Abstract
Objectives
Ki67 is the most commonly used marker to evaluate proliferative index in breast cancer, however no cutoff values have been clearly defined for high ki67 index. Cancer management should be according to loco-regional profile; therefore, we aimed to determine ki67 index in 1951 cases of intrinsic breast cancer subtypes and its association with other prognostic parameters in our set up.
Results
Triple negative breast cancers showed highest ki67 index (mean 50.9 ± 23.7%) followed by Her2neu (mean 42.6 ± 21.6%) and luminal B cancers (mean 34.9 ± 20.05%). Metaplastic and medullary breast cancers significantly showed higher ki67 index as compared to ductal carcinoma, NOS. No significant association of ki67 index was noted with any of the histologic parameters in different subtypes of breast cancer expect for tumor grade. Although, ki67 index is a valuable biomarker in breast cancer, however no independent prognostic significance of ki67 could be established in our study.
Keywords: Breast cancer, Intrinsic subtypes of breast cancer, Ki67 index, ER, PR, Her2neu
Introduction
Transition from phenotypic to intrinsic molecular breast cancer subtypes has made a paradigm shift in breast cancer treatment. With advent of new treatment regimens, it becomes important to individualize therapy according to biomarker status of the tumor. Hormone receptor and human epidermal growth factor receptor 2 (her2neu) statuses impart both prognostic and predictive impact on breast cancer management. Therefore performing estrogen receptor (ER), progesterone receptor (PR) and her2neu biomarker studies has become standard of care in breast cancer management as per American Society of Clinical Oncology (ASCO) guidelines [1]. Markers of elevated proliferation generally indicate a poor outcome in any cancer. Over the past years, there is a considerable debate over the performance and interpretation of proliferative index markers like thymidine labeling index, S-phase fraction determined by flow cytometry and immunohistochemistry (IHC). Overall, proliferative index determined by IHC correlates well with S phase fraction measured by flow cytometry [2]. Although, there is still no consensus over an optimal cutoff value used to decide chemotherapy, but several studies found that high ki67 index is associated with higher rate of relapse and worse breast cancer survival [3, 4]. It is widely accepted that cancer management should be according to loco-regional profile and therapeutic protocols should be devised accordingly, however no large-scale cancer registry is available in this part of the world. Moreover ki67 may serve as a useful marker in tailoring treatment regimen as response to chemotherapy may be altered by the proliferative activity of cancer cells [5]. Therefore we aimed to determine the ki67 in newly defined intrinsic breast cancer subtypes and its association with other prognostic parameters in our set up.
Main text
Materials and methods
The study included 1951 cases of primary breast cancers. All of these patients underwent treatment at Liaquat National hospital during January 2011 till December 2016. An approval from institutional ethical review committee was taken before conducting the study. The specimens were of trucut biopsies, breast conservative surgical specimens (wide local excision) with sentinel lymph node dissection and modified radical mastectomies (MRM).
Histopathologic characteristics including histologic type, grade, tumor size, nodal status, lymphocytic infiltration of tumor, necrosis and fibrosis were assessed by two histopathologists independently. One representative section was selected for IHC studies including ER, PR, her2neu and ki67. Antibodies for ER, PR and Her2neu IHC were purchased from DAKO and DAKO envision kit was used and stains were performed according to manufacturer’s defined protocol. Positive and negative controls were run along each batch of IHC. Only nuclear expression of ER and PR were recorded semi-quantatively and more than 1% expression was taken as positive expression [6, 7]. For Her2neu IHC, only membranous staining was considered and more than 10% strong membranous positivity was taken as positive (3+) Her2neu IHC as per CAP guidelines [8, 9]. Cases with equivocal (2+) IHC expression of Her2neu subsequently underwent FISH testing for Her2neu gene amplification. FISH testing was done using Path Vysion Her2 DNA Probe kit. Results were interpreted as amplified (positive) or not amplified (negative) according to CAP guidelines [8, 9]. For Ki-67, nuclear expression was recorded quantitatively. At-least 1000 cells were assessed to calculate an average estimate. On the basis of percentage of staining, ki67 index was further categorized into four groups, < 14%, 15–24%, 25–44%, > 45%, as can be seen in Fig. 1.
