SUMMARY
Passively administered broadly neutralizing antibodies (bNAbs) targeting the HIV envelope glycoprotein (Env) have been shown to protect nonhuman primates against chimeric simian-human immunodeficiency virus (SHIV) infection. With data from multiple nonhuman primate SHIV challenge studies that used single bNAbs, we conducted a meta-analysis to examine the relationship between predicted serum 50% neutralization titer (ID50) against the challenge virus and infection outcome. In a logistic model that adjusts for bNAb epitopes and challenge viruses, serum ID50 had a highly significant effect on infection risk (p < 0.001). The estimated ID50 to achieve 50%, 75%, and 95% protection was 91 (95% CI: 55, 153), 219 (117, 410), and 685 (319, 1471), respectively. This analysis indicates that serum neutralizing titer against the relevant virus is a key parameter of protection and that protection from acquisition by a single bNAb may require substantial levels of neutralization at the time of exposure.
Graphical Abstract
eTOC:
Pegu et al. present a meta-analysis of nonhuman primate studies that administered a single broadly neutralizing antibody (bNAb) before SHIV challenge. They find that serum neutralizing titer against the challenge virus strongly correlates with protection. Further, protection mediated by a single bNAb may require substantial levels of neutralization at exposure.
Introduction
Close to 2 million new HIV-1 infections occur annually and there remains an urgent global public health need for an effective HIV-1 vaccine that can prevent infection (UNAIDS, 2018). In the absence of an effective vaccine, alternative strategies to prevent HIV-1 infection are being actively pursued (Eakle et al., 2018). Human broadly neutralizing antibodies (bNAbs) against HIV-1 present an attractive strategy for prevention of viral infection because of their favorable safety profile and potential for long-term persistence in the circulation.
HIV-1 bNAbs are derived from the B-cells of selected HIV-1 infected donors and bind to relatively conserved regions of the envelope glycoprotein (Env) expressed on the surface of infectious virions – mediating potent virus neutralization. Detailed virologic and structural studies have determined that bNAbs target specific epitopes in Env, including the CD4 binding site, the V3-glycan supersite, the V1V2 apex, the gp 120/140 interface region and the membrane proximal region of gp41, to potently neutralize many diverse strains of HIV-1 (McCoy and Burton, 2017; Sok and Burton, 2018). Moreover, bNAbs have been shown to protect against chimeric simian-human immunodeficiency virus (SHIV) infection in nonhuman primate (NHP) models of HIV-1 infection, providing proof of concept for their potential to prevent HIV-1 infection in humans [reviewed in (Hessell et al., 2018; Pegu et al., 2017)]. Most commonly, protection in NHPs has been assessed by intravenous administration of a single bNAb, followed one day, or several days later, by a single mucosal challenge with a SHIV that would result in infection of all control animals (Hessell et al., 2007; Hessell et al., 2016; Hessell et al., 2009b; Hessell et al., 2010; Julg et al., 2017; Ko et al., 2014; Moldt et al., 2016; Moldt et al., 2012; Parren et al., 2001; Rudicell et al., 2014; Shingai et al., 2014). A variety of bNAbs have been tested, often at varying infusion doses – allowing an assessment of the amount of antibody required to achieve protection (Julg et al., 2017; Ko et al., 2014; Moldt et al., 2016; Moldt et al., 2012; Parren et al., 2001; Shingai et al., 2014).
Several HIV-1 bNAbs have entered clinical trials – including antibodies to four distinct epitopes on the HIV-1 Env (Caskey et al., 2019; Cohen and Caskey, 2018; Sok and Burton, 2018). VRC01 is a bNAb that targets the CD4 binding site of the HIV-1 envelope that has demonstrated protection against SHIV infection after passive administration in nonhuman primate studies (Gautam et al., 2016; Ko et al., 2014; Rudicell et al., 2014). It is the first bNAb to be tested for its ability to protect against HIV-1 acquisition after passive administration in humans. Two parallel randomized placebo controlled studies, the Antibody Mediated Prevention (AMP) trials, were launched in 2016, with the primary objective to assess whether intravenously-delivered VRC01 prevents HIV-1 infection in adults at high risk of HIV-1 acquisition (Gilbert et al., 2017). While the field awaits the results of the AMP trials, modeling studies have further supported the potential of passive administration of bNAbs in preventing HIV-1 infection (Huang et al., 2018).
To better understand the titers needed to achieve protection, we performed a meta-analysis of the data from passive immunization studies in NHP, as described above, that use a single infusion of a bNAb, followed by a mucosal SHIV challenge. Since bNAbs vary in their neutralization potency, and challenge SHIVs vary in their neutralization sensitivity to a given bNAb, we used the measured bNAb antibody level present in the sera of animals on the day of SHIV challenge and the in vitro neutralization sensitivity of the challenge virus to the bAb to infer the serum neutralization titer against the challenge SHIV. Predicted serum neutralization titers were then assessed for association with protective efficacy, considering other variables that included the challenge virus and the bNAb epitope targeted. We found a strong association between serum neutralization titers and protection against SHIV challenge with the estimated serum 50% neutralization titer (ID50) needed to achieve 50%, 75%, and 95% protection being 91 (95% CI: 55, 153), 219 (95% CI: 117, 410), and 685 (95% CI:319, 1471), respectively.
Results
Description of study data
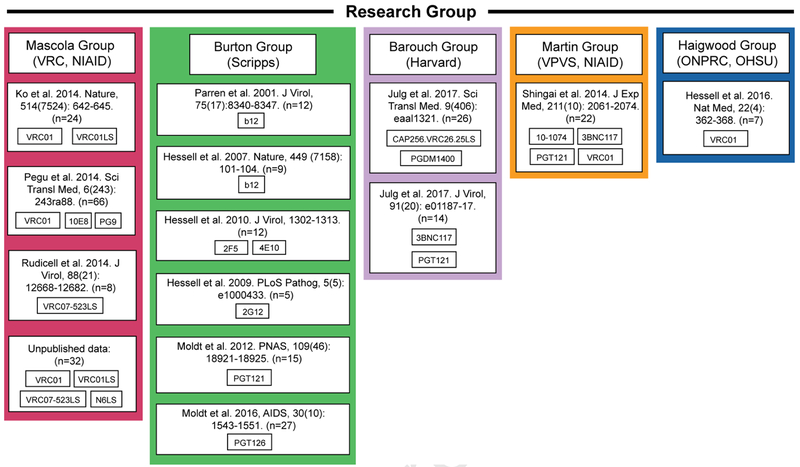
This analysis combined data from 13 published studies (Hessell et al., 2007; Hessell et al., 2016; Hessell et al., 2009b; Hessell et al., 2010; Julg et al., 2017; Ko et al., 2014; Moldt et al., 2016; Moldt et al., 2012; Parren et al., 2001; Rudicell et al., 2014; Shingai et al., 2014) and 5 unpublished studies (Table 1, Figure 1). Criteria for inclusion were: the infusion of a single dose of one bNAb, followed by a single high-dose SHIV challenge; and the availability of bNAb level data on the day of SHIV challenge. The serum bNAb level was used to predict the serum neutralization ID50 titer against the SHIV used for challenge, based on the known in vitro IC50 of the bNAb against the SHIV.
