Ngai C, Flink L, et al. Association between diet quality and measures of body adiposity using the Rate Your Plate survey in patients presenting for coronary angiography. Clin Cardiol 2018;41(1):126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 suppl 2):2889–2934. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A 2018;115(5):957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams Kenneth F., Schatzkin Arthur, Harris Tamara B., et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 Years Old. N Engl J Med 2006;355(8):763–778. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368(9536):666–678. [DOI] [PubMed] [Google Scholar]
- 28.Dong B, Peng Y, Wang Z, et al. Joint association between body fat and its distribution with all-cause mortality: A data linkage cohort study based on NHANES (1988–2011). PLoS One 2018;13(2):e0193368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tjonna AE, Stolen TO, Bye A, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116(4):317–326. [DOI] [PubMed] [Google Scholar]
- 30.Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (Lond) 2008;32(4):684–691. [DOI] [PubMed] [Google Scholar]
- 31.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol 2003;149(5):421–424. [DOI] [PubMed] [Google Scholar]
- 32.Dun Y, Liu S, Zhang W, Xie M, Qiu L. Exercise combined with rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev 2017;2017(11):e8024857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motta VF, Bargut TL, Aguila MB, Mandarim-de-Lacerda CA. Treating fructose-induced metabolic changes in mice with high-intensity interval training: insights in the liver, white adipose tissue, and skeletal muscle. J Appl Physiol (1985) 2017;123(4):699–709. [DOI] [PubMed] [Google Scholar]
- 34.Schubert MM, Clarke HE, Seay RF, Spain KK. Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl Physiol Nutr Metab 2017;42(10):1073–1081. [DOI] [PubMed] [Google Scholar]
- 35.Schleppenbach LN, Ezer AB, Gronemus SA, Widenski KR, Braun SI, Janot JM. Speed- and circuit-based high-intensity interval training on recovery oxygen consumption. Int J Exerc Sci 2017;10(7):942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazzer S, Tringali G, Caccavale M, De Micheli R, Abbruzzese L, Sartorio A. Effects of high-intensity interval training on physical capacities and substrate oxidation rate in obese adolescents. J Endocrinol Invest 2017;40(2):217–226. [DOI] [PubMed] [Google Scholar]
- 37.Ouerghi N, Fradj MKB, Bezrati I, et al. Effects of high-intensity interval training on body composition, aerobic and anaerobic performance and plasma lipids in overweight/obese and normal-weight young men. Biol Sport 2017;34(4):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez C, Ramirez-Campillo R, Martinez-Salazar C, Castillo A, Gallardo F, Ciolac EG. High-intensity interval training as a tool for counteracting dyslipidemia in women. Int J Sports Med 2018;39(5):397–406. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis 2017;16(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmer D Effect of 8 weeks of high-intensity interval training versus traditional endurance training on the blood lipid profile in humans Kinesiology. Vol PhD. Auburn: Auburn University; 2013:109. [Google Scholar]
- 41.Juneau M, Hayami D, Gayda M, Lacroix S, Nigam A. Provocative issues in heart disease prevention. Can J Cardiol 2014;30(12 Suppl):S401–409. [DOI] [PubMed] [Google Scholar]
- 42.Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012;42(7):587–605. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro PA, Boidin M, Juneau M, Nigam A, Gayda M. High-intensity interval training in patients with coronary heart disease: Prescription models and perspectives. Ann Phys Rehabil Med 2017;60(1):50–57. [DOI] [PubMed] [Google Scholar]
- 44.Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sports Med 2013;43(5):313–338. [DOI] [PubMed] [Google Scholar]
- 45.Levinger I, Bronks R, Cody DV, Linton I, Davie A. Perceived exertion as an exercise intensity indicator in chronic heart failure patients on beta-blockers. J Sports Sci Med 2004;3(YISI 1):23–27. [PMC free article] [PubMed] [Google Scholar]
- 46.Hussain SR, Macaluso A, Pearson SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev 2016;24(6):273–281. [DOI] [PubMed] [Google Scholar]
- 47.Hannan AL, Hing W, Simas V, et al. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: a systematic review and meta-analysis. Open Access J Sports Med 2018;9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes-Neto M, Duraes AR, Reis H, Neves VR, Martinez BP, Carvalho VO. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: A systematic review and meta-analysis. Eur J Prev Cardiol 2017;24(16):1696–1707. [DOI] [PubMed] [Google Scholar]
- 49.Gomes Neto M, Duraes AR, Conceicao LSR, Saquetto MB, Ellingsen O, Carvalho VO. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis. Int J Cardiol 2018;261:134–141. [DOI] [PubMed] [Google Scholar]
- 50.Tzanis G, Philippou A, Karatzanos E, et al. Effects of high-intensity interval exercise training on skeletal myopathy of chronic heart failure. J Card Fail 2017;23(1):36–46. [DOI] [PubMed] [Google Scholar]
- 51.Gaesser GA, Angadi SS. High-intensity interval training for health and fitness: can less be more? J Appl Physiol (1985) 2011;111(6):1540–1541. [DOI] [PubMed] [Google Scholar]
- 52.Bayati M, Farzad B, Gharakhanlou R, Agha-Alinejad H. A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble ‘all-out’ sprint interval training. J Sports Sci Med 2011;10(3):571–576. [PMC free article] [PubMed] [Google Scholar]
- 53.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2016;37(29):2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rognmo O, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012;126(12):1436–1440. [DOI] [PubMed] [Google Scholar]