Fig. 1.
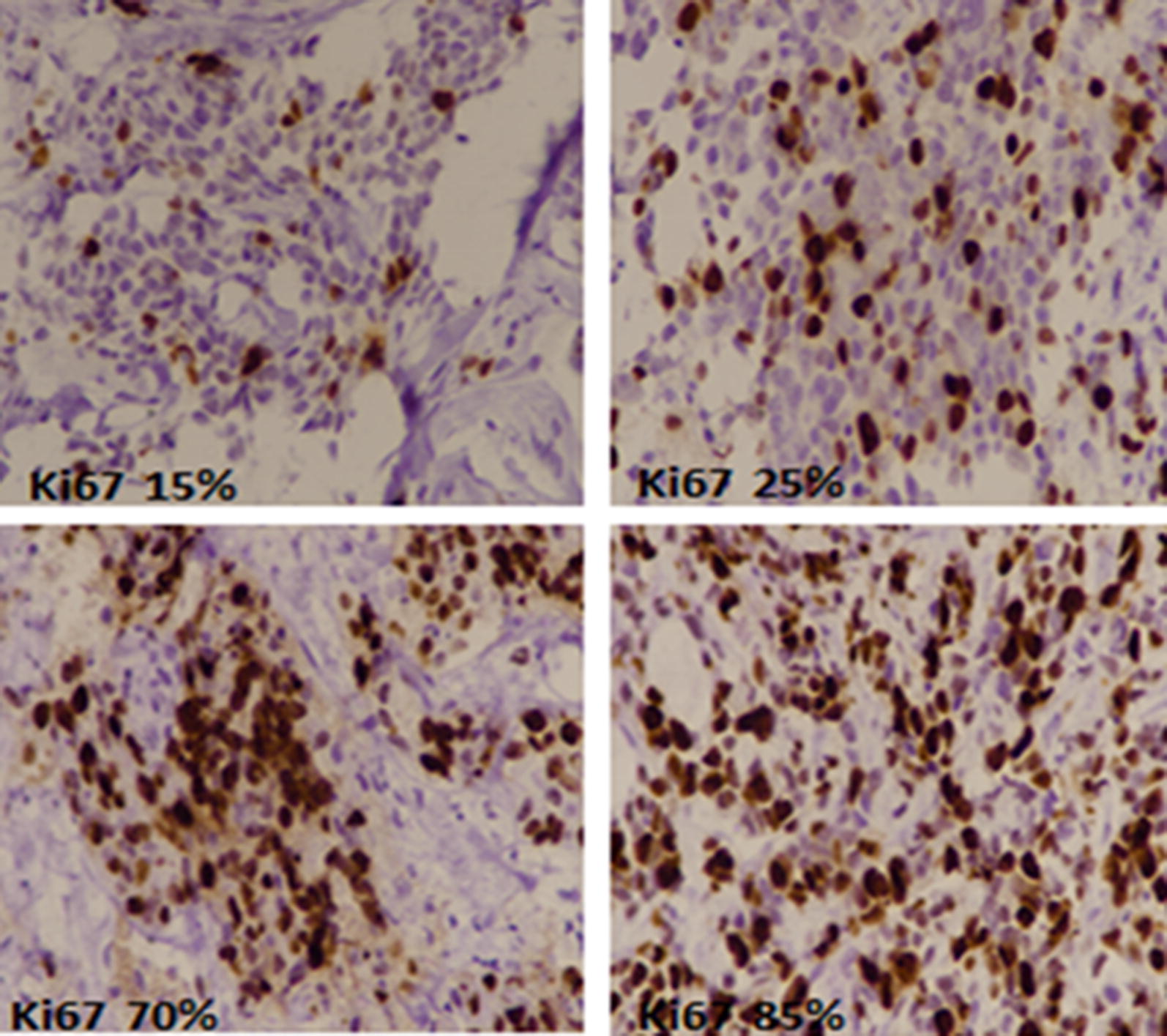
Ki67 expression in breast cancer by immunohistochemistry. Brown nuclear stain highlights ki67 positive tumor cells
Surrogate clinicopathologic definitions of intrinsic breast cancer subtypes were used as follows [10]:
Luminal A like: ER positive, PR high (> 20%).