Table 1.
| HIV-1 Env Epitope | Antibody | Challenge virus (route) | Antibody dose (mg/kg) | No. of animals (infected/total) | Reference |
|---|---|---|---|---|---|
| CD4 binding site | b12 | SHIVSF162P4 (vaginal) | 25 | 0/4 | (Parren et al., 2001) |
| 1 | 4/4 | ||||
| SHIVSF162P3 (vaginal) | 25 | 1/9 | (Hessell et al., 2007) | ||
| VRC01 | SHIVSF162P3 (vaginal) | 20 | 0/4 | (Ko et al., 2014) | |
| SHIVSF162P3 (rectal) | 20 | 0/4 | (Ko et al., 2014) | ||
| SHIVSF162P3 (oral) | (Hessell et al., 2009a) | ||||
| 5 | 1/5 | ||||
| SHIVBaL.P4 (rectal) | 20 | 0/6 | (Ko et al., 2014) | ||
| 0.3 | 16/22 | (Ko et al., 2014) | |||
| SHIVAD8EO (rectal) | (Shingai et al., 2014) | ||||
| 20 | 2/2 | ||||
| VRC01LS | SHIVSF162P3 (rectal) | 20 | 1/6 | Unpublished | |
| SHIVBaL.P4 (rectal) | 0.3 | 5/12 | (Ko et al., 2014) | ||
| SHIVBG505 (rectal) | 20 | 0/4 | Unpublished | ||
| VRC07–523LS | SHIVBaL.P4 (rectal) | (Rudicell et al., 2014) | |||
| 0.05 | 4/4 | ||||
| SHIVSF162P3 (rectal) | 20 | 0/6 | Unpublished | ||
| N6LS | SHIVBG505 (rectal) | 20 | 0/4 | Unpublished | |
| 2.5 | 2/4 | ||||
| 3BNC117 | SHIVAD8EO (rectal) | (Shingai et al., 2014) | |||
| 1 | 2/2 | ||||
| SHIV327c (rectal) | (Julg et al., 2017) | ||||
| 2 | 3/3 | ||||
| V1V2 | PGDM1400 | SHIV325C (rectal) | 2 | 0/5 | (Julg et al., 2017) |
| 0.08 | 3/4 | ||||
| CAP256.VRC26.25LS | SHIV325C (rectal) | 2 | 0/4 | (Julg et al., 2017) | |
| 0.08 | 0/4 | ||||
| PG9 | SHIVBaL.P4 (rectal) | 20 | 2/6 | (Ko et al., 2014) | |
| 0.3 | 6/6 | ||||
| V3-glycan | PGT121 | SHIVSF162P3 (vaginal) | 5 | 0/5 | (Moldt et al., 2012) |
| 0.2 | 2/5 | ||||
| SHIVAD8EO (rectal) | 20 | 0/2 | (Shingai et al., 2014) | ||
| 0/2 | |||||
| 0.2 | 2/2 | ||||
| SHIV327c (rectal) | (Julg et al., 2017) | ||||
| 2 | 1/3 | ||||
| PGT126 | SHIVSF162P3 (vaginal) | 10 | 0/5 | (Moldt et al., 2016) | |
| 0.4 | 4/5 | ||||
| SHIVSF162P3 (rectal) | 10 | 1/4 | (Moldt et al., 2016) | ||
| 0.4 | 4/4 | ||||
| 10–1074 | SHIVAD8EO (rectal) | 20 | 0/2 | (Shingai et al., 2014) | |
| 1 | 2/2 | ||||
| High mannose glycan | 2G12 | SHIVSF162P3 (vaginal) | 40 | 2/5 | (Hessell et al., 2009b) |
| MPER | 4E10 | SHIVBa-L (rectal) | 50 | 0/6 | (Hessell et al., 2010) |
| 2F5 | SHIVBa-L (rectal) | 50 | 0/6 | (Hessell et al., 2010) | |
| 10E8 | SHIVBaL.P4 (rectal) | 20 | 0/6 | (Ko et al., 2014) | |
| 0.3 | 3/6 |
Figure 1. Passive immunization studies in NHPs.
Information on the passive immunization NHP studies used for the meta-analysis, organized by research group.
Overall, data from 274 animals were available, with 242 NHPs from 13 SHIV challenge studies published between 2001 and 2017, and an additional 32 NHPs from unpublished studies (Table 1, Figure 1). Data were collected by five groups (Table 2): Mascola and colleagues from VRC, NIAID (n=130); Burton and colleagues from Scripps (n=80); Barouch and colleagues from Harvard (n=35); Martin and colleagues from the Viral Pathogenesis and Vaccine Section (VPVS), NIAID (n=22); and Haigwood and colleagues from the Oregon National Primate Research Center (ONPRC), Oregon Health & Science University (OHSU) (n=7). Information about sex is available for 198 animals (64% male, 36% female). The study dataset (Table 2) includes 16 bNAbs targeting four Env regions: the CD4 binding site, V1V2, V3-glycan, and MPER; the bNAb infusion dose ranged from 0.05 to 50 mg/kg. Eight different SHIVs were used, including three tier 1 viruses (highly sensitive to neutralization) and five tier 2 viruses (less sensitive to neutralization). Regarding route of challenge, 76%, 22%, and 2.6% of study animals were challenged by the rectal, vaginal, and oral route, respectively. The viral challenge dose ranged from 300 to 12800 TCID50, with challenges implemented at day 1 (53%), day 2 (26%), or day 5 (22%) post bNAb transfer.
Table 2.
Descriptive statistics of study dataset.