Luminal B like: ER positive, PR low (< 20%), or ER positive, Her2neu positive (3 + on IHC/amplified on FISH), any PR.
Her2neu positive (non-luminal): ER and PR negative, Her2neu positive (3+ on IHC or amplified on FISH (for 2+ IHC results)
Triple negative: ER, PR and Her2neu negative.
For data analysis, Statistical package for social sciences (SPSS 21) was used. Mean and standard deviation were evaluated for quantitative variables. Frequency and percentage were evaluated for qualitative variables. Chi square and fisher exact test was applied to determine association as appropriate. ANOVA was applied to compare difference in means among groups. P value ≤ 0.05 was considered significant.
Results
Out of total 1951 cases of primary breast cancers included in the study, 1185 cases were of trucut biopsies while 766 cases were excision specimens. Figure 2 shows association of ki67 index with intrinsic breast cancer subtypes. Triple negative breast cancers showed highest ki67 index (mean 50.9 ± 23.7%) followed by Her2neu (mean 42.6 ± 21.6%) and luminal B cancers (mean 34.9 ± 20.05%). On the other hand, luminal A cancers showed lowest ki67 index (mean 23.6 ± 19.7%). Table 1 depicts association of ki67 index categories with histologic subtypes. Metaplastic and medullary breast cancers significantly showed higher ki67 index as compared to ductal carcinoma, NOS.
Fig. 2.
Ki67 index expression in different intrinsic breast cancer subtypes, categorized into 4 sub-groups and shown at the bottom of the figure
Table 1.
Association of ki67 index with Histological subtypes
| Histologic subtype | Ki67 index category N (%) | Total | P-value | |||
|---|---|---|---|---|---|---|
| < 15% | 15–24% | 25–44% | > 44% | |||
| Ductal | 373 (22) | 311 (18.3) | 406 (24) | 605 (36) | 1695 | < 0.01 |
| Lobular | 46 (50.5) | 20 (22) | 13 (14.3) | 12 (13.2) | 91 | |
| Cribriform | 4 (57.1) | 2 (28.6) | 1 (14.3) | 0 (0) | 7 | |
| Papillary | 19 (47.5) | 10 (25) | 6 (15) | 5 (12.5) | 40 | |
| Mucinous | 23 (63.9) | 6 (16.7) | 5 (13.9) | 2 (5.6) | 36 | |
| Micropapillary | 2 (13.3) | 4 (26.7) | 5 (33.3) | 4 (26.7) | 15 | |
| Tubular | 7 (70) | 0 (0) | 1 (10) | 2 (20) | 10 | |
| Medullary | 0 (0) | 0 (0) | 1 (11.1) | 8 (88.9) | 9 | |
| Metaplastic | 4 (9.3) | 11 (25.6) | 12 (28) | 16 (37.2) | 43 | |
| Mixed Ductal &Lobular | 1 (25) | 1 (25) | 0 (0) | 2 (50) | 4 | |
| Adenoid cystic carcinoma | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 | |
| Total | 479 | 366 | 450 | 656 | 1951 | |
Fisher exact test was applied
Additional file 1: Tables S1–S4 shows association of ki67 index with various clinical and pathologic parameters according to different subtypes of breast cancer. ki67 showed significant association with tumor grade in all breast cancer subtypes.
Significant association of ki67 index was also seen with age in triple negative and luminal A subtypes. Higher ki67 index was noted in lower age groups specifically < 30 years age group. No significant association of ki67 index was noted with any of the other histological parameters or nodal stage.