| Categorical Variable | Category | N (no. of animals) | % (% among animals with information observed) | |
|---|---|---|---|---|
| Study Group | Mascola | 130 | 47.4% | |
| Burton | 80 | 29.2% | ||
| Barouch | 35 | 12.8% | ||
| Martin | 22 | 8.0% | ||
| Haigwood | 7 | 2.6% | ||
| HIV-1 Env Epitope | CD4 Binding Site | b12 | 21 | 7.7% |
| VRC01 | 57 | 20.8% | ||
| VRC01LS | 22 | 8% | ||
| VRC07–523LS | 14 | 5.1% | ||
| N6LS | 12 | 4.4% | ||
| 3BNC117 | 11 | 4% | ||
| V1V2 | PGDM1400 | 9 | 3.3% | |
| CAP256.VRC26.25LS | 12 | 4.4% | ||
| PG9 | 18 | 6.6% | ||
| V3-glycan | PGT121 | 30 | 10.9% | |
| PGT126 | 27 | 9.9% | ||
| 10–1074 | 6 | 2.2% | ||
| 2G12 | 5 | 1.8% | ||
| MPER | 4E10 | 6 | 2.2% | |
| 2F5 | 6 | 2.2% | ||
| 10E8 | 18 | 6.6% | ||
| Virus | Tier 1 | SHIVBaL | 12 | 4.4% |
| SHIVBaLP4 | 94 | 34.3% | ||
| SHIVSF162P4 | 12 | 4.4% | ||
| Tier 2 | SHIV325C | 21 | 7.7% | |
| SHIV327C | 14 | 5.1% | ||
| SHIVAD8EO | 22 | 8% | ||
| SHIVBG505 | 16 | 5.8% | ||
| SHIVSF162P3 | 83 | 30.3% | ||
| Challenge Route | Oral | 7 | 2.6% | |
| Vaginal | 60 | 21.9% | ||
| Rectal | 207 | 75.5% | ||
| Challenge dose (TCID50) | 300 | 85 | 40.3% | |
| 500 | 26 | 12.3% | ||
| 1000 | 22 | 10.4% | ||
| 2000 | 12 | 5.7% | ||
| 12800 | 66 | 31.3% | ||
| Not available | 63 | - | ||
| Sex | Female | 72 | 36.4% | |
| Male | 126 | 63.6% | ||
| Not available | 76 | - | ||
| Target Cell for Measuring in vitro | TZM-bl | 245 | 89.40% | |
| Neutralization Sensitivity | PBMC | 29 | 10.60% | |
| Day of Challenge post Ab transfer | 1 | 144 | 52.60% | |
| 2 | 70 | 25.50% | ||
| 5 | 60 | 21.90% | ||
| Continuous Variable | Median | Range | ||
| Ab Dose (mg/kg) | 5 | (0.05,50) | ||
| Ab Conc at day of challenge (ug/ml) | 25.02 | (0.11,1122.33) | ||
| ID50 titers* | 113.67 | (2.17,8010.58) | ||
| ID80 titers* | 29.05 | (0.003, 4005.29) | ||
| IIP** | 2.42 | (0.34, 8.46) |
ID50 (ID80) titers calculated as bNAb serum concentration at day of challenge divided by IC50 (IC80) of the bNAb against the challenge virus.
The instantaneous inhibitory potential (IIP) = −log10(1 − f(c)), where f(c) = cm/(IC50m + cm) = fraction neutralization at concentration c, with slope m = log(4)/(log IC80 - log IC50), and c being bNAb concentration at the day of challenge.
The in vitro neutralization potency IC50 of each bNAb against the challenge SHIV was measured using replication-competent SHIV infection of TZM-bl cells (89%) or PBMCs (11%) (Table SI). Serum bNAb level on the day of viral challenge was measured by quantitative ELISA and ranged from 0.11 to 1122 μg/mL (median 25.0 μg/mL). The predicted serum neutralization ID50 titers against the SHIV challenge virus used ranged from 2.17 to 8011 (median 114) (Table 2). Full information (including dose, challenge virus, bNAb concentration at day of challenge, protection status, and bNAb IC50 and IC80 against the challenge SHIV) for the 32 NHPs from unpublished studies is provided in Table S2.
Logistic regression modeling of infection risk as a function of ID50 titer and other covariates
The risk of infection was modeled as a function of the log 10 transformed predicted serum neutralization ID50 titer and additional covariates. Since the observed infection risk was zero for several bNAbs (CAP256.VRC26.25, 4E10, 2F5), bNAb information was modeled by the Env epitope category they target. In addition, since the observed infection risk was also zero for the SHIVBaL virus, in our analysis it was pooled together with SHIVBaLP4 virus in the logistic model. Among various combinations of covariates to adjust for, the best fitting model selected by minimum AIC models infection risk as a linear function of log10(ID50) and adjusts for epitope and virus type, with a significant interaction (p=0.005) between log10(ID50) and challenge virus (SHIVSF162P3 vs. other) (Table 3). Within this best fitting model, the overall contribution of ID50 to infection risk is highly significant (p<0.001). In particular, an increase in serum ID50 titer was associated with a significant reduction in infection risk, with an odds ratio of 0.012 per 10-fold increase in ID50 titer (p<0.001) for all SHIV challenge viruses except for SHIVSF162P3, for which an odds ratio of 0.17 per 10-fold increase in ID50 titer (p=0.002) was observed. Also, after accounting for ID50 level and challenge virus type, animals injected with bNAbs targeting MPER tend to have a significantly lower risk of infection compared to animals injected with bNAbs targeting other epitope categories (Table 3), with the caveat that these MPER bNAbs were studied against only one SHIV, which has a tier 1 neutralization phenotype but is not highly sensitive to MPER bNAbs compared to bNAbs against other epitopes.
Table 3.
Odds ratio (OR) estimates for log10(ID50) and covariates based on the model of infection risk conditional on log10(ID50), epitope of the bNAb, challenge virus, and the interaction between log10(ID50) and challenge virus (SF162P3 vs. other). See also Table S3.
| OR | 95% CI | p-value | |
|---|---|---|---|
| log10(ID50) for virus other than SHIVSF162P3 | 0.012 | (0.003,0.054) | <0.001 |
| log10(ID50) for virus SHIVSF162P3 | 0.17 | (0.057,0.51) | 0.002 |
| Epitope relative to MPER | |||
| CD4 Binding Site | 801 | (58, 16243) | <0.001 |
| V1V2 | 1030 | (43, 24887) | < 0.001 |
| V3-glycan | 4120 | (173, 97943) | < 0.001 |
| Virus relative to SHIVBaL/SHIVBaLP4 | |||
| SHIVSF162P4 | 1.98 | (0.29,13.43) | 0.49 |
| SHIV325C | 0.008 | (0,0.18) | 0.002 |
| SHIV327C | 0.83 | (0.18,3.79) | 0.81 |
| SHIVAD8EO | 0.52 | (0.12,2.17) | 0.37 |
| SHIVBG505 | 0.095 | (0.016,0.57) | 0.01 |
| SHIVSF162P3 | 0 | (0,0.016) | <0.001 |
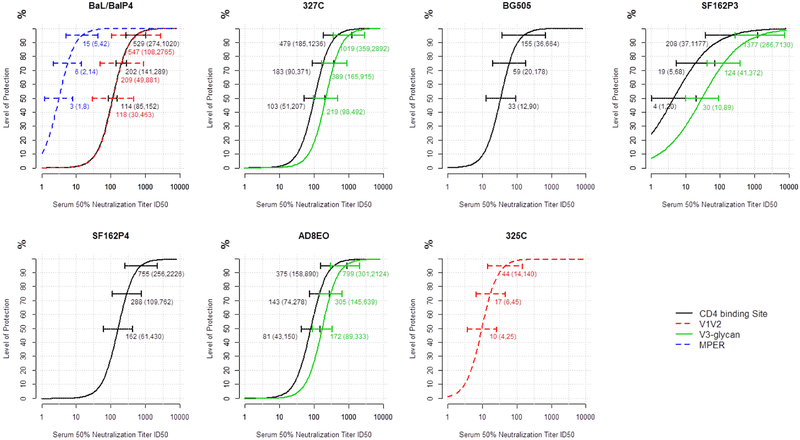
Based on the selected logistic regression risk model, the curve for protection level based on ID50 titer was constructed for various epitope and virus combinations. As shown in Table S3, there are 12 different epitope and virus combinations in the study dataset. Serum ID50 titer was a consistently strong correlate of protection across combinations, with some variability in the specific ID50 titer needed to achieve a given protection level across combinations (Figure 2 and Figure S1). For example, when challenged with SHIVBaL/SHIVBaLP4, the estimated required serum ID50 titer to achieve 50% protection was 114 (95% CI: 85, 152) for bNAbs that target the CD4 binding site and 118 (95% CI: 30, 463) for bNAbs that target the V1V2 region, but only 3 (95% CI: 1, 8) for bNAbs that target MPER; the corresponding serum ID50 titers required to achieve 95% protection were 529 (95% CI: 274, 1020), 547 (95% CI: 108, 2765), and 15 (95% CI: 5, 42) for bNAbs that target the CD4 binding site, VIV2, and MPER, respectively.
Figure 2. Association of protection with serum ID50 titer for different SHIVs.
The curves for each indicated SHIV strain depict the estimated level of protection as a function of serum ID50 titer for bNabs targeting various epitopes in the study dataset. The 95% bootstrap confidence intervals for ID50 titers required to achieve 50%, 75%, and 95% protection are represented by the three colored horizontal lines on each curve. See also Figures S1 and S2.
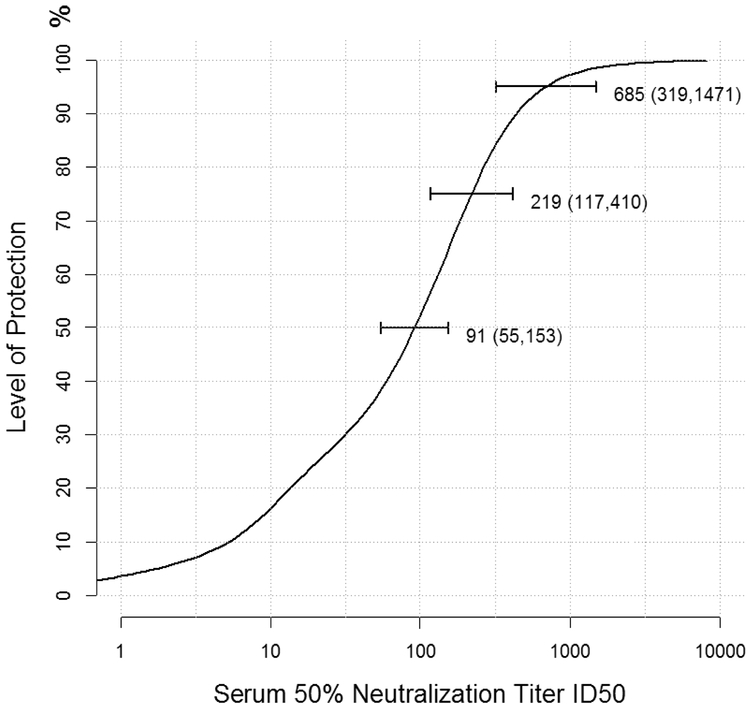
Based on the selected risk model, we estimated the overall level of protection as a function of ID50 titer, averaging over all epitope and virus combinations in the study dataset (Figure 3). The required ID50 titers to achieve 50%, 75%, and 95% protection were estimated at 91 (95% CI: 55, 153), 219 (95% CI: 117, 410), and 685 (95% CI: 319, 1471), respectively.
Figure 3. Association of protection with serum ID50 titers averaging over all epitope and virus combinations.
The 95% bootstrap confidence intervals for ID50 titers required to achieve 50%, 75%, and 95% protection are represented by the three horizontal lines on the curve. See also Figure S3.
Logistic regression modeling of infection risk as a function of instantaneous inhibitory potential (IIP), ID50 titer, and ID80 titer
Data were available from 218 animals in studies for which both IC50 and IC80 information was available. In this subset, highly significant (p<0.001) pairwise spearman correlations are observed among log10(ID50), log10(ID80) and IIP, with a strong correlation between log10(ID50) and log10(ID80) (cor = 0.84) and between log10(ID80) and IIP (cor = 0.83) and a modest correlation between log10(ID50) and IIP (cor=0.55) (Figure S2). Adjusting for epitope and challenge virus, IIP correlated with infection risk when ID50 and ID80 titers were left out of the model, with an estimated 90.6% reduction in the odds of infection per SD increase in IIP. However, IIP was not associated with infection risk when either ID50 or ID80 titer was included in the model. In models with ID50 or ID80 titer in addition to epitope and challenge virus, the reductions in the odds of infection by SHIVsfi62P3 or a challenge virus other than SHIVSF162P3 were 57.9% and 94.0% per SD increase in log10(ID50) and 75.1% and 98.5% per SD increase in log10(ID80). The model with ID80 titers has only slightly better fit compared to the model with ID50 (AIC=175.4 and 177.2 respectively); combining ID50 and ID80 titers did not further improve model fitting. The average estimated levels of protection as a function of ID50 titer, ID80 titer, and IIP are presented in Figure S3. The curve of protection vs IIP (Figure S3c) allows one to compute the fraction of neutralization to achieve a desired protection level. This analysis suggests that it is possible to achieve 50% protection with 93.7% neutralization (95% CI: 68.4%, 98.4%), 75% protection with 99.8% neutralization (95% CI: 96.8%, 99.9%) and 95% protection with >99.9% neutralization.
Super Learner analysis to identify best classification models
We conducted Super Learner analysis to further compare the predictive capacity of models based on ID50, ID80, IIP, or combinations thereof. Table S4 shows model performance in predicting level of protection for various candidate learning algorithms and the resulting Super Learner ensemble model fitted using ID50, ID80, IIP or a combination thereof, in addition to epitope and challenge virus. The top 10 best performing models and their CV-AUCs along with the predictors used to fit them are presented in Table S5. The Super Learner model fit using ID80, epitope, and challenge virus as predictors was the best performing model with a CV-AUC of 0.879 (95% CI: 0.827, 0.931). Addition of IIP as a predictor did not seem to enhance model performance. Replacing ID80 with ID50 in the Super Learner model led to similar performance with a CV-AUC of 0.875 (95% CI: 0.822, 0.928), which again was not enhanced by the addition of IIP. Combining ID50 titer with ID80 titer in the Super Learner did not improve performance compared to using either one of the two measures alone. The best performing model (random forest) with IIP and without ID50 and ID80 titers had a CV-AUC of 0.833 (95% CI: 0.772, 0.894), smaller than those for models using either ID50 or ID80 titer. The addition of any one of the three neutralization activity measures led to a substantial improvement of the CV-AUC compared to the best performing model based on epitope and challenge virus alone (CV-AUC of 0.719, 95% CI: 0.638, 0.800) (Table 4).