Discussion
In the present study, we evaluated ki67 index in different intrinsic and histologic breast cancer subtypes and found high ki67 index in her2neu and triple negative intrinsic breast cancer subtype and metaplastic & medullary histologic breast cancer types [11, 12]. All of these categories of breast cancer are uniformly considered as aggressive phenotypes of breast cancer. Moreover, significant association of ki67 index was noted with tumor grade which is considered as one of the prognostic factor in breast cancer [13, 14]. Apart from its association with tumor grade, we didn’t find any significant association of ki67 index with any other prognostic parameter including nodal metastasis. Furthermore, we also found a significantly high ki67 index (> 44%) in women < 30 years of age in triple negative and luminal B subtypes. A high frequency of young age breast cancer has been reported in previous studies conducted in this part of the world [15]. Although, lack of availability of widespread molecular tests makes it difficult to identify the genomic profile of young age breast cancer in our population; nevertheless, importance of these findings can’t be overlooked.
The association of ki67 index with prognostic profile of breast cancer has been extensively studied [16, 17]. Despite inconsistency in defining cutoff values and lack of inter-laboratory validity in ki67 results, it has been shown that ki67 index is an independent prognostic factor in breast cancer. Results of a large meta-analysis involving 64,196 patients concluded that; when using > 25% ki67 (as high ki67 index) cutoff, ki67 index is an independent prognostic factor in terms of overall survival in breast cancer patients [18]. Similarly, a meta-analysis analyzed samples from randomized controlled trials and confirmed the independent prognostic value of ki67 [19]. Another meta-analysis included 46 studies and 12,155 patients; they reported that high ki67 was associated with higher risk of relapse in both node negative and node positive disease and worse survival in breast cancer [20]. We didn’t evaluate the survival and recurrence status of patients in our study which was one of the limitations of our study.
Ki67 index in different molecular subtypes of breast cancer has been investigated in various studies. Soliman et al. reported a high ki67 index (> 15%) in 34% & 60% of her2neu and triple negative breast cancer respectively [21]. On the other hand, we found an even high ki67 in these two subtypes of breast cancer; more than 90% of her2neu and triple negative breast cancers had ki67 > 14% in our study.
St. Gallen international expert consensus on primary therapy for early breast cancer 2013, defined surrogate clinicopathologic definitions of intrinsic breast cancer subtypes taken into account percentage of PR positivity (cutoff > 20%) and ki67 index. There was a disagreement on the exact cutoffs for ki67 index. Although a cutoff value of 20% was proposed, especially for the adjuvant use of chemotherapy; however cutoff value of 14% beast correlated with gene expression definition of luminal A breast cancer [10].
Conclusion
Ki67 index is a valuable biomarker of breast cancer as higher ki67 correlates with higher tumor grade. However, no independent prognostic significance of ki67 index could be established in our study due to lack of its association with nodal metastasis or any other prognostic factor in breast cancer.
Limitations
One of the major limitations of our study was that, recurrence status of patients was not evaluated; therefore, we recommend more large-scale studies evaluating prognostic significance of ki67 in terms of tumor recurrence and disease-free survival.
Supplementary information
Acknowledgements
We gratefully acknowledge all staff members of department of Pathology, Liaquat National Hospital, Karachi, Pakistan for their help and cooperation.
Abbreviations
- ER
estrogen receptor
- PR
progesterone receptor
- ASCO
American Society of Clinical Oncology
Authors’ contributions
AAH, KAH and MI: main author of manuscript, have made substantial contributions to conception and design of study. SMK, MME, JPA, SKH, HA, NF and AK: been involved in drafting the manuscript, involved in analysis of the data, revising it critically for important intellectual content and gave final approval and revision of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was provided.
Availability of data and materials
Please contact author for data requests.
Ethics approval and consent to participate
Ethics committee of Liaquat National Hospital, Karachi, Pakistan approved the study. Written informed consent was obtained from the patients for the participation.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Atif Ali Hashmi, Email: doc_atif2005@yahoo.com.
Kashif Ali Hashmi, Email: drkhashmi72@gmail.com.