Table 4:
Top performing models and their CV-AUCs based on models with one of ID50, ID80 and IIP as predictors, in addition to epitope and challenge virus, based on the subset of 218 animals with IC80 information. See also Tables S4 and S5.
| Neutralization Activity Measure* | Algorithm** | CV-AUC | 95% CI | |
|---|---|---|---|---|
| None | SL.glm | 0.719 | 0.638–0.800 | |
| ID50 | SuperLearner | 0.875 | 0.822–0.928 | |
| ID80 | SuperLearner | 0.879 | 0.827–0.931 | |
| IIP | SL. randomF orest | 0.833 | 0.772–0.894 |
In addition to epitope and challenge virus
Algorithms are listed by the functions used in the SuperLearner R package. An exception is “SL.naivebayes”, which was a custom wrapper designed to use the naiveBayes function from the e1071 package. The SL.glmnet package was used with the lasso penalty. All tuning parameters are set to the default values of the SuperLearner package, except SL.xgboost, which we modified to fit decision stumps rather than trees.
Discussion
This meta-analysis of a comprehensive dataset of published and unpublished data from passive immunization studies using bNAbs and mucosal SHIV challenges confirms that serum neutralization titer on the day of SHIV challenge is strongly associated with the protective efficacy against the challenge virus. This finding supports the hypothesis that the serum neutralization titers against prevalent HIV-1 strains will be an important determinant of protective efficacy in human passive immunization studies, including the ongoing AMP trials. Moreover, our models allow the estimation of serum neutralization titers needed to achieve 50%, 75%, and 95% protection from infection in the SHIV challenge model, which can be compared to data from ongoing and future human studies to provide a better understanding of the predictability of NHP protection models.
The passive transfer studies in this meta-analysis all used a single mucosal SHIV challenge that aimed to produce infection in 100% of control animals. We chose this study format because it represented the largest body of NHP data, and because it allowed us to most precisely associate serum antibody level, and the inferred neutralization titer, with protection. Compared to the relative inefficiency of human sexual transmission, this NHP challenge format may represent a more stringent test of the ability of bNAbs to protect – therefore the protective sera titers arrived at in this meta-analysis may be an overestimate of the minimal titers required to mediate protection against viral challenge in the setting of mucosal exposure to HIV-1.
This meta-analysis included bNAbs to four different Env epitopes and eight different strains of SHIV. Serum ID50 titer was identified as a consistently strong correlate of protection across various combinations of epitope and virus type, although the odds ratio is less extreme for SHIVSF162P3 compared to other virus types. The less significant effect estimate of ID50 on infection risk for SHIVSF162P3 was driven by a few studies with bNAbs targeting the CD4 binding site (including b12, VRC01, VRC01LS, VRC07–523LS) and SHIVSF162P3, which had very low overall infection rate (3 infections out of 33 animals) with little association with ID50 levels. Additional data are warranted to further investigate this finding.
Comparing different epitopes included in the study, a significantly lower risk of infection was observed with bNAbs targeting MPER relative to bNAbs targeting other epitope categories, with the caveat that these MPER bNAbs were studied using only one SHIV strain, which is known to be generally neutralization sensitive, though not unusually sensitive to neutralization by MPER bNAbs. These results indicate that for MPER targeting bNAbs, effector functions other than neutralization may also contribute significantly to their protective efficacy in this model. Additional studies using challenge viruses displaying different sensitivities to MPER bNAbs would be needed to better understand the protective efficacy of this category of bNAbs.
One limitation of our analyses is that the SHIV stocks used for these mucosal challenges have generally been noted to have limited diversity, e.g. 0.4–0.5% Env diversity in the SHIVSF162P3 challenge stock (Jayaraman et al., 2007; Ng et al., 2010) and 0.3% mean genetic Env diversity in the SHIVBaLP4 challenge stock (Santra et al., 2015). It may be difficult to extrapolate these results to protection of humans from mucosal HIV exposure, especially if the HIV-1 virions present at the exposure event in humans exhibit greater genetic diversity than is observed in the challenge stocks in the NHP studies. Despite this limited diversity in the challenge stocks, divergence of viral sequences from the challenge virus sequence in each infected animal has been observed (Jayaraman et al., 2007; Ng et al., 2010; Santra et al., 2015). In addition, enumeration of Transmitter/Founder (T/F) env performed in these studies has shown that the number of T/F envs are very similar to that observed in HIV infection in humans (Keele et al., 2008). This provides some support for the use of mucosal SHIV challenge as a model for natural HIV transmission.
The levels of antibody at mucosal surfaces, where the SHIV challenge was performed, were not determined in most of these studies. This is an important issue to address since antibodies at mucosal surfaces, or in mucosal tissues, represent the first barrier to the establishment of infection. However, it is challenging to accurately and reproducibly determine mucosal antibody levels, and most NHP studies have not done this. Another important caveat is that most studies performed mucosal SHIV challenge within a day after antibody administration, while recent studies have shown that mucosal accumulation of passively transferred antibodies can take more than 2 days to peak in mucosal tissues (Ko et al., 2014; Welles et al., 2018). It has also been shown that the protective efficacy of bNAbs against mucosal SHIV challenge can involve clearance of infectious virus from distal sites of virus exposure, suggesting that antibody-mediated protection may not be solely related to action at the mucosal surface (Hessell et al., 2016; Liu et al., 2016). Overall, systemic antibody levels appear to be a robust corelate of antibody-mediated protection against infection.
The instantaneous inhibitory potential (IIP) measures the log 10 reduction in a single round of infection in the presence of a bNAb, and in capturing information about the neutralization curve slope, measures a different feature of neutralization than ID50 or ID80 titers (Jilek et al., 2012). Among a subset of studies with ID80 and IIP available, we found IIP to be positively correlate with protection against SHIV infection, as also reported previously in a repeated low-dose challenge study (22). We did find that IIP was a strong independent predictor of protection by itself but adding IIP to models with ID50 or ID80 titers did not further enhance the performance of the model to predict the level of protection compared to the performance of the model with ID50 or ID80 titer alone.
The protective serum ID50 titers reported here are similar to the titers that were observed to be protective for 5 different bNAbs in passive transfer experiments using repetitive mucosal SHIV challenge (Shingai et al., 2014) and this lends additional support to the protected titer predicted from this meta-analysis. Overall, these data suggest that the protective titers from NHP SHIV challenge studies may be predictive of efficacy in human trials of bNAbs and therefore a useful metric to measure in such trials. Of note, many published studies report neutralization using 293T-derived Env-pseudoviruses, which can be more sensitive to neutralization than the PBMC derived SHIV neutralization data that were used in this analysis (Cohen et al., 2018; Louder et al., 2005). However, the assay using 293T-derived pseudoviruses is reproducible with low technical measurement error and is therefore advantageous for developing a potential statistical correlate of prevention efficacy; this assay is being used in the first planned correlates analyses in the AMP trials. In addition, a recent study of vaccination with recombinant native-like Env trimers to induce nAbs found that a serum ID50 titer of 500 was correlated with >90% protection against mucosal SHIV challenge (Pauthner et al., 2019). This finding is similar to our result that a serum ID50 titer of 685 corresponds to 95% protection. These findings suggest that achieving such high titers should be one of the key goals of a successful prophylactic vaccine.
Our analysis suggests that serum neutralization titer is a strong predictor of protective efficacy in the NHP model of HIV infection and could be an important determinant of protective efficacy against HIV. Our findings may also inform the development of Env protein-based vaccination strategies that aim to elicit bNAbs and suggest benchmarks for the bNAb titers that a successful preventative vaccine may need to achieve. Our results further highlight the importance of evaluating serum neutralizing titers against relevant viruses in efficacy trials of bNAbs and vaccine candidates.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Materials Availability Statement: Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ying Huang (yhuang@fredhutch.org). This study did not generate new unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Rhesus macaques
Details on the 242 NHPs used in the 13 published studies can be found in the original papers (Hessell et al., 2007; Hessell et al., 2016; Hessell et al., 2009b; Hessell et al., 2010; Julg et al., 2017; Ko et al., 2014; Moldt et al., 2016; Moldt et al., 2012; Parren et al., 2001; Rudicell et al., 2014; Shingai et al., 2014). The 32 NHPs (20 males, 12 females) in the 5 unpublished studies were Outbred Indian rhesus macaques (Macaca mulatto). All rhesus monkeys were adults (> 3 years old) that did not express the class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17. All animal experiments were reviewed and approved by the Animal Care and Use Committee of the Vaccine Research Center, NIAID, NIH, and all animals were housed in a fully AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) accredited facility with stringent standard operating procedures and compliant with U.S. Animal Welfare Act (AWA) and Regulations, the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, the Guide for the Care and Use of Laboratory Animals and all applicable NIH Policies on in vivo research.
METHOD DETAILS
All method details for the 242 NHPs from the 13 published studies can be found in the original papers (Hessell et al., 2007; Hessell et al., 2016; Hessell et al., 2009b; Hessell et al., 2010; Julg et al., 2017; Ko et al., 2014; Moldt et al., 2016; Moldt et al., 2012; Parren et al., 2001; Rudicell et al., 2014; Shingai et al., 2014). Method details for the 32 NHPs in the 5 unpublished studies follow below:
Antibodies
bNAbs were prepared as previously described, as follows: N6LS: Walker et al., 2011; VRC01 and VRC01LS: Ko et al., 2014; VRC07–523LS: Rudicell et al., 2014.
Virus strains
SHIV challenge stocks were prepared as previously described, as follows: SHIVBG505: Li et al., 2016; SHIVBALP4: Pal et al., 2003; SHIVSF162P3: Harouse et al., 2001; Tan et al., 1999.
Rhesus macaque immunization and challenge
NHPs were intravenously infused with the appropriate bNAb 2–5 days before challenge. Each infusion consisted of the appropriate dose of the stock antibody diluted in PBS. For challenge, animals were inoculated intra-rectally with the dose of the SHIV challenge stock that was determined to lead to infection in control animals after a single challenge.
Measurement of antibody levels
Similar quantitative ELISA methods as those used in the published studies (Hessell et al., 2007; Hessell et al., 2016; Hessell et al., 2009b; Hessell et al., 2010; Julg et al., 2017; Ko et al., 2014; Moldt et al., 2016; Moldt et al., 2012; Parren et al., 2001; Rudicell et al., 2014; Shingai et al., 2014) were used to measure antibody levels (μg/mL) in plasma or serum samples on the day of challenge. Briefly, anti-idiotype antibodies or HIV-1 Env-based proteins were used to capture the bNAbs; complexes were detected by incubation with HRP-conjugated anti-human IgG conjugates, followed by incubation with a colorimetric substrate and spectrophotometric measurement (Ko et al., 2014).
Neutralization assays
Neutralization of replication-competent SHIV by the studied bNAbs was evaluated in vitro using TZM-bl (Li et al., 2005; Montefiori, 2009) or PBMC (Parren et al., 2001) target cells (NIH AIDS Research and Reference Reagent Program, catalog number 8129) as described. The in vitro 50% and 80% inhibitory concentration (IC) neutralization titers, IC50 and IC80, determined previously in published studies were used for analysis. For SHIVs used in unpublished studies, similar methods were used to determine the neutralization titers. For the TZM-bl target cell assay (Li et al., 2005; Montefiori, 2009), replication-competent SHIV viruses were incubated with antibody for 30 min at 37°C, after which TZM-bl cells were added. The HIV protease inhibitor indinavir was added to wells at a final concentration of 1 μM to limit infection of target cells to a single round of viral replication. Luciferase expression was quantified 48 hours after infection upon cell lysis and the addition of luciferin substrate (Promega). For the PBMC-based assay, phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMC) were used as target cells; neutralization assessment was carried out as described previously (Parren et al., 2001).
QUANTIFICATION AND STATISTICAL ANALYSIS
All analyses were based on data from individual animals that received bNAb infusion. Descriptive analyses were performed to characterize the distribution of various covariates, including study group, bNAb and epitope-targeting region, challenge virus, route and dose, sex of animal, day of challenge post-bNAb administration, and target cell type for measuring neutralization sensitivity.
Predicted ID50 titer against the challenge virus was calculated by dividing serum concentration of the bNAb at the day of challenge by the in vitro IC50 titer of the bNAb against the SHIV challenge virus. In support of this approach, predicted ID50 titers were shown to be tightly correlated with experimental ID50 titers in a repeated low dose SHIV challenge model (Gautam et al., 2016; Wagh et al., 2018) and in a phase 1 study using bNAb VRC01 (Ledgerwood et al., 2015). We also analyzed a subset of data from a previously unpublished passive transfer study of the N6LS bNAb to further establish the validity of this prediction approach. The comparison between the experimentally determined and predicted ID50 titers in plasma samples of rhesus macaques five days after intravenous administration is presented in Table S6.
Logistic regression was used to model the association of log10-transformed serum ID50 titer with infection outcome, and to assess possible effect modification by other covariates as well as to adjust for possible confounding variables. The Akaike information criterion (AIC) was used to compare the fit of different models. All p-values are two-sided and based on a Wald test. Statistical significance is declared with p-value < 0.05.
Since all control animals were infected in each of the included studies (infection rate = 100%), based on the selected model of P(infection|ID50, covariate), the % protection conditional on ID50 titer at given covariate level was computed as % protection = [1-P(infection | ID50, covariate)] × 100%. Serum ID50 titer for achieving 50%, 75%, and 95% protection was estimated conditional on covariates. A nonparametric bootstrap procedure with 1000 resamples was conducted to construct 95% confidence intervals about these estimates.