Muhammad Irfan, Email: irfanzafar892@gmail.com.
Saadia Mehmood Khan, Email: smk.state.alchemist@gmail.com.
Muhammad Muzzammil Edhi, Email: Muhammad_edhi@brown.edu.
Javaria Parwez Ali, Email: javaria_29@hotmail.com.
Shumaila Kanwal Hashmi, Email: huzaifaurwah@gmail.com.
Huda Asif, Email: huda.ha16@gmail.com.
Naveen Faridi, Email: Naveen.faridi@gmail.com.
Amir Khan, Email: dramirkhan04@gmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13104-019-4653-x.
References
- 1.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 2.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 3.de Azambuja E, Cardoso F, de Castro G, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors—a surrogate marker? Cancer. 2003;97:1321. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 5.Alba E, Lluch A, Ribelles N, Anton-Torres A, Sanchez-Rovira P, Albanell J, et al. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. Oncologist. 2016;21(2):150–155. doi: 10.1634/theoncologist.2015-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins LC, Botero ML, Schnitt SJ. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer. Am J Clin Pathol. 2005;123:16–20. doi: 10.1309/HCF035N9WK40ETJ0. [DOI] [PubMed] [Google Scholar]
- 7.McCarty KS, Jr, Miller LS, Cox EB, et al. Estrogen receptor analyses: correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.1043/1543-2165(2007)131[18:asocco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology—College of American Pathologists (ASCO/CAP) Clinical Practice Guideline Update. Arch Pathol Lab Med. 2013 doi: 10.5858/arpa.2013-0953-sa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ. Panel members: personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behranwala KA, Nasiri N, Abdullah N, et al. Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29:386. doi: 10.1053/ejso.2002.1422. [DOI] [PubMed] [Google Scholar]
- 12.Hashmi AA, Edhi MM, Naqvi H, Faridi N, Khurshid A, Khan M. Clinicopathologic features of triple negative breast cancers: an experience from Pakistan. Diagn Pathol. 2014;28(9):43. doi: 10.1186/1746-1596-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson Y, Frisell J, Sylvan M, et al. Breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. J Clin Oncol. 2010;28:2868. doi: 10.1200/JCO.2009.24.5001. [DOI] [PubMed] [Google Scholar]
- 14.Hashmi AA, Faridi N, Khurshid A, Naqvi H, Malik B, Malik FR, Fida Z, Mujtuba S. Accuracy of frozen section analysis of sentinel lymph nodes for the detection of Asian breast cancer micrometastasis–experience from Pakistan. Asian Pac J Cancer Prev. 2013;14(4):2657–2662. doi: 10.7314/APJCP.2013.14.4.2657. [DOI] [PubMed] [Google Scholar]
- 15.Hashmi AA, Edhi MM, Naqvi H, Khurshid A, Faridi N. Molecular subtypes of breast cancer in South Asian population by immunohistochemical profile and Her2neu gene amplification by FISH technique: association with other clinicopathologic parameters. Breast J. 2014;20(6):578–585. doi: 10.1111/tbj.12329. [DOI] [PubMed] [Google Scholar]
- 16.Luporsi E, André F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations forits role inthe clinical management of breastcancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroon S, Hashmi AA, Khurshid A, Kanpurwala MA, Mujtuba S, Malik B, Faridi N. Ki67 index in breast cancer: correlation with other prognostic markers and potential in pakistani patients. Asian Pac J Cancer Prev. 2013;14(7):4353–4358. doi: 10.7314/APJCP.2013.14.7.4353. [DOI] [PubMed] [Google Scholar]
- 18.Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of ki67 in breast cancer: a systematic review and meta-analysis of 64196 patients. Breast Cancer Res Treat. 2015;153:477–491. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 19.Luporsi E, André F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Azambuja E, Cardoso F, de Castro G, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soliman NA, Yussif SM. Ki67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. 2016;66:2095–3941. doi: 10.20892/j.issn.2095-3941.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please contact author for data requests.