Based on the selected model of P(infection | ID50, covariate), the overall % protection at a given ID50 titer, averaging over values of all other covariates, was estimated as 1- P(infection | ID50)= 1- ΣP(infection | ID50, covariate group) ×P(covariate group | ID50), where the sum is over all distinct covariate groups and P(covariate group | ID50) was modeled by multinomial logistic regression with a cubic spline basis for log10(ID50) with a node at its median. Again, a nonparametric bootstrap with 1000 resamples was used to construct 95% confidence intervals for ID50 titers required to achieve 50%, 75%, and 95% protection in the study dataset.
The instantaneous inhibitory potential (IIP) measures the log10 reduction in infectious events in the presence of drugs/antibodies (Montefiori 2009). In a subset of studies for which in vitro IC80 titer of the bNAb against the challenge virus was available (n=218), IIP at the day of challenge was computed using estimated Hill curves based on IC50 and IC80 values (Wagh et al., 2016) as IIP = −log10(1 − f(c)), where f(c) = cm/(IC50m + cm) = fraction neutralization at concentration c, with slope m = log(4)/(log IC80 − log IC50), and c being bNAb concentration at the day of challenge; serum 80% neutralization titer (ID80) against the challenge virus was also predicted by dividing serum concentration of the bNAb at the day of challenge by the IC80 titer of the bNAb against the challenge virus. Spearman rank correlation was assessed among ID50 titer, ID80 titer, and IIP. Logistic models were fitted with log10 transformed ID50 titer, ID80 titer, and/or IIP to evaluate and compare their performance in prediction.
ID50 titer, ID80 titer and IIP were also assessed as predictors of infection by building convex ensemble models using regression stacking (Breiman, 1996; Wolpert, 1992), also known as Super Learning (van der Laan et al., 2007), with the SuperLearner R package (van der Laan et al., 2007; Polley et al., 2011). This approach applies convex weights to combine candidate learning algorithms with the aim of minimizing the negative log-likelihood loss as a cross-validated prediction criterion. The candidate learning algorithms included lasso (Tibshirani, 1996) [with logistic link as implemented in the glmnet R package (Friedman J, 2018)], random forests (Liaw and Wiener, 2002) [implemented in the randomForest R package (Liaw and Wiener, 2018-03-25)], Naïve Bayes (John and Langley, 1995) [implemented in the e1071 R package (Meyer et al., 2017-02-02)], and boosted decision stumps via extreme gradient boosting (Chen and Guestrin, 2016) [implemented in the xgboost R package (Chen et al., 2018-01-23)]. We also included classical statistical techniques: generalized linear models and stepwise-selected generalized linear models (again with logistic link as implemented in the glmnet R package). Each candidate learning algorithm and the resulting Super Learner ensemble model was fit with log10 transformed ID50 titer, log10 transformed ID80 titer, IIP, or a combination thereof with additional covariates. The candidate learners and the Super Learner were evaluated via external 10-fold cross-validation to guard against overfitting. The cross-validated area under the receiver operating characteristics curve (CV-AUC) was used as the prediction metric. Wald-type 95% confidence intervals about CV-AUC were computed using influence function-based standard error estimates (LeDell et al., 2015).
DATA AND CODE AVAILABILITY
Data and Code Availability Statement: All data analyzed during this study is included in this or other published studies. All code used during this study is included in other published studies.
Supplementary Material
Table S2. Detailed animal, challenge, antibody, and protection status information for the n=32 NHPs from the 5 unpublished studies. Related to Table 1.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| N56LS | Generated in-house as previously described (Walker et al., 2011, Huang et al., 2016) | N/A |
| VRC01 | Generated in-house as previously described (Ko et al., 2014) | N/A |
| VRC01LS | Generated in-house as previously described (Ko et al., 2014) | N/A |
| VRC07–523LS | Generated in-house as previously described (Rudicell et al., 2014) | N/A |
| Bacterial and Virus Strains | ||
| SHIVBG505 | Generated in-house as previously described (Li et al., 2016) | N/A |
| SHIVBaL P4 | Generated in-house as previously described (Pal et al., 2003) | N/A |
| SHIVSF162P3 | Generated in-house as previously (Harouse et al., 2001; Tan et al., 1999) | N/A |
| Biological Samples | ||
| N/A | N/A | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| N/A | N/A | N/A |
| Critical Commercial Assays | ||
| N/A | N/A | N/A |
| Deposited Data | ||
| N/A | N/A | N/A |
| Experimental Models: Cell Lines | ||
| TZM-bl cells (also called JC53BL-1) | NIH AIDS Research and Reference Reagent Program | Cat. no. 8129 |
| Experimental Models: Organisms/Strains | ||
| Indian origin, outbred, young adult male and female rhesus monkeys (Macaca mulatta) that did not express the class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17 | Alphagenesis Inc., Yermasse, SC | N/A |
| Oligonucleotides | ||
| N/A | N/A | N/A |
| Recombinant DNA | ||
| N/A | N/A | N/A |
| Software and Algorithms | ||
| glmnet R package | Friedman et al., 2010 | https://cran.r-project.org/web/packages/glmnet/glmnet.pdf |
| randomForest R package | Liaw and Weiner, 2002 | Version 46–14 at https://www.stat.berkeley.edu/~breiman/RandomForests/ |
| e1071 R package | N/A | https://cranr-projectorg/web/packages/e1071/indexhtml |
| xgboost R package | Chen and Guestrin, 2016 | https://cran.r-project.org/web/packages/xgboost/xgboost.pdf |
| SuperLearner R package | Van der Laan, Polley, and Hubbard, 2007; Polley et al., 2011 | https://cran.r-project.org/web/packages/SuperLearner/index.html |
| Other | ||
| N/A | N/A | N/A |
Highlights.
Meta-analysis of NHP studies administering a single bNAb before SHIV challenge
Serum neutralizing titers correlate strongly with protection against SHIV infection
ID50, ID80, and IIP all predict protection, with ID50 and ID80 stronger predictors
Substantial levels of neutralization may be needed for protection by a single bNAb
Acknowledgments:
Support for this work was provided in part by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID), NIH. This work was also supported in part by NIAID through awards UM1AI068635 (HVTN SDMC) and R37AI054165 to PBG and awards UM1AI144462 and UM1 AI100663 to DRB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: John Mascola and Mario Roederer are listed as inventors on NIH patents, or patent applications for 10E8, N6LS, VRC01, VRC01LS, and VRC07–523LS. Dennis Burton is listed as an inventor on patents for b12, PG9, PGT121, PGT126, and PGDM1400. He is also on the Executive Advisory Board of HVTN and the SAWG of the VRC and is a paid consultant of IAVI.
REFERENCES
- Breiman L (1996). Stacked Regressions. Machine Learning. 24(1), 49–64. [Google Scholar]
- Caskey M, Klein F, and Nussenzweig MC (2019). Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med. 25(4), 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, and Guestrin C, 2016. XGBoost: A scalable tree boosting system, 22nd SIGKDD Conference on Knowledge Discovery and Data Mining ACM, pp. 785–794. [Google Scholar]
- Chen T, He T, and Benesty M (2018-January-23). xgboost: extreme Gradient Boosting. R package version 0.6.4.1. (https://cran.r-project.org/web/packages/xgboost/index.html).
- Cohen YZ, and Caskey M (2018). Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS. 13(4), 366–373. [DOI] [PubMed] [Google Scholar]
- Cohen YZ, Lorenzi JCC, Seaman MS, Nogueira L, Schoofs T, Krassnig L, Butler A, Millard K, Fitzsimons T, Daniell X, et al. (2018). Neutralizing Activity of Broadly Neutralizing Anti-HIV-1 Antibodies against Clade B Clinical Isolates Produced in Peripheral Blood Mononuclear Cells. J Virol. 92(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakle R, Venter F, and Rees H (2018). Pre-exposure prophylaxis (PrEP) in an era of stalled HIV prevention: Can it change the game? Retrovirology. 15(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J,HT, Tibshirani R, Simon N, Narasimhan B, Qian J, 2018. glmnet: Lasso and Elastic-Net Regularized Generalized Linear Models.
- Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, et al. (2016). A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 533(7601), 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PB, Juraska M, deCamp AC, Karuna S, Edupuganti S, Mgodi N, Donnell DJ, Bentley C, Sista N, Andrew P, et al. (2017). Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials. Stat Commun Infect Dis. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM., Landucci G, Forthal DN, Parren PW, et al. (2007). Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 449(7158), 101–104. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, et al. (2016). Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med. 22(4), 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Malherbe DC, and Haigwood NL (2018). Passive and active antibody studies in primates to inform HIV vaccines. Expert Rev Vaccines. 17(2), 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, and Burton DR (2009a). Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 15(8), 951–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, and Burton DR (2009b). Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5(5), e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, et al. (2010). Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 84(3), 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Karuna S, Carpp LN, Reeves D, Pegu A, Seaton K, Mayer K, Schiffer J, Mascola J, and Gilbert PB (2018). Modeling cumulative overall prevention efficacy for the VRC01 phase 2b efficacy trials. Hum Vaccin Immunother. 14(9), 2116–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P, Zhu T, Misher L, Mohan D, Kuller L, Polacino P, Richardson BA, Bielefeldt-Ohmann H, Anderson D, Hu SL, et al. (2007). Evidence for persistent, occult infection in neonatal macaques following perinatal transmission of simian-human immunodeficiency virus SF162P3. J Virol. 81(2), 822–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek BL, Zarr M, Sampah ME, Rabi SA, Bullen CK, Lai J, Shen L, and Siliciano RF (2012). A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat Med. 18(3), 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GH, and Langley P, 1995. Estimating continuous distributions in Bayesian classifiers, Proceedings of the Eleventh conference on Uncertainty in artificial intelligence Morgan Kaufmann Publishers Inc., pp. 338–345. [Google Scholar]
- Julg B, Sok D, Schmidt SD, Abbink P, Newman RM, Broge T, Linde C, Nkolola J, Le K, Su D, et al. (2017). Protective Efficacy of Broadly Neutralizing Antibodies with Incomplete Neutralization Activity against Simian-Human Immunodeficiency Virus in Rhesus Monkeys. J Virol. 91(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. (2008). Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 105(21), 7552–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, et al. (2014). Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 514(7524), 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDell E, Petersen ML, and Van der Laan M (2015). Computationally efficient confidence intervals for cross-validated area under the ROC curve estimates. Electron. J. Statist 9(1), 1583–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, DeZure A, Lynch RM, Gordon I, Plummer S, et al. (2015). Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 182(3), 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. (2005). Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 79(16), 10108–10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A, and Wiener M (2002). Classification and regression by randomForest. R news. 2(3), 18–22. [Google Scholar]
- Liaw A, and Wiener M (2018-March-25). randomForest: Breiman and Cutler’s Random Forests for Classification and Regression. Version 4.6–14. https://www.stat.berkeley.edu/~breiman/RandomForests/.
- Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, et al. (2016). Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science. 353(6303), 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, Sanders-Buell E, Zolla-Pazner S, McCutchan FE, Roser JD, et al. (2005). HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology. 339(2), 226–238. [DOI] [PubMed] [Google Scholar]
- McCoy LE, and Burton DR (2017). Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 275(1), 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F, Chang C-C, and Lin C-C (2017-February-02). e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. https://cran.r-project.org/web/packages/e1071/index.html. V 1.6-8
- Moldt B, Le KM, Carnathan DG, Whitney JB, Schultz N, Lewis MG, Borducchi EN, Smith KM, Mackel JJ, Sweat SL, et al. (2016). Neutralizing antibody affords comparable protection against vaginal and rectal simian/human immunodeficiency virus challenge in macaques. AIDS. 30(10), 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, et al. (2012). Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 109(46), 18921–18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC (2009). Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 485, 395–405. [DOI] [PubMed] [Google Scholar]
- Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, et al. (2010). Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 16(10), 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, and Burton DR (2001). Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 75(17), 8340–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner MG, Nkolola JP, Havenar-Daughton C, Murrell B, Reiss SM, Bastidas R, Prevost J, Nedellec R, von Bredow B, Abbink P, et al. (2019). Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers. Immunity. 50(1), 241–252 e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu A, Hessell AJ, Mascola JR, and Haigwood NL (2017). Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. 275(1), 296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudicell RS, Kwon YD, Ko SY, Pegu A, Louder MK, Georgiev IS, Wu X, Zhu J, Boyington JC, Chen X, et al. (2014). Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 88(21), 12669–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Tomaras GD, Warrier R, Nicely NI, Liao HX, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, et al. (2015). Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog. 11(8), e1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, et al. (2014). Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 211(10), 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D, and Burton DR (2018). Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol. 19(11), 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R (1996). Regression shrinkage and selection via the Lasso. J Roy Stat Soc B Met. 58(1), 267–288. [Google Scholar]
- UNAIDS, 2018. UNAIDS Fact sheet.
- van der Laan MJ, Polley EC, and Hubbard AE (2007). Super Learner. Stat Appl Genet Mol. 6(1), Article 25. [DOI] [PubMed] [Google Scholar]
- Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, Rist M, Rademeyer C, Yoon H, Lapedes A, et al. (2016). Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog. 12(3), e1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh K, Seaman MS, Zingg M, Fitzsimons T, Barouch DH, Burton DR, Connors M, Ho DD, Mascola JR, Nussenzweig MC, et al. (2018). Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog. 14(3), e1006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welles HC, Jennewein MF, Mason RD, Narpala S, Wang L, Cheng C, Zhang Y, Todd JP, Lifson JD, Balazs AB, et al. (2018). Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLoS Pathog. 14(12), e1007395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DH (1992). Stacked Generalization. Neural Networks. 5(2), 241–259. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Detailed animal, challenge, antibody, and protection status information for the n=32 NHPs from the 5 unpublished studies. Related to Table 1.
Data Availability Statement
Data and Code Availability Statement: All data analyzed during this study is included in this or other published studies. All code used during this study is included in other published studies.